Publisher’s version / Version de l'éditeur:
Journal of Membrane Science, 188, 2001
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Thyermostable ultrafiltration and nanofiltration membranes from
sulfonated poly(phthalazinone ether sulfone ketone)
Dai, Y.; Jian, X.; Zhang, S.; Guiver, Michael
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=17d15d9e-e74c-4ef4-9257-479b18b2520f https://publications-cnrc.canada.ca/fra/voir/objet/?id=17d15d9e-e74c-4ef4-9257-479b18b2520f
Thermostable ultrafiltration and nanofiltration membranes from
sulfonated poly(phthalazinone ether sulfone ketone)
夽
Ying Dai
a,∗, Xigao Jian
b, Shouhai Zhang
b, Michael D. Guiver
a aNational Research Council of Canada, Institute for Chemical Process and Environmental Technology,Ottawa, Ont., Canada K1A 0R6
bCollege of Chemical Engineering, Dalian University of Technology, Dalian 116012, PR China
Received 30 November 2000; received in revised form 6 February 2001; accepted 7 February 2001
Abstract
Modification of poly(phthalazinone ether sulfone ketone) (PPESK) by sulfonation with concentrated or fuming sulfuric acid was carried out in order to prepare thermally stable polymers as membrane materials having increased hydrophilicity and potentially improved fouling-resistance. The sulfonated poly(phthalazinone ether sulfone ketone)s (SPPESK) were fabricated into ultrafiltration (UF) and nanofiltration (NF) asymmetric membranes. The effects of SPPESK concentration and the type and concentration of additives in the casting solution on membrane permeation flux and rejection were evaluated by using an orthogonal array experimental design in the separation of polyethyleneglycol (PEG12000 and PEG2000) and Clayton Yellow (CY, MW 695). One UF membrane formulation type had a 98% rejection rate for PEG12000 and a high pure water flux of 867 kg m−2h−1. All the NF membranes made in the present study had rejections of ≥96%, and one had a high water flux of
160 kg m−2h−1. Several of the NF membrane formulation types had ∼90% rejection for CY. When the membranes were
oper-ated at higher temperatures (80◦C), the rejection rates declined slightly and pure water flux was increased more than two-fold.
Rejection and flux values returned to previous values when the membranes were operated at room temperature again. Mono-and divalent salt rejections Mono-and fluxes were studied on an additional NF membrane set. Published by Elsevier Science B.V.
Keywords:Membrane preparation and structure; Microporous and porous membranes; Sulfonated membrane; Ultrafiltration; Nanofiltration
1. Introduction
Currently, there is a significant interest in nanofil-tration (NF) separations which have applications as follows: (1) removal of organic compounds (100–1000 Da) from water; (2) separation of organic compounds having different MW; (3) separation be-tween divalent salts and monovalent salts; (4) sepa-rations between salts and their corresponding acids. NF separations occupy an area that lies between
夽NRCC Publication 44362.
∗Corresponding author. Fax: +1-613-991-2384.
E-mail address:ying.dai@nrc.ca (Y. Dai).
reverse osmosis (RO) and ultrafiltration (UF) and one that has become an active research area in membrane separation [1].
Polypiperazineamide, sulfonated polysulfone, polyamide, polysulfone/polyamide and cellulose ac-etate are among the materials currently used in commercial NF membrane [2]. The development of new membrane materials requires not only an un-derstanding of membrane transport phenomena, but also a knowledge of polymer chemistry, morphology, mechanical and thermal properties, and polymer in-teraction in the solute–solvent–membrane system [3]. A series of poly(phthalazinone ether sulfone ke-tone) (PPESK) copolymers containing different ratios
0376-7388/01/$ – see front matter Published by Elsevier Science B.V. PII: S 0 3 7 6 - 7 3 8 8 ( 0 1 ) 0 0 3 7 9 - 9
196 Y. Dai et al. / Journal of Membrane Science 188 (2001) 195–203
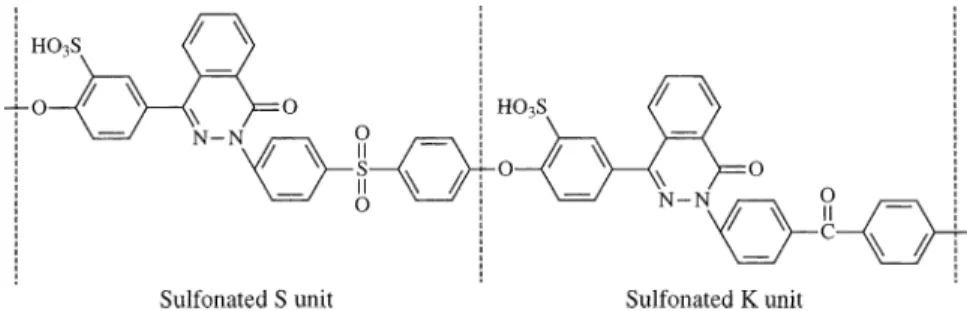
Fig. 1. Chemical structure representing a S/K 1:1 SPPESK repeat unit.
of sulfone and ketone units (S/K) were previously syn-thesized [4–6]. These polymers, whose glass transition temperatures are in the range of 263–305◦C and which
have small linear coefficients of expansion, show ex-cellent comprehensive properties and outstanding ther-mostability. In the previous investigations, we reported that dense and asymmetric membranes made from PPESK have shown good separation and permeation properties for gas and liquid separation [6,7].
Sulfonation is commonly applied to modify poly-mers in order to increase their hydrophilic and ionic character [8–11]. For example, sulfonated polysul-fones and polyetherketones have been reported to be useful in desalination, ion exchange and fuel cell membrane applications [1,12–19]. Based on our previ-ous synthetic and characterization studies of PPESK, SPPESKs were synthesized to improve the polymers’ hydrophilicity and thereby utilize this type of polymer as membranes for their applications in water treat-ment [20]. The molecular structure representing a S/K 1:1 SPPESK structural repeat unit is shown in Fig. 1. In the present paper, UF and NF asymmetric mem-branes have been prepared from SPPESK (S/K 3:1) having a 24% degree of sulfonation (DS). The effect of different DS of SPPESK, SPPESK casting solution concentration, the type and concentration of additives in the casting solution, and temperature for operation on membrane permeation flux and solution rejection were evaluated.
2. Experimental
2.1. Materials and methods
PPESK was synthesized having a S/K ratio of 3:1 and then sulfonated to different DS according to a
similar synthetic procedure reported previously [20]. Chloroform, N-methyl-2-pyrrolidinone (NMP), bu-tanone (BO), ethylene glycol dimethyl ether (EGME), diethyl ether (EE), tetrahydrofuran (THF) and other chemicals were of analytical grade and used without further purification. PEG with MW of 12000 and 2000, Clayton Yellow (CY, a sulfonated dye of MW 695 also known as Thiazol Yellow G), Na2SO4 and
NaCl were used as challenge solutes for the mem-brane characterization.
Contact angles of asymmetric membranes were determined by JY-82 Contact Angles Equipment (Chengde Experimental Equipment China) for 1 min in air. The concentration of PEG and CY were mea-sured using a Spectrophotometer-751 (Shanghai In-strument). Salt concentrations were determined by a DDS-11A Electrical Conductivity Instrument (Shang-hai Leichi Instrument China). The membrane feed solution side was stirred magnetically to reduce concentration polarization. A flat-sheet dead-end membrane module (Ecological Environment Center of Chinese Academy of Science) having an effec-tive separation area of 41 cm2 and a feed volume of 550 ml was used in all membrane flux charac-terization and separation experiments as shown in Fig. 2.
2.2. Membrane preparation
Several sheets of asymmetric membranes were cast from each of the SPPESK solutions in NMP onto glass at a temperature of 18◦
C and a rela-tive humidity of 35%. Based on previous related work [7], we chose an evaporation time of 15 s. in air. The cast solutions were precipitated by im-mersion into water at 6◦C for 36 h. This extended
Fig. 2. Experimental membrane test cell.
immersion time was to allow adequate time for the solvent to be replaced by non-solvent water. The resulting membrane sheets were rinsed after immer-sion and had a thickness of approximately 200 m each. Selections of membranes were made from SPPESK solution concentrations of 10, 12, 14, 16, 18 wt.%. The types of additives used in casting solu-tion were butanone, ethylene glycol dimethyl ether, and tetrahydrofuran at concentrations of 12, 15, 18 wt.%.
2.3. Membrane characterization
The membranes were characterized in the module after pretreatment with pure water (distilled) under 0.2 MPa pressure for 30 min. The pure water flux and the rejection of PEG and CY 100 ppm solutions were measured under a pressure difference of 0.1 MPa at a temperature of 20◦C. In some experiments, the
solu-tion fluxes were measured in addisolu-tion to the pure wa-ter fluxes. Fluxes were dewa-termined by measuring the time taken to collect 10 ml of permeate, following the passage of an initial 30 ml permeate volume. Solutes Na2SO4 and NaCl were measured at 1000 ppm
con-centration and a pressure difference of 0.25 MPa. A minimum of three membrane disks cut from the cast sheets was used to generate results for each test. The water fluxes and solution rejections given are average values.
The permeation flux (F) is calculated as F = W/At, where W is the total weight of the water or solution per-meated during the experiment; A represents the mem-brane area; t denotes the operation time. Rejection (R) is calculated as R = 1 − Cp/Cf, where Cp and Cf
are the concentrations of the permeate and the feed, respectively.
3. Results and discussions
3.1. Influence of the degree of sulfonation on UF membrane properties
A series SPPESK polymers with different DS was used to prepare a set of asymmetric membranes. Ul-trafiltration membranes were cast from NMP solutions containing 14 wt.% SPPESK with 17 wt.% EE addi-tive.
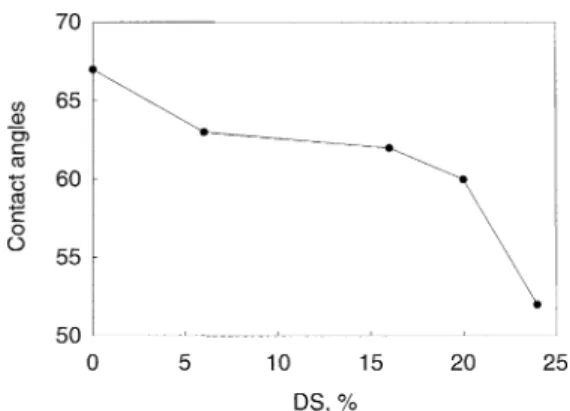
The contact angles between these SPPESK asym-metric membranes and water were determined for each DS and are listed in Fig. 3. As expected, the contact an-gles decreased with the introduction of –SO3H group
and with increasing degree of sulfonation, giving an indication of increased membrane hydrophilicity. The most significant reduction in contact angle occurred when DS > 20%.
The pure water flux and PEG12000 rejection of the above membranes were measured at 20◦C and at a
driving pressure of 0.1 MPa. The results are shown in Fig. 4. In the DS range up to approximately 14%, both the flux and solute rejection increased steadily, with the rejection reaching over 97%. At DS > 14%, a sharp increase in flux value was observed, with the rejection leveling off at 99%.
As a comparison between unsulfonated and sul-fonated polymers and their membrane properties, asymmetric membranes were prepared from PPESK and SPPESK (S/K 3:1, DS 24%). The casting formu-lation used in both membranes was 14 wt.% polymer in NMP and 12 wt.% EE additive. The flux and solute
Fig. 3. Contact angles of SPPESK UF membranes with different DS.
198 Y. Dai et al. / Journal of Membrane Science 188 (2001) 195–203
Fig. 4. The Influence of different DS on the pure water flux and PEG12000 rejection for UF membranes.
rejection data measured under the same conditions of 0.1 MPa and 20◦C for PEG12000 and PEG2000
solutes are shown in Table 1. The data shows that the sulfonated membrane SPPESK had higher pure water fluxes (70% increase) as well as higher rejec-tions than the unsulfonated PPESK membrane for both solutes. Under the casting conditions chosen, only the SPPESK membrane was capable of giving a high PEG2000 solute rejection whereas the solute rejection for the PPESK membrane was 45%.
3.2. The influence of casting solution composition on UF membrane properties
From a previous investigation, we correlated Tg,
contact angle and solubility data for SPPESKs (S/K 1:1) with different DS as shown in Table 2 [20]. The SPPESKs with DS 24 and 34% both had similarly good solubility characteristics, higher Tgs and reduced
contact angles compared with the PPESK. However, the DS 24% SPPESK had better overall processing characteristics and better solubility behavior of casting solutions where additives were used. For the higher DS 85% SPPESK, it was felt that the membranes would
Table 1
Comparison of membrane properties for PPESK and SPPESK Membrane property PPESK SPPESK (DS 24%)
R(%) for PEG12000 95 97
R(%) for PEG2000 45 99
F(pure water) (kg m−2h−1) 86 147
Table 2
Characteristics of SPPESK
DS (%) Tg (◦C) Contact angle Solvent
0 278 74 CHCl3 24 287 68 CHCl3 34 289 58 CHCl3 85 292 46 DMF 150 321 39 50% aqueous C2H5OH 200 323 – H2O
be susceptible to swelling. At even higher DS, the SPPESKs are unsuitable as membrane materials due to their solubility in water. Based on these previous S/K 1:1 SPPESK, we selected SPPESK (S/K 3:1, DS 24%) for the present membrane study.
A series of SPPESK asymmetric membranes were prepared for characterization according to an orthogo-nal design method, which is a method used to solve for multiple factors. Under fixed conditions for membrane preparation, the composition of the casting solution is a major factor that affects the membrane performance and the integrity of membrane. We designed our exper-iments by a three-factor three-level orthogonal design table. The factors are the polymer solution concentra-tion, type of additive, and concentration of additive. Each factor was varied by three levels to investigate its effect on membrane performance. The membrane set was characterized at 20◦
C under 0.1 MPa using 100 ppm PEG12000 solution as the solute. Table 3 shows the results for the three levels of each of the three factors, where R1and F1 are the average
rejec-tion rate and average flux of pure water for level 1 of all the factors, respectively. The polarity difference reflects the influence of the factors on the results.
From the membrane casting formulation vari-ables in Table 3, the rejection range for PEG12000 was 72–98% and the pure water flux range was 480–870 kg m−2h−1. In comparison with previously reported UF membrane data of PPESK [7], which had rejection ranges of 20–99% and pure water fluxes in the range of 77–500 kg m−2h−1, SPPESK UF mem-branes had generally higher PEG12000 rejection and significantly higher pure water fluxes.
3.2.1. The influence of SPPESK casting solution concentration
As the polymer casting solution concentration was increased from 10 to 14%, the rejections R1–R3 for
Table 3
Three-factor three-level orthogonal design table for UF SPPESK membranes
Membrane number Factors Results
SPPESK concentration Additive types Additive conc. R(%) (PEG12000)
F(pure water) (kg m−2h−1) Level % Level Name Level %
UF1 1 10 1 EGME 1 12 93 692 UF2 1 10 2 BO 2 15 72 804 UF3 1 10 3 EE 3 18 93 710 UF4 2 12 2 BO 1 12 77 788 UF5 2 12 3 EE 2 15 98 593 UF6 2 12 1 EGME 3 18 88 671 UF7 3 14 3 EE 1 12 86 482 UF8 3 14 1 EGME 2 15 98 867 UF9 3 14 2 BO 3 18 97 509
Average rejection rate for all factors
R1 86 93 85
R2 91 82 91
R3 94 93 93
Max R difference 8 11 8
Average flux rate for all factors
F1 732 743 654
F2 684 700 755
F3 619 595 630
Max F difference 113 148 125
PEG12000 increased from 86% to above 94% for the third level, while the water fluxes F1–F3decreased. It
can be expected that when the concentration of casting solution increases, the density of the polymer network increases, resulting in a tighter skin layer and a larger resistance to water flow.
3.2.2. The influence of different additives
The experimental results in Table 3 indicate that the type of additive is the most important factor influencing solute rejection and pure water flux be-cause it generates the largest maximum difference for the three factors tested. Of the three additives selected, EGME had the best combination of rejec-tion and water flux, having the highest rejecrejec-tion of 93% as well as the highest flux of 743 kg m−2h−1.
EE gave a similarly high rejection for PEG12000 but had the lowest water flux. BO gave the low-est rejection of 82% but a reasonably good water flux.
The mode of action of the additive on the casting is complicated, but two likely factors to be considered
are the additive volatility and difference in solubility coefficients between polymer, solvent and additive. A volatile additive such as EE (bp 34.5◦C) evaporates
easily in air during the pre-gelation evaporation pe-riod. However, different results of the additive order were obtained in the case of unsulfonated PPESK membranes [7], most likely due to the difference in solubility coefficients between the polymer and the additives.
3.2.3. The influence of the additive concentration
Table 3 reveals that within the experiment range, the higher additive content results in higher rejection rate (R1–R3) and higher water flux (F1–F2). The
mem-brane dense-layer structure could be loosened by in-creasing the additive concentration, because the num-ber of pores increases without an obvious change in the pore size. However, the flux decreased significantly for 18% additive concentration in level 3, so under the above conditions of preparation, the best performance was obtained when the additive concentration was not greater than 15%.
200 Y. Dai et al. / Journal of Membrane Science 188 (2001) 195–203
3.3. SPPESK NF membrane
Another group of SPPESK (S/K 3:1, DS 24%) NF asymmetric membranes were prepared according to the three-factor three-level orthogonal design table, utilizing data from the previous UF membrane results. The factors used for the NF membrane set were also the same as for the UF membrane set, i.e. polymer casting concentration, type of additive, and concen-tration of additive. In this case, the SPPESK polymer concentration was changed to a higher range in or-der to achieve denser skin layers necessary for NF membrane formation. Three different types of addi-tive were used: EE, THF/EE (1:2 vol.%), and THF, and the concentration range of the additives was the same as for the UF membrane set. The NF membrane set was characterized at 20◦C under 0.1 MPa pressure
for pure water flux and PEG2000 (100 ppm) and CY (100 ppm) solutes.
Table 4 shows the results for the three levels of each of the three factors. Only the rejections of CY were used to calculate the differences in R1–R3in the
Table 4
Three-factor three-level orthogonal design table for NF SPPESK membranes
Membrane number Factors Results
SPPESK concentration Additive types Additive concentration R(%) (PEG2000)
R(%) (CY)
F(pure water) (kg m−2h−1)
Level % Level Name Level %
NF1 1 14 1 EE 1 12 97 67 147 NF2 1 14 2 THF/EE 2 15 97 65 169 NF3 1 14 3 THF 3 18 96 80 160 NF4 2 16 2 THF/EE 1 12 98 82 68 NF5 2 16 3 THF 2 15 96 87 20 NF6 2 16 1 EE 3 18 99 90 30 NF7 3 18 3 THF 1 12 99 88 16 NF8 3 18 1 EE 2 15 96 89 48 NF9 3 18 2 THF/EE 3 18 99 91 45
Average rejection rate for all factors
R1(CY) 70 85 78
R2(CY) 86 81 80
R3(CY) 89 79 87
Max R difference 19 6 9
Average flux rate for all factors
F1 159 84 77
F2 46 70 84
F3 36 94 84
Max F difference 123 24 7
results, since those of PEG2000 for the different levels are too close to analyze.
3.3.1. The influence of casting solution concentration
With increasing polymer casting solution concen-tration from 14 to 18%, the rejections for PEG2000 were maintained above 96%, but the rejections R1–R3
for CY increased from 70 to 89%. The water fluxes
F1–F3 correspondingly decreased significantly from
159 to 36 kg m−2h−1. The pure water fluxes were much lower in the second and third level than in the first level. For the polymer concentration range se-lected for the fabrication of NF membranes, the con-centration factor resulted in the largest maximum R and F differences, suggesting that it is the factor hav-ing the greatest effect.
3.3.2. The influence of the additive type
For the preparation of the NF membrane set, THF, EE and THF/EE (1:2) were used as additives. As shown in Table 4, the rejection for CY decreased in order of EE > THF/EE > THF and the pure water
Table 5
The effect on membrane properties of thermal treatment at 100◦C
Membrane number Before thermal treatment After thermal treatment
R(CY) (%) F(solution) (kg m−2h−1) R(CY) (%) F(solution) (kg m−2h−1)
NF4 84 56 85 57
NF9 84 11 88 12
flux decreased in order of THF > EE > THF/EE. The highest water flux and the lowest rejection for CY was obtained for membranes prepared with THF as the ad-ditive. The more volatile EE resulted in membranes having the highest rejection and reasonably good wa-ter flux. In the NF membrane set studied, the additive mixture THF/EE did not give good results.
3.3.3. The influence of the of additive concentration
Table 4 reveals that within the experiment range of 12–18% concentrations, a higher additive content is beneficial in increasing the NF membrane perfor-mance. For levels 1–3, R1–R3for CY increased from
78 to 87%, but at the same time the fluxes F1–F3also
increased from 77 to 84%. This result is somewhat different from the UF membrane set summarized in Table 3, where the solute rejection increased and the flux F3was lower than F1. Under the range of
mem-brane formulations studied, the best performance was for a 18 wt.% additive concentration.
3.4. The effect of membrane operation temperature
The thermal stabilities of two membrane types of composition NF4 and NF9 were tested by exposure of the membranes to boiling water for a period of 15 min. The CY solute rejections and the solution fluxes (not pure water fluxes) were first measured at 20◦C before
Table 6
The effect of membrane operation temperature Operating temperature (◦C) NF3 NF7 NF7 (repeat test) R(CY) (%) F(solution) (kg m−2h−1) R(CY) (%) F(solution) (kg m−2h−1) R(CY) (%) F(solution) (kg m−2h−1) 20 84 72 89 8 90 7 40 77 104 83 11 86 10 60 70 141 81 13 78 14 80 69 182 74 17 72 18
temperature treatment, then measured again at 20◦C
following treatment so as to determine whether the membrane performance was restored to original val-ues. The results shown in Table 5 indicate that for both NF4 and NF9 compositions there was no observed loss in CY solute rejection and solution flux following treatment at 100◦C.
The effect of different operational temperatures on CY rejections and the solution fluxes was measured for four different temperatures. For this test, two mem-brane types having NF3 and NF7 composition were selected for testing at operation temperatures in the range of 20–80◦C. The results in Table 6 show a trend
that rejection for CY decreased but the solution flux in-creased more than two-fold within the operation tem-perature range increase. One membrane type NF7 was subjected to a repeat test cycle to determine the repro-ducibility of the data for each operational temperature. The data for the second cycle was closely similar to the first cycle, indicating that the membrane operates predictably under each temperature range.
3.5. The properties NF asymmetric membranes for separation salt
Another set of three NF asymmetric membranes were prepared having different formulations in order to test for mono- and divalent salt rejection. The polymer
202 Y. Dai et al. / Journal of Membrane Science 188 (2001) 195–203
Table 7
The performance of NF asymmetric membrane for separation salt
Membrane number SPPESK concentration (%) Additive types 12% R(%) F(pure water) (kg m−2h−1)
Na2SO4 NaCl
NF10 22 EE 42 13 5
NF11 22 THF/EE 41 13 10
NF12 29 THF/EE 68 27 2
casting concentration of the solution was increased to 22 and 29% and the additive concentrations for EE and THF/EE were fixed at 12%, with the expectation of making tighter membranes capable of salt rejection. Three NF membrane types (NF10, NF11, and NF12) were prepared and their salt rejection (1000 ppm) and pure water fluxes measured under 0.25 MPa are listed in Table 7.
The membranes showed an expected Donnan effect arising from the interaction of ions with the charged membrane, since the rejection for divalent salts was greater than monovalent salts. The membrane set M1–M3 were not optimized for best performance but were prepared to demonstrate the utility of low DS SPPESK membranes for NF as well as UF applica-tions. This work was also performed as a basis for higher performance thin film composite membranes using high DS SPPESK.
4. Conclusions
UF and NF asymmetric membranes have been pre-pared successfully from SPPESK (S/K 3:1) having a DS of 24%. The effects of SPPESK casting concen-tration and the type and concenconcen-tration of additives in the casting solution on membrane permeation flux and solute rejection were evaluated using a three-factor three-level orthogonal design. Three solutes were used for the evaluation: PEG12000, PEG2000 and Clayton Yellow dye (MW 695). For SPPESK UF membranes operating at 0.1 MPa pressure and room temperature, one membrane formulation type UF8 had a 98% re-jection rate for PEG12000 and a pure water flux of 867 kg m−2h−1. All the NF membranes made in the
present study and operating under the same conditions had rejections of ≥96%, and NF3 had one of the best water fluxes of 160 kg m−2h−1. NF membrane
formu-lation types NF6–NF9 had ∼90% rejection for CY.
When the membranes were operated at higher tem-peratures (80◦C), the rejection rates declined slightly
and pure water flux was increased more than two-fold. Rejection and flux values returned to previous values when the membranes were operated at lower temper-atures again. Mono- and divalent salt rejections were studied on an additional NF membrane set NF10 to NF12. For one membrane NF12, the Na2SO4 and
NaCl rejection rates were 68 and 27%, respectively, while the solution flux was 2 kg m−2h−1under an op-eration pressure as low as 0.25 MPa at room tempera-ture.
Acknowledgements
Part of this work was supported by the National Science Foundation of China (NSFC).
References
[1] R.J. Petersen, Composite reverse osmosis and nanofiltration membranes, J. Membr. Sci. 83 (1993) 81.
[2] W.R. Bowen, A.W. Mohammad, Characterization and prediction of nanofiltration membrane performance — a general assessment, Trans. IchemE.: Part A 76 (1998) 885. [3] D.R. Lloyd, L.E. Gerlowski, C.D. Sunderland, Poly(aryl ether)
membranes for reverse osmosis, J. Membr. Sci. 153 (1981) 327.
[4] X.G. Jian, Y.Z. Meng, H.B. Zheng, Preparation of poly(phthalazinone ether sulfone), Chinese Patent 93109180.2 (1993).
[5] X.G. Jian, Y.Z. Meng, H.B. Zheng, Preparation of poly(phthalazinone ether ketone), Chinese Patent 93109179.9 (1993).
[6] X.G. Jian, Y. Dai, L. Zheng, R.X. Xu, Application of poly(phthalazinone ether sulfone ketone)s to gas membrane separation, J. Appl. Polym. Sci. 71 (1999) 2385.
[7] X.G. Jian, Y. Dai, G.H. He, Preparation of UF and NF poly(phthalazinone ether sulfone ketone) membranes for high temperature application, J. Membr. Sci. 161 (1999) 185.
[8] A. Bunn, J.B. Rose, Sulphonation of poly(phenylene ether sulphone)s containing hydroquinone residues, Polymer 34 (1993) 1114.
[9] M. Ueda, H. Toyota, T. Ouchi, J.I. Sugiyama, K. Yonetake, T. Masuko, T. Teramoyo, Synthesis and characterization of aromatic poly(ether sulfone)s containing pendant sodium sulfonate groups, J. Polym. Sci. Part A: Polym. Chem. 31 (1993) 853.
[10] H.S. Chao, N.Y. Watervliet, D.S. Kelsey, N.J. Hillsborough, Process for preparing sulfonated poly(aryl ether) resins, US Patent 4,625,000 (1986).
[11] A. Noshay, L.M. Robeson, Sulfonated polysulfone, J. Appl. Polym. Sci. 20 (1976) 1885.
[12] N. Sivashinsky, G.B. Tanny, Ionic heterogeneities in sulfo-nated polysulfone films, J. Appl. Polym. Sci. 28 (1983) 3235. [13] R. Nolte, K. Ledjeff, M. Bauer, R. Mülhaupt, Partially sulfonated poly(arylene ether sulfone) — a versatile proton conducting membrane material for modern energy conversion technologies, J. Membr. Sci. 83 (1993) 211.
[14] D.R. Lloyd, L.E. Gerlowski, C.D. Sunderland, J.P. Wightman, J.E. McGrath, M. Igbal, Y. Kang, Synthetic membranes, in: A.F. Turbak (Ed.), ACS Symposium Series 153, American Chemical Society, Washington, DC, 1981, Vol. 1, Desalination, Chapter 22, pp. 327–350.
[15] S.M.J. Zaidi, S.D. Mikhailenko, G.P. Robertson, M.D. Guiver, S. Kaliaguine, Proton conducting composite membranes from polyether ether ketone and heteropolyacids for fuel cell applications, J. Membr. Sci. 173 (2000) 17.
[16] Y. Matsumoto, M. Sudoh, Y. Suzuki, Separation of bonito extract by composite UF membranes of sulfonated polysulfone coated on ceramics, J. Membr. Sci. 157 (1999) 139.
[17] S. Koter, P. Piotrowski, J. Kerres, Comparative investi-gations of ion-exchange membranes, J. Membr. Sci. 153 (1999) 83.
[18] J. Kerres, W. Cui, R. Disson, W. Nenbrand, Development and characterization of crosslinked ionomer membranes based upon sulfinated and sulfonated PSU crosslinked PSU blend membranes by disproportionation of sulfinic acid groups, J. Membr. Sci. 139 (1998) 211.
[19] D. Möckel, E. Staude, M.D. Guiver, Static protein adsorp-tion, ultrafiltration behavior and cleanability of hydro-philized polysulfone membranes, J. Membr. Sci. 158 (1999) 63.
[20] Y. Dai, X.G. Jian, X.M. Liu, M.D. Guiver, Synthesis and characterization of sulfonated poly(phthalazinone ether sulfone ketone) for ultrafiltration and nanofiltration membranes, J. Appl. Polym. Sci. 79 (2001) 1685.