Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Technical Translation (National Research Council of Canada), 1982
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=cfea5749-1a83-4d9c-a2e7-61311e0974f7 https://publications-cnrc.canada.ca/fra/voir/objet/?id=cfea5749-1a83-4d9c-a2e7-61311e0974f7 For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20386638
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Improvements in waterproofing membranes by modifying bitumen with
thermoplastic polymer
Authors/Auteurs: Title/Titre:
NATIONAL RESEARCH COUNCIL CANADA CONSEIL NATIONAL DE RECHERCHES CANADA
TECHNICAL TRANSLATION 2011 TRADUCTION TECHNIQUE
S. Piazza, et al.
Improvements in Waterproofing Membranes by Modifying Bitumen with Thermoplastic Polymer. Reference/Reference:
Translator/Traducteur:
Poliplasti, 27 (257): 91-102, 1979. S. Nowacki.
Canada Institute for Scientific and Technical Information
Institut canadien de l'information scientifique et technique
Ottawa, Canada K1A OS2
The Division of Building Research of the National Research Council Canada has been actively involved in develop-ing test methods for and evaluatdevelop-ing hundreds of new bituminous
roofing materials to contribute to the development of suitable
standards for the materials' properties and application methods.
This work is carried out through membership on Technical
Committees of the Canadian General Standards Board; the standards
concerned are CGSB 37-GP-50 through -60M.
This paper, translated from Italian, is a valuable
contribution to the understanding of the mechanism of modification
of bitumen with polymers and thermoplastic rubbers. It is hoped
that it will be useful to English-speaking architects, engineer
designers as well as the roofing material manufacturers.
The Division thanks S. Nowacki who translated this
report and H.O. Laa1y of the DBR Materials Section who checked
the translation for technical accuracy and H.E. Ashton who
re-translated the paper.
OTTAWA
November 1980
C.B. Crawford
F
- - - -セ MセN __N⦅N⦅セMMM⦅NPOLIPLASTI, 27(257): 91-102, 1979
S. Piazza, A. Arcozzi, F. Balestrazzi of ANIC, S. Donato Milanese C. Verga of ASSORENI, S. Donato Milanese
Improvements in Waterproofing Membranes by Modifying Bitumen with Thermoplastic Polymer
Abstract: Waterproofing of roofs with impermeable membranes based on
bitumen modified with high modulus materials is now widely employed in Europe. The mechanical and viscoelastic behavior of bitumen, either at low or at high temperatures, are appreciably improved by adddition of
thermoplastic polymers such as styrene-butadiene-styrene rubbers and
atactic polypropylene. Such materials can exhibit a complementary use but
to obtain the optimum properties it is necessary that compatible bitumens be used and that a proper mixing of the bitumen and thermoplastic polymers be achieved.
Bitumen was one of the first thermoplastic materials used by man (1). Archaeologists have revealed its wide use certainly before 3000 B.C. in Mesopotamia and in the Indus Valley, as a binder in the construction of walls and roads and as a waterproof lining in temple baths and water
cisterns. Noah's Ark was caulked with bitumen and Pharaohs were embalmed
with it. Bitumen came from lake asphaltite and the Dead Sea.
Most probably, the roof was the first structural element made by man in order to satisfy one of his first vital necessities, that is,
protection from the implacable forces of nature and temperature extremes. Bitumen, with its exceptional properties of being at the same time a waterproofer, a preservative and a binder, was known to the ancients who found a widespread use for it in the construction of their homes and roads.
In Europe, the first materials employed on a large scale for waterproofing were the molten asphalts composed of a mixture of fillers and
bitumens (2). Such materials occur also in nature in the form of sands
impregnated by residues of crude oil which had distilled over the centuries and formed asphaltic rocks.
Introduced in Germany during the second half of the last century were multiple layer systems consisting of sheets of paper or similar
continuous support alternating with coatings of the straight waterproofing
material.
*
The tar used was soon replaced by petroleum asphalt, eitherunmodified or mixed with ground limestone or asbestos fibers.
To enlarge the field of applicability of petroleum bitumen, it was necessary to raise its softening point and to decrease its
penetration; this led to the origination of oxidized bitumen. At present,
bitumen is widely used and still may be found in nature although most of the total needs for this material come from the oil industry, where it is an important product.
The development of waterproofing systems for dwellings and industrial buildings in our country (3) is similar to that occurring in other European countries, the only difference being due to the presence of
a large clay and cement tile manufacturing industry in Italy. The
*
Editor's Note: In today's terms - bituminous built-up roofing (BUR)p
-3-evolution of traditional roof originated in the need for lighter structures and from the tradition of terraced roofs, especially in southern Italy, of
the type common to all hot climate countries. The vigorous post-war
rebuilding program increased the use of lighter and more elastic skeleton structures combined with components that were completely different either
technologically or materially. The combination of these factors introduced
the parameter of thermal expansion as an agent of mechanical degradation, whereas, on the solid structures of the past this effect was barely
noticeable because of the substantial thermal inertia and the relative homogeneity of the structural components.
These factors emphasized dramatically the need for a waterproof membrane that would provide the mechanical functions and performance as well as resist stresses of a physical, chemical and thermal nature (4),
(5).
With time, hot asphalts were replaced by multilayer, tar-based waterproofers, then came distilled bitumen followed by oxidized bitumen interlaid with roofing felt and later alternating with inorganic-based sheets.
Since these methods competed with the traditional materials, such as clay and asbestos-cement tiles, they had to become fully reliable to remain in use.
Gセゥエィ the advent of thermal insulation, industry searched for new solutions such as products with better weather resistance and greater reliability.
This, together with scarcity of labour willing to carry out the work connected with preparing a traditional, multi-layer waterproofing, favored the development of membranes that were hot-applied as sheets reinforced with glass mats or other materials (e.g., woven glass or non-woven polyester) covered with a mixture based on asphalt with other
ingredients.
What is Required of a Waterproof Covering
Some authors, in particular G. Croyere (6) and J.Y. Meynard (7), have investigated the causes of wear and cracking which occur in the
impermeable covering of a roofing system.
It would be ideal for the covering if it moved only within the elastic part of its stress-strain characteristics, that is, it would be deformed elastically and not plastically and returns to its original shape. According to the above authors, mathematical analysis of elastic work
theory produced an applicable equation of the form: X.. MA
セ
where:
x -
is the width of a growing crack,
-5-exceeding its elastic limit. i.e., M is a modulus,
A - is the value of the elastic limit (up to this value
there is 100% recovery and above it there is permanent
deformation),
セ - is the frictional stress as defined by Coulomb's law:
セ
=
R=
N tg セ + Cwhere:
C - is the adherence and N is the load normal to the surface.
Since the width X of a crack is controlled by the substrate,
optimization of this equation may be achieved by diminishing セ through
using an independent or a semi-independent mode of laying. whereas the value of M and/or A may be increased by selection of suitable materials
(i.e •• reinforcing and binder). For instance. use of a system comprised of
a reinforced film with bitumen requires that, since the modulus of the bitumen is very low, the membrane be strengthened by a strong
reinforcement. i.e •• having a much higher modulus. However, this is true
only up to a point, since the stresses are transmitted by joints which, as
a rule, are made of materials having low mechanical resistance. Otherwise,
there is a trend to increase the number of plies.
Another possibility is to increase the elastic limit which. however. applies only to materials with a marked rubberlike elasticity. such as vulcanized or thermoplastic elastomers or the like which, even when blended with bitumen. retain their elastic elongation characteristics.
-6-The question arises whether bitumen alone is capable of satisfying such requirements.
What is Bitumen
At present there is much greater knowledge of the nature,
composition and rheological behaviour of bitumen than was available in the
not-so-distant past. The bulk of the information was collected chiefly
from applications of bitumen containing aggregates in road construction and to tunnel and reservoir linings.
It appears that the best way of describing bitumen is to define it as a material comprised of complex hydrocarbons of high molecular weight and other compounds containing oxygen, nitrogen and sulfur, and soluble in
carbon disulfide. Not all bitumens are mutually compatible (2). For
example, derivatives of South American crudes have a chemical composition different from analogous products obtained from the Middle East or
Caucasian crudes.
The methods of production and the nature of additives employed to bring bitumen within the requirements of standard specifications modify, to a substantial degree, the composition of bitumens.
Basic research by M.H.B. Hayes, M. Stacey and J. Standley (8)
indicates that the viscous, high boiling point mixtures contain, beside the aliphatic, aromatic and naphthenic hydrocarbons, small quantities of
ps
-7-hydrocarbons were shown to be paraffins, olefins, cycloparaffins and asphaltenes with highly conjugated systems.
Fractionation of bitumen with n-paraffins (e.g., n-hexane or a double fractionation with n-heptane followed by n-pentane) permits
separation of the asphaltenes and asphaltic resins, which precipitate, from the maltenes (i.e., polar resins and oils).
Bitumen behaves as a colloidal substance in which the asphaltene is the nucleus or center, the polar resins form a protective phase around the nucleus and the whole (asphaltenes and resins) is suspended in oils
(9). Although it is not easy to describe the chemical nature of the
various phases, it may be said that the nucleus has more aromatic-naphthenic characteristics, the resins are less aromatic and more naphthenic while the oils, in which the asphaltenes and resins are
dispersed, are more of the paraffinic type. The percentage variations in
the constituents are responsible for the bitumen properties.
With regard to the mechanical behaviour of bitumen, its rheology and its viscoelastic characteristics as a function of temperature, of loading (frequency) and stress, we rely on basic works of various authors who attempted to correlate the viscoelastic properties of bitumen with
results obtained from commonly-used and easily-performed empirical tests.
Van Der Poel (10) clearly explained that Young's modulus does not apply to bitumen, since its application is limited to cases where there is
a linear relation between the stress and strain, and the latter must be
independent of the time of loading. For viscoelastic materials the ratio
of stress to strain defined as rigidity is dependent on not only the time of testing but also the temperature, according to the equation:
s
=
£(t,T) E
where:
a - is a constant applied load, which during a time t and at a
temperature T, induces a deformation E.
The validity of this relation is limited to very small values of
E, since at higher values the behaviour of these materials is no longer
linear.
Recently, Dobson (11) pointed out that bitumen behaves as a
viscoelastic substance. In fact, under fast loading rates and at low
temperatures, bitumen behaves like an elastic glass, whereas under slow
loading rates and high temperatures it displays viscous flow. In
intermediate conditions, i.e., those occurring in practice, bitumen exhibits a combination of viscous and elastic properties.
Heukelom (12) further developed the Van Der Poel theory and prepared graphs for easy application of laboratory results to practice.
-9-These graphs make it possible to account for the behaviour of bitumen under various conditions of application.
In their fundamental work Isayev et al. (13)t by application of the theory of linear viscoelasticity and the principle of time-temperature superposition t confirmed that the rheological behaviour of bitumen is very
similar to that of polymers. Furthermore t they showed that the relaxation
of bitumens tends to a relaxation-time distribution more complex than Gaussian.
It may be of interest to recall the experiment by Heukelom and more recent work by Hayes t Stacey and Standley (14) that demonstrated that bitumen subjected to a mixing process no longer has its original properties. The changes are due to evaporation t oxidation and dehydrogenation t which bring about increases in the average molecular weight because of the loss of low boiling point components and the reactions of free radical and anionic polymerization.
All this work demonstrated t and various authors have recently emphasized t that the stress resistance of unmodified bitumen is limited by its thermal sensitivitYt brittleness at low temperatures and ageing t which
manifests itself by hardening and by increased viscosity. Sometimes
failure may be caused by stresses of short duration as well as alternating stresses.
Modification of Bitumen with Polymers
Recently because of its limited characteristicst bitumen was the
object of numerous investigations aimed at improving its physical and
mechanical properties. Several approaches were triedt such as chemical
treatment and addition at different levels of various polymers (epoxy
resinst polyurethanest polyolefinest polysulfidest elastomers). In
particulart the addition of natural rubberst latexes and synthetic rubbers
was tried for quite a long time and with a certain measure of successt but
without obtaining convincing and definite results.
Van Beem and Brasser (15) clearly indicated the criteria for the proper selection of such polymers:
a) the polymer must improve as much as possible the
susceptibility to temperature-loading ratet the resistance to permanent
deformations and the resistance to fracture over the widest range of
temperaturest loading rates or timest and strains.
b) the polymer and bitumen compatibility must be such as to exclude any tendency to separate either while maintained at high temperatures or in normal service.
c) the polymer must have only a slight influence on the viscosity of molten bitumen so that the rubber-bitumen mixture can be applied like a conventional bitumen.
If amorphous linear polymers having a solubility parameter
between 8 and 9 and a molecular weight below 106 are added to bitument they
p
-11-dispersion at a much lower temperature. This is the case with
polyisobutylenes, polybutadienes, polyisoprenes, natural rubbers and
styrene-butadiene rubbers. These polymers, once dispersed in bitumen,
which at normal temperatures is a poor solvent, are not very effective in increasing the viscosity of bitumen at its critical working temperature, i.e., between 50 to 600C, at which temperatures there is a tendency to separate.
Cross-linked polymers, such as vulcanized rubbers, are not
readily miscible with bitumen. As a rule, these mixtures are coarse
dispersions of partly swollen rubber particles in bitumen. Better results
may be obtained by mixing linear non-vulcanized rubbers, of relatively low molecular weight, with bitumen and subsequently vulcanizing the rubber in
situ. This method, however, may create serious control problems during the
manufacturing process.
Perhaps a more promising method, from the control aspect, would
be that of cold curing a mixture based on epoxies and polyurethanes. The
use of this method would have to be limited to very special cases.
Which method would be the best to follow? It appears that the
considerations recently expressed in a study by Hameau and Druon (16) have clearly outlined the problem.
It is noted that the degree of peptization, similar to a micelle (asphaltenes), controls the sol or gel structure of the bitumen and
therefore its rheological behaviour. For example, it has been established that the asphaltenes obtained by oxidation, which differ from natural
spherical asphaltenes by their ellipsoidal form and particularly by a wider range in molecular weights, display a certain tendency to increase the elasticity of bitumen by enhancing the formation of gel structures.
It is therefore necessary to create within the bitumen a more structured gel having a higher molecular weight or, better yet, to produce within the body of bitumen a second three-dimensional structure having the elastic properties of an elastomer by means of polymers that can be either inserted into or placed beside the bitumen structure without disturbance. In this connection, consideration must be given not only to the phenomena of interfacial tension, polarity, aromaticity and configuration, but also to modification of the viscoelastic and thixotropic properties of bitumen.
If a significant decrease in bitumen brittleness at low
temperatures is required, the glass-transition point of bitumen must be
much lower. It follows that polymers that are too crosslinked, too
branched or even too polar are unsuitable, as are those that have much
steric hindrance. Preference must therefore be given to lightly
cross-linked polymers or those with moderate intermolecular forces of the Van der Waals type and, to obtain suitable elastic properties, polymers having macromolecular chains sufficiently long to permit rotation and increased flexibility should be used.
-13-Simi1ar1Yt to attain substantial elastic deformations t only polymers with an atactic configuration or a limited amount of structural regularity should be used.
If the polymers have a low molecular cohesion (e.g. t
po1yethy1ene t po1ybutadiene and natural rubber) they must be added in sufficiently high proportions to improve substantially the mechanical
strength of bitumen. Converse1Yt when structures with a strong molecular
cohesion t such as polystyrene or polyvinyl acetate t are added t it should be expected that the intrinsic strength of the resulting bonds will be sharply increased.
With regard to thermal sensitivitYt when the objective is to increase the plasticity range of bitumen t either its softening point can be raised (e.g. t by adding products with high molecular weight) or its low temperature brittleness point lowered.
All these alternatives must be related to the characteristics of the bitumen and the conditions of app1ication t which should be modified as
little as possible. In our opinion t a positive solution is to use
thermoplastic elastomers of the S-B-S (styrene-butadiene-styrene) type and atactic polypropylene.
Modification with Thermoplastic Elastomer S-B-S
Known by their trade names t Europrene Sol T and Europrene AG t and produced by ANIC t styrene-butadiene block copolymers are linked together to
form a symmetrical, radial structure (Fig. 1), composed of four chains with
the polystyrene phases at the ends (17, 18). Since there is an absolute
mutual incompatibility between polystyrene and polybutadiene, these two types of blocks are separated into two distinct but well-dispersed phases
linked chemically to form a three-dimensional structure (Fig. 2). At
temperatures below 70 to 80°C the polystyrene regions, being rigid and resistant to deformation owing to the presence of physical bonds (Van der Waals forces), act as crosslinks for the polybutadiene phase.
At higher temperatures the styrene areas soften causing plastic
behaviour. Since there is no chemical bonding the polymer has excellent
solubility properties. Furthermore, the polybutadiene phase (T
g
approximately -900C) is responsible for the flexibility of the polymeric
mass and ensures a typically elastomeric behaviour, e.g., high resiliency
and an optimum resistance to low temperatures. Figure 3 shows an electron
microscope view of an S-B-S polymer dispersed in a polypropylene matrix (rubber was treated with Os04' osmium tetroxide).
The network structure of such polymers may be destroyed by suitable solvents or by heating the polymer to a temperature above the
glass transition point of the polystyrene blocks. The structure with all
its original properties may be restored by eliminating solvents or by
cooling the polymer below the glass transition of polystyrene. Presumably
what occurs with the bitumen is that, when the polymer is dispersed in hot bitumen, the cohesion of the polystyrene blocks diminishes and may
-15-thermoplastic rubber allows the mixture to behave like a viscous liquid and, at the same time, because of the relatively low molecular weight of the S-B-S chains, it increases the viscosity only slightly greater than
that of bitumen at the same temperature. When cooled, the polystyrene
segments associate and, when the temperature falls below the glass
transition point, they act as crosslinks. Only if the polymer has been
thoroughly mixed with the bitumen and the elastomeric segments remain well dispersed at ambient temperatures, will the structure be continuous and
rubbery and form entirely within the bitumen.
In other words, these advantageous properties may be obtained only with a suitable degree of compatibility between bitumen and
thermoplastic resin and by preparing the mixture with a certain type of bitumen.
According to fundamental study by Van Beem and Brasser (15) on the subject of choosing the most suitable bitumen, the solvent power of the bitumen for thermoplastic rubber at temperatures above 140°C depends
chiefly on the aromaticity of maltenes and even on the content of asphaltenes.
The calculation of the aromaticity index, according to the Corbett method (19), is given by the equation:
Ia
=
1.31 +ゥN{セM
13.2H% - 54Jd4
e% - is the carbon content,
H% - is the hydrogen content, and d
42 0- is the density of the maltenes.
According to the above authors, to obtain a thermally stable bitumen-rubber blend the following condition must be satisfied:
Ia セ 0.28 + 0.004A
where:
A - is the content of asphaltenes extracted from bitumen by n-heptane.
We agree with these authors that the study of bitumen as a
solvent for the two-phase block copolymer is a very complex problem, since the same bitumen, as has already been noted, is a complex system composed of high molecular weight asphaltenes dispersed in low molecular weight
hydrocarbons of a very variable chemical nature. Thus, modern theories on
solvent-polymer systems are not readily applicable in this case.
As manufacturers of waterproofing sheets and membranes and therefore interested in a rapid and correct solution of this problem, we have used and still use empirical methods that relate precisely to
practice.
Table 1 presents results of chemical analyses of the most
important bitumens available in Italy, as well as evaluation tests of bitumen-thermoplastic rubber mixtures obtained after mixing one hour at
p
-17-controlling variations in the penetration, softening point, and mandrel flexibility and by microscopic examination (some of such microphotographs
are shown in Figures 4 to 8). The purpose of the above was to compare
bitumen-thermoplastic rubber compounds with differing homogeneities and
with various types of bitumen. Examples of the effect of increasing
quantities of thermoplastic rubber from 5 to 15 parts per hundred of an 80-100 and a 180-200 grade bitumen on the mechanical and viscoelastic
properties of the bitumens are given in Tables II and III. Figures 9 to 12
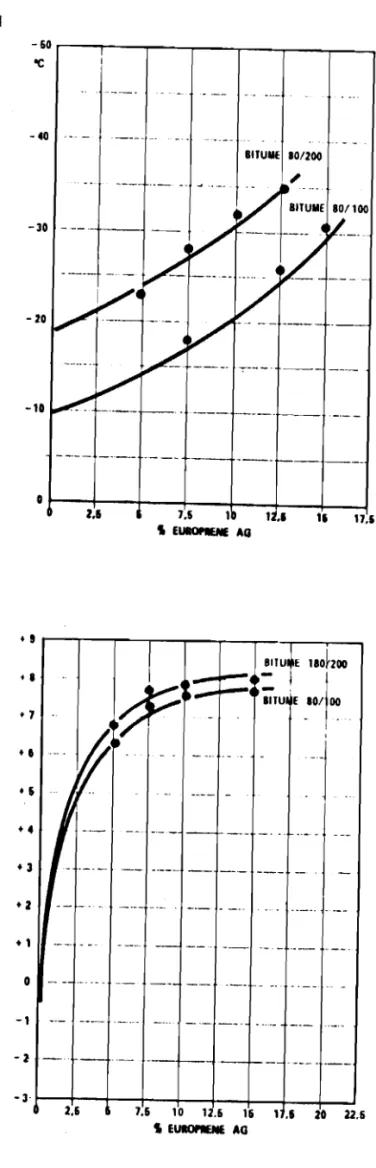
show the change in penetration, softening point, FRAASS breaking point and penetration index with increasing content of thermoplastic rubber.
To illustrate better the behaviour of thermoplastic rubber, some test results are shown on a Heukelom diagram (Fig. 13).
It is clearly evident that addition of the optimum quantity of a thermoplastic rubber substantially improves the physical-mechanical
properties of bitumen; in particular, the penetration values are lower, the softening points are considerably elevated and the low temperature resistance markedly improved, thus showing a more elastomeric than visco-elastic behaviour.
At the low temperatures it was possible to verify the effect of higher and lower compatibility of the bitumen with the rubber and of
thorough mixing which, if not well done at the melting temperature of
bitumen, definitely causes the low temperatures resistance to be lower than the predicted level.
The microscopic tests, although not easy to interpret and to correlate in all cases, substantially agree with the opinions of Van Beem and Brasser (15) and Delme (9), that the optimum solution is to employ bitumen having sufficient aromaticity to dissolve rubber and subsequently
to prevent separation during storage at high temperatures. At room
temperature polystyrene blocks, similar to those in the pure polymer, form containing the least possible quantity of bituminous components thus being able to form sufficiently solid crosslink networks for the polybutadiene
portions. Under such conditions, the polymeric fractions should remain
finely dispersed in the bitumen. The best properties, especially at low
temperatures, are obtained when one oberves with the microscope a fairly fine dispersion of globules of bitumen in an almost continuous bulk of bitumen and polymer.
Bitumens with too high or too low aromaticity are unsuitable since the first kind gives rise to a single phase compound in which the polystyrene blocks appear dissolved even at room temperature, while bitumens with low aromaticity yield mixtures unstable during storage and with a coarse dispersion, visible even to the naked eye.
Bitumens with a high content of heavy asphaltenes (insoluble in n-heptane) are to be avoided.
Some recent observations by Hameau and Druon (16) are of particular interest concerning the mixing stage.
Polymer may be present in a blend either in its swollen form (i.e., after having absorbed light fractions of bitumen up to 300% by
-19-weight) or in the dissolved state as a true or a colloidal solution, both
of which can be present simultaneously. The ratio between the dissolved
and the swollen phases determines the reinforcing properties of polymers and is a function of the mixing conditions, such as temperature, physical state of the polymer, mixing method and duration, etc., as well as of the nature and the concentration of the components.
In attempts to improve mixing, experiments used rubber in powder form (size below I mm), high temperatures (around 2000C), and application of vigorous mechanical mixing for the necessary but shortest possible time (to avoid any degradation of either polymer or bitumen).
Waterproof roofing membranes containing the optimum quantity of thermoplastic rubber are widely used in Europe, especially in northern climate countries, and their use is still increasing, although it is only
3-4 years since initial introduction. Certainly, not all problems have
been resolved. For instance, in some cases there may be a certain
tackiness to the finished sheets caused by the rubber and the phenomenon of
diminished cohesion sometimes revealed after prolonged storage. There may
be a slightly greater tendency to creep in comparison with membranes
manufactured with atactic polyproylene (APP). Such phenomena may be
overcome with suitable mixtures and methods of preparation but, nevertheless, may become evident in warmer climates.
Modification With Polypropylene
Now the possibility of modifying bitumen with atactic
polypropylene will be discussed, although such processes have been known in all practical details in Italy for more than fifteen years (20).
Large supplies of relatively moderately priced atactic
polypropylene, a by-product of isotactic polypropylene manufacture, have stimulated its use so much that now over 65% of the waterproofing market in Italy comprises prefabricated membranes made of polyproylene-modified
distilled bitumen. It is known that linear crystalline thermoplastic
polymers, such as polyethylene and polyproylene, may be associated at room temperatures and are thus able to improve the rigidity and the mechanical
resistance of bitumens. Because atactic (amorphous) polypropylene is the
type that seems to dissolve better at higher temperatures, industrial operations must be carried out so as to prevent separation during hot
storage and, once the dispersed state has been obtained, cooled to a coarse dispersion (similar to a mineral filler-bitumen system).
We were able to make a large series of experiments on this subject since ANIe are the owners of a large production plant in Ragusa, Sicily (see Figs. 22 to 26).
From the examination of sheets made with various types of bitumen and atactic polypropylene, it was observed that the best properties are obtained only at operating temperatures where the polypropylene appears to
-21-continuous phase appears to be bitumen with polypropylene islands the
behaviour is not homogeneoust which can cause phase separation (see Figs.
14t 15).
Observations on mixing bitumen with thermoplastic rubber for the optimum quantity of rubber showed the best results at approximately 7 to 10
parts of rubbert but the quantity of polypropylene required to impart
better properties to bitumen seemed considerably higher (about 25 to 30 parts).
The behaviour of thermoplastic rubber and of atactic polypropylene blended with bitumen was recently examined in our
laboratories with the object of obtaining from the experiments a clearer
picture of the effects of the degree of dispersiont the type of polymer and
the concentrationt on the viscoelastic characteristics of the membrane.
Investigations on the Viscoelastic Behavior of Compounds for Membranes
As shown in Table IV six mixes were prepared by heating 180/200 grade bitumen to 2000C and adding powdered thermoplastic rubber or APP in small pieces with constant stirrer speed.
The condition of the resulting mixtures was examined by an optical microscope using sheets with a uniform thickness of 4 rom obtained
by pouring into a metallic mold and conditioning at 1500C. Test pieces
were conducted at 210C and at a speed of 80 mm/min. noting the maximum load and elongation at break.
The low temperature flexibility test was carried out according to the Aschimici-IGLAE method (21), progressively lowering the temperature and using a 20 rom diameter mandrel.
For the torsional creep tests, a Clash
&
Berg torsiometer wasused according to ASTM D 1043-72. The test consists of subjecting a
specimen to a constant torsional stress and measuring the deformation,
i.e., measuring the torsional angle at time intervals and various temperatures.
The microscopic examination showed that when APP was added the particle size decreased with increased mixing time and a solution did not form in the bitumen but a more or less fine dispersion.
The mechanical property tests demonstrated that the strength and elongation at break are substantially higher for compounds with
thermoplastic rubber; for those with APP the maximum loads occur at low
elongations. Low temperature test results clearly showed, as already
known, the improved properties of membranes containing thermoplastic rubber.
However, the most important findings came from the torsion
MセM⦅NセMMMセMMMMMMMMMMMM
-23-the Maxwell ideal model must be considered since it helps to interpret certain characteristics of deformation by separating the elastic component from the viscous component.
As shown schematically in Figure 16, the Maxwell model consists of two elements one of which is elastic and is represented by a spring
having one end rigidly fixed. The other element is viscous and is
represented by a piston submerged in a fluid.
If, beginning at time t , the system is subjected to a constant
o
stress, a, it reacts by deforming, the first being the elastic element,
i.e., the spring, which elongates by a finite amount; subsequently the cylinder starts to move along the piston, which is permitted by the viscous
creep of the fluid. The two deformations differ in that the elastic (the
spring) is reversible while the viscous (the piston) is irreversible and the deformation is permanent.
The deformation per unit stress, i.e., the creep compliance J, may be represented as a function of time, as shown in Figure 17 (dotted
segments). The line parallel to the ordinate indicates the instantaneous
deformation of the elastic component; the sloping section represents the deformation of the viscous component.
The slope of the second portion is inversely proportional to the
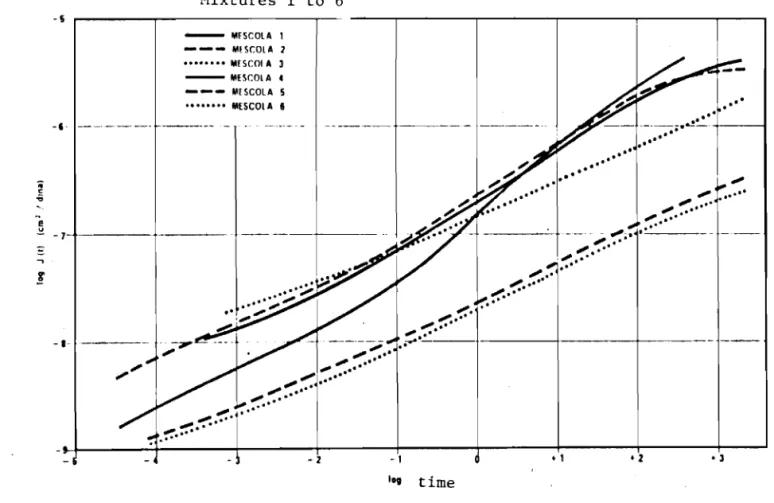
can be seen by comparing it to the continuous lines corresponding to mixture No.5.
On our test pieces we measured the compliance J with time under a
constant stress, 0, and a temperature range between -17 0C and +200C.
Figure 18 shows the change in compliance, J , (elastic component) as a
o
function of temperature for the six mixtures. Figure 19 shows the behavior
of the viscosity, n, as a function of temperature. The values, J and n,
o
were calculated from the curves of J(t) against t, the slopes of which are inversely proportional to the viscosity of the viscous element of the model whereas the intercept with the origin is equivalent to the compliance of the elastic element (J ).
o
From the examination of such Figures it can be seen that mixtures with APP have a compliance, J , that is lower than that with thermoplastic
o
rubber and that the degree of dispersion favourably influences the behavior
of blends with either APP (above OOC) or thermoplastic rubber (below OOC).
A higher content of thermoplastic rubber increases the
recoverable elastic component of the compliance over the complete range of investigated temperatures.
With regard to the dependence of viscosity on temperature, there
is a clear difference between the two polymers: mixtures with APP are
-25-temperatures above
oDe,
while blends with thermoplastic rubber tend toseparate at temperatures below OOC.
Another approach was based on the studies of Isayev et ale (13) as well as work carried out in our research laboratories (22-24), i.e., by employing the general concepts of viscoelasticity applicable to polymeric materials and, in particular, the principle of time-temperature
superposition.
The log J/log t curves, obtained at various temperatures, were superimposed by sliding the curves along the time axis to obtain the "master curves", using the curves at 200C as the reference (see Fig. 20).
These curves cover a very large time span from 10-4 to 103 s.
Figure 21 shows instead the time-temperature shift factors
utilized to plot the master curves as a function of temperature. As can be
seen, curves 1, 2 and 3 present an anomaly at temperatures below SOC to
-loDe,
i.e., there is a slowing down, a levelling off or a decrease of theshift factors as the temperature decreases. Such behavior is anomalous for
a homogeneous system, as the decrease in rigidity with a lowering
temperature is not understood, but it suggests a physico-chemical change of
the system. The hypothesis that can be made is that it indicates the
phenomenon of separation of the thermoplastic rubber and bitumen compound due to different behaviors at low temperatures.
In other words, at lower temperatures the thermoplastic rubber is probably present as a continuous phase in which the bitumen, now completely
rigid, acts as a filler. This phenomenon does not occur in the case of
curves related to mixtures with atactic polypropylene. Instead, another
type of phenomenon is observed at temperatures above lODC, e.g., the shift factor tends to decrease very little with increasing temperature,
suggesting again the phenomenon of separation. Apparently polypropylene
increases the solubility and by solvation prevents the progressive
softening of the mixture. This phenomenon appears analogous to that
confirmed by the use of polymers to confer viscostatic properties to lubricants.
The log J/log t curves confirm that atactic polypropylene
provides to compounds a good level of compliance whether at short or long
test times. Consequently, it is believed that at room temperature
polypropylene mixtures exhibit little creep but become brittle at low temperatures.
Conversely, mixtures with thermoplastic rubber possess elevated compliance characteristics at slow loading rates and tend, therefore, to
undergo creep. At slow loading rates or at low temperatures they remain
sufficiently flexible to permit postulation of the absence of brittleness. If our conclusions are correct, atactic polypropylene and thermoplastic rubber can be used as complementary additives for bitumen (25).
-27-Two already mentioned conditions, that the bitumen composition be adequate and that optimum mixing of polymers and bitumen be obtained,
result basically from the application of such mixtures. In the range of
investigated temperatures and with compounds that we prepared, we found the behavior more typical of a viscoelastic liquid than of a viscoelastic solid, i.e., the viscous component is at a very high level while the elastic component is relatively low.
The authors thank Eng. Pasquini and Signors Riva and Vitali for their valuable co-operation.
Bibliography
(1) The Asphalt Handbook, Manual Series n. 4, July 1962, 1-2.
(2) Gorgati, R., Waterproofing of Structures, 1974, Bologna.
(3) De Marchi, A., Journees d'Etudes AlE, Venice, 12-13 May 1977,
Evolution of Waterproofing Systems in Italy.
(4) Croce, S., Impermeabilizzare, No.1, December 1975, 7-10,
"Waterproofing Systems."
(5) Dubini, F., Impermeabilizzare, No.2, February 1976, 11-14, "Technical
Selection of Protective Waterproofings."
(6) Croyere, C., Convention on New Techniques in Waterproofing, Coperture,
No.7, 7-10, Milan, 13 November 1975.
(7) Meynard, J.Y., Journees d'Etudes AlE, Venice, 12-13 May, 1977, Theory
(8) Hayes, M.H.B., Stacey, M., Standley, J., Fuel, 1972, vol. 51, Jan
27-31, "Studies on bitumen: Part 1. Characterization of bitumen by use of
physical methods."
(9) Delme, R., Impermeabilizzare, 1977, 28-33. "Solprene: Radial
Thermoplastic Rubbers for Modifying Bitumen."
(0) Van Der Poel, C., J. Appl. Chem; , 4 May, 1954, 221. "A general system
describing the viscoelastic properties of bitumens and its relation to routine test data."
(11) Dobson, G.R., Journal of the Institute of Petroleum, vol. 58, No. 559,
Jan. 1972, 14-24. "On the development of rational specifications for the
rheological properties of bitumens."
(12) Heukelom, W., Journal of the Institute of Petroleum, vol. 55, No.
546, Nov. 1969, 404-417. "A bitumen test data chart for showing the effect
of temperature on the mechanical behavior of asphaltic bitumens."
(13) Isayev, A.I., Zolotarev, V.A., Vinogradov, G.V., Rheol Acta, 1975,
135-144. "Viscoelastic properties of bitumens in continuous and cyclic
deformation. "
(14) Hayes, M.H.B., Stacey, M., Standley, J., Fuel, 1972, vol. 51, Jan.
32-37. "Part 2, Changes in bitumen during weathering."
(15) Van Beem, E.J., Brasser, P., Journal of the Institute of Petroleum,
March 1973, vol. 59, No. 566, 91-97. "Bituminous binders of improved
quality containing Cariflex thermoplastic rubbers."
(16) Hameau, G., Druon, M., Bull. Liaison Labo. P. et Ch., 81, Jan.-Feb.
1976, Ref. 1756, Rilem, 121-129. "On the physico-mechanical study of
l
-29-(17) Piazza, S., Pizzamiglio, G., Palma, C., Danmarks Gummiteknologiske
Forening, Annual General Meeting 1975, Lyngby 75-06, 12-13. Europrene Sol
T, thermoplastic elastomers.
(18) Ferrando, G., Piazza, S., Palma, C., Reunion AFICEP, Paris, 16 October
1975. Thermoplastic rubbers, Europrene Sol T, used to modify traditional
plastic materials.
(19) Corbett, L.W., Anal. Chem. 1964, vol. 36, 1967-1971. "Densimetric
method for characterizing asphalt."
(20) Gorgati, R., Symposium on roofing technology, Washington, 1977, 21-23
September, Paper No. 13, 93-95. The modified bitumen one layers in Italy
and Europe: origins and growth.
(21) Aschimici - IGLAE, Impermeabilizzare, No.4, 1976. "Standards for
Membranes."
(22) Verga, C., Battiato, G., La Bella, C., National Research Council-Highway Research Board 53rd Annual Meeting Washington, January 1974. Laboratory evaluation of rheological behavior of an asphalt concrete containing a BSR elastomer.
(23) Verga, C., Battiato, G., La Bella, C., Ronca, G., Proceedings of the
2nd Meeting of Italian Society of Rheology, Siena, May 1973. Viscoelastic
analysis of the deformation behavior for flexible pavement models.
(24) Verga, C., Battiato, G., La Bella, C., Association of asphalt paving
technologists, 1975 Annual Meeting Phoenix, Arizona. Asphalt cements
improvement: the influence of a carboxylated SBR elastomer investigated by
(25) Arcozzit A.t Balestrazzit F. t Pasquinit F. t ANICt S. Donato Mil.
27-6-1977. Viscoelastic characteristics of some compounds for bituminous
31
-FIGURE CAPTIONS
Fig. 1
Fig. 2
Fig. 3
Molecular structure of Europrene Sol T. Crosslink structure of Europrene Sol T.
Electron microscope view of an S-B-S polymer dispersed in a polypropylene matrix and treated
with Os04 (100,000 X).
Figs. 4 to 8 - Bitumen thermoplastic rubber mixtures of
various degrees of homogenization and with
different types of bitumen (125 X).
Fig. 9 Change in penetration with increasing content
of Europrene AG.
Fig. 10 Change in softening point with increasing content
of Europrene AG.
Fig. 11 Change in FRAAS brittle fracture point with
increasing content of Europrene AG.
Fig. 12 Change in penetration index with increasing content
of Europrene AG.
Fig. 13 Change in viscoelastic properties of two bitumens as
a function of the content of thermoplastic rubber,
Europrene AG.
Figs. 14, 15 - Optical micrographs showing mixtures of atactic
Fig. 16
polypropylene with various types of bitumen (150 X).
Ideal Maxwell model
J Deformation of the spring
o
n
-
Viscosity of fluid around pistonFig. 17 Deformation (J) as a function of time (t) for the
ideal Maxwell model (dotted line) compared with the
behavior of mixture No.5 (solid line).
Fig. 18 Compliance as a function of temperature for the six
mixtures of bitumen and thermoplastic polymers.
Fig. 19 Viscosity as a function of temperature for the six
compounds of bitumen and thermoplastic polymers.
Fig. 20 Master curves log J/1og time for the six compounds
The reference
Fig. 21
of bitumen and thermoplastic polymers.
temperature for the curves is 20°C.
Shift factor as a function of temperature for the six
compounds of bitumen and thermoplastic polymers.
Figs. 22 to 26 - Views of the ANTC plant in Ragusa for the
セ 33
-Fig. 3 Electron microscope view
of an sMbセs polymer dispersed ina a
polypropylene matrix and treated
with 0804 (100.000 X).
_ ..•- iGolGAャutセdiene
tHtHt
kャセセヲエゥゥᆪエ\ieMolecular structure of Europrene Sol T.
poャセャャエャAャᆪi|ャe '!;OCKS
poゥNGwGャZadャ・セ sQゥTセカGョ
Fig. 1
Fig. 2 Crosslink structure of
Figs. セ to 8 - Bitumen thermoplastic rubber mixtures of various degrees of homogenization
!
I 3,---,
9
200イMMBBtBBMMMイMMNNLNNNNNMセMMNNMM
...--..
lao セM⦅ ..
-Fig. 9 Change in penetration with increasing
content of Europrene AG.
120-100· so aD 40 - - - -00 20 M⦅N⦅セMM 17.5 7.5 10 12,5 , EUIOPlIENE AG llt--t--+--+--+--t---l----l o ... IU ME 10/100 .
-セ
セQQ
anME 180)200 ao-100.. 110 セN 130 _ _-...._ _...--_-.,.._ _.,..-_-,._ _..,..._--, "C 120 - -_Nセ .•セMM _.Change in softening point with increasing content of Europrene AG. Fig. 10 10. 40 _ .. -_ . . . . -5 7. 1 12;5 , EUROPllENE AG 2. 30+-_-+_--'+-_-+__+-_---+__KM⦅セ o
-40
Fig. 11 Change in FRAAS brittle
fracture point with increasing content of Europrene AG.
-10 BITUIII: 10/200
,
. --- --_. MセM ._._-11 11.5 1.5 1 1 .1 セ EUIlOPtlENE AQ oイMMZゥZ[MMMイMᄆMセᄆMMMKMMMKMMMj 12•
I
-
Xiャuセe
110boo•
.. t --
I
10/)00Nセ
,-
';TUA'E 1 .. -I
セ
,•
.. ,---
..., , _.. 5I
, - ---- - .. 4 -- -"-'--
--
_
... .---- --. - ----- _._-- --
- ,----
- -. --_.-.. "---- -." -'-_
.. ..-. .._-. .---
--- ---- --- --,. - - セMM-
-----_.
-
- .•.._-- _..- _ .--
._
. .. .. .--- .. -.-- ' . - --.-- -------
-_. -_.-.----
.. _.-' - - . -.._-
, .---- ._-2.5 1.5 10 tz.s 1& 1 .5 20 22.& o +3 +:I -1 + 1-3-Fig. 12 Change in penetration index
with increasing content of Europrene AG.
セjMMセャャゥゥャャゥゥャMwMlャャ
セRP Rセ セDo セo
@ セャイオャャャャe 901100
IlIII BIWllllf S0/10@,10 ph.HJilOI'iiEIIllE All A セijuiiiヲR 30/100'セU pI>,p", eBIWIIIIE 1$01200 IIIIIIWME 180nOO • 10 ph.euセHIiGャャeiGャe AG - 37-ill!l'O 108000 11111101011110 vセセmゥuエmA (ot)
Fig. 14, 15 - Optical micrographs showing mixtures of atactic polypropylene
with various types of bitumen (150 X).
セ セM
セ QセQ]Zエ][エセセ
セ セiス I---++-I--+---+' セ セqMゥMMMMjMセMKMMKMMKセセ QZセセセエ]セェ[セセセセ
m
セoャャM MセMiMMKMMMKMiMMMiMNT。 セセ
eooセ
1Fig. 13 Change in viscoelastic properties of two bitumens as a function of
Fig. 16 Ideal Maxwell model
J Deformation of the spring
o
n - Viscosity of fluid around piston
o - Applied force
TEMPO
'0
17 16
Fig'. 17 Deformation (J) as a function of time (t ) for the ideal Maxwell
model (dotted line) compared with the behavior of mixture No. 5 (solid line).
- 39 + 1
-
-ャMMMMMMKMMMMMMMセMMMMMMェ
,
7 - - - . - .·
5 -C 10·•
7,
5 c . mixtures 1 to 6 10' ----w-
--
L---t
---" ::...::.::- • IIIESCOlA 1 - - - -- __ セセセ⦅]⦅M 7 - . ""SCOlA 2 - - - -• - - -- • MESCOLA J - - - -I - - • MfSCOlA C - - - _ . -MMN⦅M⦅NセMM C - - __ - _ • MESCOlA 5 _ _ _ _ A MESCOlA • --- --
- - - セセMMMMMMM - - ._---i 'lI <,-
!
10',
• 7 ,,
5 C 101L--+- - - - +-- - - t-- - - +-- - - +---l
-17 - 10 • 10 • 20 TEMPlIIA TUlIA 'CFig. 18 Viscosity as a function of temperature for the six compounds
---_
...
⦅MMMMMMMMMMMMMMMMMMMMMMMMMMセBBB
• ZO • 10 o lE_U1UIA 'C Mixtures 1 to 6 • ME;COlAゥャャᄋセ]セセセセ セZ
• MESCOlA Z . -A MESCOl-A J -• MESCOIA 4 - - - . -• MESCOlA 5 _ • MEKOLA • .. 1-_ _--' ,__ -- 0 - 1 Z- . -• - - - - " - - ! - - - 1 1•
i - - - . 4 40 -QPセ I 1 I - - -i 1 -4 lOll _·
- - . " 1 .•
i BセMKMMMNBLZ 4'9
セセセ Viscosity as a function of temperature for the six
41 -20 Mixtures 1 to 6 -5 f + + -• 3 +2
--
MMMMᄋFMAセᄋii
....
"...'
.
' .' .'.'
,.
"..
, +1 fill' ••••• filii"セ ••••"
.
セM - 'fIIII'- • • _,,-"---セ..
,,
....
,(////J.I'"".1 ",
filii' •••• セ..,.
,.' - 1 109 time - 2 - IiIfSCOlA 1 - - - IiI!SCOLA 2 ••••••••IIlISCOIA 3 . . . .-- MfSCOlA 4 - - - MISCOLA 5 •••••••• MESCOlA I - 1--I· . -...e .;;e
セ -7 -!Fig. 20 Master curves log J/10g time for the six compounds of
bitumen and thermoplastic polymers.
temperature for the curves is 200
e.
- Tセ -5 21 4 :- J J -10 TEMPERA TUR«' 'C +10 +20
QセQセセセスQ Shift factor as a function of temperature for the six compounds of bitumen and thermoplastic polymers.
43
-23
26
Fig. 22 to 26 Views of the ANIC plant in Ragusa for the production of
Type of Bitumen A B C D E F G H I L M
Grade 60/70 180/200 60/7 0 60/70 80/100 80/100 60/70 60/70 60/70 60/70 80/100
Penetration at 25°C, 0.1 mm 70 188 67 67 89 87 58 64 67 85 90
Softening Point IP, °c 50.5 40.5 50.0 51.0 49.0 47.5 50.2 50.7 49.7 47.0 47.0
Composition Asphaltenes Insoluble in n-heptane, 13.38 10.52 14.61 15.18 12.83 8.91 8.82 16.44 12.57 15.03 23.15 • % wt. Insoluble in n-pentane, 10.43 8.48 9.26 8.45 10.26 11.26 12.39 10.04 10.96 13.26 10.04 % wt.
Paraffins and Naphthenes, 14.53 14.74 15.18 15.54 14.89 7.71 8.20 6.63 13.98 14.48 6.28
.,..
% wt.
.,..
Resins (polar) , % wt , 36.95 34.63 33.48 32.91 33.68 36.99 30.37 39.05 36.92 29.26 32.70 Aromatics, % wt. 24.71 31.63 27.47 27.92 28.34 35.13 40.22 27.84 25.57 27.97 27.83 Bitumen/Europrene AG** 100/10 mixed 1 h at 200°C Penetration at 25°C, 0.1 mm 28 53 31 30 36 37 28 26 28 34 37Softening Point IP, °c 110 107 104 101 96 106 108 102 108 117 90
Mandrel Bend Test, °c -15 -30 -10 -10 -10 -15 -15 -10 -15 -10 -15
Visual examination of surface: rough smooth rough rough rough smooth smooth rough smooth rough rough
*
**Using ASTM Method D 2007/75 modified for bitumens.
Europrene AG is the name of a block synthetic rubber of the (SB) X type,
produced by ANIC in their Ravenna plant and supplied in powder form.
il
I
Table II - Influence of Europrene AG on the properties of an 80/100 bitumen.
MMMNNNNNLLセ
Bitumen 80/100 100 100 100 100 100 100 100 5 (1) (1 ) 10(1) 12.5(1) 15(2) (3 ) Europrene AG-
7.5 Atactic Propylene-
-
-
-
-
-
25 Penetration at 25°C, 0.1 mm 92 58 53 48 46 45 27 Softening Point I P , °c 45.6 94 104 112 115 117 113Fraass breaking point, °C -10 -15 -18 -20 -26 -31 -14
Penetration index -0.6 6.5 7 . 3 7.6 7 . 7 7 .8 6.3 Viscosity at 150°C, cSt 160
-
-
3437-
-
1122 .e-175°C, V1 Viscosity at cSt 61-
-
1705-
-
325 Viscosity at 200°C, cSt 34-
-
920-
-
135 (1) Nixed 1 hour at 200°C. (2 ) HixeJ 1 hour at 210°C. (3 ) Nixed 8 hours at 200°C.Bitumen 180/200 100 100 100 100 100 100
Europrene AG
-
5 (1) 7.5(1) 10(1) 12.5(1) 15(2)Penetration at 25°C, 0.1
mrn
188 80 72 60 53 50Softening point IP, °c 40 89 99 106 112 115
Fraass breaking point, °c -19 -24 -28 -32 -35 -37
Penetration index -0.2 6.9 7.6 7.8 7.9 8.0 Viscosity at 150°C, cSt 127
-
-
5158 Viscosity at 175°C, cSt 52-
-
1597 Viscosity at 200°C, cSt 29-
-
850 .l>-0' I (1) Mixed 1 hour at 200°C. (2 ) Mixed 1 hour at 210°C. MMMMMMMセMMBBMMMMMMMMMMMMBBセN
j!47
-Table IV - Mixing test on mixtures of bitumen 180/200 with
thermoplastic rubber or atactic polypropylene
I
セQゥク composition Tensile FlexibilityProperties
セ
:-rix Mixing Elonga- Max. Initial
No. Polymer % time tion Load Crack Fracture
min. !J.L/L kg/cm2