Design and application of a genetically-encoded probe for
peroxiredoxin-2 oxidation in human cells
By
Troy Frederick Langford
M.S. Chemical Engineering Practice
Massachusetts Institute of Technology, 2015
B.S. Chemical Engineering
Columbia University, 2013
SUBMITTED TO THE DEPARTMENT OF CHEMICAL ENGINEERING IN PARTIAL
FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
DOCTOR OF PHILOSOPHY IN CHEMICAL ENGINEERING
AT THE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
SEPTEMBER 2018
2018 Troy Frederick Langford. All rights reserved.
The author hereby grants to MIT permission to reproduce
and to distribute publicly paper and electronic
copies of this thesis document in whole or in part
in any medium now known or hereafter created.
Signature redacted
Signature of author...
ro
dc
Langford
Department of Chemic
gineering
Certifiedby...Signature
redacted
Hadley D. Sikes
Associate Professor and Esther and Harold E. Edgerton Career Development Professor of
Chemical Engineering
Thesis Supervisor
Signature redacted
e y ...MASSAkCHUSETTS INSTITUE
OF TECHNOLOGY Robert T. Haslam
Chairm
MAY
0,32019
LIBRARI S
Patrick S. Doyle
Professor of Chemical Engineering
in, Committee of Graduate Student
1
MITLibraries
77 Massachusetts Avenue Cambridge, MA 02139 http://Iibraries.mit.edu/ask
DISCLAIMER NOTICE
Due to the condition of the original material, there are unavoidable
flaws in this reproduction. We have made every effort possible to
provide you with the best copy available.
Thank you.
The images contained in this document are of the
best quality available.
ABSTRACT
Hydrogen peroxide (H202) is a well-known oxidant species commonly produced in eukaryotic organisms as a result of cellular metabolism that plays a central role in numerous processes in cells, and dysregulation of this species can result in a number of different disease states in human cells. In the case of cancer, elevated metabolism is believed to result in higher rates of H202 production in these cells, as well as more susceptibility to H202-induced apoptosis than normal cells. To this end, researchers have identified several therapeutic compounds that are believed to kill cancer cells via the intracellular elevation of one or more oxidants. However, due to the limitations of current tools for detection of these species, little is known about which therapeutic compounds induce toxicity via elevation of specific oxidants, which would aid in the
identification of susceptible tumors to these treatments.
Currently, the main limitation of genetically-encoded tools for detection of H202 in these
applications is the low sensitivity to H202. Most genetically-encoded probes for this species used in human cells utilize H202-responsive domains with reaction rate coefficients nearly two orders of magnitude lower than other, more reactive peroxidases in the cell, such as peroxiredoxins (Prxs). In this regard, several studies have demonstrated that Prxs should react with the majority of intracellular H202 on the basis of a high reaction rate coefficient with H202 and intracellular abundance.
In light of these studies, research in the field of redox biology has shifted to focus more on Prxs' role as natural sensors of H202 fluctuations in human cells. To this end, the first part of my thesis project focuses on the development of a genetically-encoded probe for H202-mediated human Prx2 oxidation in human cells. The second part of my thesis focuses on the application of this probe in a high-throughput screen to identify small-molecule cancer therapeutics that act through H202-mediated mechanisms. Together, this thesis lays the foundation for a new class of
genetically-encoded sensors that enable specific, sensitive measurement of H202 perturbations in human cells in response to redox-based therapeutics, which will facilitate the advancement of these therapeutic compounds in the future.
Thesis Supervisor: Hadley Sikes
Title: Associate Professor and Esther and Harold E. Edgerton Career Development Professor of Chemical Engineering
ACKNOWLEDGEMENTS
The work contained in this thesis document would not have been possible without the guidance and support of my advisor, friends, lab-mates, and family. From the bottom of my heart, thank you to each and every one of you.
To my thesis advisor Hadley Sikes, thank you for believing in me and giving me the opportunity to work in your lab. Words cannot describe how much I appreciate all the time you devoted to my work, as well as all the valuable feedback that you gave me every step of the way in this process. You taught me how to think carefully and critically about my work, and always encouraged me to push forward with even when I felt uncertain about my progress. Without this help, I would not have accomplished nearly as much as I did. To my family, thank you for your unconditional support, and believing in me even when I didn't always believe in myself. Your constant words of encouragement helped me stay positive even when I felt at my lowest over this five-year period.
To my friends at MIT, thank you all for the fun experiences in Cambridge and Boston over the past five years. I will always remembers all the good times we had at barbeques, game nights, and other social events, and I hope I have the opportunity to share many more experiences with you all in the future.
Last but not least, to Katie Browning, thank you for standing by my side throughout this journey, and dragging me out of lab just long enough to remind me that there is much more to
life than graduate school. I have sincerely enjoyed all of our movie dates, last-minute day trips, and lazy days over the past several years, and I look forward to many more in the years to come.
TABLE OF CONTENTS A B S T R A C T ... 2 ACKNOWLEDGEMENTS... 3 L IST O F T A B L E S ... 7 L IST O F F IG U R E S ... 8 1. IN T R O D U C T IO N ... 11 1.1. A b stract...1 1 1.2. Hydrogen peroxide as a key mediator of human cell function... 11
1.3. Pro-oxidant small-molecule cancer therapeutics ... 13
1.4. Current methods for detection of hydrogen peroxide... 15
1.5. Overview of peroxiredoxin catalytic cycle... 18
1.6. Overview of thesis objectives... 19
2. PEROXIREDOXINS AS A SENSITIVE MEASURE OF H202 LEVELS IN HUMAN C E L L S ... 2 1 2 .1. A b stract...2 1 2 .2 . Introdu ction ... 2 1 2.3. Materials and Methods ... 22
2.3.1. Overview of kinetic model... 22
2.3.2. Intracellular generation of H202 with D-amino acid oxidase (DAAO) and detection of HyPer fluorescence...23
2.3.3. Gel electrophoresis and Prx2 western blots...23
2 .4 . R esu lts...2 4 2.4.1. Comparison of reactivity of different molecular targets for H202 in cytosol of human epithelial cells ... 24
2.4.2. Implications of Prx-mediated H202 clearance for H202-mediated signal transduction in cytosol of human epithelial cells ... 26
2.4.3. Measurement of Prx2 oxidation in response to DAAO-mediated generation of intracellular H202 ... .. . . .. . . .. . . . .. . . .. . . .. . . .. . . .. . . 29
2 .5 . D iscu ssion ... 30
3. DESIGN AND CHARACTERIZATION OF GENETICALLY-ENCODED PROBE FOR HUMAN PEROXIREDOXIN-2 TO MONITOR THE ACTION OF REDOX-BASED CANCER THERAPEUTICS... 33
3 .1. A b stract...3 3 3 .2 . Introdu ction ... 33
3.3. Materials and Methods ... 34
3.3.1. Construction of fluorescent probe. ... 34
3.3.2. Cell culture and production of stable cell line for expression of probe...35
3.3.3. Characterization of FRET probe reactivity in human cells. ... 35
3.3.4. Characterization of cytosolic FRET probe dose response to H202. ... 35
3.3.5. Production of intracellular hydrogen peroxide from D-amino acid oxidase...36
3.3.6. Treatment with auranofin and piperlongumine. ... 36
3.3.7. Selenocystine assay for thioredoxin reductase activity. ... 36
3.3.8. Gel electrophoresis and peroxiredoxin-2 oxidation western blots. ... 37
3.3.9. Fluorescent measurements and image analysis. ... 37
3.3.10. Statistical Analysis. ... 38
3.4.1. Construction of genetically encoded probe to measure human Prx2 oxidation and characterize the sensitivity of the purified probe to various oxidants and
environm ental factors ... 38
3.4.2. Expression of the fluorescent construct in the cytosol of human epithelial cells and characterization of the response of the probe to various H202 bolus additions..40
3.4.3. Characterization of the response of the probe to intracellular H202 generation as well as redox-active small molecules... 44
3 .5 . D iscu ssion ... 49
4. APPLICATION OF GENETICALLY-ENCODED PROBE FOR PEROXIREDOXIN-2 OXIDATION IN HIGH-THROUGHPUT SCREEN FOR H202-MEDIATED CANCER T H E R A P E U T IC S ... 52
4 .1. A b stract...52
4 .2 . Introdu ction ... 52
4.3. M aterials and M ethods ... 53
4.3.1. Generation of stable cell line for expression of Prx2-based probe and design of high throughput screens... 53
4.3.2. Gel electrophoresis and peroxiredoxin-2 immunoblots ... 54
4.3.3. Cell viability assay in response to small-molecule compounds ... 54
4.3.4. Mitochondrial localization and depolarization assays...55
4.3.5. Immunocytochemistry of S-glutathionylated proteins ... 55
4.3.6. Cell viability assay in response to small-molecule compounds in different hum an cancer cell lines ... 55
4.3.7. Fluorescent signal quantification and statistical analysis ... 55
4 .4 . R esu lts...56
4.4.1. Design and implementation of the high-throughput screens ... 56
4.4.2. Three compounds identified from high-throughput screen for small molecule compounds that elevate intracellular H202... . . . . .. . . . .. . . .. . . . .. . . .. . . . 58
4.4.3. Six compounds identified from high-throughput screen for small molecule compounds that inactivate human Prx2...60
4.4.4. Increased peroxiredoxin hyperoxidation observed in response to selected compounds from the high-throughput screen...62
4.4.5. Increased protein s-glutathionylation observed in several selected compounds from high-throughput screen ... 63
4.4.6. Cytotoxicity of screen compounds in different human cancer cell lines...66
4 .5 . D iscu ssion ... 67
5. MEASUREMENT OF PEROXIREDOXIN-2 AND PEROXIREDOXIN-3 OXIDATION IN PATIENT-DERIVED TUMORS ... 71
5 .1. A b stract...7 1 5.2 . Introdu ction ... 7 1 5.3. M aterials and M ethods ... 73
5.3.1. Tum or m odel ... 73
5.3.2. Extraction of intracellular protein from patient-derived xenograft model tumor ... 7 3 5.3.3. Gel electrophoresis and Prx oxidation western blots ... 73
5 .4 . R esu lts...73
5.4.1. Effect of lysis-induced oxidation on Prx oxidation status in human tumor sam p les ... 7 3 5.4.2. Effect of alkylating agent and lysis conditions on measurement of Prx2 and Prx3 oxidation in xenograft model GIST ... 74
5.5. Discussion...78
6. CONCLUSION S AND FUTURE DIRECTIONS ... 80
6.1. Abstract...80
6.2. Kinetic models for H202 clearance H202-mediated signal transduction in HeLa cells. ... 8 0 6.3. Peroxiredoxin-based sensors for H202 in hum an cells ... 80
6.4. Application of the genetically-encoded probe for identification of H202-mediated cancer therapeutics...81
6.5. Measurement of Prx2 and Prx3 oxidation status in patient derived gastrointestinal strom al tum ors (GISTs)... 82
7. REFERENCES ... 83
8. APPENDIX A ... 102
8.1. MATLAB Code for H202 clearance model in the cytosol of HeLa cells...104
8.2. Additional simulation details...108
8.3. Sensitivity analysis plots...111
8.4. Calculation of concentration of reduced and oxidized STAT3 ... 118
8.5. Calculation of relative rates of reaction...118
8.6. Calculation of diffusion controlled rate constant...119
8.7. MATLAB code for STAT3 oxidation simulation (Model A): ... 120
8.8. MATLAB code for STAT3 oxidation simulation (Model B): ... 124
9. APPENDIX B ... 130
LIST OF TABLES
Table 1.1. Advantages and disadvantages of main non-peroxiredoxin based probes for
detection of intracellular H 20 2... . . .. . . .. . . .. . . . .. . . . .. . . .. . . . .. . . 17
Table 2.1. Reactivity of different intracellular species with H202 found in the cytosol of HeLa
c e lls...2 5
Table 2.2. Effect of intracellular concentration of H202-reactive species on effective rate coefficient from the m odel... 26 Table 8.1. Reactions studied in the kinetic model of intracellular hydrogen peroxide
con sum p tion ... 102 Table 8.2. Initial concentration of species in kinetic model ... 103 Table 8.3. Initial concentrations of species in Prx-dependent H202 reduction pathway and
STAT3 redox relay pathway simulated in kinetic model. ... 108 Table 8.4. Rate coefficients used for key reaction expressions in Prx2-dependent H202
reduction pathway and Prx2-STAT3 redox relay pathway used in kinetic model.... 108 Table 8.5. Concentrations of oxidized and reduced STAT3 in response to 1.25 x 10-1 mol
H 20 2/cell...109 Table 8.6. Summary of values used in the estimation of the diffusion-limited reaction
coefficient for the reaction between Prx2-SS and reduced STAT3...111 Table 8.7. Effect of different reduced Trx initial concentrations on predicted STAT3
oxidation rate coefficient from the m odel...117 Table 8.8. Effect of different reduced Trx initial concentrations on relative rate of
Prx2-STAT3 reaction compared with Prx2-Trx reaction ... 117 Table 8.9. Effect of STAT3 copy number on calculated concentration of oxidized STAT3 at
t= 12 0 s...1 17 Table 10.1. Signal fold change for compounds in first high-throughput screen...140 Table 10.2. Signal fold change for compounds in second high-throughput screen...145
LIST OF FIGURES
Figure 1.1. Schematic representation of major sources and sinks of intracellular H202 in
hum an cells. ... 13
Figure 1.2. Biological basis of preferential death of cancer cells upon addition of H2 02-m ediated therapeutics... 14
Figure 1.3 Oxidation of reactive peroxiredoxin thiol group to sulfenic acid...18
Figure 1.4 Catalytic cycle of peroxiredoxin-2...19
Figure 2.1. Kinetic model for H202 clearance in the cytosol of HeLa cells...24
Figure 2.2. Overview of expanded kinetic model for H202 clearance in cytosol of HeLa ce lls. ... 2 7 Figure 2.3. Simulated STAT3 oxidation over time... 27
Figure 2.4. Sensitivity analysis of expanded kinetic model...28
Figure 2.5. Simulation of HyPer and Prx response to DAAO-mediated cytosolic H202 gen eration ... 2 9 Figure 2.6. HyPer and Peroxiredoxin-2 oxidation over time in response to DAAO-mediated intracellular H 202 generation... 30
Figure 3.1. Design of genetically-encoded probe for H202-induced Prx2 oxidation. ... 39
Figure 3.2. Characterization of purified fluorescent construct. ... 40
Figure 3.3. Characterization of fluorescent construct in the cytosol of HeLa cells...41
Figure 3.4. Concentration-dependent kinetics and magnitude of the fluorescent response to stim ulation w ith H 20 2... 43
Figure 3.5. Effect of fluorescent Prx2 probe on H202 peroxidase activity of native Prx2 in the cytosol of H eLa cells. ... 44
Figure 3.6. Sensitivity of Prx2-based probe compared to HyPer in response to D-amino acid oxidase (DAAO)-generated cytosolic H202.... . .. . . . .. . . .. . . .. . . . 45
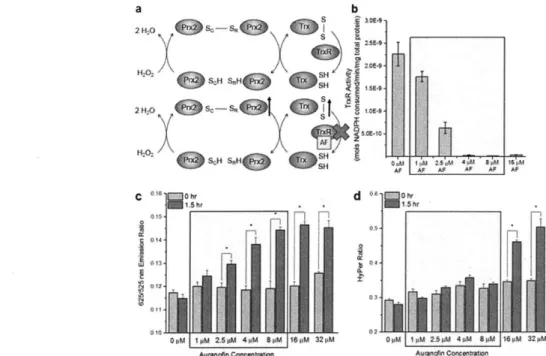
Figure 3.7. Effect of thioredoxin reductase inhibitor auranofin (AF) on response of fluorescent probe... 47
Figure 3.8. Response of Prx2-based probe and HyPer to the redox therapeutic compound piperlongum ine (PL)... 48
Figure 4.1. High-throughput screens to identify compounds that act through H202-mediated toxicity m echanism s... 57
Figure 4.2. Summary of results from high-throughput screen for small molecule compounds that elevate intracellular H202 with fluorescent, genetically-encoded sensor for human peroxiredoxin-2 oxidation in live Hela cells...59
Figure 4.3. Summary of results from high-throughput screen for small molecule compounds that inactivate human Prx2 with fluorescent, genetically-encoded sensor for human peroxiredoxin-2 oxidation in live Hela cells...61
Figure 4.4. Hyperoxidation of peroxiredoxin proteins by DHT and PB. ... 63
Figure 4.5. Intracellular glutathione content and protein S-glutathionylation in response to selected compounds from screens...65 Figure 4.6. Cytotoxicity of selected compounds from screens in HeLa, A549, and HT-29
Figure 5.1. Effect of lysis-induced oxidation on Prx oxidation status in human tumor samples.
... 7 4
Figure 5.2. Effect of alkylating agent identity and alkylating agent concentration on Prx dimerization status in GIST biopsies...75 Figure 5.3. Effect of freeze-thaw process on Prx dimerization status in homogenized GIST
b iop sies. ... 76
Figure 5.4. Comparison of oxidized and reduced Prx2 and Prx3 levels in patient-derived xenograft GIST biopsies and HeLa cell samples ... 77 Figure 8.1. Sensitivity of oxidized STAT3 concentration to decreases in key model reaction
rate coefficients...112 Figure 8.2. Sensitivity of oxidized STAT3 concentration to increases in first 11 model
reaction rate coefficients...113 Figure 8.3. Sensitivity of oxidized STAT3 concentration to increase in last 11 model reaction
rate coefficients...113 Figure 8.4. Sensitivity of oxidized STAT3 concentration to decrease in first 11 model reaction rate coefficients...114 Figure 8.5. Sensitivity of oxidized STAT3 concentration to decrease in last 11 model reaction
rate coefficients...114 Figure 8.6. Sensitivity of oxidized STAT3 concentration to increase in first 12 model species
initial concentrations...115 Figure 8.7. Sensitivity of oxidized STAT3 concentration to increase in last 10 model species
initial concentrations...115 Figure 8.8. Sensitivity of oxidized STAT3 concentration to decrease in first 12 model species
initial concentrations...116 Figure 8.9. Sensitivity of oxidized STAT3 concentration to decrease in last 10 model species
initial concentrations...116 Figure 9.1. Normalized peak fold change of Prx2 based probe and HyPer in response various
H 20 2 bolus additions...130 Figure 9.2. Fluorescent response of Prx2 probe after stimulation with subsequent bolus
addition s of H 20 2... . .. . . . .. . . . .. . . . .. . . .. . . . .. . . .. . . . .. . . .. . .130
Figure 9.3. Fluorescent signal from Prx2-based probe over time upon exposure to a 20 IM H 20 2 b o lu s. ... 13 1 Figure 9.4. Hyperoxidized Prx western blot of whole cell lysates from HeLa cells stably
expressing the Prx2-based probe. ... 131 Figure 9.5. Prx2 western blot of whole cell lysates from HeLa cells stably expressing the
Prx2-based probe. ... 132
Figure 9.6. Blue Native PAGE of cytosolic fraction of HeLa cell lysates followed by western blotting for Prx2-based probe molecules. ... 132 Figure 9.7. DAAO-mediated-H202 production rate in HeLa cell lysate. H202 production in
cell lysate for seven different D-alanine concentrations...133 Figure 9.8. Fluorescent signal from Prx2-based probe and HyPer on the minutes timescale
after bolus addition of either D-alanine or medium only to cultures of cells expressing D -am ino acid oxidase. ... 133 Figure 9.9. Auranofin-induced oxidation of Prx2 in HeLa cells. ... 134
Figure 9.10. Selenocystine assay for measurement of thioredoxin reductase activity in HeLa cell lysates treated w ith auranofin...134 Figure 9.11. Response of Prx2-based probe to the redox therapeutic compound
piperlongumine in HeLa cells with or without additional cytosolic catalase. ... 135 Figure 9.12. Estimation of intracellular probe concentration from purified protein
fluorescence standard curve and lysate probe fluorescence. ... 135 Figure 10.1. Signal fold change from fluorescent, genetically-encoded probe for human
peroxiredoxin-2 oxidation immediately after 4 hours of incubation with library com p oun ds...136 Figure 10.2. Over-expression of catalase and sulfiredoxin in the cytosol of HeLa cells. ... 136 Figure 10.3. Glutaredoxin-based assay for detection of reversible protein S-glutathionylation.
... 13 7
Figure 10.4. Cytosolic protein S-glutathionylation in response to small-molecule cancer therapeutics identified in high-throughput screen...137 Figure 10.5. Fraction of live cells after 24 hours of incubation with 10 IM SMER3 for
KEAP-1 null p53/kRas mouse model cell line or a control p53/kRas mouse model cell
lin e ... 13 8
Figure 10.6. Characterization of thioredoxin reductase inactivation of dihydrotanshinone and
p lum b agin . ... 138
Figure 10.7. Prx2 oxidation in HeLa cells in response to various concentrations of epirubicin.
1. INTRODUCTION 1.1. Abstract
Hydrogen peroxide (H202) is a key oxidant species in biological organisms that can affect
numerous different processes in human cells such as homeostasis, growth, apoptosis, and necrosis. It is thought that dysregulation of this species can result in the occurrence of several disease states in human cells such as cancer. In that respect, it is believed that cancer cells exhibit much higher rates of production of intracellular H202 compared to normal cells, and that certain cancer cell lines have higher susceptibility to small-molecule compounds that further elevate intracellular H202. However, due to limitations of current tools to detect this
species in human cells, it remains difficult to measure slight changes in H202 levels in these
systems, as well as the effects of small molecule species that perturb the levels of this species. This chapter gives an overview of the biology of H202 in human cells, as well as current
cancer therapeutics that are believed to act through modulation of intracellular H202 and other oxidants. Current tools used to detect this species in biological organisms are also
highlighted. The chapter ends with an overview of the objectives of this thesis, which aims to lay the foundation for the next generation of ultra-sensitive H202 sensors.
1.2. Hydrogen peroxide as a key mediator of human cell function
For many years, researchers have used the term "reactive oxygen species" or "ROS" to describe a plethora of different oxygen-based species that act in biological organisms that include superoxide (02-), hydrogen peroxide (H202), hydroxyl radical (OHO), and many
others- 3. While this term ostensibly suggests that these species have similar functions in biological organisms, several studies have demonstrated that these species act in distinct ways in human cells due to their disparate chemical properties 1 4'. For example, negatively charged species such as 02 cannot passively diffuse across the lipid bilayers that surround many intracellular compartments due to the presence of polar chemical groups on the outer
surface of membranes that repel other charged species; as a result, charged species such as 02- typically only act in the compartment from which they originate.
In humans cells, mitochondria produce 02- as a natural by-product of aerobic respiration',. In this process, several proteins work in tandem to pass electrons through the various
components of the electron transport chain (located in the inner mitochondrial membrane of the cell), which results in the formation of a proton gradient across the membrane and creation of ATP. During this process, electrons can escape from iron-sulfur clusters and flavin groups located at the different sites, and combine with molecular oxygen to form 02- .
Electrons can also escape from the electron transport chain as they move between the different sites. Ubiquinone (also known as coenzyme
Q)
acts to shuttle pairs of electrons from complex I and complex II in the mitochondrial electron transport chain to complex III. Upon donation of the first electron from either complex I or complex II to complex III, the radical species known as ubisemiquinone forms'. As this protein waits to deliver the second electron to complex III, molecular oxygen can react with the radical species to formsuperoxide in the mitochondrial matrix9. In addition to these instances of natural electron leakage, defects in the mitochondrial electron transport chain or small-molecules that interfere with this process can result in further leakage of electrons from the electron transport chain, which can result in even more 02- formation8'1 0.
In addition to this process, cells can also control 02- production through the action of enzymes such as NADPH oxidases (NOXs)'1' 1,12. These enzymes localize to the plasma membrane as well as other biological membranes that surround intracellular compartments
such as the endoplasmic reticulum, mitochondria, and nucleus, and they facilitate the
reduction of molecular oxygen to superoxide (through oxidation of NADPH) as part of large multi-protein complexes". In normal cells, these enzymes can facilitate activation of cellular proliferation reaction pathways in response to growth stimuli such as epidermal growth factor and platelet-derived growth factor; however, in certain cancer types these enzymes can facilitate cell survival through the inhibition of reaction pathways that promote tumor cell apoptosis, and researchers have also linked their activity to other tumor-supportive processes
such as glucose uptake and angiogenesis'3
. Aside from these NOX-mediated processes, human cells can also generate intracellular 02- through the action of several other enzymes,
such as xanthine oxidases and certain monooxygenases (e.g. cytochrome P450)1"1 4.
Upon 02 formation, nearby superoxide dismutase rapidly converts this species to H2027'8,
which proceeds at rates that approach the diffusion-controlled limit for this reaction pair5. In
addition to this process, cells can also directly generate H202 in the endoplasmic recticulum
as a result of disulfide bond formation during folding of newly expressed proteins1. Once
H202 forms, it can react specifically with certain thiol groups on proteins to selectively alter
their function'6-18. H202 first reacts with the thiol group to form water and a sulfenic acid
group, which can then form a disulfide bond upon condensation with another thiol group19'20
These disulfide bonds can alter the three-dimensional structure of a protein, which can lead to
changes in function721 22. In many cases, these changes can lead to activation/deactivation of
the protein function, as occurs in certain kinases, phosphatases, and transcription factors232
At low intracellular levels of H202, this species typically promotes pro-growth pathways,
whereas at higher intracellular levels, H202 can lead to apoptosis or necrosis1,17,18,26,2 7
.
Ateven higher intracellular concentrations of H202, this species can react with Fe+2, which can
then form OH* through Fenton chemistry; once OH. forms, it can react readily with many
biomolecular species, such proteins, lipids, and nucleic acids in the context of the cell1. This
uncontrolled reactivity results in widespread oxidation of biological molecules, disruption of key cellular functions, and eventually cell death'".
In order maintain intracellular H202 concentrations below toxic levels, cells utilize a
powerful network of antioxidant species. In the cytosol of human cells, these antioxidant species include glutathione (GSH), glutathione peroxidases (Gpxs), and peroxiredoxins
(Prxs)28,29. In addition to these species, several other intracellular redox-regulated proteins
such as several kinases, phosphatases, and transcriptions factors, have cysteine residues that
can also react with H202 in theory"'6. Upon formation of H202 in the cell, these species
engage in a kinetic competition in which only the most reactive species consume H202, and
of these species, peroxiredoxins 1 and 2 (Prx 1 and Prx2) consume the vast majority of
cytosolic H202 on the basis of the combination of their high second order-rate coefficient for
their reaction with H202 as well as their intracellular abundance, 29. A depiction of some of
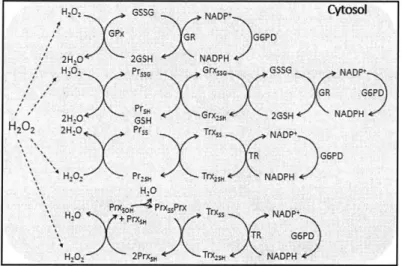
the major sources and sinks for H202 in human cells is shown in Figure 1.1 (adapted from
H202 -Miocoora ten 02 2 H20 (:: H D, Liid HAN Key Electron =Transport =a Dismutase soNADPH Oxidase = Peroxiredoxin Pr =H, 20-reactive Protein
Figure 1.1. Schematic representation of major sources and sinks of intracellular H202 in human cells.
In healthy cells, the sources and sinks of intracellular H202 maintain a balance and ensure tight control of the intracellular level of H202. Dysregulation of either of these components can lead to several disease states in cells. In particular, dysregulation of intracellular H202 has been suggested to play a role in diabetes30, cardiovascular disease3 1, neurodegenerative diseases32,33, and several other debilitating conditions. In addition to these disease states, H202 has been suggested to play a major role in cancerous tumor formation and growth
8,13,34,35. Due to the fact that many cancer cells exhibit elevated metabolism compared to
normal cells, they are believed to produce higher amounts of free electrons from the mitochondrial electron transport chain, and consequently also higher fluxes of both 02- and H20 2
3 5
. These oxidants can also result in formation of dangerous radical species such as OH*, which can damage DNA and result in mutations to proteins that can cause further increase in oxidant fluxes36
. In order to combat the elevated rates of productions of these oxidants, many cancer cells also upregulate key antioxidant species such as peroxiredoxins, thioredoxin reductase, and glutathione reductase, which are believed play key roles in the defense against oxidant-induced cell death'0'37-4.
1.3. Pro-oxidant small-molecule cancer therapeutics
In light of the fact that many cancer cells are believed to have much higher rates of
production of oxidant species such as 02~ and H202, and as well as an elevated dependence on various antioxidant proteins for survival, many researchers have theorized that small molecule compounds further increase production of oxidant species from intracellular sources such as the mitochondria, or target the anti-oxidant defense systems, could form the basis of an effective anti-cancer strategy42. Several researchers have hypothesized that these small-molecule compounds can elevate intracellular levels of key oxidants species above the toxicity threshold for cancerous cells, while non-cancerous cells, which are thought to produce much lower levels of these oxidant species, remained relatively unharmed by the therapeutic compound10. The general idea behind these pro-oxidant cancer therapeutics is shown below in Figure 1.2.
Homeostasis
Tumorgenesis
Death
Normal
Basal
cells
Cancer
cells
Basal
I
H
20
2-mediated
therapeutic
Intracellular
H202Figure 1.2. Biological basis of preferential death of cancer cells upon addition of
H202-mediated therapeutics.
Over the past several years, several studies from different research groups have suggested that oxidative species play a role in the toxicity mechanism of several different small-molecule compounds. For example, previous studies have suggested that several
anthracyclines (e.g. doxorubicin and daunorubicin) induce oxidant production in human
cancer cells4 5, and other studies have shown that the well-known anti-cancer agents cisplatin
and arsenic trioxide both elevate oxidants in human cancer cells46'47. In addition to these
examples, many polysulfide-based compounds can also increase intracellular oxidant levels
through reactions with reactive disulfides or thiol groups48'49, and several studies have
demonstrated that many naturally-derived small-molecule compounds can also cause
elevation of intracellular oxidants in human cells5 -54.
While the exact mechanism of action of most of these small-molecule compounds remains unknown, many of these compounds contain reactive pharmacophores which are thought to
generate intracellular oxidant species through a process known as "redox-cycling" 1,43. In this
process, specific reductase enzymes within the cell catalyze the one-electron reduction of quinone species to a semiquinone radical species that can then react with molecular oxygen to
form O2 .43. Paraquat and other related compounds act through this mechanism, but due to
their indiscriminate action in both cancerous and non-cancerous cells these compounds have
experienced very limited success in the clinic' 43. Other enzymes can detoxify quinone-based
species through conversion of the quinone to a stable hydroquinone species that does not contain a free electron; however, certain hydroquinone species can also undergo subsequent
conversion to semi-quinone species, which can then react with molecular oxygen to form 02~
". One of these reductase enzymes, NADPH:quinone oxidoreductase 1 (NQO1), has received much attention in recent years due to apparent dependence that many cancer cell have on this
enzyme. In particular, several research groups have noted overexpression of this enzyme in
the tissue of several different forms of cancer" 58, and other groups have linked NQO 1
activity in these cells to enhanced cellular proliferation, metastasis, and poorer prognosis
55,59,60. In light of these observations, several research groups have hypothesized that
small-molecule quinone species that act through NQO 1-mediated mechanisms may have enhanced anti-cancer potential due to their ability to stimulate the production of large amounts of O2~
and H202. Several studies have shown that the small molecule P-lapachone induces selective
cancer cell death through NQO 1-mediated oxidant production6
1,62
, but poor solubility in aqueous solvents and unfavorable bioavailability has somewhat limited its therapeutic potential of this compound. Recently, however, Zhao et al. reported a novel NQO 1-mediated
small-molecule therapeutic that has more potency than f-lapachone and better solubility in aqueous solvents63
Many small-molecule compounds that induce oxidant formation in cells also contain reactive electrophilic groups that affect cellular function through reaction with molecular targets at nucleophilic sites. Many of these small-molecule species can react with intracellular antioxidant species that contain reactive cysteine residues, such as Prx or TrxR, and form covalent adducts at the site of the cysteine residue4 364. Upon inhibition of these antioxidant species, oxidants can accumulate inside the cell, which can eventually result in cell death. Over the past several years, a handful of studies have proposed small-molecule inhibitors of Prxs that act through this covalent modification mode of action61,66, but these compounds
likely have reactivity with many other species in cells3764. In particular, many of these compounds have a very high affinity for TrxR, which contains a selenocysteine residue that reacts much more readily with electrophilic compounds than most cysteine residues67, and has led to further investigation of the therapeutic potential of TrxR inhibition. As with NQO 1, several studies have demonstrated that many types of cancer contain elevated levels of this TrxR67-70, and researchers have hypothesized that cancer cells heavily rely upon this enzyme to combat the elevated levels of oxidants that result from rapid cellular proliferation67'71. Previously, many research groups have utilized the gold-based compound auranofin as an inhibitor of TrxR and an anti-cancer agent72
-74
. However, several studies have revealed that this compound reacts with several other targets in cells75'76, which can result in toxic side effects non-cancerous cells exposed to this compound. Other groups have identified several other inhibitors for this enzyme77
-79, but none of these compounds have achieved widespread
success in the clinic. Recently, however, Stafford et al. employed a high-throughput screen approach to identify small-molecule compounds with higher specificity for the cytosolic form of the enzyme67, which could have much lower off-target toxic effects in normal cells. Despite the numerous accounts of small-molecule compounds that reportedly elevate oxidant species in human cells, very few of these compounds have had much success in terms of clinical benefit to patients. While many of these compounds display well-defined reactivity with certain molecular targets in isolated systems with purified proteins, these compounds likely undergo several different reactions in the context of the human body. As a result, researchers have very little information about which of these compounds enact cancer cell toxicity through elevation of specific intracellular oxidants, and which compounds enact toxicity through other means. This lack of information limits clinicians' ability to develop optimized treatment regimens for patients with tumors with potentially higher susceptibility to oxidant-mediated therapeutics. As a result, only a small subset of patients respond positively upon administration of these small-molecule therapeutic compounds.
1.4. Current methods for detection of hydrogen peroxide
Currently, researchers measure H202 in biological contexts via one of two approaches:
small-molecule detection systems and genetically-encoded (i.e. protein-based) detection systems. Small-molecule detection systems can be further divided into colorimetric assays for detection of extracellular H202 and small-molecule probes for measurement of intracellular H202. Most colorimetric assays for detection of extracellular H202 rely upon horseradish peroxidase, which can oxidize several different substrates in the presence of H202. Many of
these horseradish peroxidase-based assays use either 1 0-acetyl-3,7-dihydroxyphenoxazin (Amplex Red) or azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as substrates, which results in the formation of either a red or green oxidation product, respectively8 -83.
Due to the ease of detection of these colored products, researchers have extensively used these assays to measure extracellular H202 in various systems82-84. However, due to the
irreversible nature of these assays, these assays do not report on changes in H202 levels but
rather accumulation of H202 over time.
In contrast to these colorimetric systems, small-molecule sensors typically include chemical probes that contain a fluorescent core as part of the structure that changes fluorescence upon reaction with oxidants. The oxidized molecule can then undergo reduction by intracellular reductants (e.g. glutathione) in order to regenerate the original sensor molecule. Examples of . this type of sensor include dichlorodihydrofluorescein (DCFH2), dihydrorhodamine, and
several other compounds0,81. Importantly, many of these probes do not react directly with specific oxidant species in cells. Instead, these probes undergo an oxidation process mediated by a number of different free radical species and metal catalysts80 81. As a result, these probes do not detect intracellular levels of specific oxidant species, but rather reflect changes in the intracellular concentrations of a number of different oxidant species in cells, as well as the concentrations of metal catalysts species that mediate the oxidation reaction. Several of these probes also produce 02 (and therefore also H202) as part of their reduction mechanism,
which can lead to signal amplification and misinterpretation of probe fluorescence80'81 More recently, several alternative small-molecule sensors were developed to avoid some of the issues of other previous small-molecule probes. Most notably, the Chang group developed a series of small-molecule probes (e.g. Peroxy Green) that contain a boronate group that dissociates from the rest of the probe structure upon oxidation with specific oxidant species to reveal a fluorescent core 5. While these boronate probes, do react with H202 in isolated
systems, subsequent characterization of these probes revealed that they also react with peroxynitrite and hypochlorous acid 5'8 6. More importantly, the rate constant for reaction of these probes with H202 (k 1 I
M-1s-1
) is nearly ten thousand times slower than the rate constant for the reaction with hypochlorous acid (k ~ lx104 M-1s-1) and one million times slower than the rate constant for the reaction with peroxynitrite (k ~ 1x106 M-s-1)80. As a result, these probes likely have a much higher selectivity for peroxynitrite and hypochlorous acid over H202. In addition to the boronate-based probes, several fluorescein-based probes
have been developed based on a similar mechanism of action7'88. However, these probes also react relatively slowly with H202 compared to other species, and thus have similar issues as the boronate probes80.
In addition to the low specificity for H202 of most small-molecule probes, these intracellular
probes must compete with several other intracellular species for the oxidant of interest. In the case of H202, small-molecule probes must compete with H202-sensitive transcription factors,
kinases, phosphatases, and scavengers, which can react with intracellular H202 with second order rate coefficients as high as lx 10' M-1s-' and with relatively high specificity'. In light of this fact, several research groups started to develop fluorescent probes for different oxidants based on these oxidant-sensitive protein domains. In 2006, Belousov et al. reported the first protein-based sensor for H202, dubbed HyPer, which the authors based on the reactive
domain of the bacterial transcription factor OxyR8 9. In order to make a functional fluorescent probe based on this reactive domain, the authors of this study inserted a circularly permuted yellow fluorescent protein domain (a type of fluorescent protein in which the original N- and C-termini of the fluorophore are fused together and the P-barrel structure is broken open to introduce new termini) between the two halves of the OxyR reactive domain. Upon H202-mediated oxidation of the reactive thiol group on the first half of the OxyR domain to a sulfenic acid moiety, a disulfide bond can form between the sulfenic acid group and adjacent
thiol group on the other half of the OxyR domain89'90. A conformational change in the circularly permuted fluorophore domain (abbreviated here as cpYFP) ensues as a result of this disulfide bond formation, which causes a change in the fluorescent properties of the fluorophore89 9 0. While this design presented a significant advantage of prior probes due to the specificity of reaction with H202 and the large signal fold change upon oxidation with
H202, the circularly permuted structure of the fluorophore resulted in sensitivity to
environmental factors such as pH, which could lead to misinterpretation of the signal89 9 1. While HyPer still remains a useful tool for the study of H202 in biological organisms, the use of pH controls remains crucial for rigorous interpretation of the signals obtained from this probe.
In order to mitigate this disadvantage, several research groups developed alternative genetically-encoded probes for H202 based on other designs. Most notably, the Dick group developed several alternate H202 probes based on the oxidation-sensitive fluorophore ro-GFP292
. In their first design, they fused the yeast peroxidase OrpI with roGFP2; upon H202 -mediated oxidation of the reactive thiol group on Orp 1, a disulfide bond forms between the newly formed sulfenic acid group and a thiol group on roGFP2, which eventually yields a change in the roGFP2 structure, which results in a change in the fluorescent properties of the
92 Wieti
protein .While this design solved the issue of pH sensitivity, this design (as well as HyPer's design) contained a responsive domain that reacted with H202 with a rate coefficient nearly two orders of magnitude lower than that of native peroxiredoxins, and thus did not have the requisite sensitivity to compete with the ultrasensitive H202 scavengers found in human cells9 3. More recently, Enyedi et al. developed two different FRET-based sensors for H202 for application in human cells (dubbed OxyFRET and PerFRET)94, but these probes also utilized responsive domains with low reactivity towards H202, and thus suffer the same issues
as OrpI-roGFP2.
Table 1.1. Advantages and disadvantages of main non-peroxiredoxin based probes for detection of intracellular H202
Probe Small-molecule or Advantages Disadvantages
Protein-based?
DCFH2 Small-molecule Easy to use; large signal fold Not specific to H202; can change artificially amplify signal
through generation of oxidant species
Peroxy Green Small-molecule Easy to use; higher specificity to High reactivity with both H202 compared to DCFH2 hypochlorous acid and
peroxynitrite
HyPer Protein-based Facile intracellular localization of Low sensitivity to H202; probe; large signal fold change; high pH sensitivity very high specificity for H202
OxyFRET and Protein-based Facile intracellular localization of Low sensitivity to H202; PerFRET probe; high specificity for H202; low signal fold change
low pH sensitivity
Orpl-roGFP2 Protein-based Facile intracellular localization of Low sensitivity to H202 probe; large signal fold change;
high specificity for H202; low pH sensitivity
1.5. Overview of peroxiredoxin catalytic cycle
This observation has led to the recent re-examination of the molecular targets of H202 in cells. Prxs have emerged as possible candidates for the basis of a more sensitive genetically-encoded sensor for H202 on the grounds of their relatively high rate coefficient for their reaction with H202 and intracellular abundance in many cell types91. 2-cysteine Prxs owe their high rate of reaction with H202 to the reactive cysteine residue in the active site of the enzyme9 5. The general mechanism for the reaction between H202 and the reactive cysteine residue in human Prx2 is shown in figure 1.3 (adapted from Wood et al.95). As seen in the figure, a catalytic base first removes the hydrogen from the thiol group on the catalytic cysteine. Next, the reactive thiolate group (which is stabilized by a neighboring amine group) attacks the oxygen in hydrogen peroxide to form a sulfenic acid group. Finally, the remaining oxygen from the hydrogen peroxide abstracts a hydrogen to regenerate the base and form water95.
n
Base: Base-H H Base: H
0 0
H HH
Cys - Sc H2N+ Cys - Sc---H2N+ Cys - ScOH H2N+
--Arg )\-Arg hArg
H2N H2N H2N
Figure 1.3 Oxidation of reactive peroxiredoxin thiol group to sulfenic acid.
After reaction with hydrogen peroxide and formation of the sulfenic acid in the catalytic cysteine of the peroxiredoxin, the sulfenic acid moiety then undergoes condensation followed by reduction. The complete catalytic cycle of 2-cysteine Prxs is shown in figure 1.4. First, a disulfide bond typically forms between the sulfur atom of the catalytic cysteine residue and the sulfur atom of the resolving cysteine along with the release of water. For human Prxl and Prx2, this cysteine originates from an adjacent Prx monomer, whereas for human Prx5, this cysteine residue originates from the same Prx monomer95. After disulfide bond, the oxidized peroxiredoxin dimer can then undergo reduction by either thioredoxin or another redox-regulated protein as part of a "redox-relay" complex22
The oxidized cysteine residue of Prxs can also undergo reaction with an additional molecule of H202 to form a "hyperoxidized" Prx20. After oxidation of the thiol group on the catalytic cysteine residue to a sulfenic acid group, the protein undergoes a conformational change that positions the sulfenic acid group near the thiol group of the resolving cysteine20
. This
disulfide bond locks the enzymes into the locally unfolded structure, and prevents any further reaction with H202; however, due to the relatively low rate coefficient for this process, the oxidized enzyme can react with another molecule of H202 before disulfide bond formation to form a sulfinic acid moiety, or with two molecules of H202 to form a sulfonic acid species96. The sulfinic acid can undergo reduction to a sulfenic acid via the action of sulfiredoxin, but the sulfonic acid species cannot undergo reduction, which can lead to the accumulation of hyperoxidized Prx species.
HH
202 H2 H202 H20rx2 H QI- 1 H C S ,H
Local
Reduced conformational Sutfiredoxin +
Thioredoxin change ATP
H20
Figure 1.4 Catalytic cycle of peroxiredoxin-2. Sc designates the catalytic thiolate group, SR designates the resolving thiol group, Prx-ScH designates the reduced form of the
peroxiredoxin, and Prx-ScOH designates the oxidized form of peroxiredoxin, and Prx-SC-SR-Prx designates the disulfide linked peroxiredoxin dimer.
Due to the relatively high reactivity of Prxs compared to other peroxidases, several new genetically-encoded probes for H202 have been developed with reactive domains based upon Prxs. In particular, two groups have developed new probes based on yeast and plant Prxs (designed for use in yeast and plant cells, respectively) 97-100, and the creators of the former probe recently demonstrated that this probe detects intracellular H202 below the detection limit of HyPer in yeast cells97. This observation suggests that a genetically-encoded probe for H202 based on a human Prx could also function as a sensitive detector of H202 in human cells.
1.6. Overview of thesis objectives
Aim 1: Evaluate reactivity of potential molecular targets of H202 with kinetic model of H202 clearance in HeLa cells
Aim 1.1 Use kinetic model of H202 clearance in the cytosol of human epithelial cells to compare the pseudo-first order rate coefficients of several potential molecular
targets of H202
Aim 1.2 Assess potential of Prx2 oxidation as a sensitive measure of intracellular H202 levels in HeLa cells
Aim 2: Build genetically-encoded probe to study human Prx2 oxidation in live human epithelial cells.
Aim 2.1 Construct genetically-encoded probe to measure human Prx2 oxidation, purify the fluorescent construct, and characterize the sensitivity of the purified probe to various oxidants and environmental factors (e.g. pH)
Aim 2.2 Express the fluorescent construct in the cytosol of human epithelial cells and characterize the response of the cytosolic probe to a range of hydrogen peroxide (H202) bolus additions
Aim 2.3 Characterize the response of the probe to intracellular H202 generation as well as redox-active small molecules and compare the fluorescent response of the probe to currently available genetically-encoded H202 probes.
Aim 3: Evaluate feasibility of high-throughput screen with genetically-encoded probe and screen cancer therapeutic library for compounds that act through H202-mediated toxicity mechanisms
Aim 3.1 Determine optimal parameters for high-throughput screen with genetically-encoded probe
Aim 3.2 Design high-throughput screen to identify small-molecule compounds that act through H202-mediated toxicity mechanisms in human epithelial cells
Aim 3.3 Determine effect of increased intracellular H202 on cytotoxicity with cell viability experiments
Aim 3.4 Characterize effects of increased intracellular H202 on Prx2 oxidation status and protein-glutathione conjugation status
Aim 4: Develop methodology to test the Prx oxidation status of patient-derived gastrointestinal stromal tumors (GIST) as a means to identify tumors with potential
susceptibility to H202 mediated therapeutics
Aim 4.1 Measure the relative fraction of oxidized Prx2 protein and oxidized Prx3 protein in patient-derived xenograft GIST models both with and without functional
succinate dehydrogenase
Aim 4.2 Determine effects of various pre-treatment methods on observed fraction of oxidized Prx species in patient-derived xenograft GIST samples.
2. PEROXIREDOXINS AS A SENSITIVE MEASURE OF H202 LEVELS IN HUMAN CELLS
2.1. Abstract
Human cells tightly control intracellular levels of H202 through a complex network of
oxidation and reduction reactions. While several intracellular species contain thiol groups that can react with H202, the relative reactivity of each of these species determines which
molecular target will consume the majority of H202. That said, numerous studies have
demonstrated that addition of H202 to cell culture results in oxidation of many relatively
unreactive thiols such as those on protein phosphatases or kinases. Researchers in the field have proposed several theories on how H202 can oxidize these less reactive species in the presence of highly reactive antioxidant species such as glutathione peroxidases and peroxiredoxins (Prxs), but the details of the molecular mechanisms of these processes
remains unclear. In this chapter, we first used a current kinetic model of H202 clearance from the cytosol of HeLa cells to calculate the relative reactivity of different molecular targets towards H202, which revealed that Prxs should consume the majority of H202 on the basis of a high second-order rate coefficient for their reaction with H202 and their intracellular abundance. We then modified this kinetic model to test a hypothesis related to peroxiredoxin-mediated signal transduction in HeLa cells. Finally, we used non-reducing SDS-PAGE followed by Prx2 western blots to demonstrate the sensitivity of Prx2 towards intracellular H202 generation. Together, these result in this chapter lay the groundwork for the
development of an ultrasensitive genetically-encoded probe for H202 for application in human cells, which is described in more depth in chapter 3.
2.2. Introduction
Elucidation of the molecular mechanisms through which hydrogen peroxide (H202) regulates cellular function remains a major challenge in the field of redox biology. Despite the
widespread presence of oxidation-sensitive cysteine residues in proteins throughout cels2 3
-25,101, H202 reacts far too slowly with the vast majority of these proteins to compete with
highly reactive antioxidant proteins that co-exist throughout the cell'62 1, such as glutathione peroxidases, peroxiredoxins, and catalase (which exists in the peroxisome)2 ,102. In recent years, a number of studies have suggested that peroxiredoxins consume essentially all of the H202 found in the cytosol on the basis of the combination of its high rate coefficient for reaction with H202 and its cytosolic abundance1',2 9,103-05
In spite of these studies, it is still unclear how oxidized peroxidase species can transmit these H20 2 signals to other proteins. One hypothesis is that oxidized peroxidase proteins can
oxidize secondary target proteins via a disulfide exchange reaction22,1 10'. In particular, Jarvis et al. demonstrated that human peroxiredoxin- 1 (Prx1) can transiently oxidize ASKI in response to a bolus addition of H202, and Sobotta et al. showed that that human
peroxiredoxin-2 (Prx2) can oxidize the transcription factor STAT3 in human cells in order to inhibit gene transcription in response to stimulation with H20209'110. In addition, Sobotta et
al. demonstrated that oxidized STAT3 forms disulfide-linked dimers and tetramers upon
oxidation by Prx2, and used non-reducing western blots to observe the dynamics of oxidized oligomer formation as a function of time in response to a bolus addition of H202. However,
the oxidation-reduction steps and molecular mechanisms that control the dynamics of these processes are not completely understood.
Kinetic models represent a powerful means to study the dynamics of these processes under specific conditions, as well as test hypotheses about different mechanisms that describe the
same reaction pathway. By representation of reaction steps mathematically and comparison of predicted dynamics with experimental data from the same system under the same set of conditions, one can estimate unknown parameters in the model consistent with the assumed mechanism. Currently, several mathematical models that describe the network of oxidation and reduction reactions involved in H202 metabolism inside human cells have been
reported28,29,102. However, none of these models explicitly consider disulfide exchange reactions between "first-responder" antioxidant proteins such as Prx 1 and Prx2 and other redox-regulated proteins.
In this study, we first utilized a current kinetic model of H202-clearance in the cytosol of HeLa cells to calculate the relative reactivity of different molecular targets for H202 found in these cells. We then modified this model to include reactions that describe H202-mediated STAT3 oxidation and reduction. We considered two cases to connect the data of Sobotta et al. to this model. First, we assumed that STAT3 oligomers formed rapidly after the initial
reaction between STAT3 and oxidized Prx2. Thus, the number of oxidized STAT3 oligomers formed equaled the number of Prx2-STAT3 mixed disulfides formed at each point in time (i.e. Prx2-mediated STAT3 oxidation was the slowest step in this process and limited all subsequent reactions). We then used this model to determine the expected dynamics of Prx2-mediated oxidation of STAT3 in response to external H202 stimulation under these
assumptions, as well as an estimate for the rate coefficient of STAT3 oxidation consistent with the model. Second, we assumed that oligomerization of STAT3 post-oxidation is the slower process and used a diffusion-limited value as an upper bound for the rate constant for reaction of oxidized Prx2 with reduced STAT3. We then used the predictions from each set of assumptions to estimate the relative rates of Prx2-mediated oxidation of STAT3 compared to other reactions that competed for the oxidized Prx2 substrate in the system, as well as the implications of these rates for H202-mediated signal transduction in human cells. In the final part of this study, we demonstrated the utility of non-reducing SDS-PAGE and Prx2 western blots to measure Prx2 oxidation in response to cytosolic H202 generation by the enzyme
D-amino acid oxidase. 2.3. Materials and Methods
2.3.1. Overview of kinetic model
In order to predict the concentration of different species in the cell in response to a transient increase in intracellular H202, a kinetic model for H202 oxidation and reduction reactions in
Jurkat cells originally developed by Adimora et al.28, and later adapted to human epithelial cells by Lim et al.2 9, was utilized. In all simulations with this initial model, the initial
concentration of cytosolic H202 was set to 100 nM and the model system was simulated for a total of 0.1 seconds with a step size of 0.0001 seconds.
In order to predict the concentrations of oxidized and reduced STAT3 in response to bolus addition of H202, the model was expanded to include reactions that describe H202 uptake
from the extracellular media as well as Prx2-mediated oxidation of STAT3 and Trx-mediated reduction of STAT3. Currently, it is not known whether the sulfenic acid form or the
disulfide-linked form of Prx2 reacts with STAT3 in the cytosol. For that reason, two expanded models were utilized: one in which the disulfide-linked form of Prx2 (Prx2-SS) reacted with STAT3, and one in which the sulfenic acid form (Prx2-SOH) reacted with STAT3. These models will be referred to as model A and model B (Figure 2.2). After
10-14 mol H202/cell (50 iM H202 bolus addition in a cell density of 4x106 cells/mL), and the
concentrations of all species of interest were plotted as a function of time.
In order to model the concentrations of the oxidized and reduced forms of HyPer, the original clearance model was expanded to include reactions that describe oxidation and reduction of HyPer as described previously1 . After inclusion of the additional reactions in the model, the system was simulated in response to various H202 generation rates, and the fraction of reduced HyPer and Prx was calculated after the system reached steady state. Additional details of the simulations used in this chapter are described in Appendix A.
2.3.2. Intracellular generation of H202 with D-amino acid oxidase (DAAO) and detection of HyPer fluorescence
In order to continuously produce H202 in the cytosol of HeLa cells, D-amino acid oxidase (DAAO), which we previously14
cloned into the pLJM I-EGFP construct (Addgene plasmid # 19319), was stably expressed under control of the CMV promoter in the cytosol of HeLa cells containing HyPer with the procedure described above. Cells were grown in the selective media for a period of 2 days before the cells were passaged again. Two days before the experiment, 1.75 x 105 cells were seeded onto the surface of a 6-well plate. The day of the experiment, cells were washed once with PBS (lx) and supplemented with 1 mL of RPMI 1640 (lx) containing 5 iM flavin adenine dinucleotide (FAD). Before addition of D-alanine (the DAAO substrate) to the wells, images in each fluorescent channel were acquired for each well. After these images were acquired, D-alanine (dissolved in RPMI 1640) was added to the wells to reach final D-alanine concentrations of 25 mM and 10 mM in a total volume 2 mL; as a control, an equivalent volume of RPMI 1640 with no D-alanine was added to a separate cell sample. Images were acquired in each fluorescent channel in each well after 1, 2, 4, and 6 hours.
For HyPer images, the following two filter sets were used: for short wavelength HyPer excitation, a 415/6 nm excitation filter (Semrock) with a 525/40 nm emission filter (Semrock); for long wavelength HyPer excitation, a 488/6 nm excitation filter with a 525/40 nm emission filter (Semrock). A 250 ms exposure time at 10% lamp intensity was used to acquire the images in both channels. ROIs were determined in images from both fluorescent channels as above. In order to calculate the ratiometric HyPer signal (i.e. the HyPer ratio) in each ROI at each time point, the background-subtracted fluorescence intensity from the 488 nm excitation channel was divided by the background-subtracted fluorescence intensity from the 415 nm excitation channel in ImageJ.
2.3.3. Gel electrophoresis and Prx2 western blots
For each experiment in which western blots were used, the adherent cells were first washed twice with PBS (Ix). Next, the liquid in each well was aspirated and replaced with 2 mL of 100 mM methyl methanethiolsulfonate (MMTS) in order to block any free thiol groups as described previously. The plates were then incubated on ice for a period of 20-30 minutes, and washed twice with 1 mL PBS. Next, 100 ptL of 1% Triton x-100 supplemented with lx HALT protease cocktail was added to lyse the cells. The lysed cells were then re-pelleted at 12,000 g for 10 minutes. The supernatant of the solutions was then collected and the protein content in the solution was assessed via the BCA assay. 25 pig of protein was then loading into a tris-tricine acrylamide gel and subjected to SDS-PAGE under non-reducing conditions (i.e. with no