HAL Id: jpa-00248713
https://hal.archives-ouvertes.fr/jpa-00248713
Submitted on 1 Jan 1991
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
EXAFS of nebulized solutions
C. Landron, D. Ruffier, Ph. Odier, J. Coutures, Dominique Bazin, H. Dexpert
To cite this version:
C. Landron, D. Ruffier, Ph. Odier, J. Coutures, Dominique Bazin, et al.. EXAFS of nebulized
solutions. Journal de Physique III, EDP Sciences, 1991, 1 (12), pp.1971-1975. �10.1051/jp3:1991243�.
�jpa-00248713�
LPhys. III France 1
(1991)
1971-1975 DtCEMBREI991, PAGE 1971Classification
Physics
Abnmcts78.70 4~68
Sho" Communication
EXAFS
of
nebulized
solutions
C.
Landron(I
),
D.Ruttier(I),
Ph.Cdier(I
),
J-PCoutures(I),
D.Bazin(~)
and H.Dexpert(~)
(~ Centre de Recherches sur la
Physique
des muteslbmp6ratures,
45071 Orleans Cedex 2, France (~) Laboratoire pour l'Utilisation duRayonnement
Electromagn6tique,
91405 Orsay Cedex, France(Received 31Mqy 1991, revbed 27 September1991, accepted I
October1991)
Abstract. The first EXAFS results on nebulized solutions are
presented.
High quality spectrafrom in siw X-ray absorption experiments are performed on micrometer-sized spheres of nickel
(II)
bromide in 95fb alcoholic or in aqueous solution via ultrasonic nebulization. The structural informa-iion given by the X-ray absorption complete the data given by other
absorption
spectroscopies. Thestrong dissociation of the precursors is shown and the first coordination sphere of the nickel atom is
evidenced. It is formed by nickel atoms surrounded by
hydrating
water molecules.The
optical
properties
of smallparticles,
which have been reviewedby Campillo
and Linill,
have been the
subject
of a great number of studiesduring
thepast
years:absorption
(using
visibleor infrared
radiation)
and fluorescence are twotechniques
among thepowerful
spectroscopies
inaerosol
analysis. They
use radiation with awavelength larger
than the size of thedroplets.
Aerosol is considered as a stabledispersion
of smallparticles
into a gasphase.
The firsttheory
concerning
the
optical
behavior of a mist is based on the solution of the Maxwell'sequation
forscattering
andabsorption
ofelectromagnetic
wavesby
a dielectricsphere by
Mie [2] and morerecently by
Kerker[3]. The
propagation
of ahigh intensity
laser beamthrough
anabsorbing
aerosol cloud has beendescribed
by
Walsh et al. [4]. Inspite
of an extensive number of availablespectroscopic analysis,
there remain some unresolved structural
problems
at the nanometer scaleconcerning
atomizedproducts.
Our aim is todevelop
an bt situabsorption technique adapted
to nebulized solutionsby
extending
thespectroscopic
studies of aerosols to the smallwavelengths
into theX-ray
range.A schematic
diagram
of ourexperimental
arrangement is described infigure
I. A detaileddescription
of the ultrasonic nebulizer used toproduce gels
of zirconiaparticles
[5j and furtherstudied
by
EXAFS can be found elsewhere [6j. Nickel(II)
bromide dissolved in a 959b ethanolsolution is introduced in the reservoir. The nebulization chamber is then circulated with helium
during
five minutes. A mist of the solution is thusgenerated by
the atomizer which activepart
is constituted
by
apiezoelectric
transduceroperating
at afrequency
of 1.6 MHz. Thediame-ter of the
droplets
isgiven by
the relation d =(xa/4pf~)~'~
where a and p arerespectively
the surface tension and the
density
of the solution andf
is thefrequency
of the transducer,d
1972 JOURNAL DE PHYSIQUE III N°12 X RAY -FLUORESCENCE UETECTUR MIST CIRCULATOR i ' GAS FILLER ULTRASONIC TRANSDUCER
Fig.
I. Outline of theexperimental
set up used for theX-ray absorption
measurements of a mist ofmicrometer-s12ed
droplets
of solutions. The apparatus is composed of an ultrasonic nebulizerproducing
the mist from a 5 cm
high
geyser generated from the solution. The spray chamber isdesigned
to promote a goodmixing
and transport of the aerosol towards the analysis cell where the droplets are irradiated in varioussituations. A dose circuit
permits
to keep a constant level in the solution and a stabledensity
ofparticles
infront of the
X-ray
source.the
X,ray
irradiation cell for XASexperiments.
We note twoimportant advantages
of theultra-sonic nebulizer: first the aerosol h
practically monodispersed,
secondly
both aerosolproduction
rate and carrier gas flow rate can be
independently
varied.The
typical
EXAFS measurements, at roomtemperature,
were carried out at LURE(Orsay)
on the Ni
K-edge (8250-9000
eV energyrange).
Thesynchrotron
radiation wasprodded
by
the1.85 GeV storage
ring
of DCI. TheX-ray
beam of the EXAFS IV station was monochromatizedby
a doubleBragg
reflection(ill)
of Sicrystals.
Theintensity
of thepositron
beam was about300 mA~ We used an air-filled ionization chamber to measure the
photon intensity
before thetarget. In order to avoid
charging
of thedroplets
underX-ray
irradiation,
we have used heliumat
atmospheric
pressure as a carrier gas which furthermore has theadvantage
of a lowX-ray
absorption
cross section. We note a smallersignavnoise
ratio for those data obtained with anair filled cell. A fluorescence detector is used for measurement of the
absorption
coefficient,
theescape
depth
ofphotons being
in the range of a few micrometers. The fluorescence detection consists of aplastic
scintillatorcoupled
with ahigh
gain
photomultiplier.
In order to reduce thephoton
fractioncontributing
to thebackground
of thespectra
at the NiK-edge,
we used a cobaltfilter
placed
in front of the scintillator that absorbs the scatteredphotons.
Theadvantage
of the fluorescence detection is wellrecognized
for the local structuralstudy
of very dilutesolutions,
itis in addition fair
adapted
for ourgeometrical
configuration.
Four
samples
have been examinedby
EXAFS: two molar solutionsprepared by dissolving
NiBr~
in 9596 ethanol or in water and twocrystallized compounds:
Ni andNiBr~-2H20.
Thesepowders
have been used as standards fordetermining
thephase
shifts and theamplitude
factorsin the
analysis procedure
of the EXAFSspectra. NiBr2
crystallizes
in aclose-packed
cubic struc-ture built fromlayers
of octahedral NiBr6 coordination groups eachsharing
anedge
common with six othersadjacent
groups. The Br~ anions are characterizedby
apyramidal
coordination.N°12 EXAFS OF NEBULIZED SOLU~ONS 1973
molecules such that the Ni-O distance b 0.20 nm, the four Br~ ions are located at 0.26 nm from
the Ni atoms. The oxide NiO [8] which has a Nacl structure where Ni and O atoms altemate
in a
simple
cubicpacking,
each atombeing
surroundedby
six others at the vertices of aregular
octahedron.
I
rd=
0
~ £0
50
100
(nm~~)
Fig.
~ ln siw experimental EXAFS oscillationsX(k)
above the Ni K~dge of a nebulized molar solutionofNiBr~ in 95fb ethanol.
The mathematical treatment of the
spectra
is based on theprocedure previously
described [6]. The data were firstcalibrated,
averaged
and normalized. Thebackgrounds
were subtracted and the EXAFS oscillations were Fourier transformed. Thek-space
used for the Fourier transformwas limited
by
km~
= 26 nm~ and kmax = 120 nm~ ~. The least squaresfitting
method forcoor-dination shell determination has been
employed
with values ofphases
andamplitudes
calculatedaccording
to theprocedure
of McKale et al. [9]. The first coordination shell of the nickel ions isformed
by
bromide ions and oxygen issued from H20 molecules. The Br~ are too close to eachother to be resolved on the Radial Distribution Function. The free
parameters
used in the fitprocedure
were the interatomic distance, the coordination number and theDebye-Waller
factorwhile the
K,edge
position
shift was fixed. Thereliability
factor of least squaresfitting
of the data for the foursamples
were similar.Figure
2 shows the fine structures of theabsorption
above theNi
K-edge
of a nebulized molar solution ofNiBr2
in 9596 ethanol. We note that thisspectrum
1W4 JOURNAL DE PHYSIQUE III N°12 t t i i t t i t i Ii iii fit - ~~ ~ i,i iii ~ i, i ,, , 1, 6~ ~( ' -,i j i ,, U-ii ii ' ii i' ii ii @ ii # i' i( i i( i ii if i' ii ii ii ii ii ii ii 11 0 0.1 0.2 0.3 0! 05 0.6 RInml
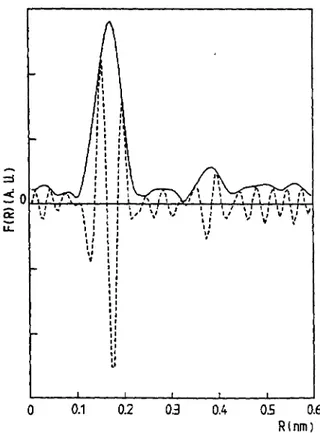
Fig.
3. Module(full line)
andimaginary
part(dotted line)
of the Fourier transform corresponding to thek~-weighted
EXAFS spectrum of a nebulized molar solution of NiBr~ in 95fb ethanol. Thepseudo
radialdistribution functions are uncorrected for the phase shift of the Ni-O and Ni-Br
pairs.
two sub-shells are
required
to fit EXAFS data of both aqueous and alcoholic solutions as well as for that of theNiBr2-2H20
standard. The Ni-Br contribution is included in a shoulder of the Ni-Opeak
illustrated in thefigure
3 for the nebulized solution ofNiBr2
in ethanol. Themajor
contrlution of the
peak
appears at a distance R = 0.205 nm of the nickelcation,
it is attributedto the oTygen atoms of the water molecules in accordance with the
X-ray
diffraction results ofCaminiti and Cucca [10] on aqueous Ni-Br solution. The Radial DbtrAution Function
analysis
shows a Ni-Br bond at 0.254 nm. This distance b smaller than the distance
RNI-B,
= 0.262 nm foundby
Caminiti and Cucca andhigher
than the dhtanceRN;-Br
= 0.251 nm foundby Ludwig
et al [11]. An achievement of thin structural
investigation
concems the contraction of the bonds in the nebulized solutions with an increase of theDebye-Waller
factors.This
study
confirms the octahedral coordination of the nickel cation in the alcoholic nebulizedsolution as well as in the aqueous nebulized
solution,
similarly
as in thedihydrate
bromide. Inspite
of the strong dissociation of the solution, the Br~ anions arereplaced by
water moleculesin the first coordination
sphere
as seen in table I. Our results are ingood
agreement with the Differential AnomalousScattering
data ofLudwig
et al[I I]
on 4 M aqueous NiBr2 solution andEXAFS results of
Lagarde
et al. [12] on the same solution.we have tested for the first time the
applicability
of ~ situX-ray
Absorption
Spectroscopy
tosuuctural characterization of micrometer,sized
droplets
of a mkt. We cansafely
conclude that th>technique
is well suitable forgiving
some detailed local information about atomic dhtancesN°12 EXAFS OF NEBULIZED SOLU~ONS 1W5
lhble I. Ni-O and Ni-Br bond
lengths,
nickel coord~ation numbers andDebye-Wauer factor
obtained ~y simulation
ofEK4FS
spec~afor
thefollowing
samplks:
1)
clyswlliied
NiOpowder 2)
nebufited molar solution
of
NiBr2
in 9596ethanol,
3)
nebufited nickel(II)
bromide in a normal aqueous solution4) clysta#ized NiBr2-2H20 powder.
Sample
1 2 3 4 sub shell I Ni ODist.,(nm)
0.209
0.205 0.206 0.209 Coord. Num. 6 5.7 5.6 2a,(nm)
0.~~l6l 0.@~68 0.0071 0.0%4 sub shell 2 Ni BrDist.,
(nm)
0.254 0.253 0.255 Coord. Num. 0.7 0.8 4 a, 0.lXl9 0.0084 0.@J62and coordination numbers in such
samples.
Because of the
large
surface/volume ratio of eachdroplet,
the thermal and chemicalexchanges
are fast and efficient. Therefore thin reactor should be very useful for on fine studies such as evolution of the local structure of the precursor
during
thepowder
elaboration process.Refemnces
[1] CAMPILLC AJ. and LIN
H.B., Absorption
and fluorescence spectroscopy, inOptical
effects Associated with Small Particles, S. Ramaseshan Ed.(Word
Publishing
Co.,Singapore,
1988).[2] MIE G., Ann Phys. 2s
(1908)
377.[3] KERKER M., The
scattering
of light and otherelectromagnetic
radiation(Academic
Press, New York, 1969).[4] WALSH J.L. and ULRISH PB., Laser beam
propagation
in theatmosphere,
J.W Strohbehn Ed.(Springer
Verlag,
New York, 1978).[~
DUIIOIS B., RUFHER D. and ODtER P,L Am Cermn Soc. 72(1989)
713.[q
LANDRON C., RUFFIER D., Dullols B., ODIER P, BONNIN D. and DEXPERr H., Phys. Status Sofidi 121(1990)
360.[7JWEIGELD., Bull Soc. Chim Fmnce10
(1963)110.
[8] WELLS A-E, Structural
inorganic
chemistry(Oxford University
Press, London, 1W5).[9] MCKALE A-G-, VEAL B.W, PAULIKAS A-P, CHAN S-K and KNAPP G-S.,L Am. Cermn Soc. 110
(1988)
3763.
[10] CAMINm R. and CuccA P, Chem Phys. Lett. 89
(1982)
110.[11] LUDWG KE, WARIIURrON WK and FONTAINE A~, L Chem Phys. 87