HAL Id: hal-02348026
https://hal.archives-ouvertes.fr/hal-02348026
Submitted on 8 Nov 2019
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Cytochrome P450 dependent metabolism of fluindione
in vitro in rat and human microsomes and in vivo in rat.
Patrick Dansette, Assia Hessani, Virginie Siguret, Catherine Boisson-Vidal,
Jean-Luc Boucher, Blandine Dizier
To cite this version:
Patrick Dansette, Assia Hessani, Virginie Siguret, Catherine Boisson-Vidal, Jean-Luc Boucher,
et al..
Cytochrome P450 dependent metabolism of fluindione in vitro in rat and human
mi-crosomes and in vivo in rat..
MDO-JSSX joint confrerence, Oct 2018, Kanazawa, Japan.
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/328027170
INTRODUCTION : Fluindione (2-(4'fluoro-phenyl Cytochrome P450
dependent metabolism of fluindione in vitro in rat and human microsomes
and in vivo in rat
Poster · October 2018 DOI: 10.13140/RG.2.2.30474.31680 CITATIONS 0 READS 31 13 authors, including:
Some of the authors of this publication are also working on these related projects:
ThrombotrispyView project
Arene oxide formation and enzymatic reactionsView project
Patrick M Dansette
French National Centre for Scientific Research
180PUBLICATIONS 6,290CITATIONS SEE PROFILE Assia Hessani Université Paris-Sud 11 10PUBLICATIONS 46CITATIONS SEE PROFILE Catherine Boisson-vidal
French National Centre for Scientific Research
80PUBLICATIONS 2,047CITATIONS
SEE PROFILE
All content following this page was uploaded by Patrick M Dansette on 02 October 2018.
INTRODUCTION: Fluindione (2-(4’fluoro-phenyl)-phenylindan1,3-dione) is an anti-vitamin K (AVK) marketed in 1967 in France and in Luxembourg (1-3). It is still the leading AVK in France in 2016 (around 800.000 patients, 82% of the market). However very little is known on the metabolism of this drug. In order to better understand the pharmacokinetics of this drug (4,5), we started to study the in vitro and in vivo metabolism of fluindione. The results below show a series of new metabolites both in vitro and in vivo.
METHODS: In vivo : Sprague Dawley rats weighting 200- 400 g were injected IP with fluindione (5 mg/mL solution in sodium bicarbonate) at 5 or 20 mg/kg. Blood was obtained at the tail at selected time and urine was collected.
In Vitro : Rat or human liver microsomes or recombinant Cytochrome P450 (CYP) (Supersomes Gentest) were incubated with fluindione (100 and 200 µM) for 10 to 60 min in presence of NADPH generating system. Reactions were stopped with one volume of CH3CN/AcOH 9:1, centrifuged at 13000 g and
supernatants were analyzed by HPLC/MS on a LCQ Advantage system or an Exactive Orbitrap (Thermo) using negative ESI. The metabolite M2 was obtained from semipreparative incubations, purified by HPLC and analyzed by NMR on a Bruker 500 MHz. RatFluindione05 #591RT:8.34AV:1SB:16 6.69-6.91 , 6.01-6.24NL: T:FTMS {1;1} - p ESI Full ms [200.00-500.00] 100 R e la ti ve A b un d a n ce 245.07138 C913C6H8O2F 239.05132 C15H8O2F 100 R e la tiv e A b u n d a n ce 253.04842 C13H7O3N3 Fluindione13C 6/12C M3 12C M3 13C 6/12C
Cytochrome P450 dependent metabolism of fluindione
in vitro in rat and human microsomes and in vivo in rat.
Patrick M. Dansette
1, Assia Hessani
1, Virginie Siguret
2,3, Catherine Boisson-Vidal
2, Jean-Luc Bouche r
1and
Blandine Dizier
2with the participation of master students : Carole Berndt, Caroline Moreau, Idir Hamdidouche,
Slimane Moktari, Antoine Bienne, Shannon Pecnard and Victoria Rutman.
1
LCBPT, CNRS UMR 8601, Université Paris Descartes, Sorbonne Paris-Cité, PARIS, France
2
INSERM UMR-S-1140 "Innovations thérapeutiques en hémostase" Faculté de Pharmacie, Université Paris
Descartes, Sorbonne Paris-Cité, PARIS, France
3
Service d'hématologie biologique (Pr A. Veyradier) - Hôpital Lariboisière, PARIS, France
Human liver microsomes : Incubation with human liver microsomes showed that
metabolite M2 was formed in presence of NADPH. Control without NADPH were blank. Its formation was inhibited by benzyl imidazole (50 µM) implicating cytochrome P450 catalysis. An impurity, 2-hydroxy-fluindione slowly increased on storage of incubations. It’s a known oxidation product of fluindione by air.
Recombinant human P450: The formation of M2 was studied using insect cell
microsomes expressing recombinant CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4 and 3A5. CYP1A2, 2B6, 2C9, 2D6, 3A4 and 3A5 could make this metabolite. CYP2C9 and CYP1A2 had best affinities (about 30 µM) as shown in Table1.
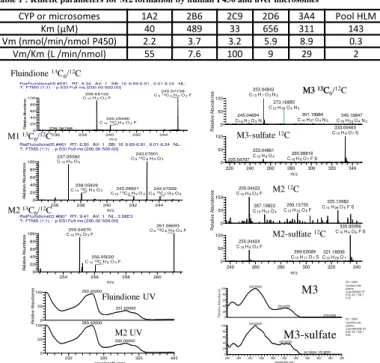
Table 1 : Kinetic parameters for M2 formation by human P450 and liver microsomes
CYP or microsomes 1A2 2B6 2C9 2D6 3A4 Pool HLM
Km (µM) 40 489 33 656 311 143
Vm (nmol/min/nmol P450) 2.2 3.7 3.2 5.9 8.9 0.3
Vm/Km (L /min/nmol) 55 7.6 100 9 29 2
Bruker 500 MHz.
Synthesis:13C
6labeled fluindione was synthetized from13C6labeled phthalic
anhydride (6). 3-(4-Fluoro-phenyl)-4-hydroxy-coumarin (7), 3-(4-Fluorophenyl)-4-hydroxy-isocoumarin (8), (4-hydroxyphenyl)-indane-1,3-dione (9) and 2-hydroxy-fluindione (10) were synthetised by modification of known methods.
RESULTS :
In vivo : Rats were treated by 5 and 20 mg/kg fluindione13C
6/12C 1/1 mixture
Plasma : Analysis of plasma showed doubly labeled compounds with a
doublet in the mass spectrum : fluindione F (m/z = 239/245, C15H9O2-) and
metabolites: M1 (m/z = 237.0558/243.0760) corresponding to defluorination and hydroxylation, and identified by comparison with an authentic to 2-(4-hydroxyphenyl)-indane-1,3-dione and M2 (m/z = 255.0467/ 261.0668) corresponding to C15H8O3F thus addition of an oxygen to fluindione. M2 was less polar than fluindione and had a UV spectrum nearly superimposable with that of fluindione (see below). A minor metabolite M3 also less (m/z = 253.0499/259.0700) corresponding to C15H9O4 thus fluindione defluorinated and dihydroxylated .
Urine : Analysis of urine showed the same metabolites in low amount plus
series of more polar metabolites. They corresponded to sulfate esters: S2 was relatively abondant ( m/z = 335.0006, C15H8O6FS ) its MS and MS2 showed fragmentation at m/z = 255.0446 with loss of SO3. S3 had a m/z = 333.0046 in
MS and its MS2 spectrum showed fragmentation at m/z = 253.0499 with loss of SO3. Thus we believe that S2 and S3 are the sufate esters of M2 and M3.
Plasma and Human Urine: A poly-medicated volunteer took orally 10 mg of fluindione. Fluindione could be detected in plasma at 1, 2 and 6 hours. However the metabolites could only be found in very low amount (limit of detection).
Figure 2: Selected mass and UVspectra of fluindione and its metabolites
Synthesis of some potential metabolites and13C
6labeled fluindione:
In order to characterize M2, the chemical synthesis of several candidate molecules was performed: 2-hydroxy-fluindione, 2-(4’-fluorophenyl)-4-hydroxy-coumarin, 2-(4’-fluorophen yl)-4-hydroxy-isocoumarin, 4’-hydroxy-phenindione . Metabolite M2 did not correspond to any of the synthetized products. However M1 was identified as 4’-hydroxy-phenindione .
RatFluindione03 #667RT:9.41AV:1NL:3.08E3
T:FTMS {1;1} - p ESI Full ms [200.00-500.00] 254 256 258 260 m/z 0 20 40 60 80 100 R e la ti v e A b u n d a n c e 261.06693 C913C6H8O3F 255.04675 C15H8O3F 256.05020 C1413C H8O3F RatFluindione05 #461RT:6.50AV:1SB:16 6.69-6.91 , 6.01-6.24NL: T:FTMS {1;1} - p ESI Full ms [200.00-500.00] 236 238 240 242 244 m/z 0 20 40 60 80 100 R e la ti v e A b u n d a n c e 243.07601 C913C6H9O3 237.05582 C15H9O3 238.05929 C1413C H9O3 242.06821 C1013C5H9O3 244.07939 C813C7H9O3 236 238 240 242 244 m/z 0 20 40 60 80 100 R e la ti ve A b un d a n ce C15H8O2F 240.05480 C1413C H8O2F 236.36768 220 240 260 280 300 320 340 m/z 0 50 100 0 50 100 R e la tiv e A b u n d a n ce 301.19984 C14H27O4N3 273.16885 C12H23O4N3 345.16847 C18H23O4N3 245.04694 C16H7O2N 333.00465 C15H9O7S 253.04861 C15H9O4 225.54707 290.99816 C10H8O7F S 240 260 280 300 320 340 m/z 0 50 100 0 50 100 R e la tiv e A b u n d a n ce 255.04423 C15H8O3F 325.13982 C14H26O5F S 293.13755 C13H22O6F 267.15822 C15H23O4 335.00056 C15H8O6F S 255.04424 C15H8O3F 299.02089 C12H11O7S 321.18936 C15H29O7 M1 13C 6/12C M2 13C 6/12C M3-sulfate 12C M2 12C M2-sulfate 12C 200 220 240 260 280 300 320 340 360 380 400 wavelength (nm) 0 20 40 60 80 100 0 20 40 60 80 100 R e la tiv e A b so rb an ce 249.00000 299.00000 379.00000 249.00000 302.00000 370.00000 347.00000 NL: 1.03E5 fluindione-rats- plasma-urine14#7828 RT: 6.52 AV: 1 SB: 1 6.42 NL: 1.80E4 fluindione-rats- plasma-urine14#10398 RT: 8.66 AV: 1 SB: 1 8.54 250 300 350 400 wavelength (nm) 0 50 100 0 50 100 R e la ti ve A b s o rb a n c e 283.00000 331.00000 283.00000 330.00000 Fluindione UV M2 UV
M3-sulfate
M3
Studies in Vitro:Rat liver microsomes: Incubation of rat liver microsomes with 100 µM
fluindione showed formation of a major metabolite identical to M2 with a m/z = 255.0446 and a UV spectrum identical to that of fluindione. A semi-preparative incubation using 60 mL incubation medium allowed to isolate by HPLC about 0.3 mg of metabolite. Its proton NMR spectrum showed a disymetrical molecule with all the ring A proton different. The fluorophenyl ring was not modified. We could not abtain a 13C spectrum for further identification. M2 was compared to synthetic 3-(4-fluoro-phenyl)-4-hydroxy-coumarin, 3-(4-fluoro-phenyl)-4-hydroxy-isocoumarin, 2-(4-hydroxy-phenyl)-indane-1,3-dione and 2-hydroxy-fluindione which were all different. Thus until now we don’t have the structure of M2.
Figure 1 :Proton NMR spectra of fluindione and metabolite M2
References: 1) Molho D. and Boschetti E. (1963), French Patent 1.369.396 2) Geiger K et al.US patent (1963), US patent 3,090,813. 3) Shapiro L. et al. (1961) J Org Chem 26, 3580-81. 4) Comets E, et al. (2012) Clin Pharmacol Ther. 91(5):777-86 and references therein. 5) Moreau C, et al. (2014) Thromb Haemost. 111(4):705-12. 6) Oskaja, V.and Vanags, G. (1961) Lat PSR Zin Akad Vestis3, 67-76. 7) Zhou ZZ et al. (2013) Chem Pharm Bull (Tokyo) 61(11), pp 1166-1172. 8) Faizi DJ, et al. (2016) J Am Chem Soc. 138(7):2126-9. 9) Marinov M. et al. (2014) J. Structural Chem. 55, 3, 446-455.
.
CONCLUSION:
- Fluindione is metabolized in rat and human microsome to oxidized metabolites corresponding to either an oxidation of the B ring of the indanedione (M2, m/z=255), or oxidation of the fluorophenyl with defluorination (M1, m/z =237) and both reactions (M3, m/z = 253).
- These oxidations are P450 dependent and human recombinant CYP2C9 and CYP1A2 have good affinities for formation of M2 also formed by CYP3A4. In vivo in rat, the primary metabolites are found in plasma, but in urine one find the sulfate esters of M2 and M3.
- A tentative experiment in a human volunteer showed only fluindione and M2 in plasma and only traces of fluindione in urine.
- The metabolism of fluindione is mediated by several cytochrome P450 which explain why CYP2C9 is not a genetic determinant for its posology.
- Finally the structure of M2 and M3 remain to be characterized.
Fluindione Metabolite M2
View publication stats View publication stats