DOI: 10.5897/SRE2017.6544 Article Number: 51A363355338 ISSN 1992-2248
Copyright©2018
Author(s) retain the copyright of this article http://www.academicjournals.org/SRE
Scientific Research and Essays
Full Length Research Paper
Histological changes in the larvae of the domestic
mosquito Culex pipiens treated with the
entomopathogenic fungus Beauveria bassiana
BENZINA Farida
1*, HAMID Sonia
1,2, MOHAND-KACI Hakima
1, BISSAAD Fatma
1and
HALOUANE Fatma
11
Laboratory of Valuation and Conservation of Biological Resources, Department of Biology, Faculty of Science, University of M’hamed Bougara, Boumerdes, Algeria.
2
Department of Biology, Faculty of Science, University of Akli Mohand Oulhadj, Bouira, Algeria.
Received 17 October, 2017; Accepted 6 December, 2017
The Culicidae are biting insects that are most harmful to people. They are almost all blood-suckers, and are responsible for the spread of many important diseases such as malaria, yellow fever and elephantiasis. Entomopathogenic microorganisms occupy an important place among the alternative methods of fighting against pests insect. The fungus, Beauveria bassiana is an entomopathogenic agent naturally present in the ecosystems and offers a very interesting potential for controlling populations of mosquitoes. Our study aims at showing the 4th stage larvae histological changes of the domestic mosquito Culex pipiens, treated with the entomopathogenic fungus B. bassiana in suspension which corresponds to a dose of 107 spore/mL. In fact, after developing the individuals in the fungal solution, histological study was carried out on the following parts; cuticle, intestine, adipose tissue and hemolymph. The histological studies showed many alterations and malformation in the treated 4th instar larvae body and tissues. The infection affected the different parts of the body, especially the cuticle, the adipose cells, and midgut, in addition to the development and the colonization of B. bassiana inside tissues. The application of B. bassiana on the cuticle of the larvae was dependent on an apparent disturbance in the structure of the cuticle or the degeneration of its different parts. The fungus infection does not stop at the level of the body walls, so it even goes to adipose tissue, epidermal cells and intestine. Based on these results, B. bassiana could be suggested as suitable biocontrol agent against C. pipiens.
Key words: Beauveria bassiana, Culex pipiens, cuticle, histological study, midgut, adipose tissue.
INTRODUCTION
Mosquitoes (Diptera: Culicidae) are zoonotic vectors responsible for many infectious diseases of medical and
veterinary importance such as filariasis, malaria, and arboviruses (Goddard, 2008; Mullen and Durden, 2009).
*Corresponding author. E-mail: benzinafarida@yahoo.fr.
Author(s) agree that this article remain permanently open access under the terms of the Creative Commons Attribution License 4.0 International License
region in 1994, as isolated cases of encephalitis in humans with fatal cases were reported by Zientara (2001).
Control of these diseases depends on the understanding of the transmission mechanisms of the disease, which are generally complex because of the indirect mode of transmission (Richards et al. 2008) and means of struggles such as insecticides, UV lamps, ultrasound devices, dermal repellents, and mosquito nets that are used to remove these unwanted insects with variable success (Matthew et al., 2007).
For several years, the control methods used sporadically in Algeria consist of spraying chemicals (Berchi and Laouadi, 2007). However, the widespread use of these products quickly revealed several difficulties such as the resistance phenomena, the ecosystems imbalance as well as adverse effects on humans, animals, plants, air and soil pollution, along with the lack of specificity and persistence effect for non-biodegradable insecticides, which are the most frequent (Rajkumar and Japanesan 2005). To avoid these problems, research is oriented towards the discovery of new methods. Therefore there is considerable interest in the exploitation of naturally occurring entomopathogenic fungi for control of different insects including mosquitoes (Ragavendran and Nataraja, 2015).
Fungal diseases in insects are common and widespread. There are more than 700 species of entomopathogenic fungi currently known (Goettel et al., 2000). Entomopathogenic fungi infect their hosts through the cuticle, penetrate them and spread through the body and after the fungus has killed the host, it can grow out of the host cadaver and produce more spores, increasing the chance for others to be infested (Mul et al., 2009).
The Integrated Pest Management (IPM) is being promoted and entomopathogenic microorganisms are being increasingly studied in a biological control context for their ability to infect and kill their hosts with varying degrees of selectivity (Becker et al., 2010). Biological control includes Beauveria bassiana (Bals.-Criv.) Vuill (Clavicipitaceae), a fungus considered among the most primitive entomopathogenic microorganisms in the worldwide.
Biological material
Insects (Culex pipiens)
The insect used in this study is an autochthonous mosquito species, that originated from Boumerdes region (North Algeria), and emerges from rearing in laboratory. It is identified by the team of Medical Entomology of the Laboratory of Ecology of Vector Systems at the Pasteur Institute of Algiers (Pr. Bitam I.).
Fungal strain
This strain was isolated from the soil of Boumerdes region (Algeria). The soil samples were previously dried and calibrated by sieving. To prepare the soil suspensions, 10 g of each sample was diluted in 90 mL of sterile distilled water (Ulacio et al., 1997), followed by a series of decimal dilutions of each sample. After preparation of the dilutions, vigorous stirring with a Vortex was carried out in order to allow a good homogenization of the suspensions.
Moreover, the PDA media (Potato infusion: 200 g; Dextrose: 20 g; Agar: 20 g; Distilled water: 1 L; Final pH: 5.6±0.2) were inoculated by plating in Petri dishes at a rate of 5 μL per dilution. The plates were incubated at 28°C in the dark and observed daily for three weeks. Its identification was carried out in the Department of Botany of the National High School of Agronomy, based on the macroscopic and microscopic characters (Pr. Keddad).
Harvesting pre-imaginary stages
Mosquito surveys were carried out in Boumerdes, where the climate is Mediterranean (cold and wet winters and hot and dry summers). It is considered as an ecosystem favorable to the spread of mosquitoes.
Harvesting of the pre-imaginary stages was carried out by the dipping method, which entails collection of the water from the deposit by means of a ladle (bucket) and filtration of the latter with tulle of net. Thus, the larvae and nymphs were put in bins containing heel water and brought back for breeding at the Laboratory of Valorisation and Conservation of Biological Resources at the University of Boumerdes.
Rearing of mosquitoes
The eggs are harvested and placed in tanks filled with water from the gite, in which the hatching larvae will develop. Larvae and nymphs brought to the laboratory were raised in the insectarium under controlled conditions (average temperature of 24°C and
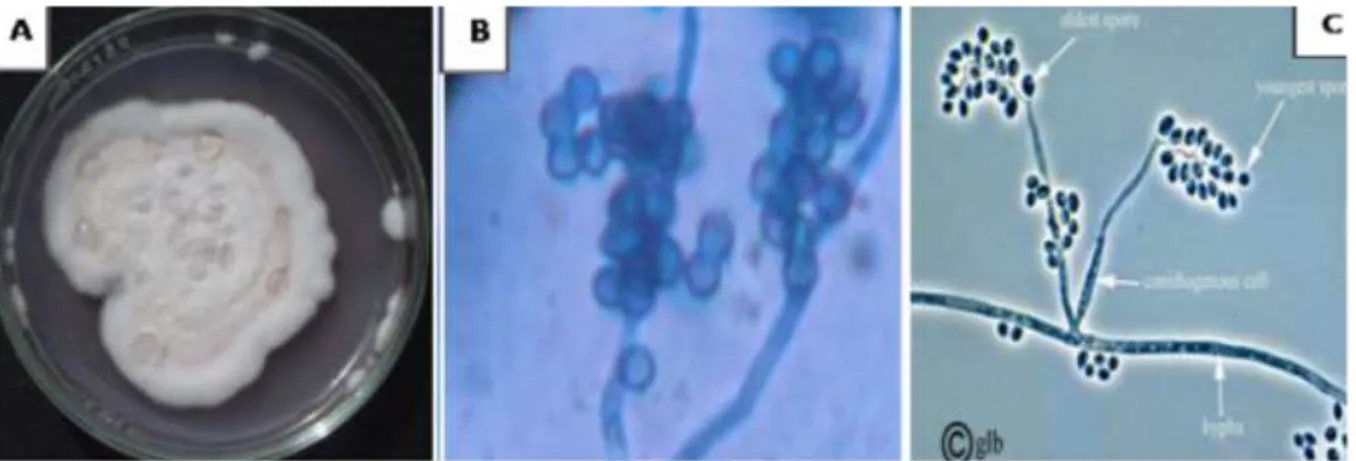
Figure 1. Macroscopic (A) and microscopic (B) characters of isolated Beauveria bassiana observed under a light
microscope at (GX100). (C) Representative schemea of the genus Beauveria.
mean humidity of 65%). The larvae were placed in mosquito net-covered breeding tanks lined up on the pallias. Each morning they were fed with dried biscuit powder; thereafter, the nymphs were collected and put in a crate covered with mosquito netting.
Preparation of the entomopathogenic solution
From the colonies aged 8 to 15 days, explantas are removed and placed in an Erlenmeyer flask containing 500-ml of sterile distilled water, which is sealed to avoid contamination.
To allow maximum release of the spores, the Erlenmeyer flask is stirred for 30 min by adding 2 to 3 drops of Tween 80 reagents. Meanwhile, the spore concentration is evaluated using a hematite cell of Malassez.
Dose used
In order to carry out this study, a dose of 0.33 × 107 spores/mL has
been used. This dose was chosen according to the works carried out by various microbiological control researchers. Wang and Powell (2002), Meikle et al. (2007), Halouane (2008), Fan et al. (2012), Hamid et al. (2013) and Khaleil et al. (2016) obtained satisfactory results by treating various types of insects, with a fungal
suspension of B. bassiana (107 spores/ml). The selected dose is
applied to the fourth larval stages of C. pipiens.
Type of treatment
In order to administer the fungal suspension to our insects, the dip mode was chosen, which consists of developing the individuals in the fungal solution at the rate of twenty individuals (with three repetitions) for 15 min, and then they were put back in their usual habitat (the gite water).
Control individuals were distributed in the same manner and counted as the treated individuals, except that they were developed in sterile distilled water and then returned to their usual habitat (Water of gite).
Histological study
The histological study was carried out at the level of the Cytology Service Laboratory of CHU Pr Nefissa-Hamoud of Hussein Dey,
Algiers. The study was conducted on 4th stage C. pipiens larvae,
brought into contact with spore suspension (107 spores/mL) of B.
bassiana. The body parts studied includes cuticle, intestine,
adipose tissue and hemolymph.
According to Martoja and Martoja (1967), the histological techniques aim at obtaining thin and colored sections of biological material, observable under an optical microscope. The biological material undergoes different treatments before being analyzed under a microscope (Venteo and Velot, 2010).
96 h after treatment, the individuals were dissected to be immediately fixed in alcoholic Bouin solution. Control larvae, placed in distilled water, were sampled at the same time intervals and were also fixed. Before initiating the histological technique steps, the heads and respiratory siphons were cut with a scalpel blade. The parts were then placed in little boxes which were then passed successively through ethanol baths of 70° to 100°, in order to dehydrate them, or in other words remove the intra- and extracellular water. They were then immersed in three xylene containers in order to remove traces of ethanol and lighten the parts. Once dehydrated, the parts were enclosed in paraffin to achieve permeation into the studied tissue as complete as possible. The impregnation of paraffin takes place in an oven at a temperature of 58 to 60°C. The blocks were then prepared and cut using a microtome in 5 μ thickness. The obtained sections were stained, fixed between slide and cover slip and observed under an optical microscope to determine the various anomalies that may have appeared in the different parts of the treated C. pipiens larvae.
RESULTS AND DISCUSSION
Macroscopic and microscopic aspects of Beauveria sp
B. bassiana produces white cottony colonies. Its conidia
or spores are supported by long zigzag filaments which are transparent hyphae. They are produced on short spikes giving a convex appearance to the conidiogenous cells (Figure 1).
The criteria on which our identification was based resemble those of Lipa (1975) who described the genus
Beauveria as follows; It produces whitish to yellowish
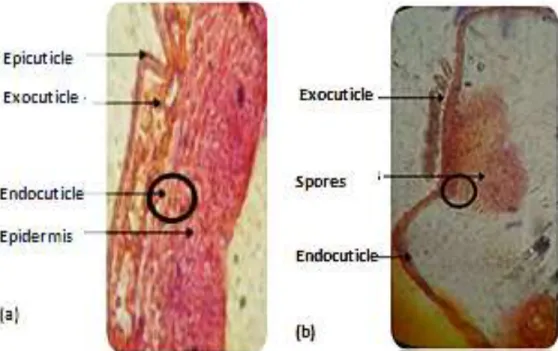
Figure 2. Structure of the cuticle of Culex pipiens control larvae (a) and Spores of B. bassiana
surrounding the cuticle of Culex pipiens larvae treated with B. bassiana (b) (G10 × 40).
hyphae. The latter are transparent and septal with a diameter of 2.5 to 25 μm. Conidia are produced on short heads called conidiophores giving conidiogenous cells a spiny appearance. Under aerobic conditions, the fungus produces conidiospores of spherical shape (1 - 4 μm diameter). However, the fungus has the ability to grow in anaerobic condition where it produces oval shaped blastospores (2 - 3 μm diameter). Blastospores are as infectious as conidiospores.
Results of histological study
Many histological changes in most insects due to the infection with different entomopathogenic fungi were revealed using light and electron microscopy by many investigators (Scheneider et al., 2013; Gabarty et al., 2014; Khaleil et al., 2016; Ragavendran et al., 2017). The insects cuticle, with its underlying epidermis, forms the integument. It plays both the role of exoskeleton and barrier between the environment and the animal. It covers the whole surface of the body and invaginates in the gastrointestinal tract and the respiratory system. From a structural point of view, two major layers of the outer epicuticle, thin, non-chitinous, and thicker and chitinous internal procuticle (exocuticle and endocuticule) are recognizable according to chemical composition (Grassé, 1949; Wigglesworth, 1972; Raccaud Schoeller, 1980; Retnakaran et al., 1996). The untreated and fungal treated larvae of C. pipiens were histologically examined after 96 h by light microscope. The microscopic examination of 4th instar larvae of C. pipiens in this study
revealed that the B. bassiana spore suspension brought about massive disintegration and deformation of the moustiquo body and tissues. Also, it revealed the development and the colonization of the fungus inside the insect. The untreated 4th instar larvae (Figure 2a) showed the normal structure of the mostiquo’s body with normal intact cuticle which clearly differentiated into epicuticle and endocuticle layers. On the other hand, the treated individuals semi-thin section showing many abnormalities in the insect's body as appeared in (Figure 2b). There is a disorganization of the cuticle which appeared not differentiated into its epicuticle and endocuticle layers and become black-spotted indicating direct attack of the fungus on the defense system of the insects (Khaleil el al., 2016). In the treatment of Spodoptera littoralis larvae with five strains of entomopathogenic fungi, many malformations were observed in the cuticle (Amer et al., 2008). The alterations in the host’s cuticle is probably through physical damages owing to the mycelium growth as well as the beginning of the sporulation process of the fungus or may be due to biochemical degradation (Benserradj and Mihoubi, 2014).
As mentioned by Haitham and Alleddin (2012), the analysis showed great effects at all parts of the body including wall, intestine and adipose tissue, as was the destruction of the wall of the body, specifically the larval epicuticle which is degraded into small pieces, in addition to the development of large masses of spores and the appearance gaps which are regions of crossing the fungal spore (Figure 2). By comparing with the control which has an intact wall (Figure 2a), this destructuring has engendered, as an external sign, a disturbed and
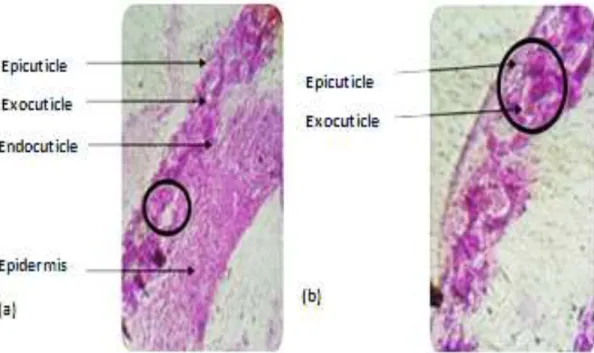
Figure 3. Cross section at (a) the epidermis of treated larvae showing (b) epidermal cell degeneration.
Figure 4. Degeneration of the different parts of cuticle of Culex pipiens larvae (a) control (G10x40) and
(b) treated (G 10×100).
blocked moult.
The effect of histological changes in larvae has affected even epidermal cells or the case of caryolysis-type necrosis has been detected (Figures 3 and 4).
Experimental infestations of Anopheles gambiae and
Mansonia uniformis by Wuchereria bancrofti were carried
out; some histological aspects of the interactions between W. bancrofti and these two hosts are approached; within the both hosts, the larva destroys the parasitized muscle. These muscular injuries are spreading gradually as intramuscular filarial development goes on; and also, from the epidemiological point of view,
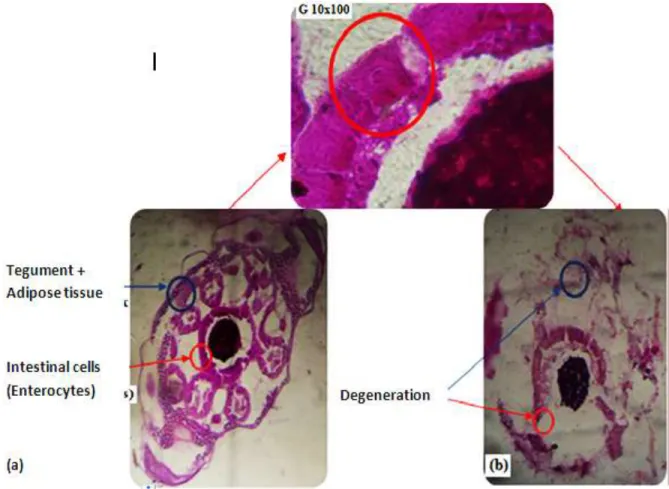
Figure 5. Cross section of the larvae of Culex pipiens (a) control and (b) treated.
these lesions instigate a flying handicap important for the two mosquitoes (Brunhes and Brunhes, 1972).
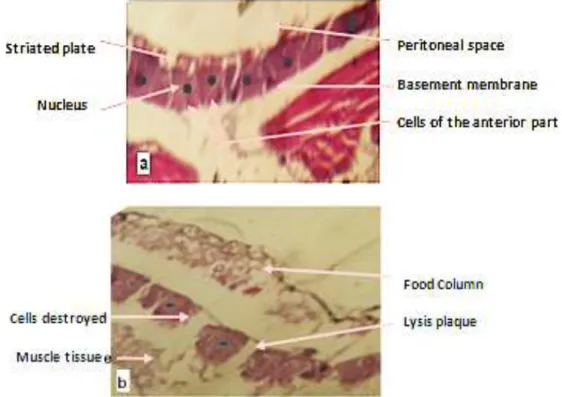
The cross section in the gastrointestinal tract showed the effect of the fungus on the muscular layers, which causes a change in the thickness of these layers compared with those of the controls, as the disappearance of the supports of the muscle layer, the same for intestinal cells where degradation caused by invasion of the fungi was estimated (Figure 5).
Histopathological changes in the intestine of C. pipiens are similar to those observed by Kellen et al. (1965) on
Culex tarsalised with Bacillus sphaericus, including cell
degeneration of the anterior stomach and vacuolation, which invades cells during all stages of the poisoning of
Culex (Figure 6).
This research results can probably mention that the effect of the fungus on the muscular layers and the intestinal cells is considered a good control on the colonization of the digestive canal by the fungus as it was recorded (Boucias and Pendland., 1998; Miranpuri and Khachattourians, 1991; Darzynkiewicz et al., 1997).
Histological and ultrastrucutural alterations in the midgut of Aedes albopictus larvae infected with Bacillus
thuringiensis var. israelensis (Bti) were observed by Silva
et al. (2008). After Bti ingestion, the intestinal cells
showed a disorganization of the basal processes, dilatation and fragmentation of the rough endoplasmic reticulum, with intense cytoplasmic vacuolization. There were concentric dense laminas accumulated in the cytoplasm, and these residual membranous bodies were seen greatly increased in size after 60 min.
However, neem (Azadirachta indica) products were applied to mosquito (Culex quinquefasciatus) larvae and nymphs. The histopathological study reveals that neem products act upon larval digestive system after ingestion, whereas for nymphs only contact effects are shown (Seye et al., 2006).
According to Zerroug et al. (2017), histological study on the 4th stage C. pipiens’ larvae treated with Eucalyptus
globulus leaves aqueous extract shows different and
progressive damage to the larvae's intestinal tissue, causing the mixing of gut cells content with hemolymph, which is responsible for larval mortality.
The changes that were observed in the gastric caeca and midgut cells, are explained by the fact that this portion of the digestive tract, which is responsible for digestion in insects, is in direct contact with the toxic elements and so causing death. The fact that this section is the major food absorption site in mosquitoes is confirmed by Seye et al. (2006) in their study, where
Figure 6. Cross section in the anterior stomach of (a) control larvae (G10x40) and(b) infected
larvae.
hungry mosquito larvae are fed with fructose and glucose; and after a few hours, massive glycogen deposits appear inside the cells of the midgut’s posterior portion, indicating that it is the major absorption site.
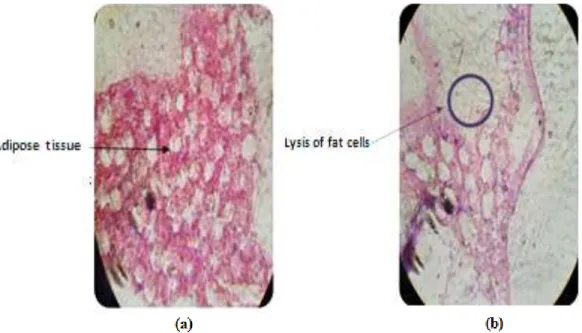
Fungal infection does not stop at the level of the walls of the body, therefore it even completes the adipose tissue where it has been shown that the fat cells of the larvae have lost their functional specialization because of the multiplication of the spores at the level of the latter, as well as the release of lipase enzymes by the fungus, which have as a function the digestion of the adipose tissues as well as their effect on the plasma membrane of the adipose cells. It causes the lysis and the overlap of the latter which form large gaps in size, different in the tissue (Figure 7).
Histology of C. quinquefasciatus larvae exposed to LC50 of fatty acid methyl esters (FAMEs) obtained by
transesterification of vegetable oils, was performed and changes in the midgut and fat body morphology were identified (Ribeiro-Netoa et al., 2017).
In the colonization events observed by Toledo et al. (2010) in the planthopper Peregrinus maidis (Hemiptera: Delphacidae) traited by B. bassiana and Metarhizium
anisopliae, the formation and multiplication of hyphal
bodies by both fungal species inside the host's body was noted. The host's whole body was invaded by hyphae between five and six days post-inoculation, and body fat was the most affected tissue.
Infection in the hemolymph tissue caused, on the one
hand, the appearance of the hemolymph cell nucleus swelling, and on the other hand, a morphological change of the latter, to the spherical form (Figure 8) reported by Miranpuri and Khachattourians (1991).
Lepidopteran larvae of Galleria mellonella were infected with fungus Candida albicans strains, in which the whole larvae were then examined for tissue changes by histological techniques. Various degrees of pathogenicity (strain- or inoculum-related), and the infection time course are described (Perdoni et al., 2014). Meanwhile the examination of histological sections of various parts of the digestive tract showed some changes in larvae of Locusta migratoria treated with Pseudomonas
fluorescens especially at mesenteron level with alteration
of the peritrophic membrane (Oulebsir-Mohandkaci and Doumandji-Mitiche, 2012).
In the same context, the results reported by Zayed et al. (2009) on the effect of Citrus limon and Allium sativum oils against C. pipiens larvae, reveal that there was clear damage to the epithelial cells of the midgut. Intestinal tissue, muscles and cuticle were the most severely damaged by the treatment, as well as the separation of the midgut cells from their basal membrane.
This research results show similarities with those observed by Al-Mehmadi and Al-Khalaf (2010) in their research on the mode of action of the Bacillus sphaericus bacteria against C. pipiens larvae's digestive tract. The first changes are observed in the midgut and posterior stomach.
Figure 7. Adipose tissue cross section of control (a) and (b) treated C. pipens larvae showing the onset
of degradation of fat cells (G10x40).
Figure 8. Tissue of treated larva showing swelling of (b) hemolymphatic cells compared to (a) control.
Conclusion
In recent years, several studies have been conducted to examine the bioefficacy of natural products against many arthropod pests including mosquitoes.
The application of the entomopathogenic fungus B.
bassiana on the larvae of the 4th stage of C. pipiens
induces disturbances in all parts of the body, including the structure of the cuticle, adipose tissue, haemolymph and intestine, witnessing the effectiveness of this fungus towards the larvae of the domestic mosquito C. pipiens.
This study reports the histopathological alterations in C.
pipiens after infection with B. bassiana and contributes to
a better understanding of the mode of action of this fungus used as bioinsecticide against mosquito larvae.
These results suggest the possibility of carrying out new tests under natural conditions and should be done on a larger scale (in the field) to confirm the results presented in this study.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
Al-Mehmadi RM, Al-Khalaf AA (2010). Larvicidal, histological effects of
Melia azedarach extract on Culex quinquefasciatus Say larvae
Amer MM, El-Sayed TI, Bakheit HK, Moustafa SA El-Sayed YA (2008). Pathogenicity and genetic variability of five entomopathogenic fungi against Spodoptera littoralis. Res. J. Agric. Biol. Sci. 4(5):354-367. Bawin T, Seye F, Boukraa S, Zimmer JY (2015). La lutte contre les
moustiques (Diptera: Culicidae): diversité des approches et application du contrôle biologique. The Canadian Entomologist. 147(4):476-500.
Benserradj O, Mihoubi I (2014). Larvicidal activity of entomopathogenic fungi Metarhizium anisopliae against mosquito larvae in Algeria. Int. J. Curr. Microbiol. Appl. Sci. 3:54-62.
Berchi S, Louadi K (2007). Characterization of resistance of Culex
pipiens L., 1758, to malathion (organophosphorus) and detection of
resistance genes (Diptera, Culicidae). Bulletin of the Entomological Society of France. 112(1):99-104.
Becker N, Petric D, Zgomba M, Boase C, Madon MB, Dahl C, Kaiser A
(2010). Mosquitoes and Their Control. 2nd Ed. Springer-Verlag Berlin
Heidelberg. 577p.
Boucias DG, Pendland JG (1998). Principles of Insect pathology. Kluwer Academic of Entomo pathogenic fungi Beauveria bassiana and Metarhizium anisopliae to Ixodidae Tick species. Dermacentor
variabilis, Rhipicephalus sanguineus, and Ixodus scapularis. J. Med.
Entomol. 41(4):705-711.
Brunhes MJ, Brunhes J (1972). Histological study, in Mansonia
uniformis Thcobald and Anopbeles gambiae B Giles, the evolution of Wubereria bancrofti Cobbold and the interactions between wired and
these two hosts. Cah. O.R.S.T.O.M., Ser. Ent. Med. Parasitol. 3:217-233.
Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F (1997). Cytometry in Cell Necrobiology: Analysis of Apoptosis and Accidental Cell Death (Necrosis). Cytom. 27:1-20.
Fan Y, Borovsky D, Hawkings C, Ortiz-Urquiza A, Keyhani NO (2012).
Exploiting host molecules to augment myco-insecticide
virulence. Nature Biotechnol. 30:35-37.
Faradj C, Elkohli M, Lyagoubi M (2006). Gonotrophic cycle of Culex
pipiens (Dipterae: Culicidae), potential vector of the West Nile virus,
in Morocco: estimation of the duration in laboratory. Bull. Sociol. Pathol. Exot. 119-121.
Gabarty A, Salem HM, Fouda MA, Abas AA, Ibrahim AA (2014). Pathogencity induced by the entomopathogenic fungi Beauveria
bassiana and Metarhizium anisopliae in Agrotis ipsilon (Hufn.). J.
Radiat. Res. Appl. Sci. 7(1):95-100.
Goddard J (2008). Infectious Diseases and Arthropods. 2nd ed. Totowa,
NJ: Humana Press. 251p.
Goettel MS, Inglis GD, Wraight SP (2000) Fungi. In: Field Manual Technique in Invertebrate Pathology, Lacey LA and Kaya HK (eds.). (Kluwer Academic Publisher, Netherlands: pp 255-282.
Grassé PP (1949). Treaty of Zoology. Anatomy, systematic and biology.
Ed. Masson and Cie, Paris, T. IX, 1117 P.
Haitham MAH, Alleddin AH (2012). Histological study of Culex pipiens
pipiens larvae and adults infected with Beauveria bassiana. Rev.
Baghdad Sci. 9(2):187-193.
Halouane F (2008). Research on the entomopathogenic Beauveria
bassiana (Bals.) Vuill. (Sordariomycetes- Clavicipitaceae): Bioécologie, production and application on Schistocerca gregaria (Forskàl, 1775) and Locusta migratoria (Linné, 1758) (Orthoptera, Acrididae). Doctoral thesis on Agronomic Science; Agronomic National Institut of EL-Harrach, Algeria; P 290.
Hamid S, Halouane F, Bissaad FZ, Benzina F (2013). Study about the effect of Beauveria bassiana (Vuillemin IN 1912) on the aquatic stages of Culex pipiens (LINNÉ, 1758). Int. J. Bio-Technol. Res. 3(3):31-42.
Khaleil M, El- Mougith A, Hashem H, Lokma N (2016). Biocontrol Potential of Entomopathogenic Fungus, Trichoderma Hamatum against the Cotton Aphid, Aphis Gossypii. J. Environ. Sci. Toxicol. Food Technol. 10(5):11-20.
Kellen WR, Cklarc TB, Lindegren JE, Ho CB, Rogoff MH, Singer S (1965). Bacillus Sphericus Neile as a pathogen of mostiquitos. J. Invert. Pathol.. 7:442-448.
Kosone K, Ito M, Kosuge K, Kanayam A (2008). Distribution of Culex
pipiens (Diptera: Culicidae) complex in Yokohama Japan. Proceeding
of the sixth international conference on urban pests, 497. Lipa JJ (1975). White muscardines (Beauveria sp.). In: An outline of
Insect Pathology. Foreign Sci. Publ. Dept NCSTEI, Warsaw, Poland. pp. 139-142.
Martoja R, Martoja Pierson M (1967). Animal histology techniques. Ed.
Masson and Cie, Paris Vol: V. 331p.
Matthew T, Read A, Taylor L (2007). Can fungal biopesticides control malaria. Nature Microbiol. Rev. 5:377-383.
Meikle W, Mercadier G, Girod V (2007). The micro-fungus, new hope in biological fighting against Varroa destructor. BEES & Cie. N° 118.3. Miranpuri GS, Khachattourians GG (1991). Infection sites of the
entomopathogenic fungus Beauveria bassiana in the larvae of the mosquito Aedesaegypti. J. Entomol. Exp. Appl. 59(2):19-27.
Mul M, Niekerk T, Chirico T, Maurer J, Kilpinen O, Sparagano O, Thind B, Zoons J, Moore D, Bell B, Gjevre AG, Chauve C (2009). Control methods for Dermanyssus gallinae in systems for laying hens: Results of an international seminar. World’s Poult. Sci. J. 65:589-599.
Mullen G and Durden L (2009). Medical and veterinary entomology.2nd
ed. Academic Press. 637p.
Oulebsir-Mohandkaci H, Doumandji-Mitiche B (2012). Study of the biological impact of Pseudomonas fluorescens. on haemolyphatic metabolites and digestive tract histology of L5 larvae of Locusta
migratoria (Linnaeus, 1758). Lebanese Sci. J. 13(2):99-115.
Perdoni F, Falleni M, Tosi D, Cirasola D, Romagnoli S, Braidotti P, Clementi E, Bulfamante G, Borghi E (2014). A histological procedure to study fungal infection in the wax moth Galleria mellonella. Eur. J. Histochem. 58(3):24-28.
Raccaud Schoeller J (1980). Insects, Physiology and development. Ed. Masson, Paris, 269p.
Ragavendran C, Nataraja, D (2015). Insecticidal potency of Aspergillus
terreus against larvae and pupae of thre emosquito species Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti.
Environ. Sci. Pollut. Res. 22:17224-17237.
Ragavendran C, Mariappan T, Nataraja D (2017). Larvicidal, Histopathological Efficacy of Penicillium daleae against Larvae of
Culex quinquefasciatus and Aedes aegypti Plus Biotoxicity on Artemia nauplii a Non-target Aquatic Organism. Front. Pharmacol.
8:1-14.
Rajkumar S, Japanesan A (2005). Oviposition detterent and skin repellent activities of Salanum trilobatum leaf extract against the material vector Anopheles stephensi. J. Insect. Sci. 5(15):1-3. Ressiguier P (2011). Contribution to the study of the blood meal of
Culex pipiens. Exercise Thesis, National School of Toulouse-ENTV.
P 80.
Retnakaran A, Pallis SR, Tomkins WL, Primavera MJ, Brownwright A (1996). The regulation of chitin synthesis and deposition in an insect, the spruce budworm, at the biochemical and ultrastructural level, pp. 174-182 in Stevens W.F., Rao M.S. and Chandrkrachang S., Chitin and Chitosan, Environmental friendly and versatile biomaterials, Ed. Asian Institute of technology, Bangkok, Thailand.
Ribeiro-Netoa JA, Pintoa MEA, Ferreiraa VV, Tibúrciob JD, Varottia FP, Azevedoc DO, Siqueira-Filhod EP, Serrãoe JE, Santos Limaa LA, Alvesa SN, Ribeiro-Neto JA (2017). Larvicidal activity of vegetable oils and esterified compounds against Culex quinquefasciatus (Diptera: Culicidae). Ecotoxicol. Environ. Safety.143:57-61.
Richards SL, Ghosh SK, Zeichner BC, Apperson CS (2008). Impact of source reduction on the spatial distribution of larvae and pupae of Aedes albopictus (Diptera: Culicidae) in suburban neighborhoods of a Piedmont community in North Carolina. J. Med. Entomol. 45(4):617-28.
Savage HM, Ceianu C, Nicolescu G, Karabatsos N, Lanciotti R, Vladimirescu A (1999). Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996, with serologic and molecular characterizations of a virus isolate from mosquitoes. Am. J Trop. Med. Hyg. 61:600-611.
Schneider LCL, Silva VC, Pamphile JA, Cone H (2013). Infection, colonization and extrusion of Metarhizium anisopliae (Metsch) Sorokin (Deuteromycotina: Hyphomycetes) in pupae of Diatraea
saccharalis F. (Lepidoptera: Crambidae), J. Entomol. Nematol.
5(1):1-9.
Seye F, Ndione RD, Ndiaye M (2006). Comparative study of two neem products (oil and powder) on preimaginal mosquito stages Culex
quinquefasciatus (Diptera : Culicidae). Afr. Sci. 02(2):212-225.