ANALYSES OF TAU AND RADIXiN: TWO ACCESSORY
PROTEINS OF THE CYTOSKELETON IN MAMMALIAN
CELLS
BY
MICHAEL D. HENRY
B.S., University of Georgia 1989
Submitted to the Department of Biology in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Biology at the
Massachusetts Institute of Technology February 1996
0 1995 Massachusetts Institute of Technology All rights reserved
Signature of Author
Certified by
Tlhesis'Advisor: Protessor .vrank Solomon Accepted by
Chairman of the~Graduate Committee: ProfessorFrank~Solomon
OF TE CHNOLOGY
NOV 2 2 1995
1
ANALYSES OF TAU AND RADIXJN: TWO ACCESSORY PROTEINS OF THE CYTOSKELETON IN MAMMALIAN CELLS
by
Michael D. Henry
Submitted to the department of Biology on October 10, 1995 in partial fulfillment of the requirements for the degree of Doctor of Philosophy in
Biology ABSTRACT
Tau and radixin are two proteins that have been implicated in organizing the microtubule- and microfilament-based cytoskeletons respectively. Here we have examined the structure and function of these proteins in cultured mammalian cells. To test tau function, we inhibited its expression in
embryonal carcinoma P19 cells with antisense RNA. The results show that inhibition of tau expression does not inhibit the ability of P19 cells to
undergo morphological differentiation into neurons- a process that is known to depend on microtubule function.
Radixin is a member of the ezrin-radixin-moesin (ERM) protein family and is known to localize in cells to cortical structures in which there is a close
apposition between the plasma membrane and underlying microfilament-rich cytoskeleton. To define the regions of the radixin molecule that specify its subcellular localization, we expressed full-length and truncated
versions of the molecule in cultured mammalian cells and determined their localization. Exogenous full-length radixin localized in a manner similar to endogenous ERM proteins. Moreover, expression of full-length radixin was correlated with the disappearance of endogenous moesin from cortical structures suggesting that these two ERM proteins compete for localization in cortical structures. Localization of the full-length molecule depended on distinct determinants in both the carboxy- and amino-terminal domains of the protein. High level expression of the carboxy-terminal
domain of radixin had deleterious effects on cells including the induction of abnormal cortical processes. Neither the full-length molecule nor its
amino-terminal domain had these effects on cells. These results suggested that the amino-terminal domain of radixin modulated the function of the carboxy-terminal domain in the context of the full-length molecule. This hypothesis was tested in vitro. We found that the amino- and carboxy-terminal domains interact with one another with high affinity in solution. This inter-domain interaction could inhibit intermolecular binding of other proteins from cell extracts. Taken together, these studies have suggested an overall model for the molecular organization of radixin which might explain its localization and function in dynamic cortical cytoskeletal structures involved in cell motility.
Thesis Advisor: Dr. Frank Solomon Title: Professor of Biology
ACKNOWLEDGEMENTS
I would like to thank the following people for specific contributions to the work presented in each chapter:
CHAPTER TWO and APPENDIX TWO: Drs. Jon Dinsmore and Arthur Lander, Jill Hahn, Karl Yen, and Sean Walsh for experimental guidance and contributions.
CHAPTER THREE: Dr. Charo Gonzalez Agosti for experimental
contributions. The work presented in this chapter is published: Henry et al. 1995.
CHAPTER FIVE: Most of the experimental work in this chapter was performed by Dr. Margaret Magendantz. Dr. Arthur Lander also
contributed to the experiments presented in this chapter. My contribution to this work was in aid of providing an intellectual framework for some of the experiments. The result of our collaboration is published: Magendantz
et al., 1995.
APPENDIX THREE: Most of the experimental work presented in this appendix was performed by Nancy-Lorena Torres, an undergraduate whose work in the lab I had the great privilege of supervising.
I owe a tremendous debt of gratitude to the following people:
To past and present members of the Solomon lab: Julie Archer, Letty Vega, John Dinsmore, Adam Grancell, Vida Praitis, Bettina Winckler, Adelle
Smith, Jill Hahn, Jim Fleming, Etchell Cordero, Karl Yen, Nancy Torres, Charo Gonzalez, and especially Margaret Magendantz for copious
instruction, insightful conversation, rewarding collaboration, and, of course, indecent amounts of chocolate.
To classmates: Mike Brodsky, John Crispino, Juli Klemm, Sumati Murli, Eric Schmidt, Tracy Smith, and especially Brian and Brenda Kennedy without whose friendship these years would have lacked a significant amount of vitality, croquet, and flyfishing.
To the members of my thesis committee: Richard Hynes for guidance and support in my career both during and after MIT, Tyler Jacks for teaching me to keep a shrewd Yankee eye toward the bottom line of any avenue of inquiry, and Arthur Lander for witty banter and for encouragement to be clever in the design of experiments and parsimonious in the interpretation of such.
To mi professor6 Frank Solomon: who instilled in me, among other first principles, an appreciation of the beauty and power of a controlled
experiment, a taste for the kickin' barbeque to be had at J&E's, and most of all, a sense of the profound humanity of the scientific enterprise.
To Sloane Henry: whose limitless patience, forbearance and love made these years possible and to whom I dedicate this work.
TABLE OF CONTENTS
Title Page 1
Abstract 2
Ackowledgements 3
Table of Contents 4
List of Figures and Tables 7
CHAPTER ONE:
Modulation of cytoskeletal form and function by accessory proteins.
Summary 10
Cytoskeletal Form and Function in Animal Cells 11 Molecular Mechanisms for Organization of the Cytoskeleton 17 Identification and Characterization of Tau and Radixin: Two
Accessory Proteins of the Cytoskeleton in Cultured Mammalian
Cells 25
CHAPTER TWO:
Antisense inhibition of tau protein expression in embryonal carcinoma P19 cells.
Summary 31
Introduction 32
Materials and Methods 35
Results 39
Discussion 58
CHAPTER THREE:
Molecular dissection of radixin: Distinct and interdependent functions of the amino- and carboxy-terminal domains.
Summary 64
Introduction 65
Materials and Methods 68
Results 74
Discussion 110
CHAPTER FOUR:
Deletion analysis of radixin's carboxy-terminal domain.
Summary 115
Materials and Methods 116
Results 120
Discussion 137
CHAPTER FIVE:
Summary 141
Introduction 142
Materials and Methods 143
Results 146
Discussion 156
CHAPTER SIX:
Intermolecular interactions of radixin.
Summary 159
Introduction 160
Materials and Methods 162
Results 165
Discussion 177
CHAPTER SEVEN:
A model for the molecular organization of radixin.
Summary 183
The Model 184
Unresolved Issues 189
Testing the Model 193
APPENDIX ONE:
Isolation of a euploid embryonal carcinoma P19 cell line.
Summary 196
Materials and Methods 197
Results and Discussion 198
APPENDIX TWO:
Characterization of a cell-substratum adhesion deficient embryonal carcinoma P19 cell line.
Summary 205
Materials and Methods 206
Results 208
Discussion 228
APPENDIX THREE:
Expression and localization of HA-radixin constructs in embryonal carcinoma P19 cells.
Summary 233
Introduction 234
Materials and Methods 236
Results 237
Discussion 244
LITERATURE CITED
LIST OF FIGURES AND TABLES FIGURES:
1-1 Protein sequence comparison of ERM proteins and band 4.1 family members. p. 29
2-1 Tau mRNA expression in EC P19 cells. p. 41 2-2 Antisense constructs. p. 43
2-3 RA differentiation of pGKBA-derived cell lines. p. 47
2-4 Tau protein expression in RA-induced pGKBA-derived cell lines. p. 5 1
2-5 RA differentiation of pCXN2-derived cell lines. p. 55
2-6 Tau protein expression in RA-induced pCXN2-derived cell lines. p. 57
3-1 Localization of endogenous ERM proteins in NIH-3T3 cells. p. 77 3-2 Stable expression of HA-radixin constructs in NIH-3T3 cells. p. 79 3-3 Localization of full-length HA-radixin polypeptides in NIH-3T3 cell
lines. p. 83
3-4 Localization of truncated HA-radixin polypeptides in NIH-3T3 cell lines. p. 85
3-5 Displacement of endogenous moesin from cortical structures by HA-radixin polypeptides in NIH-3T3 cells. p. 87
3-6 HAC-RAD, but not HAC-RADC, displaces endogenous moesin from cleavage furrows in NIH-3T3 cells. p. 89
3-7 Moesin protein expression in NIH-3T3 cell lines expressing HA-radixin proteins. p. 89
3-8 Endogenous ERM protein expression and localization in HtTA-1 cells. p. 93
3-9 Transient expression of HA-radixin constructs in HtTA-1 cells. p. 95 3-10 Localization of HA-radixin polypeptides in transiently transfected
HtTA-1 cells. p. 97
3-11 Optical sectioning of HtTA-1 cells transiently expressing HAN-RADC. p. 99
3-12 Localization of HA-radixin in cleavage furrows of transiently transfected HtTA-1 cells. p. 103
3-13 Localization of HA-radixin constructs in the ventral cytoplasm of transiently transfected HtTA-1 cells. p. 105
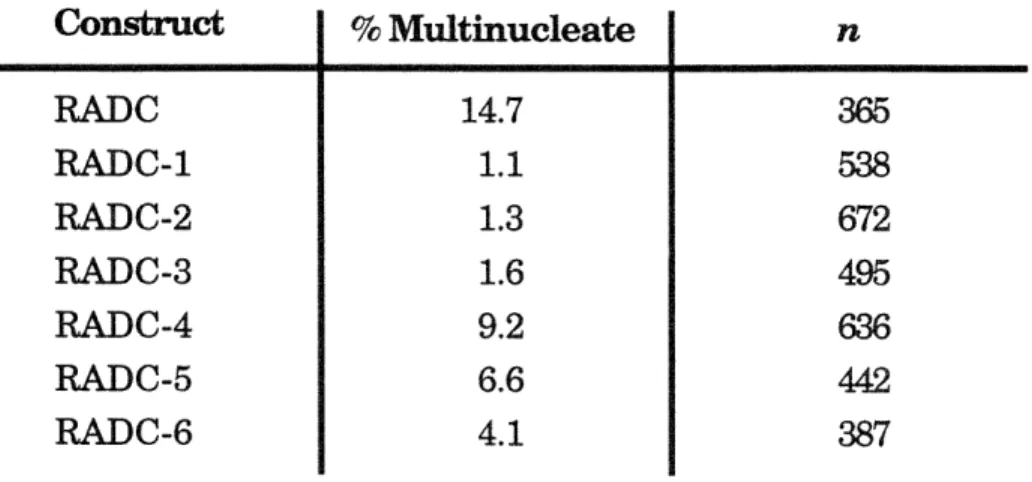
3-14 Expression of the carboxy-terminal HA-radixin constructs results in an increased number of multinucleated cells. p. 107
3-15 Transient expression of HA-radixin proteins in NIH-3T3 cells. p. 109 4-1 Expression of radixin carboxy-terminal domain deletion constructs
in HtTA-1 cells. p. 123
4-2 Effects of carboxy-terminal domain deletion constructs on cortical structures. p. 125
4-3 Subcellular localization of radixin carboxy-terminal domain deletion constructs. p. 129
4-4 Radixin carboxy-terminal domain deletion constructs do not displace moesin from cortical structures. p. 133
4-5 The carboxy-terminal domain of moesin induces abnormal cortical structures in HtTA-1 cells. p. 135
5-1 Immunoblot analyses of column eluates. p. 149
5-2 Affinity co-electrophoresis of the N- and C- domains of radixin. p. 153 5-3 Co-expression of the amino-terminal domain does not suppress the
effects of expression of the carboxy-terminla domain in HtTA-1 cells. p. 155
6-1 Detergent fractionation of HA-radixin polypeptides. p. 167 6-2 Cellular fractionation of HA-radixin polypeptides. p. 167
6-3 The carboxy-terminal domain of radixin binds F-actin in solution. p. 171
6-4 Scatchard analysis of data from C-6 actin binding experiment. p. 173 6-5 Co-immunoprecipitation of cellular proteins with radixin and its
domains. p. 175
7-1 A model for the molecular organization of radixin. p. 185
Al-1 Karyotype analysis of embryonal carcinoma P19 lab stock. p. 201 A1-2 Characterization of P19-A4- a euploid embryonal carcinoma P19 cell
line. p. 203
A2-1 RA-induced differentiation of P19 cell line TA3A. p. 211 A2-2 Extended culture of TA3A. p. 213
A2-3 Loss of cell-substratum adhesion in TA3A cells is a specific effect of exposure to RA. p. 215
A2-4 Adherence properties of TA3A cells on a variety of culture substrata. p. 217
A2-5 Development of TA3A phenotype after exposure to retinoic acid. p. 221
A2-6 TA3A phenotype is independent of culture density. p. 223 A2-7 Expression of neuron-specific markers by retinoic acid-induced
TA3A cells. p. 225
A2-8 Neurite extension in aggregates. p. 227
A3-1 Expression of HA-radixin constructs in uninduced and RA-induced P19 cell lines. p. 239
A3-2 Co-localization of HA-radixin proteins and ezrin in uninduced P19 cell lines. p. 241
TABLES:
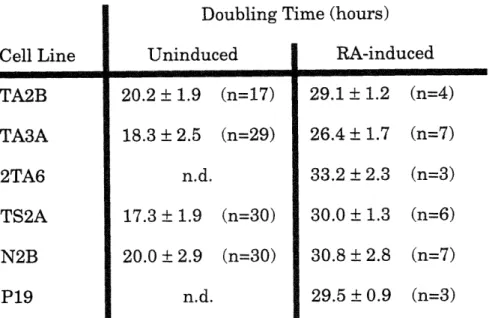
2-1 Growth of tau antisense lines. p. 49
4-1 Effects of radixin carboxy-terminal domain deletion constructs on cytokinesis. p. 131
CHAPTER ONE:
Modulation of cytoskeletal form and function by accessory
proteins.
SUMMARY
The importance of the cytoskeleton in cell shape and motility has been recognized for many years. Recent advances in molecular biology have begun to define the molecular components of the cytoskeleton, yet a detailed understanding of how these molecules affect cytoskeletal form and function remains elusive. One of the central mysteries is how a relatively
simple set of cytoskeletal polymers can assume the varied roles required in distinct cell types. One working hypothesis is that non-covalent
associations of other proteins with cytoskeletal polymers play crucial roles in organizing the cytoskeleton for diverse functions. These cytoskeletal modulating proteins are collectively referred to as accessory proteins. In this chapter, we will illustrate the range of cytoskeletal diversity, provide
some examples of the roles that accessory proteins play in the cytoskeleton, and introduce two accessory proteins -tau and radixin- which are the focus of this work.
CYTOSKELETAL FORM AND FUNCTION IN ANIMAL CELLS
Metazoa are characterized by a variety of differentiated cell types. Often the most readily observable hallmark of the differentiated state is an asymetric morphology. Because lipid membranes are fluid, other
intra-and extra-cellular determinants are required for the establishment intra-and maintenance of these asymetric morphologies. Extraction of membranes with non-ionic detergents reveals an insoluble matrix that retains the
pre-extracted cell shape (Webster et al., 1978; Ben-Ze'ev et al., 1979). Among the components of this matrix are the cytoskeletal polymers- microfilaments, microtubules, and intermediate filaments. The cytoskeleton is not,
however, simply a collection of rigid elements passively filling space within the amorphous membrane. Indeed, forces generated by motor-driven
sliding of polymers against one another or by polymerization of the polymers themselves underlie many motile cell behaviors.
Pharmacological agents that specifically disrupt microfilament and microtubule arrays demonstrate that these two cytoskeletal polymers are required for cell morphogenesis and motility. Below, we briefly describe the cytoskeletal polymers and a few of the specialized organelles formed by these polymers in living cells.
The cytoskeletal polymers- The cytoskeleton is composed of three distinct fiber systems: microtubules, microfilaments, and intermediate filaments. Since the focus of this study is on the microtubule and
microfilament components of the cytoskeleton, we have largely restricted further comment to these two systems. Initially, microtubules and
microfilaments were described microscopically and thereby differentiated according to the diameter of the fiber. Later, molecular analysis revealed that each of these fibers is a polymer composed of identical, repeating protein subunits. The 24 nm-diameter microtubules are polymers
composed of the heterodimeric unit of oc- and B- tubulin (Dustin, 1978). As the name implies, microtubules are tubes. The heterodimeric tubulin subunits are arrayed into thirteen longitudinal rows (the protofilaments) which form the walls of the tube. With few exceptions, this basic structure of microtubules is conserved in all eukaryotic cells. It is thought that the tubulin heterodimers join in a head-to-tail fashion within the
protofilament. This arrangement confers a structural polarity on the microtubule lattice which is evident in the properties of microtubule ends.
One end of the microtubule (the plus end) grows faster than the other end (the minus end) during microtubule polymerization. Minus ends of microtubules also tend to be associated with proteinaceous structures in cells, called microtubule organizing centers, from which many
microtubules might radiate.
The 7 nm-diameter microfilaments are composed of a "string" of actin "beads". Monomeric actin is conventionally referred to as G-actin while the polymeric form is called F-actin. The structure of G-actin is known at atomic resolution (Kabsch et al., 1990). Like microtubules, microfilament ends exhibit a functional polarity. In this case, the faster growing end is called the barbed end and the slower growing end is called the pointed end based on the way that the S1 myosin fragment decorates F-actin. Again, as for microtubules, the basic structure of microfilaments is remarkably conserved among all eukaryotes.
Cytoskeletal organelles- Microtubules and microfilaments are typically arrayed into organelles that carry out specialized functions.
Often, the activities of one or more cytoskeletal organelles are coordinated to achieve complex cellular behavior. During mitosis, the microtubule-based spindle is charged with segregating the chromosomes. Following
chromosome segregation, the contractile ring of microfilaments serves to separate the mitotic cell into two daughter cells. Little is known about how these two organelles communicate with one another, but the fidelity with which chromosome segregation precedes cell division at each mitosis indicates that such communication must exist. Before one can dissect the higher order relationships among cytoskeletal organelles, it is important to understand the structure and function of individual organelles. Below we will describe in more detail a few of the cytoskeletal organelles that are relevant to these studies.
Microtubule organelles- A particularly striking microtubule
organelle is found within the neuritic processes of nerve cells. Bundles of microtubules course through the neurite parallel to its long axis. In axons, the microtubules exhibit a uniform polarity with the plus ends of the
microtubule all towards the distal tip of the neurite (Heidemann and
McIntosh, 1981). At least one function that these axonal microtubules serve is to transport macromolecules from the cell body through the neurite to its
tip. This function is vital since axons can be quite long and are devoid of protein synthetic machinery. Early evidence that axons served a transport function came from Weiss and Hiscoe (1948) who observed "damming" on
the proximal side of constricted nerve fibers. Later, work defined distinct classes of transported molecules using radioactive tracers (Willard et al., 1974). One of these classes, the fast component, moves at the rate of 2-4 pm per second and represents vesicles and small granules transported along microtubule tracks. At the other extreme, the slow component moves at about 0.002-0.01 gm per second and among its constituents is tubulin (Hoffman and Lasek, 1975). More recent experiments have demonstrated that the tubulin moves through the neurite in a coherent phase consistent with the view that polymer is the transported form of tubulin (Reinsch et al., 1991). In addition to this transport function, the neuritic arrays of microtubules also support the structure of neuronal processes. This is demonstrated by treatment of neurons in culture with microtubule depolymerizing drugs which results in the rapid retraction of neurites (Yamada et al., 1970; Solomon and Magendantz, 1981).
Microfilament organelles- The cortical actin cytoskeleton is marked by a close apposition or a physical union of microfilaments with the plasma membrane. Among other roles, this organelle lends shape and structural integrity to the pliable plasma membrane. Clear examples of this function are found in the membrane skeleton of mammalian erythrocytes and in the brush border microvilli of intestinal epithelial cells (Marchesi, 1985;
Mooseker, 1985). In these two cell types, respectively, the cortical cytoskeleton maintains the form of plasma membrane to withstand
tremendous shear forces present in circulatory system or to effect a greater surface to volume ratio for nutrient absorption in the gut. The cortical cytoskeleton in these two cell types are particularly well characterized because of their comparative simplicity and availability in large quantities for biochemical analysis.
In other cell types, the cortical cytoskeleton plays important roles in dynamic membrane structures, including those associated with cell
motility. For instance, cultured animal cells exhibit a number of motile surface protrusions (Abercrombie, 1970). Filopodia are fingerlike
projections that extend up to several microns from the cell body and are often in contact with the culture substratum. When observed over time, these structures may alternately grow, shrink until they are resorbed by the cell body, detach from the substratum, and make new attachments
elsewhere. This sort of behavior is suggestive of a role for filopodia in exploring the extracellular environment. Another, motile cortical structure is the lamellipodium. This structure is characterized by a
flattened sheet of membrane that spreads out over the culture substratum. While this structure appears to move away from the cell body, there are also
extensions -membrane ruffles- that appear to move back toward the cell body. Often, moving cells have a single, well defined lamellipodium that is oriented at the front of the cell in the direction of movement. This type of lamellipodium is referred to as the leading edge.
Both filopodia and lamellipodia are rich in microfilaments, but these microfilaments are less stereotyped in organization compared to the stable membrane skeletons of mammalian erythrocytes or intestinal brush border microvilli. Microfilaments in filopodia tend to be parallel bundles whereas those in lamellipodia form an orthogonal meshwork (Small et al., 1982). Electron microscopic inspection of fibroblast leading edges reveals that filopodia and lammellipodia are a continuum of microfilaments rather than distinct organelles- the lattice of microfilaments in the lamellipodium is consolidated into the bundled array present in the filopodium. In both structures, the barbed ends of the cortical microfilaments are oriented toward the plasma membrane (Small, 1978).
That filopodial and lamellipodial activity is often polarized in the form of a leading edge initially suggested that these structures played a key role in the translocation of cells across surfaces. Indeed, treatments that
disrupt microfilaments cause these type of cortical cytoskeletal structures to collapse and cell crawling to cease (Albrecht-Buehler and Lancaster,
1976). The molecular mechanisms underlying the protrusion of filopodia and lamellipodia are not yet clear. Among the current models for actin based motility in animal cells is one that posits actin polymerization as a
driving force for membrane protrusion (reviewed in Condeelis, 1993). The essence of this model is that thermal vibration in the plasma membrane
allows for the interposition and subsequent addition of actin monomers onto the barbed ends of anchored microfilaments. This newly polymerized actin results in displacement of the membrane. This model is consistent with the force generating capacity of actin polymerization as calculated from thermodynamic considerations and thermal oscillation properties of biological membranes. Recently, support for this model of actin based motility was garnered from an unexpected source. Studies on the invasive pathogenic bacteria Listeria and Shigella reveal that these organisms move about the cytoplasm of host cells by polymerizing host actin into a structure called a comet tail (Tilney et al., 1992). Observation of the actin dynamics in comet tails indicated that propulsion is driven by polymerization proximal to the bacterium at a rate that is consistent with the rate of movement of the bacterium and, interestingly, the rates of membrane protrusive activity seen in cultured cells (Theriot et al., 1992).
Composite cytoskeletal organelles- The classification of the cytoskeletal organelles described above as strictly microtubule and
microfilament organelles is somewhat arbitrary since often more than one type of polymer occupies any cellular compartment. However, in some cytoskeletal organelles, the functions of more than one polymer type may be integrated to accomplish a particular task. These we have designated composite cytoskeletal organelles. One example of a composite organelle is the neuronal growth cone. At the distal tip of growing neurite there exists a motile domain called the growth cone. Growth cones possess many of the same motile actin-containing cortical cytoskeletal features, like the
filopodia and lamellipodia described above. Evidence indicates that the growth cone filopodia play a role in transducing signals from the local environment of the growth cone (Davenport et al., 1993). In fact, in preparations of grasshopper limbs, where resolution of growth cones is possible during neurite outgrowth, growth cone filopodia are observed to reach out and touch guidepost cells that direct growth cone steering events. Disruption of filopodial microfilaments with cytochalasin results in
disoriented axonal outgrowth (Bentley and Toroian-Raymond, 1986). As mentioned above, the neurite shaft trailing the growth cone is filled with microtubules that play a role in macromolecular transport to the growth cone. High resolution imaging of microtubules revealed that they also
extend well into the growth cone and occupy the same compartments with microfilaments (Tanaka and Kirschner, 1991; Sabry et al., 1991). These growth cone microtubules displayed several interconvertible arrangements and could sometimes be detected deep in lamellipodia where they closely apposed the membrane. Interestingly, in the same study, just prior to growth cone turning, microtubules were often consolidated in the future
direction of growth. This provocative result suggests that microtubles and microfilaments may cooperate at sites where growth cone steering
decisions are being made.
Another example of a composite cytoskeletal organelle is the marginal band of avian erythrocytes. In mature erythrocytes, a band
consisting of 10-14 microtubule profiles trace an elliptical orbit around the cell periphery (Miller and Solomon, 1984). Microfilaments are also
concentrated in the region of the marginal band microtubules (Kim et al., 1987). One role for the marginal band is in the morphogenesis of these cells. During development chicken erythrocytes change from an initial spherical form to a lentil-shaped mature form. Concomitant with this shape change, initially diffuse microtubule arrays are first bundled then the bundles are consolidated into the final position of the marginal band. Treatment of developing erythrocytes with cytochalasin to disrupt
microfilaments results in abnormal process formation in the immature spherical cells (Winckler and Solomon, 1991). Taken together, these
observations have suggested a mechanism for the morphological transition in chicken erythrocytes in which the relatively rigid microtubules pushing from within are constrained by the plasma membrane and cortical
cytoskeleton. The net effect of these opposing forces is that microtubules take the path of least resistance which is to circle the equator of the ellipse in a bundle.
MOLECULAR MECHANISMS FOR ORGANIZATION OF THE
CYTOSKELETON
In the preceding section, we described a few of the many known cytoskeletal organelles. From these examples, it is clear that microtubules and microfilaments may adopt a wide variety of structural and functional arrangements. What are the molecular determinants of cytoskeletal
organization? Over the years, several models have emerged that explain, in part, how the cell might achieve the complex molecular relationships
present in the cytoskeleton. In this section, we will briefly describe and illustrate the salient points of each of the predominant paradigms. At the
outset however, we would like to stress that these models should not be considered as absolutes. Indeed, there are examples to support each of the models presented. An extant challenge in molecular cell biology is to understand how these mechanisms are integrated within the cell.
Modulation of polymer dynamics- In vitro assembly reactions of purified tubulin and actin demonstrate that monomers are in an
equilibrium with polymer. Under defined conditions in solution, there exists a concentration of tubulin and actin such that a steady state is reached between the monomeric and polymeric pools- polymerization is balanced by depolymerization. This is termed the critical concentration. Above the critical concentration, there is net polymer assembly and below this value there is net disassembly. The critical concentration for the fast-growing barbed ends of microfilaments is around 1gM while for the slower growing pointed ends this value is near 8 gM under physiological
conditions. For microtubules, the critical concentration is around 14 gM. However, Mitchison and Kirschner (1984a) found that when the minus ends of microtubules are sequestered by organizing centers (centrosomes), microtubule growth is supported at lower concentrations. Direct
observation of individual microtubules emanating from organizing centers after dilution below the critical concentration led to a surprising finding. Some microtubules continued to grow while others disappeared. To explain this unusual behavior, Mitchison and Kirschner (1984b) proposed the
dynamic instability model for microtubule growth. Microtubule dynamics are governed by four parameters- polymerization rate, depolymerization
rate, transition from growing to shrinking (catastrophe), and transition from shrinking to growing (rescue). The essence of the dynamic instability model is that newly polymerized tubulin subunits have a guanosine
triphosphate molecule bound- the so-called GTP cap. After addition to the polymer, the GTP is hydrolyzed to guanosine diphosphate. Since the off rate of GDP-tubulin is much higher than that for GTP-tubulin, when a microtubule loses its GTP cap, catastrophe occurs and depolymerization is rapid.
These in vitro studies have relevance for microfilaments and
microtubules in cells. Since estimates of the cellular concentration of actin range from 150 to 900 gM are well above the critical concentration of actin assembly, one would expect most of the actin to be polymerized. Moreover, under these conditions, there would seem to be little opportunity for the sort of dynamic actin polymerization evident in the motile cortical structures described above. However, several non-actin protein factors influence microfilament polymerization in the cell. Due to the activities of monomer sequestering proteins, like thymosin 14, and barbed-end capping proteins, such as gelsolin, blocking actin polymerization, a considerable portion of the actin in the cell is maintained as G-actin (Carlier and Pantaloni, 1994). This pool of G-actin, which exhibits a focal distribution in cultured cells
(Cao, et al., 1993) may be available for localized F-actin assembly (Cassimeris et al., 1992). Profilin plays a more complex role in actin
dynamics. Genetic experiments support an essential role for profilin in actin-based functions in yeast and Drosophila and in intracellular motility of Listeria (Haarer, et al. 1990; Cooley, et. al., 1992; Theriot, et al., 1994). Biochemical data indicates that profilin may facilitate transfer of thymosin 14 sequestered G-actin to uncapped barbed ends, but the precise role of
profilin in animal cells is unclear. For instance, overexpression of profilin in CHO cells results in an increase in F-actin, whereas microinjection of
profilin in fibroblasts leads to microfilament disassembly (Finkel et al., 1993; Cao et al., 1992)
In cells, as in the test tube, microtubules are highly dynamic structures. Co-existing with in the same cell are both growing and shrinking microtubules (Schultze and Kirschner, 1988). While the
dynamic instability model for microtubule assembly is widely accepted as a plausible mechanism to explain, in part, microtubule dynamics in cells,
certain aspects of this model remain to be firmly demonstrated. For instance, although there is some direct evidence for a GTP-cap on microtubules in vitro, there is no direct evidence for this structure
occurring in vivo (Mitchison, 1993). Analogous to the microfilament barbed end capping proteins, other proteins such as pericentrin and y- tubulin occur in association with the minus ends of microtubules at organizing centers effectively blocking subunit addition at this end. In contrast to the monomer sequestering activities regulating the actin cytoskeleton, there are as yet no proteins that have been shown to sequester assembly
competent tubulin dimer. However, recent evidence indicates that Rbl2p, a protein that binds to B-tubulin in yeast cells, could play an important role in microtubule assembly by regulating dimer formation (Archer et al., 1995).
In conclusion, factors that modulate the polymer dynamics of cytoskeletal proteins most certainly influence when and where cytoskeletal polymers form in cells.
Polymer molecular heterogeneity- Another potential mechanism for regulating the cytoskeleton exists in the form of molecular heterogeneity of polymer proteins. Such heterogeneity comes in two basic forms- protein isotypes encoded by distinct genes and post-translational modifications. There are at least six actin isoforms that are encoded by separate genes (Vandekerckhove and Weber, 1978). These include smooth muscle a- and y-actin, cardiac and skeletal muscle a- actin and non-muscle B- and y- actin. In certain non-muscle cells, there may be differential sorting of actin isoforms. Using isoform specific antisera, DeNofrio et al. (1989) showed
that in microvascular pericytes B- and y- actin is enriched in motile cellular domains whereas a- actin tended to be distributed in stress fibers. One possible mechanism for this sorting could be the translation of localized
B-actin mRNA (Lawrence and Singer, 1986). However, the functional basis for F-actin sorting is unclear at present.
Higher eukaryotic organisms have around 6 a-tubulin and 6
B-tubulin genes each. These have been relatively well conserved throughout evolution (Sullivan, 1988). In general, different tubulin isotypes, even
highly evolutionarily divergent isotypes, may coassemble in vivo arguing that isotypes are not sufficient to specify particular microtubule organelles (Bond et al., 1986). However, certain isotypes may confer special properties
to microtubules. For instance, Antarctic fishes, which require cold-stable microtubules, also have unique a-tubulin genes (Detrich et al., 1987).
Tubulins are also the targets for extensive post-translational modifications. Among these are de-tyrosination, acetylation, and
polyglutamylation (reviewed in Joshi and Cleveland, 1990). Again though, the functional significance of these modifications is not certain. It is known that axonal arrays of microtubules are stable in the presence of depolymerizing drugs and also tend to be de-tyrosinated. However, enhanced stability of these microtubules does not seem to be a direct
consequence of de-tyrosination (Khawaja, et al., 1988).
Lower eukaryotic organisms present a much simpler set of tubulin isotypes and post-translational modifications and also afford the possibility
of testing function by gene replacement. Saccharomyces cerevisiae has two a-tubulin genes and one B-tubulin gene. One of the two a- tubulin genes is essential, although overexpression of the non-essential a-tubulin can compensate for the loss of the essential gene (Schatz et al. 1986). Indeed, yeast are quite sensitive to the relative levels of a- and B-tubulin (Katz et al.,
1990; Weinstein and Solomon, 1990). The role of post-translational modifications of tubulin has also been investigated by genetic analysis. Replacement of the single Tetrahymena themophila a-tubulin gene with a mutant form incapable of being modified at an evolutionarily conserved acetylation site had no effect on these cells (Gaertig et al., 1995). These rigorous tests of tubulin function in these simple organisms argue against an essential role for both specific isotypes and post-translational
modifications of tubulin. While not definitive, the accumulated evidence thus far points toward molecular heterogeneity playing an auxiliary role in specifying cytoskeletal organization.
Polymer accessory proteins- Another mechanism by which the cytoskeleton can be modulated is by the action of non-covalently associated proteins- the accessory proteins. Both microtubules and microfilaments have characteristic sets of accessory proteins. We have already mentioned several such proteins in the discussion of the regulation of actin polymer dynamics- monomer sequestering proteins and barbed end capping
proteins. For the microfilament cytoskeleton, there exists a wide range of accessory proteins. A full accounting of this long list is well beyond the
scope of this introduction. Therefore, we will just briefly describe the
classes of microfilament accessory proteins in non-muscle cells to illustrate the range of their activities.
Microfilament accessory proteins can be broadly separated into motor and non-motor proteins. Motor proteins might play important roles in cell motility by mediating the sliding of microfilaments relative to one another
and/or to a substratum over which a cell is walking. A relative to myosin II, the mechanoenzyme responsible for muscular contraction, myosin I was initially identified as an actin-stimulated ATPase activity from Acanthamoeba (Pollard and Korn, 1973). Subsequently, this enzyme was
shown to support ATP-dependent movement of F-actin in in vitro motility assays (Albanesi et al., 1985). Myosin I's from a host of non-muscle cell types now comprise a large family of molecules. The function of myosin I has been investigated in cells. Disruption of myosin heavy chain
expression in Dictyostelium by antisense inhibition or gene disruption leads to mutant phenotypes in these cells (Knecht and Loomis, 1987; De Lozanne and Spudich, 1987). These mutants are defective in cytokinesis and a developmentally regulated morphogenetic event. These phenotypes are consistent with myosin I playing a key role in motile cell behavior. However, the cells still move. Individual cells walk across substrata exhibiting the classic (wild-type) motile behaviors such a extension of membrane ruffles, pseudopods, and other cortical cytoskeletal structures. There may be other myosin I-like molecules in these cells that can
compensate for the reduction or loss of myosin I. Alternatively, myosin I might be required for some motile behaviors and not others.
Non-motor microfilament accessory proteins come in a variety of flavors. These include crosslinking proteins such as filamin which can link actin filaments into an orthogonal network forming an actin gel in solution (Wang and Singer, 1987). Other crosslinking proteins arrange microfilaments into parallel bundles. Still other classes of microfilament accessory proteins can nucleate F-actin polymerization and sever pre-existing microfilaments. Examples of the last three classes can be found in a single molecule. Villin is a sort of molecular jack-of-all-trades which takes its name from the source of its initial purification in intestinal brush border microvilli. In biochemical studies with purified villin, this molecule can sever, crosslink into bundles, and cap the barbed ends of actin
filaments or nucleate the assembly of new filaments (Matsudaira and Janmey, 1988). The particular activities that villin exhibits at any one time
are regulated by calcium and phosphoinositides. Transfection of CV-1 cells with a full-length villin cDNA construct induces the presence of long,
intestinal microvilli-like processes suggesting that villin plays an
important role in microvilli formation (Friederich et al., 1989). One final class of microfilament accessory proteins are those which stabilize F-actin by binding along the sides of microfilaments. An example of this type is tropomyosin. Tropomyosins occur in a wide variety of muscle and non-muscle tissues. Biochemical studies have demonstrated that tropomyosin can protect actin filaments from severing (Fattoum et al., 1983).
Endogenous and microinjected tropomyosins localize to stress fibers (Pittenger and Helfman, 1992). However the role of these proteins in the establishment or maintenance of these relatively stable F-actin structures remains to be elucidated.
By way of comparison to microfilaments, microtubules may seem rather bereft accessory protein functions. Microtubules do have motor proteins- dynein and kinesin. Cytoplasmic dynein was first identified as an ATPase activity from sea urchin eggs (Weisenberg and Taylor, 1968).
Later, this protein was found associated with microtubules from bovine brain preparations and this form was shown to translocate to the minus
ends of microtubules in an ATP-dependent manner in vitro (Paschal et al., 1987). The presence of a minus-end directed motor in neurons has led to speculation that this motor plays a role in retrograde axonal transport (Vallee et al. 1989). More is known about kinesin. This protein was first purified as an ATP-binding protein from squid giant axons (Vale et al., 1985). In vitro motility assays of the purified protein revealed an ATP-dependent plus end-directed motor activity. Like myosins, kinesins
represent a large family of related proteins. Mutant alleles of kinesin heavy chain homologs in Aspergillus nidulans, Saccharomyces cerevisiae, and Drosophila melanogaster support a role for kinesin in mitotic or meiotic
spindle function (reviewed in Endow and Titus, 1992). Other experiments support a role for kinesin in fast axonal transport. Treatment of cultured neurons with kinesin antisense oligonucleotides resulted in a reduction of neurite length and aberrant distribution of growth cone localized proteins (Ferreira et al., 1992). A more pertinent finding is that antibody
perturbation studies show that kinesins play essential roles in anterograde vesicular transport in squid giant axons (Brady et al., 1990).
Microtubules also have non-motor accessory proteins. These
activities include proteins or protein complexes that nucleate microtubule assembly at microtubule organizing centers. As mentioned in the section on polymer dynamics, the centrosome is slowly yielding to molecular and cellular analysis. Microinjection of anti- y-tubulin antibodies inhibits reassembly of microtubules after drug-induced depolymerization (Joshi et al., 1990. Immunodepletion of cell extracts with anti-y-tubulin antibodies blocks the ability of sperm centrsomes to nucleate microtubule growth in a cell free system (Felix et al., 1994; Stearns and Kirschner, 1994). Likewise, depletion of pericentrin leads to the same effect, although y-tubulin is
recruited from the cell extract to the nucleating centrosome indicating that y-tubulin is not sufficient for nucleation (Doxsey et al., 1994). There is recent evidence for an activity, katainin, that severs microtubules in vitro (McNally and Vale, 1993). Such a protein could be of tremendous
importance for organizing the cytoskeleton; however, little is known about the in vivo function of this protein at present. By far the most extensively studied non-motor microtubule associated proteins are the so-called fibrous microtubule-associated proteins (Wiche et al., 1991). Weisenberg (1972) demonstrated that microtubules could be taken through successive rounds of polymerization and depolymerization in vitro. Since then, others have used this technique as a means for enrichment of proteins that specifically associate with microtubules. These studies initially defined a class of proteins called microtubule-associated proteins or MAPs (for example Weingarten et al., 1975). Over time, this group of proteins came to include MAPlA, MAP1B, MAP2, MAP4 and tau. These proteins all shared the features of high affinity binding to microtubules. Other, perhaps weaker microtubule binding MAPs-the chartins- were identified as proteins that co-align with microtubules in cells and are extracted along with tubulin by calcium (Duerr et al., 1981). Instead of reviewing the current evidence for the in vivo function for all of these MAPs, we will restrict comments below to two MAPs- tau and MAP2- that are germane to the present study.
The examples of accessory proteins above demonstrate the many ways in which these molecules can modulate and specify the organization of the cytoskeleton. In the work presented here, we have endeavored to
understand further the cellular functions of accessory proteins. Below, we will introduce the particular accessory proteins that are the focus of the present study.
IDENTIFICATION AND CHARACTERIZATION OF TAU AND RADIXiN: TWO ACCESSORY PROTEINS THE CYTOSKELETON IN
CULTURED MAMMALIAN CELLS
Tau- As mentioned above, MAP2 and tau were identified by virtue of their ability to co-assemble with microtubules. Among the other
demonstrated biochemical properties of tau and MAP2 is stimulation of microtubule polymerization and microtubule bundling (Cleveland et al.
1977). In neuronal cells tau and MAP2 occur in the neuritic processes with enrichment of tau in axons and MAP2 in dendrites (Matus et al., 1981; Peng et al., 1986). Taken together these observations suggested that tau and MAP2 might mediate the bundling of the microtubule arrays found in those
organelles. Transfection of tau and MAP2 into cultured cells induces the presence of bundles of microtubules (Kanai et al., 1989; Lewis et al., 1989). Molecular cloning revealed that these two proteins have related amino-acid sequences in their carboxy-terminal domains (Lee et al., 1988; Lewis et al., 1988). These conserved motifs were shown to be capable of promoting
microtubule assembly in vitro (Joly et al., 1989). Ultrastructural analysis of tau and MAP2 by immunoelectron microscopy indicated that these
molecules project from the surface of the microtubule lattice (Hirokawa et al., 1988 a,b). These results along with the biochemical data fueled
speculation that these projection domains mediated lateral interactions between microtubules. Indeed, transfection studies in cultured cells using
MAP2 deletion constructs identified a short hydrophobic segment responsible for the observed bundling properties of MAP2 in cells and suggested that MAP2 molecules dimerized via this domain (Lewis et al., 1989). However, subsequent to this study, these authors provided evidence that cast doubt on their earlier data (Lewis and Cowan, 1990).
Furthermore, more recent studies also argue against MAP2-mediated microtubule bundling by dimerization of MAP2 molecules (Burgin et al., 1994). What mechanisms might account for microtubule bundling? One hypothesis put forth by Chapin et al. (1991), is based on the observation that taxol- a microtubule stabilizing drug- induces microtubule bundling. This theory holds that bundles are an energetically favorable state for stable microtubules. If MAP2 and tau do not directly mediate microtubule
bundling in cells, what are their functions? We have addressed this question for tau in Chapter Two.
Radixin- For a number of years, the Solomon laboratory has used the chicken erythrocyte as a model system to study the molecular specification of the cytoskeleton. As described above, these cells are available in bulk, the cytoskeleton -the marginal band- in this cell is rather simple, and
microtubules and microfilaments are though to play a critical role in the morphogenesis of this cell type (Winckler and Solomon, 1991). That
accessory proteins are critical for the specification of the marginal band is demonstrated by the following experiment. In detergent extracted cells, the marginal band microtubules can be selectively depolymerized by a
temperature shift so that endogenous tubulin can be separated from the remaining cytoskeleton. When exogenous calf brain tubulin is added to these erythrocyte cytoskeletons in a buffer that supports polymerization, the marginal band microtubules re-form in detail- the correct number of
microtubules course around the original equator of the extracted cell (Swan and Solomon, 1984). This experiment argues that polymer-extrinsic factors specify the structure of the band. Birgbauer and Solomon (1989) conducted an immunological screen for non-tubulin marginal band proteins. They reasoned that MAPs could play a role in specification of the band and so used cycled chicken brain microtubule proteins as an immunogen for the
production of monoclonal antibodies. Antibodies were screened for specific localization to the marginal band. One antibody, 13H9, emerged that
fulfilled the criteria set forth in the screen. This antibody recognized an 80 kDa protein on Western blots of chicken cytoskeletal proteins and also
stained cortical cytoskeletal structures in fibroblasts and neurons (Goslin et al., 1989; Birgbauer, 1991). Through the considerable efforts of a number of members of the Solomon laboratory, the identity of the 13H9 antigen was
established. mAb 13H9 recognized an epitope conserved in all members of the ezrin-radixin-moesin (ERM) protein family.
The ERM proteins were being isolated and characterized
concomitantly with the work on the marginal band from the Solomon laboratory. At that point in time, the ERM proteins were thought to be involved in mediating interactions between the cytoskeleton and plasma membrane. This hypothesis stemmed from two findings: 1) the proteins
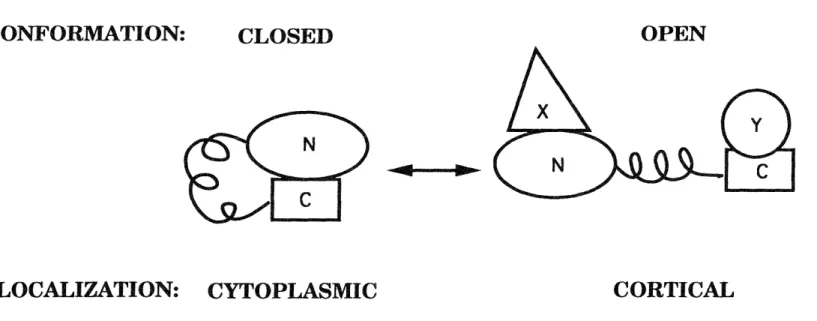
were localized to cortical cytoskeletal sites, and 2) molecular cloning revealed that the amino-terminal domains of ERM proteins are similar to the amino-terminal domain of band 4.1 (Fig. 1-1). All of the members of the band 4.1 superfamily share the property of being closely associated with the plasma membrane. Other studies implicated the amino-terminal domain of band 4.1 in binding to the integral membrane protein glycophorin (Leto et al., 1986). Sequencing of the distinct genes that encode ERM proteins in different laboratories revealed that these proteins are highly similar to one another and created the unfortunate situation where the proteins are named individually rather than as isoforms of one another.
The similarity of the amino acid sequences of ERM proteins hampered immunological efforts to determine the cellular and tissue
distribution of ERM proteins. Winckler et al. (1994) developed mono-specific anti-peptide antisera that have shed light on this issue. The results from this study indicated that radixin and ezrin localize to the position of the marginal band, with radixin being the predominant species. In a
developmental series, radixin localized to the position of the marginal band only after the microtubules had arrived there, making it unlikely that this protein is involved in the establishment of marginal band microtubules. In fact, during development, radixin is more closely co-localized with F-actin. Data indicate that the arrival of radixin in the band is correlated with the resistance of marginal band microfilaments to drug-induced
depolymerization, supporting a possible role for radixin in stabilization of the marginal band. In Chapters Three through Seven, we have extended the studies on radixin with an emphasis on understanding its function in cultured mammalian cells.
Figure 1-1. Protein sequence comparison of ERM proteins and band 4.1 family members. Ezrin and moesin are compared to full-length radixin, and the amino-terminal domains of band 4.1 family members are
compared to the amino-terminal domain of radixin using the BestFit
sequence comparison program. Percent similarity is indicated at the right of the figure. Actual or predicted structural features of radixin are
Radixin: A member of the ERM protein family
and Band 4.1 superfamily
The ERM proteins
band 4.1 homology alpha helix PPP bindingF-actin % similarity toradixin 100
87
89
The Band 4.1 superfamily % similarity to radixin N-domain Band 4.1 Merlin Talin rn-rn
H-EM10 PTPH1 PTPaseMEG Radixin Ezrin MoesiniI
52 78 47 73 55 51 29m pq
pq
c IeNICHAPTER TWO:
Antisense inhibition of tau protein expression in embryonal
carcinoma P19
cells.
SUMMARY
Utilizing the properties of embryonal carcinoma P19 cells, we have studied the role of the microtubule-associated protein tau during neuronal differentiation. P19 cells can be induced to form either neural cultures (including neurons and glia) in response to retinoic acid (RA) or muscle cultures in response to dimethyl sulfoxide (DMSO). Because P19 cells can assume a number of different cell fates, like the early embryonic cells from which they were derived, these cells may provide a system to assess tau function during early stages of neuronal differentiation. To test tau function in P19 cells, we have expressed an antisense RNA element to inhibit specifically tau protein expression in these cells. Our results indicate that inhibition of tau expression, unlike inhibition of another microtubule-associated protein, MAP2, in these same cells, has no gross
effect on the expression of a differentiated neuronal morphology.
INTRODUCTION
The dramatic changes in cell shape that occur during cellular
differentiation are often manifested in changes in cytoskeletal organization. Considerable effort has focused on defining the molecular basis of the
regulation of cytoskeletal organization during cellular differentiation. Neurons are a particularly attractive model system for the study of cellular specification of cytoskeletal organization because their striking
morphologies play an explicit role in nervous system function. The axons and dendrites, the most prominent morphological features of neurons, contain dense, longitudinal bundles of microtubules. Drug interference
experiments indicate that these microtubules are necessary for both the establishment and maintenance of neuritic processes. Since microtubule associated proteins (MAPs) are known to bind to and stabilize microtubules in vitro and in vivo, and since the expression patterns of some MAPs are modulated during neuronal differentiation, MAPs may be significant deter-minants of neuronal cell shape (Matus, 1990; Wiche et al., 1991).
We have investigated the function of the neuronal MAP tau. Tau was initially identified on the basis of its ability to co-assemble with tubulin in vitro, and to promote that assembly reaction (Weingarten et al., 1975;
Cleveland et al., 1977). Tau stabilizes microtubules by modulating dynamic instability (Dreschel et al., 1992)Immunolocalization revealed that tau is concentrated in axons of mature neurons (Binder et al., 1985), in the
somatodendritic domain of developing neurons (Kosik and Finch, 1987) and even in glial cells (Papasozomenos and Binder, 1987). Tau is encoded by a single gene, conserved among vertebrates, but the protein exists as a set of polypeptides generated by both alternative splicing (Himmler, 1989) and at least one type of covalent modification (Lindwall and Cole, 1984). During normal development there is a shift from expression of lower molecular weight tau isoforms to higher molecular weight isoforms (Kosik et al.,
1989). In Alzheimer's disease patients, tau in brain tissue is abnormally phosphorylated (Grundke-Iqbal et al., 1986). It is also a principal
constituent of the paired helical filaments associated with neurofibrillary tangles- a pathological hallmark of the disease (Lee et al., 1991). However, the role of tau in this disease is unclear.
Several groups have assayed tau function in model cell culture systems. Introduction of tau into non-neuronal cells by either
microinjection of the protein (Drubin and Kirschner, 1986) or transfection with tau cDNA (Kanai et al., 1989; Lewis et al., 1989) shows that exogenous tau decorates microtubules and promotes tubulin polymerization.
Expression of tau in moth ovary cells induces the formation of long, microtubule-filled processes (Knops et al., 1991). In two neuronal cell systems, disruption of tau expression with antisense oligonucleotides affects process extension. In primary cerebellar neurons, the treated cells initiated neurites but failed to elaborate axons (Caceres and Kosik, 1990; Caceres et al., 1991). The processes of nerve growth factor-induced PC12 cells eventually retracted their neurites in response to antisense
oligonucleotides (Hanemaaijer and Ginzburg, 1991). In another study in PC12 cells, nerve growth factor-induced transfectants expressing tau antisense RNA showed a decrease in mean neurite length compared to controls (Esmaeli-Azad et al., 1994). Taken together, the results are consistent with tau playing a central role in stabilizing neuritic microtubule arrays.
We have investigated tau protein function in embryonal carcinoma P19 cells by inhibiting its synthesis through the constitutive expression of a tau antisense transcript. P19 cells can be induced to differentiate into neural cells with RA or into muscle cells with DMSO (McBurney and
Rogers, 1982; McBurney et al., 1982). RA-induced P19 cultures include cells that possess many characteristics of neurons, such as the extension of neuritic processes and the expression of neuron-specific biochemical markers (Jones-Villeneuve, 1983; McBurney et al., 1988); and also contain cells resembling several non-neuronal cell types (Jones-Villeneuve et al., 1982).
P19 cells are an attractive model system for the study of tau protein function. P19 cells are relatively uncommitted precursor cells since they can be driven down either neural or muscle differentiation pathways.
Therefore, the possibility exists to examine tau's contribution to early stages of differentiation. Since the expression of many genes are induced de novo by RA or DMSO in P19 cells, antisense RNA to these genes expressed in the uninduced state would lack a target and so not be expected to have effects on uninduced cells. This feature would allow for the selection of cell lines
without antisense RNA expression imposing an additional selective pressure. Previously, others in this lab used an antisense strategy in P19 cells to examine the role of another microtubule component, MAP2, in neuronal differentiation. The results demonstrated that MAP2 expression is required for two of the phenotypes associated with the RA-induced
differentiation of these cells: the extension of neurites and decreased cell proliferation (Dinsmore and Solomon, 1991). Here we show that inhibition of tau expression has consequences different from those associated with MAP2 inhibition. The reduction in tau levels do not affect neuronal morphology or cell growth as a response to RA in P19 cells.
MATERIALS AND METHODS
Cell culture. The EC cell line P19 (McBurney, 1982) was kindly
provided by Dr. M. McBurney (University of Ottawa, Ottawa, Canada). P19-A4, a euploid P19 subclone isolated during these studies and described in Appendix One, was used for some experiments where indicated. Routine culture and induction of P19 cells were done as previously described
(Rudnicki and McBurney, 1987). The RA and DMSO inductions were performed as modified by Dinsmore and Solomon (1991). Briefly, 2.4 X 106 cells were plated in 100 mm bacteriological grade dishes in MEMa + 2% fetal calf serum supplemented with 0.5 gM all-trans retinoic acid (Sigma). Under these conditions, cells do not adhere to the culture dish and instead form aggregates suspended in the medium. Medium described above was replaced after 2 days incubation. After 4 days incubation, cell aggregates were harvested and trypsinized to form a single cell suspension. From this
suspension, 3 X 106 cells were plated in tissue culture grade dishes in MEM(a) + 7.5% calf serum/2.5% fetal calf serum. Normally, cells treated in this manner have fully elaborated neurites and expressed
neuron-specific biochemical markers 3 d after plating on tissue culture plastic. Cells were photographed and harvested for biochemical analysis at this time unless otherwise noted. Cells were examined with a Nikon Diaphot inverted phase microscope. All phase micrographs were recorded on
Kodak Plus-X-Pan film, ASA125, developed in Microdol-X (Kodak).
DNA constructs and transfection. The vector pGEM7-KJ1-Sal (described in Dinsmore and Solomon, 1991) was modified by partial
digestion with BamHI, blunting the ends with the KIenow fragment of E. coli DNA polymerase and ligating with T4 DNA ligase to eliminate a
BamHI site 3' of the phosphoglycerate kinase (PGK) polyadenylation signal sequence. This construct, named pGKBA, was used in these studies. The pCXN2 vector was provided by Dr. Jun-ichi Miyazaki and is described in (Niwa et al., 1991).
The F13 murine tau cDNA clone was kindly provided by Dr. G. Lee (Brigham and Women's Hospital, Boston, MA). The F13 clone contains 186 bases of 5' untranslated sequence and 562 bases (54%) of coding sequence of
a tau cDNA from 6-day-old mouse (Lee et al., 1988). F13 was digested with