© Miles Ownby, 2020
Phosphorus removal and recovery from wastewater via
nano-enhanced adsorptive media
Mémoire
Miles Ownby
Maîtrise en génie chimique - avec mémoire
Maître ès sciences (M. Sc.)
ii
Résumé
L’augmentation rapide de la population mondiale et des pratiques industrielles et agricoles ont exacerbé l’épuisement des nutriments essentiels pour la croissance des plantes, phosphore en particulier, étant lui-même une ressource non-renouvelable. Après des années d’exploitation agricoles et miniers écologiquement laxistes, la société se trouve coincée entre une pénurie croissante d'éléments nutritifs et la fréquence croissante de proliférations d'algues nuisibles (HAB) causées par la lixiviation de phosphore dans les systèmes aquatiques. Toutefois, ceci présente une opportunité de développer des nouvelles technologies permettant d'éliminer, de récupérer et de réutiliser le phosphore provenant de cours d'eau pollués. L'une de ces technologies est l'adsorption nano-renforcée. Cette étude a évalué le potentiel de désorber le phosphore d'une résine échangeuse d'ions hybridée avec des nanoparticules d'oxyde de fer pour quatre solutions de régénérations différentes en utilisant une approche de plan d’expériences. Des
nouvelles solutions de régénération utilisant un mélange KOH / K2SO4 et une
solution alcaline de NH4OH se sont révélées comparables à la solution "témoin" de
KOH et de H2SO4. Parmi les 4 méthodes de régénération étudiées, la solution de
NH4OH présente le potentiel le plus élevé car il s’agit d’un déchet valorisé. Son efficacité de désorption est comparable à celle de la solution de contrôle et elle n’a démontré aucune perte de la longévité de la résine après cinq cycles d’adsorption et de désorption. Sur la base des données du plan d’expériences, une série de modèles de régression a été développée pour permettre de mieux comprendre la concentration de phosphore attendue d'un processus de régénération, en tenant compte de la chimie de régénération, du volume de traitement, de la vitesse de rinçage et de la résistance de la solution alcaline. Les solutions de post-désorption de régénération riches en nutriments semblent prometteuses pour une utilisation ultérieure. Les travaux futurs devraient inclure le développement de modèles de procédé afin de mieux comprendre les mécanismes de cette désorption. Dans l’ensemble, la technologie d’adsorption nano-améliorée offre une solution rentable et durable au problème du phosphore dans les applications de traitement des eaux usées à travers le monde.
iii
Abstract
Rapid increases in the world’s population and to-date industrial and agricultural practices have exacerbated the depletion of essential nutrients in today’s society. After years of environmentally lax agricultural and mining processes, society finds itself trapped between increasing nutrient shortage and the increased frequency of harmful algal blooms (HABs) caused by phosphorus leaching into water systems. New technologies that allow for removal and subsequent recovery and reuse of phosphorus from polluted streams is imperative. One such technology is nano-enhanced adsorption, which may allow to produce a valuable nutrient-rich solution upon desorption of the saturated media. This study evaluated the potential of four regeneration chemistries to desorb phosphorus from a commercially available ion exchange resin hybridized with iron-oxide nanoparticles using a Design of
Experiments (DoE) approach. Novel regeneration solutions using a KOH/K2SO4
blend and a recovered NH4OH alkaline solution proved to be comparable to the
"control" solution of KOH and H2SO4. Among the four regeneration methods studied,
using the NH4OH solution shows the highest potential because: i) it is a valorized
waste stream, ii) it showed a desorption efficiency comparable to the control solution, and iii) it did not demonstrate any dampening of the resin longevity after five adsorption and desorption cycles. Based on the DoE data, a series of regression models was developed to generate understanding with regard to expected phosphorus concentration from a regeneration process considering the regeneration chemistry, the treatment volume, the rinse speed, and the strength of the alkaline solution. Nutrient-rich regeneration solutions post-desorption show promising for subsequent use as hydroponic fertilizers or precursors for the P fertilizer industry. Future work should include the development of mechanistic process models to gain an even better understanding of the mechanics behind the desorption. Overall, the nano-enhanced adsorptive technology proposes a cost-effective and sustainable solution to the phosphorus problem in wastewater treatment applications across the globe.
iv
Table of Contents
RÉSUMÉ ... II ABSTRACT ... III TABLE OF CONTENTS ... IV LIST OF FIGURES ... VI LIST OF TABLES ... VII ACKNOWLEDGEMENTS ... VIIIINTRODUCTION ... 1
1. LITERATURE REVIEW ... 8
1.1REGULATIONS REGARDING PHOSPHORUS EFFLUENT LIMITS ... 8
1.2CURRENT PHOSPHORUS REMOVAL STRATEGIES ... 9
1.3NOVEL METHODS IN P RECOVERY ... 12
1.3.1 Precipitation ... 12
1.3.2 Adsorption ... 15
1.4NANO-ENHANCED ADSORPTIVE MEDIA ... 20
2. DESCRIPTION OF MASTER’S PROJECT ... 27
2.1OBJECTIVES AND ORIGINALITY ... 27
3. EXPERIMENTAL METHODOLOGY ... 30
3.1PHASE I–EXPERIMENTAL PLANNING ... 30
3.2PHASE II–REGENERATION CHEMISTRY SELECTION ... 33
3.3PHASE III–MICROCOLUMN CONSTRUCTION AND SYNTHETIC WASTEWATER PREPARATION ... 35
3.4PHASE IV–RESIN CHARACTERIZATION ... 37
3.5PHASE V–DESORPTION AND REGENERATION EXPERIMENTS ... 38
3.6PHASE VI–MODEL DEVELOPMENT ... 39
4. RESULTS ... 41
4.1RESIN CHARACTERIZATION ... 41
4.2REGENERATION EXPERIMENTS ... 43
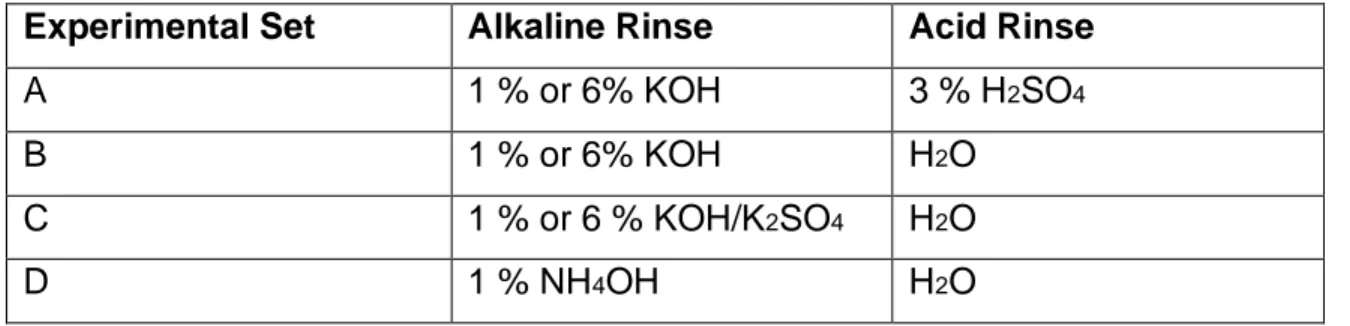
4.2.1 Experimental Set A (KOH + H2SO4)... 46
4.2.2 Experimental Set B (KOH + H2O) ... 46
4.2.3 Experimental Set C (KOH/K2SO4 + H2O) ... 46
4.2.4 Experimental Set D (NH4OH + H2O) ... 47
4.3RESIN LONGEVITY ... 47
4.4REGRESSION MODELS ... 48
4.5COMPOSITION OF RECOVERED NUTRIENT SOLUTIONS ... 51
5. DISCUSSION ... 52
5.1RESIN CHARACTERIZATION ... 52
5.2PHOSPHORUS DESORPTION... 53
5.2.1 Experimental Set A (KOH + H2SO4)... 53
5.2.2 Experimental Set B (KOH + H2O) ... 53
v
5.2.4EXPERIMENTAL SET D(NH4OH+H2O) ... 55
5.3REGRESSION MODELS ... 56
5.3.1 Model B: Base wash ... 58
5.3.2 Model B: Acid wash ... 59
5.3.3 Model B: Combined wash ... 60
5.3.4 Model C: Base wash ... 61
5.3.5 Model C: Acid wash ... 62
5.3.6 Model C: Combined wash ... 63
5.3.7 Model D: Base wash ... 64
5.3.8 Model D: Acid wash ... 65
5.3.9 Model D: Combined wash... 65
5.3.10 Regression Model Summary ... 66
5.4VALORIZATION POTENTIAL OF RECOVERED NUTRIENT SOLUTIONS ... 67
5.5 SCALE-UP STUDY AND ECONOMIC FEASIBILITY ANALYSIS ... 68
5.6 LIMITATIONS OF THIS STUDY AND SUGGESTIONS FOR FURTHER RESEARCH ... 71
CONCLUSION ... 73
vi
List of Figures
FIGURE 1. EXAMPLE OF A BREAKTHROUGH CURVE ADAPTED FROM COOK ET AL.(2017).THE BLACK LINE REPRESENTS THE EFFLUENT CONCENTRATION OF THE TARGET MOLECULE AND THE RED LINE
REPRESENTS THE INFLUENT CONCENTRATION OF THE TARGET MOLECULE... 17
FIGURE 2. CHEMICAL SIMILARITIES BETWEEN ARSENIC AND PHOSPHORUS (PADUNGTHON,2013). ... 20
FIGURE 3. SYNERGY EFFECT BETWEEN METAL OXIDE NANOPARTICLES AND POLYMERIC ION EXCHANGE RESIN (ADAPTED FROM PADUNGTHON,2013)... 22
FIGURE 4. ORTHOPHOSPHATES FORM MONO AND BIDENTATE INNER-SPHERE COMPLEXES WITH THE IRON OXIDES ON THE NANO-ENHANCED ADSORPTIVE RESIN.LAB=LEWIS ACID-BASE INTERACTION. (BLANEY ET AL.,2007) ... 23
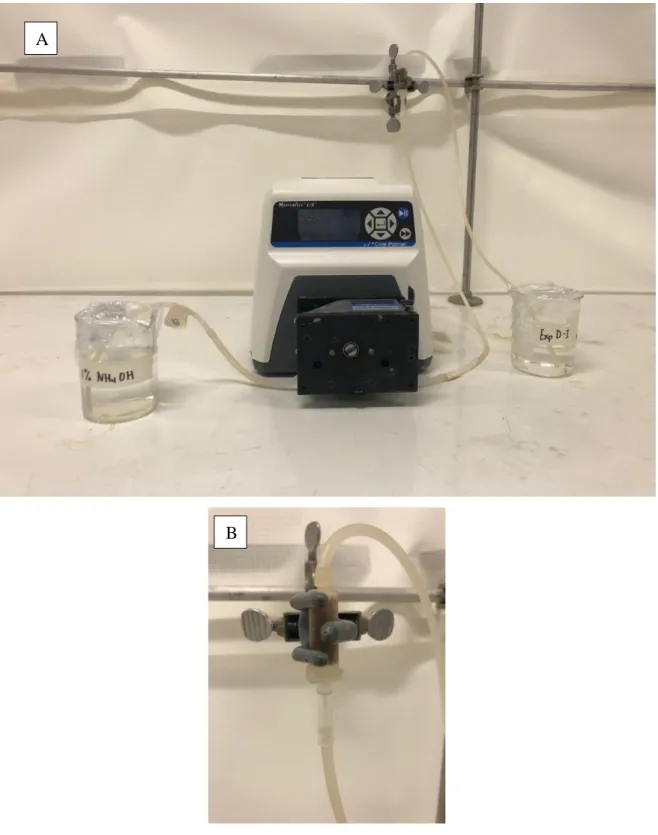
FIGURE 5. A)MICROCOLUMN SET-UP SHOWING THE INFLUENT AND EFFLUENT COLLECTION RECEPTACLES AND A PERISTALTIC PUMP USED AND B) A CLOSE-UP OF THE MICRO-SCALE COLUMN ... 36
FIGURE 6. GRAPH OF [PO4-P] IN EFFLUENT WITH RESPECT TO BED VOLUMES TREATED. ... 43
FIGURE 7. A)DESORPTION EFFLUENT CONCENTRATION VS. REGENERATION CHEMISTRY AND CONDITIONS. B) P RECOVERY EFFICIENCY VS. REGENERATION CHEMISTRY AND CONDITIONS. ... 45
FIGURE 8. COLUMN ADSORPTION QUANTITIES OVER 5 CYCLES. A)EXPERIMENTAL SET C; B)EXPERIMENTAL SET ... 48
FIGURE 9. RESIDUAL AND NORMAL Q-QPLOT FOR EXPERIMENTAL SET B-BASE. ... 59
FIGURE 10. RESIDUAL AND NORMAL Q-QPLOT FOR EXPERIMENTAL SET B-ACID. ... 60
FIGURE 11. RESIDUAL AND NORMAL Q-QPLOT FOR EXPERIMENTAL SET B–COMBINED. ... 61
FIGURE 12. RESIDUAL AND NORMAL Q-QPLOT FOR EXPERIMENTAL SET C-BASE... 62
FIGURE 13. RESIDUAL AND NORMAL Q-QPLOT FOR EXPERIMENTAL SET C-ACID. ... 63
FIGURE 14. RESIDUAL AND NORMAL Q-QPLOT FOR EXPERIMENTAL SET C-COMBINED. ... 64
FIGURE 15. RESIDUAL AND NORMAL Q-QPLOT FOR EXPERIMENTAL SET D-BASE... 65
FIGURE 16. RESIDUAL AND NORMAL Q-QPLOT FOR EXPERIMENTAL SET D-ACID. ... 65
vii
List of Tables
TABLE 1. TRIGGER LEVELS OF TOTAL PHOSPHORUS IN CANADIAN WATER SYSTEMS (ENVIRONMENT
CANADA,2004). ... 5
TABLE 2. KEY FOR EXPERIMENTAL SET REGENERATION CHEMISTRY FORMULATION. ... 31 TABLE 3. 23 FULL-FACTORIAL EXPERIMENTAL DESIGN CONDUCTED THREE TIMES (EXPERIMENTS A,B, AND
C) ACCORDING TO VARYING REGENERATION CHEMISTRY. ... 32
TABLE 4. 22 FULL-FACTORIAL EXPERIMENTAL DESIGN CONDUCTED ONE TIME (EXPERIMENT D) ACCORDING TO VARYING REGENERATION CHEMISTRY. ... 33 TABLE 5. MINIMUM, MAXIMUM, AND AVERAGE CONCENTRATIONS OF PHOSPHORUS (MG PO4-P/L) FOR ALL
EXPERIMENTS IN EACH PHASE (BASE AND ACID) AND IN THE COMBINED RECOVERY PROCESSES. ... 44
TABLE 6. REGRESSION COEFFICIENTS FOR EACH FACTOR AND INTERACTION FOR EACH PHASE OF EACH EXPERIMENTAL SET.SIGNIFICANCE LEVELS ARE INDICATED AS FOLLOWS:(.)0.1,(*)0.05,(**) 0.01, (***)0.001. ... 50
TABLE 7. FACTOR AND INTERACTION CODES USED IN LINEAR REGRESSION MODELING... 57
TABLE 8. IONIC SPECIES CONCENTRATIONS IN VILLE DE QUÉBEC MUNICIPAL EFFLUENT AFTER PRIMARY AND SECONDARY TREATMENT. ... 69
viii
Acknowledgements
I am extremely thankful for all of the support that I have had during my experience in Québec. The constant support and listening ear of my family has made an enormous impact on my master's experience. When times were tough and I was struggling to find motivation, my family quickly helped me put things into perspective and provide me with sound advice on how to continue. The same goes for my friends that I've made here. Joining the Club de Course à Pied de l'Université Laval has been one of the most formative experiences since moving to Québec. They helped me integrate into society, train for my first marathon, and relieve the stress of day to day work with fun and challenging practices several times a week. I don't know that I have ever been part of a team so quick to support and encourage runners of all skill levels. The three runners in my group, Jordane, Pierre-Charles, and Yassine, quickly became like brothers to me and helped me find my place in Québec. I would be remiss as well to not mention Nicolas, my first Québec friend. From the get-go he has been there to support and encourage my endeavors and is always down for a weeknight beer and nachos when things get stressful. And of course, my forever gratitude goes out to Dr. Céline Vaneeckhaute and the entire BioEngine team. They have become like a second family and provide unending support and advice. I will sincerely miss the socials, the discussions over lunch, and the genuinely fun experiences we had in the lab. What I initially expected to be a group of colleagues quickly became the group of my best friends in Québec. I would be remiss if I didn't highlight the work done by David-Alexandre Desrosiers in particular to help me complete my lab work. He could always be counted on for a laugh and made work significantly more fun and efficient. I would also like to thank my co-advisors Dr. Lotfi Khiari and Dr. Dominic Larivière. Their wisdom and guidance helped solidify different aspects of my wide-ranging project and inspired an even stronger passion for agro-chemistry. I couldn't be more thankful for my experience at Université Laval with BioEngine and Dr. Vaneeckhaute. It has made me the engineer that I am today, and the relationships and experiences that I've made during my time here will follow me for life.
Introduction
Phosphorus’ earliest contribution to life occurred around 3.5 billion years ago when it played a crucial role in both cell membrane stability and photosynthesis in the first oxygen-reducing bacteria, the very same that began releasing oxygen into Earth’s atmosphere (Butusov and Jernelöv, 2013). Since that time, phosphorus continues its role in the structure, energy, and functionality of most living organisms on Earth, thereby playing a supporting yet crucial role in the Earth’s major biodiversity events. From the membranes that surround each of our cells to the genetic code that makes up everything about who we are, human life is intricately and irrevocably intertwined with phosphorus.
As societies grew more intelligent and began to sustain themselves, eventually industrializing food production, they realized the power of phosphorus and harnessed it for their benefit. Phosphorus does not hold influence in merely the animal kingdom. It is a major component of the stem, leaf, and root structure of plants, and thus vital in any agricultural endeavor. Even thousands of years back, humans understood that their intervention into the natural phosphorus cycle could be used to produce more food. For example, evidence of controlled burns and manure reuse dates back thousands of years (Sutton, 2013). Augmenting the soil with nutrients such as nitrogen, potassium, and phosphorus allowed humans to produce far more than previously possible. These explosions in technology led to ever-increasing production capabilities to match the growing demand for food, growing ever greater with the global population increase.
With this rapid increase in industrialization and human population since the turn of the industrial revolution, civilization finds itself in increasingly dire need of the Earth’s resources to sustain its way of life in terms of production and consumption. Since 1960, the use of synthetic phosphorus fertilizers has tripled, damaging further and further the natural phosphorus cycle (Sutton, 2013). Mining, fertilizing, and growing
2
without abandon has pushed Earth nearer and nearer to the steep cliff into environmental detriment. Since phosphorus is primarily obtained from mining operations, the operations themselves leading to environmental issues, it is considered a non-renewable resource. Furthermore, fertilizers used in excess before the advent of precision agriculture lie dormant in the soils and water systems still today, posing the threat of nutrient leaching and poisoning of groundwater, watersheds, and marine environments.
Since the dawn of humanity, civilizations have strategically placed themselves near water sources as providers of food, fertile land, and water itself, and while societies have progressed immensely, the dependence on water continues today, as
evidenced by the fact that about half of the world’s population lives within 200
kilometers of a coastline (Sutton, 2013). That means that half of the world population is vulnerable to the adverse effects caused by excess phosphorus in water systems.
Water makes up a vital part of the water, energy, and food security nexus that affects the global society. Security issues involving food, water, and energy are irrevocably intertwined in such a global society, and the agricultural industry is situated right at the center of this nexus. Because of the intertwined nature of these three sectors of society, damage to the global water supply has implications for food and energy security all across the globe. Water quality in Quebec affects the entire population. According to the 2018-2030 Quebec Water Strategy, the St. Lawrence river is the primary source of drinking water for some 2.5 million Quebeckers while groundwater is the primary source for some one million other Quebeckers (Quebec Water Strategy, 2018). Those in Montreal, Quebec City, and other more urban settings may not feel the same level of water insecurity than those in rural, particularly northern rural, settings may feel. In fact, communities in the north of Quebec struggle to have access to clean water or to make their water drinkable to such a point that it is explicitly mentioned as a problem to remedy in the Quebec Water Report. Among the other aggressive goals set forth in the report are that by 2030, all municipalities in southern Quebec will need to have secure access to drinking water and that over
3
90% of municipalities across Quebec will need to meet wastewater treatment effluent standards (Quebec Water Strategy, 2018).
The soluble reactive phosphorus component of dissolved phosphorus is a known contributor to harmful algal blooms and eutrophication when this nutrient pollutant overloads fresh waterways. Eutrophication, from the Greek eutrophos meaning "well-nourished", describes the enrichment of water bodies in nutrients, notably phosphorus and nitrogen (Kahn and Asari, 2005; "Eutrophication", 2019). Mixed single-cell algae species in water typically have an elemental composition of carbon, nitrogen, and phosphorus in an atomic ratio of 106 carbon, 16 nitrogen, and 1 phosphorus (Bartsch, 1972). As evidenced by this ratio, phosphorus is the limiting element in the growth and proliferation of algal species. When a water system is exposed to high concentrations of phosphorus, the algal life grows at an abnormally fast rate and dominates the water system, increasing the suspended material in the water and negatively affecting its aesthetic. As the algae die, bacteria that decompose the biomass deplete the dissolved oxygen in the water system, leading to mass death of aquatic species. Moreover, the abundance of phosphorus in a water system can also cause the proliferation of cyanobacteria. These organisms appear as a blue-green scum on the surface of water bodies. They cause the same issues as algal blooms, but when the cyanobacteria die, they release harmful toxins (neurotoxins, hepatotoxins) that can lead to blood poisoning and paralysis and respiratory failure in animals that drink from the water sources (Carmichael, 1994).
The pathways of phosphorus pollution are numerous, including wastewater treatment plant discharge (municipal and industrial), farm fertilizer losses due to erosion, runoff, drainage or leaching as indicated above, urban runoff (stormwater pollution), and phosphate rock fertilizer production. The fact that phosphorus is a vital nutrient for plant, animal and human life and also a finite resource makes the phosphorus pollution problem so vexing. As global leaders in science become increasingly aware of the threat that the paradox between rapid phosphorus depletion and an excess in the environment poses to agriculture, the food industry,
4
coastal economies, the environment, and to water quality, the need for renewable infrastructure development and nutrient recycling practices becomes clear. Changes towards a more sustainable balance of the nexus will require great participation by all of society, urban and rural. Industries such as the wastewater treatment sector have made great strides in responsible nutrient management, but still some smaller-scale industries, such as local agriculture, are struggling to be incentivised to take on these sustainable practices (Sutton, 2013). Ironically, these small-scale farming communities are often the ones that will feel most strongly the effects of nutrient toxicity in their environments.
In Canada, and Quebec in particular, agriculture is a major industry that draws both local and migrant workers. In 2015, the industry employed 55,866 people, about 42,000 of which were farm operators themselves (Statistics Canada, 2016). The communities around which those farms are located, comprising over a quarter of the population, benefit equally from clean water resources (Guimond and Jean, 2015). Like many countries across the world, fertilization processes in Canada and Quebec have been heavily regulated after years of poor nutrient management. However, the excess nutrients caused by surplus fertilization stay in the soil and aquatic environments, lying dormant and unusable until they are solubilized and can harm the environment. These sources of phosphorus are called legacy phosphorus
because they comprise the legacy left to today’s rural communities by older
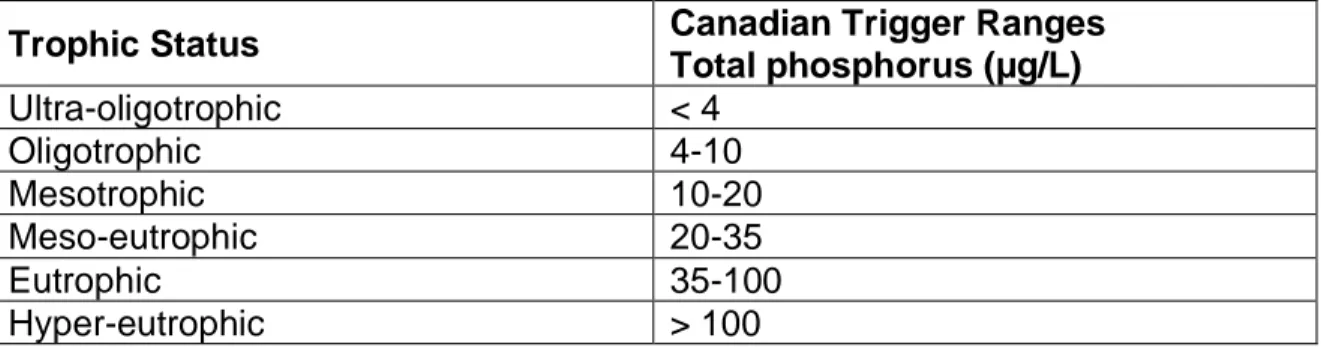
generations. Efforts to assuage the nutrient excess in the environment have led to the development of regulations by the government of Canada and the provincial Quebec government (Environment Canada, 2004). The trigger values in Table 1 show the limits for different characterizations of water in Canadian ecosystems based on their level of P and subsequent risk of nutrient toxicity.
5
Table 1. Trigger levels of total phosphorus in Canadian water systems (Environment Canada, 2004).
Trophic Status Canadian Trigger Ranges Total phosphorus (µg/L) Ultra-oligotrophic < 4 Oligotrophic 4-10 Mesotrophic 10-20 Meso-eutrophic 20-35 Eutrophic 35-100 Hyper-eutrophic > 100
These regulations, categorizing water bodies according to their phosphorus toxicity potential, were developed by the government of Canada after a lengthy investigation into the different regulations across the globe. As one can see in the previous Table 1, the phosphorus limit to qualify a body of water as ultra-eutrophic is only 0.1 mg/L and above. Common wastewater treatment processes often experience phosphorus levels of 4 to 16 mg PO4-P/L, and so must greatly reduce the amount of phosphorus in their effluent before releasing it into any surrounding bodies of water (Tchobanoglous et al., 2001). The discharge regulations will be discussed in more detail in the literature review below.
Furthermore, with the Québec action plan on climate change comes the banning of organic waste landfilling and incineration from 2022 onwards. Herewith, the presence of large-scale centralized anaerobic digesters for municipalities and urban areas is increasing, as is subsequently the production of digestate, a phosphorus-rich residual product of anaerobic digestion (Quebec Water Strategy, 2018). As defined by the Ontario Ministry of Agriculture, Food, and Rural Affairs (OMAFRA), anaerobic digestion is the process by which organic materials in an enclosed vessel are broken down by micro-organisms, in the absence of oxygen (OMAFRA, 2019). The goal of this process is both the management of organic waste and the production of bio-sourced natural gas or energy alternatives. In regions with nutrient excess problems, the production of digestate can present a serious issue that requires steep fees and complicated storage and shipping networks to resolve. To facilitate the digestate's handling, the digestate is generally separated into its solid and liquid
6
phase, each containing a significant amount of phosphorus (Hjorth et al., 2010; Tchobanoglous et al., 2001; Vaneeckhaute et al., 2017). The solid fraction is generally transformed into a soil conditioner, but the liquid fraction containing the residual dissolved phosphorus is more problematic (Vaneeckhaute et al., 2017). The regulations imposed by the Quebec Water Strategy (2018), tightening effluent standards and the growth of the anaerobic digestion industry will force the need for nutrient removal and recovery technologies.
Although the pressure on farmers to install and manage a farm-scale digester will present a new set of challenges, the added benefits of waste reduction, odor control, and energy production plus the benefit of recycled nutrient products may pose convincing arguments for implementation in these cases. Moreover, in large-scale, municipal waste treatment centers, anaerobic digestion treatment trains could provide the nutrient-rich product streams to industries or agricultural sites in the region in exchange for their organic waste, and as such promote the circular economy.
Different methods by which to reduce the phosphorus introduced into water systems already exist, but many struggle to achieve so low a concentration as is required, without the addition of large amounts of chemical salts and/or the generation of a phosphorus-rich waste that still cannot be reused (Tchobanoglous et al., 2001). However, when a chemical treatment approach is attempted for ultra-low discharge standards (<0.5 mg/L total phosphorus), the stoichiometric requirement of metal salt to phosphorus increases overwhelmingly and is costly. Larger wastewater treatment applications with higher phosphorus concentrations usually include an enhanced biological phosphorus removal process and anaerobic digestion (Tchobanoglous et al., 2001). These current practices focus primarily on phosphorus removal and not recovery. Phosphorus recovery technologies such as struvite and calcium phosphate precipitation exist, but they are not feasible for small and medium-scale wastewater treatment plants (Vaneeckhaute et al., 2018). As society progresses towards a more circular economy, more widely applicable phosphorus removal and
7
recovery efforts must be developed. One such process is the use of adsorption for phosphorus removal and recovery.
This project uses adsorption to solve the nutrient excess-shortage paradox. Adsorption is widely popular for removal of contaminants from wastewater due to its potential low cost, efficiency, and simplicity (Seader et al., 2006). The focus of this project will be the use of nanoscale enhanced adsorbents engineered for the removal and recovery of dissolved phosphorus. The proposed perspective considers a fully closed nutrient management loop that helps simultaneously reduce the demand for mined nutrient products and increase the access that industries have to nutrients already in the environment, all while substantially reducing the risk of nutrient poisoning events such as eutrophication.
Phosphorus use, or nutrient recycling, needs will determine any reconcentration processes that will take place post-desorption. The phosphorus can then be redistributed to the consumers, such as greenhouse and agricultural industries, or chemical industries for reuse in further processes.
The development of a new technology capable of removing phosphorus from polluted waste streams and recovering it for downstream use will close the nutrient loop and contribute greatly to the circular economy that is becoming ever more pertinent today.
8
1. Literature review
The increasing awareness of the need for excess phosphorus removal from the environment and the dwindling mineral phosphate resources has led to increases in novel phosphorus recovery strategies in recent years. Many of these strategies center around common nutrient-rich and heavily regulated industries such as wastewater treatment and agricultural operations. The goal of these technologies is the recovery of phosphorus from polluted water sources as bio-based fertilizer. This advancement would not have come about without the increased knowledge and stricter regulation on the part of the government, however. The following review will introduce the regulations that inspired the development of novel phosphorus removal and recovery strategies (Section 1.1) and then move into a discussion of standard phosphorus removal and recovery strategies in a wastewater treatment context (Section 1.2). Finally, it will discuss more novel phosphorus recovery strategies developed in recent years (Section 1.3), leading to the specific application of nano-enhanced adsorptive media (Section 1.4).
1.1 Regulations regarding phosphorus effluent limits
Every community across the planet generates waste, be it solid, liquid, or airborne. How society deals with that waste, however, is a constantly evolving subject. Oftentimes, these waste streams carry harmful toxins, an overabundance of nutrients, pathogens, and other chemical and biological products unsavory for society. Thus, it is the goal of wastewater treatment to remove, destroy, or valorize these pollutants. Wastewater treatment as it is known in the United States today truly took form in 1972 when Congress passed the Clean Water Act (CWA) (Clean Water Act, 1972). The implementation of these acts saw the tightening of regulations regarding water quality across the United States and the framework for the permitting structure and total maximum discharge limit (TMDL) development structure seen today in the United States. In the present-day United States, as regulated by the Environmental Protection Agency (EPA), each state must develop its own total
9
maximum daily loads for state waters for different pollutants. The EPA, however, has recommended TMDLs for streams not emptying into a reservoir (i.e. closed water body such as lake, pond, etc.) of 0.1 mg PO4-P/L, for streams emptying into a
reservoir of 0.05 mg PO4-P/L and for reservoirs of 0.025 mg PO4-P/L (EPA, 1986).
With respect to phosphorus, there are currently 4,177 TMDLs across the United States for various bodies of water and watersheds. These include all TMDL classifications mentioning phosphorus (phosphorus, phosphate, ortho-phosphates, organic phosphate, etc.). There are also 391 other TMDLs that mention "nutrients" or "eutrophication" without explicitly mentioning phosphorus, but that can be assumed to include phosphorus in the regulations (EPA, 2018). These TMDLs span from the deep ocean waters off the coast of Hawaii to the Great Lakes and have a
minimum range of 0.5 µg PO4-P/L (33 U.S.C. 1313, 1994).
In Canada, the modern wastewater regulations were set forth in the 1985 amendment to the Fisheries Act, originally passed in 1868 (1985). Today, the member states of Canada have adopted different maximum allowable phosphorus levels for discharge into receiving water bodies with respect to the environmental
conditions. For example, in Alberta, the limit is 50 g/L total phosphorus while in
British Columbia, a range of 5-15 g/L is used for all receiving water systems. Some provinces, such as Manitoba, employ different maximum phosphorus levels depending on the type of water body (0.025 g/L in lakes and 0.05 g/L in running water). In Quebec and Ontario, the maximum total phosphorus level in rivers is 30 g/L while in lakes, the maximum level is determined by the quantity of total
phosphorus regularly found in the lake (10 or 20 g/L, depending) (Environment
Canada, 2004). It is apparent that across North America, regulations are becoming more and more strict in efforts to reduce the frequency of HABs.
1.2 Current phosphorus removal strategies
Conventional wastewater treatment plants (WWTPs) involve generally a combination of unit processes, either physical or chemical, that remove a number of
10
pollutants from the influent stream and thus "clean" the water. In order to remove phosphorus from the wastewater, WWTPs will often use chemical precipitation or biological fixation.
Chemical precipitation occurs when trivalent metal ions are introduced into the wastewater solution in the form of a salt which then go on to form complexes with a low solubility. In the case of aluminum (Al(III)) or iron (Fe(III)) addition, a metal salt such as aluminum sulfate or ferric chloride is added to the wastewater, at which point it will dissociate into its respective anions and cations. The metal cation then reacts
with the phosphate ions in solution to form the insoluble complexes AlPO4 or FePO4,
depending on the salt used. The solids are settled in the primary or secondary clarification stage and removed as solid sludge. This strategy focuses only on removing phosphorus from the wastewater stream and not on recovering it in a useable form (Tchobanoglous et al., 2001). All of these complexes are insoluble and thus present poor potential as land-applied fertilizers down the road. Moreover, strict solid sludge application standards exist that prevent the application of wastewater solids with elevated levels of heavy metals, pathogens, and the like (EPA 1993,1994). If not land applicable, the sludge is incinerated according to methods set out in the Standards of Performance for Sewage Treatment Plants (40 CFR Part 60, Subpart O) and the National Emission Standard for Beryllium and Mercury (40 CFR Part 61, Subparts C and E, respectively) of the EPA.
The other common method for phosphorus removal from wastewater is the biological phosphorus removal process. This process normally follows a primary clarification step where the larger solid particles are settled and removed and is generally favored for its lower capital cost and production of sludge volume (Tchobanoglous et al., 2001). The wastewater then moves into an aeration tank in which biomass is grown using the dissolved pollutants such as phosphorus and nitrogen. The general formula for biomass growth in a biological treatment operation is
𝑣1(𝑂𝑟𝑔𝑎𝑛𝑖𝑐 𝑀𝑎𝑡𝑒𝑟𝑖𝑎𝑙) + 𝑣2𝑂2 + 𝑣3𝑁𝐻3 + 𝑣4𝑃𝑂43− 𝑚𝑖𝑐𝑟𝑜𝑜𝑟𝑔𝑎𝑛𝑖𝑠𝑚𝑠
11
where vi = the stoichiometric coefficients depending on feedstock and organisms,
and the general formula for biomass accounting for P is used (Tchobanoglous et al., 2001). Phosphorus generally makes up about 2% of the bacterial cell's dry mass, so in large-scale applications, the biological treatment of sludge can remove a significant amount (1g phosphorus / 10g biodegradable soluble chemical oxygen demand in the wastewater (Tchobanoglous et al., 2001).
In enhanced biological phosphorus removal processes (EBPR), phosphorus accumulating organisms (PAOs) that metabolize polyphosphates in the wastewater accumulate these molecules in an aerobic state and are subsequently filtered out of the wastewater, taking the phosphorus with them. These processes work best when alternating anaerobic and aerobic tanks are used. In the anaerobic phase, the PAOs metabolize polyphosphates to make adenosine triphosphate, ATP, eventually used in the formation of polyhydroxyalkanoates (PHAs), a common source of energy and carbon storage for microorganisms. In this process, they release orthophosphates into the wastewater. In the subsequent aerobic stage, the microorganisms metabolize the accumulated PHAs to polyphosphates, absorbing a large fraction of the orthophosphates previously released into the wastewater. The most well-understood PAO is Candidatus Accumulibacter phosphatis (Accumulibacter), and it is understood to be the predominant PAO in EBPR processes. The PAOs are known to be better equipped to survive starvation period, and thus outcompete other heterotrophic forms of bacteria in the wastewater (Cydzik-Kwiatkowska and Zielińska, 2016). These systems, however, are prone to malfunction in the presence of glycogen accumulating organisms (GAOs), thought to outperform the PAOs under certain pH and temperature ranges (Sevior et al., 2003). This has been demonstrated in a number of studies, although the evidence is hard to corroborate with such a wide range of communities for either process (Cech and Hartman, 1990, 1993, Liu et al. 1996, 1997). Studies on phosphorus recovery efforts in EBPR processes relied mostly on the subsequent digestion or destruction of the sludge to solubilize the accumulated phosphorus into the aqueous solution followed by struvite precipitation via metal salt addition (Machnicka et al. 2008, Britton et al. 2005). The
12
struvite precipitated had application as a slow-release land-applied fertilizer. However, Britton et al. (2005) discuss the variable and unpredictable morphology of the struvite crystals. Moreover, struvite precipitation seems only economically feasible in EBPR plants having streams with high phosphorus concentration (>50 kg/d; Vaneeckhaute et al., 2018), and if it can reduce high maintenance costs related to nuisance struvite scaling.
Another conventional phosphorus removal technology is the use of membrane bioreactors (MBRs). The integration of biological treatment as well as traditional mechanical solid-liquid separation makes this technology effective for wastewater treatment processes. Moreover, the drastically reduced footprint gives MBRs an advantage over traditional processes that require multiple settling tanks. Its primary application in a nutrient context is strictly the removal of pollutants from wastewater. Few studies exist, however, focusing on the recovery of nutrients removed from wastewater treatment systems via MBR. In one study, Lou et al. (2016) saw phosphorus recovery in the form of insoluble calcium phosphate granules after the phosphorus and calcium rich microfiltration permeate was pH adjusted. However, further research into the phosphorus recovery potential of this technology would need to be conducted before this technology could be deemed common in phosphorus recovery processes. Furthermore, MBR operation can get expensive, and the capital cost of installation may not render this technology viable for smaller-scale wastewater treatment plants. The process is energy-intensive and may require the addition of chemicals or process downtime to prevent or remediate fouling, adding to the operational costs (Tchobanoglous et al., 2001; Seader et al., 2006).
1.3 Novel methods in P recovery
1.3.1 Precipitation
13
As already briefly indicated above, one method of precipitative nutrient recovery is the formation of struvite for phosphorus capture. Discovered first during sludge digestion tests by Rawn et al. in 1937, struvite (MgNH4PO4:6H2O), commonly referred to as scale, is a crystallized soft mineral that is relatively stable and resistant to weathering (Lee et al. 2009). Rawn et al. (1937) noticed the buildup of 96% pure magnesium ammonium phosphate within the pipes of their wastewater treatment system and determined that its presence was severely restricting their system flow. Struvite fouling continues to plague wastewater treatment processes to this day, leading to issues of pump failure, reduction in system efficiency, and in severe cases, full piping replacement (Doyle and Parsons, 2002). Halting plant operation to descale the pipes and equipment is time consuming and quite costly. As is now known, struvite, or magnesium ammonium phosphate (MAP), is formed when equal molar quantities of magnesium, ammonium, and phosphate bind according to the following reaction (Bouropoulos and Koutsoukos, 2000):
𝑀𝑔2++ 𝑁𝐻4+ + 𝐻2𝑃𝑂4−+ 6𝐻2𝑂 → 𝑀𝑔𝑁𝐻4𝑃𝑂4⋅ 6𝐻2𝑂 + 2𝐻+
Several pilot-scale studies exist that test the large-scale feasibility of recovering the struvite as a fertilizer in a variety of circumstances and locations. Generally, the formation of struvite occurs in alkaline conditions around a pH of 10, usually reached through addition of NaOH (Adnam, 2003; Hallas et al., 2019). This explains why struvite fertilizers generally work best in acidic and neutral pH soil. At alkaline pH’s, the struvite tends to degrade rapidly and can release the elements into the soil and water systems rapidly (Kataki et al., 2016). All of the studies also showed a high removal capacity of phosphorus, on average exhibiting a removal between 80% and 90% (Adnam, 2003; Hallas et al. 2019; Want et al., 2018; Zhao et al., 2019). Because magnesium was the limiting reactant in these systems, the operators had to supplement the waste stream with magnesium salts in order to achieve such a high value of phosphorus removal (Adnam, 2003; Hallas et al., 2019; Britton et al., 2005; Moerman et al., 2009). The studies all discussed the variability in crystal size, growth behavior, and morphology due to the varying conditions experienced by a wastewater treatment plant. Moreover, even with the addition of the chemical salts,
14
all of the studies saw trace amounts of phosphorus left in the effluent that were not able to be captured by struvite precipitation (Adnam, 2003; Hallas et al., 2019; Britton et al., 2005; Moerman et al., 2009). Moerman et al. (2009) found between 11 and 12
mg/L of residual PO4-P in their effluent, a level significantly higher than the effluent
limits set forth by Environment Canada, all in the range of micrograms per liter (Environment Canada, 2004). Hallas et al. (2019) studied a representative sample of four small-scale municipal wastewater treatment facilities across the US and found that even when using sparging to replace the addition of sodium hydroxide to adjust the pH, struvite production was only economically feasible in one of the four plants. While struvite buildup poses an issue for many industrial applications, recent work has shown that due to its stability and elemental composition, it is applicable as a land-applied fertilizer, in many cases showing a significantly greater biomass yield per unit mass of fertilizer product than synthetic fertilizers (Prabhu and Mutnuri, 2014; Uysal et al., 2014; Ulysal and Kewu, 2013). Due to its low solubility in acidic and neutral soils, struvite presents little risk of leaching into water systems. Other studies, however, show a reduced nutrient availability in struvite and thus recommend supplementing synthetic fertilizers with struvite or acidifying soil before struvite application (Kataki et al., 2016). Several studies have found that the struvite created from precipitation processes is chemically stable and effective as a slow-release land fertilizer (Lee et al., 2009; Vaneeckhaute et al., 2016; Li et al., 2003; Yetilmezsoy et al., 2009; Massey et al., 2009). Moreover, Hutnick et al. (2013) showed significant phosphate(V) removal through struvite precipitation from a phosphorus mineral fertilizer company for reuse in fertilizer production. Thus, according to these studies, crystals formed in struvite precipitation processes can be strewn on agricultural land where they will be slowly weathered, releasing bio-available nitrogen and phosphorous for the plants to take up. The slow-release
nature due to struvite's low solubility (0.001692 g/100 mL H2O at 25ºC) is what gives
it an advantage as a fertilizer in some cases, as it can be applied in moist environments without great risk of phosphorus leaching and does not burn plant roots when over-applied (Zhang et al., 2017; Li et al., 2003; Negrea et al., 2010; Bhuiyan et al., 2007).
15
As far as non-fertilizer applications, the struvite precipitation process has not yet been proven effective in real-world applications. According to Li et al. (2019), there exist few real-world scale ups of struvite precipitation and its applications into non-fertilizer applications. However, studies exist that discuss the potential for precipitated struvite in dye absorption, radioactive waste cleanup, and harmful chemical sequestration (Foletto, 2013; Wagh, 2016; Blumberg, 2009).
Overall, while struvite is an effective means by which to reduce phosphorus in wastewater, its application is only economically feasible in large scale operations with high phosphorus concentrations where maintenance costs for descaling operations would be more expensive than the struvite recovery, and even still, it generally cannot reach phosphorus effluent limits low enough to satisfy regulations (Kabbe, 2016). Adsorption (Section 1.3.2) could remedy these problems with its versatility in size and its ability to capture even trace amounts of phosphorus.
1.3.1.2 Calcium phosphate precipitation
As an alternative for struvite precipitation, calcium (Ca(II)), lime (Ca(OH)2) could be
added to the wastewater, which interacts with the alkalinity to raise the pH of the water, and then reacts with phosphorus to precipitate in the form of the insoluble
Ca10(PO4)6(OH)2, also known as hydroxylapatite (Tchobanoglous et al., 2001). The
calcium phosphate compound has significant land application potential. However, it faces the same problem as struvite, in that it has a very low solubility and thus requires acidification or other treatment of soils prior to application (Bolan et al., 1994; Gorazda et al., 2012; Zhang et al., 2010; Bauer et al., 2007). Moreover, obtaining a pure and stable product remains challenging (Vaneeckhaute et al., 2017).
16
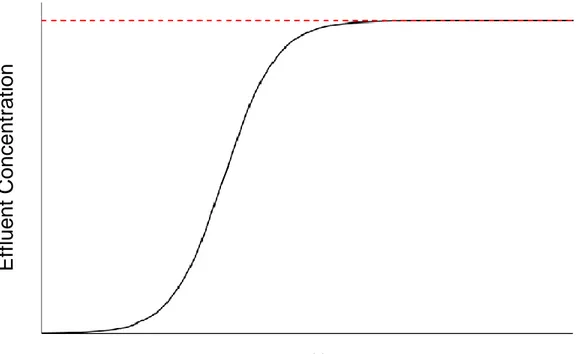
Adsorption is the process by which a target compound (the adsorbate) adheres to the surface of a solid or of an immiscible fluid (the adsorbent). In the case of phosphorus recovery from wastewaters, the adsorbate will be phosphate (PO43-) being removed from the wastewater by an adsorbent, typically a highly porous bead, flake, pellet or powder packed into a column. Following the adsorption of adsorbate onto the adsorbent, the latter will become saturated after a certain period of time, depending on its capacity, traditionally reported in g adsorbate per g adsorbent. Once enough of the adsorbate has fixed onto the active sites of the media, the adsorbent will become saturated and the system will begin to experience breakthrough. Consequently, the concentration of the effluent leaving the adsorption process, depicted by the solid black line in Figure 1 (adapted from Cook et al. (2017)) will rapidly increase towards that of the influent, denoted by the dashed red line. At this point, the saturated media must be replaced with fresh media or the adsorbate must be removed using a variety of chemical and/or physical means. In environmental processes, adsorption is becoming increasingly popular due to its simplicity, the potential use of residual materials as adsorbent, and the potential for downstream valorization of saturated resin or desorbed adsorbate (Kędziora et al., 2014; Choi et al., 2012; Panagiotou et al., 2017). The following sections will review the most prominent technologies available for phosphorus removal and recovery via adsorption.
17
Figure 1. Example of a breakthrough curve adapted from Cook et al. (2017). The black line represents the effluent concentration of the target molecule and the red line represents the influent concentration of the target molecule.
1.3.2.1 Zeolite
Naturally occurring zeolite is an inexpensive mineral of microporous aluminosilicate structure with high cation (ex. Ca2+, Mg2+, K+, Na+) exchange capacity and stability at varying environmental conditions (Wan et al., 2017). Its combined ion exchange and adsorptive capabilities give it a strong potential for application in the water treatment industry, as has been proven by several studies (Kędziora et al., 2014; Choi et al., 2012). Wan et al. (2017) showed up to 98.28% ammonium recovery with simultaneous calcium phosphate precipitation in a wastewater sample. In their tests, ammonium in activated sludge was adsorbed onto the surface of the zeolite by cation exchange, in turn releasing a high proportion of calcium ions into the solution. These free calcium ions bound with the orthophosphates in solution to form amorphous calcium phosphate precipitates. The stable zeolites could be applied to land as a nitrogen fertilizer for crops, but the amorphous calcium phosphate precipitates could not be applied without alternative soil amendments. They would in turn be used for phosphate storage to be solubilized and used when needed according to the study (Wan et al., 2017). While this study removes the phosphorus from entering the
E
ff
lue
nt
C
on
cen
tr
at
ion
Time (t)
18
sludge, it does not solve the problem of recovering the phosphorus for further use, since it generates an insoluble complex.
Similarly, Hermassi et al. (2016) used calcium-loaded zeolite produced from fly ash to adsorb phosphates from treated wastewater. Both the calcium and the aluminum and ferric oxides in the framework of the zeolite bound the phosphorus via inner and outer-sphere complexes and moderate ion exchange between calcium and sodium in solution led to the formation of calcium-phosphate mineral, or brushite
(CaPO3(OH):2H2O(s)) precipitation. The brushite desorbed from the zeolite using
sodium bicarbonate and could be used as a land-applied fertilizer (Hermassi et al., 2016). However, only partial desorption was achieved due to the strong bond between phosphates and the active sites on the zeolite, decreasing the marketability and thus feasibility of implementation into a real-world system.
1.3.2.2 Biochar
Biochar, a residual product from anaerobic thermochemical processing of lignocellulosic biomass, has also been studied for its adsorption capacity (Kizito et al., 2017). It is also produced as a by-product of wastewater treatment solid sludge incineration. In comparison with activated carbon, a common material used in adsorption and filtration, biochar is cheaper to produce ($51-381 per ton biochar versus $800-$2500 per ton activated carbon) and generates a smaller carbon footprint during its production while providing similar adsorption capacity for phosphates and ammonia from wastewater (Huggins et al., 2016). Due to its larger pore size, biochar showed less fouling and thus higher adsorption rates of phosphate
and ammonium at high concentrations (18 mg PO4-P/L) than activated carbon did in
both batch experiments and continuous column experiments. The nutrient-rich biochar was then intended to be applied to agricultural land as both a nutrient source and a soil amendment. Biochar is an effective organic fertilizer, but it contains more compounds besides only phosphorus, potentially including heavy metals (Huggins et al., 2016).
19
The efficacity of biochar as a phosphorus adsorbent, however, is disputed. Studies conducted by Namasivayam et al. (2004), Barghava et al. (1993), and Chen et al. (2011) show very low phosphorus adsorption capacities (<5 mg P/g biochar), while Yao et al. (2012) reported no significant phosphorus adsorption onto any of 13 samples of biochar. To remedy the low phosphorus adsorption potential of natural biochar, multiple studies exist that investigate the use of upgraded biochar samples, usually with a metal oxide dispersed on the surface and in the pore structure, for phosphorus adsorption (Yao et al., 2012; Tan et al., 2016; Yang et al., 2013; Fang et al., 2014, 2015; Jung et al., 2015, 2016). These upgraded biochar samples generally demonstrate a higher adsorption capacity and performance than their natural counterparts, but the addition of metal oxides increased the production cost and time to produce the biochar, nullifying the original claim that biochar is inexpensive to produce.
1.3.2.3 Other residuals as adsorbent materials
Novel nutrient recovery technologies aim to valorize other waste sources in parallel with nutrient recovery. One such study, conducted by Panagiotou et al. (2017), sought to understand the nutrient removal capacity of calcined eggshells. Eggshells are “biomineralized composites of calcite crystals embedded in an organic framework of protein fibres” that have shown to have sufficient surface area to effectively remove phosphorus (98%) from anaerobic digestion dewatering leachate samples (Panagiotou et al., 2017). However, the phosphorus adsorbed and bound to the egg shell particles was difficult to both characterize and desorb, complicating the scale up potential of this means of nutrient recovery (Panagiotou et al., 2017).
Many other applications center on the treatment of the sludge in which much of the phosphorus from the wastewater treatment is captured. Sludge pyrolysis and incineration are among two used to produce bio-crude and energy-rich syngas (Houillon et al., 2005; Hospido et al., 2005). Svanström et al. (2005) studied
20
supercritical water oxidation of sludge, allowing for energy and nutrient recovery without the emission of harmful greenhouse gases. The phosphorus was recovered by extraction post-processing in the form of ferric phosphates with poor land application potential due to the strength of the iron-phosphate bond (Svanström et al., 2005). These processes are useful for other purposes besides agricultural land application, but do not consider the recovered nutrients as the primary focus of the process. Moreover, in all of these applications, the phosphorus-rich waste product is generally simply stored in an inert waste facility and thus not reused at all (Houillon et al., 2005; Hospido et al., 2005; Svanström et al., 2005).
1.4 Nano-enhanced adsorptive media
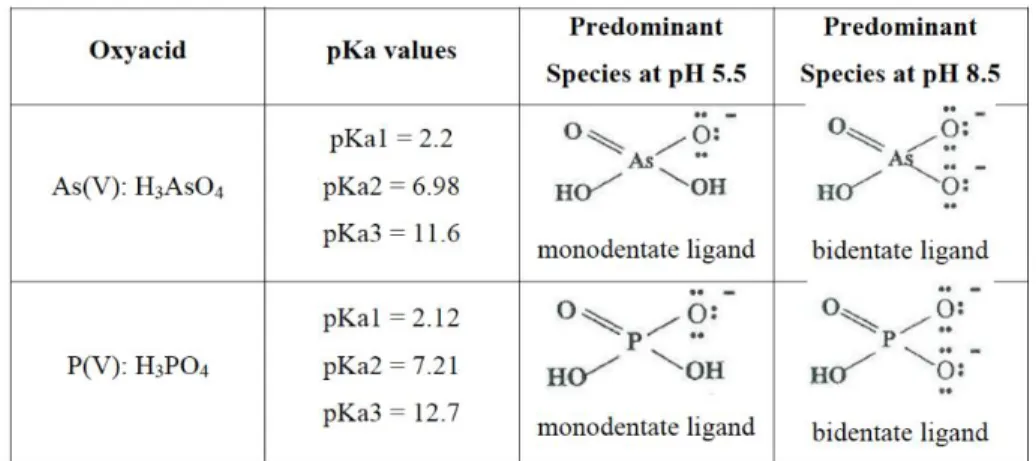
The previously mentioned studies aim to remove phosphorus, normally in the orthophosphate form, from waste streams. It is well established in science that phosphorus behaves similar to arsenic in the structure and the size of molecules that it forms as well as in its affinity to iron oxides (Jeong, 2005; Dixon, 1996; Makris, 2005). Figure 2 shows the chemical similarities between phosphorus and arsenic (Padungthon, 2013).
Figure 2. Chemical similarities between arsenic and phosphorus (Padungthon, 2013).
Previous testing into arsenic removal from water systems via iron oxide adsorption was feasible yet strongly inhibited by the presence of phosphates (Jeong, 2005; Dixon, 1996). The weakness of this technology is actually the strength of the
nano-21
enhanced adsorptive media technology for P recovery. The FerrIX A33e resin, i.e. a strong anionic exchange resin impregnated with iron-oxide nanoparticles, developed by Purolite, Inc. is originally marketed as an arsenic removal technology. However, the knowledge that phosphates and arsenates bind similarly to iron oxides made the FerrIX A33e resin possibly applicable for phosphate removal. It is worthy to note that a study conducted by Wilfred Laurier University showed that phosphates adsorbed to iron oxides more strongly than arsenic and were even used to desorb layers of dihydrogen arsenate and dimethylarsinic acid from an iron oxide adsorbent (Tofan-Lazar, 2012).
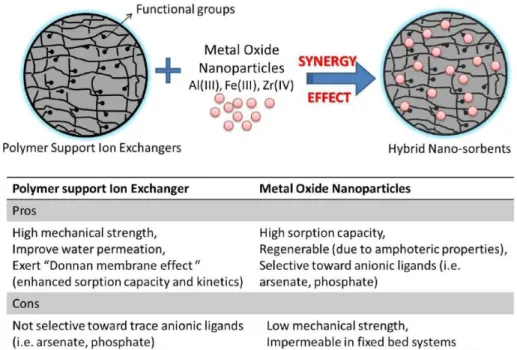
The study into the FerrIX resin’s capability to adsorb phosphorus will potentially lead to another accepted use for the FerrIX resin as well as its implementation potential in a broad range of industries. The FerrIX A33e resin is constructed of polystyrene cross-linked with divinylbenzene and impregnated with iron oxide nanoparticles. It is a brown spherical bead resin with particle sizes from 300 to 1200 micrometers. The optimal operating pH range is between 4.5 and 8.5. The ion exchange resin acts as a resilient host for the iron oxide nanoparticles. The active adsorption agents for the removal of phosphorus are the nano-iron oxides, but without a host media, they would wash away in the system and toxify the environment. The synergy between the ion exchange resin and the nano-scale iron oxide particles is summarized in Figure 3 (adapted from Padungthon, 2013). The resulting resin is also referred to as a hybrid ion exchange nano-adsorbent resin.
22
Figure 3. Synergy effect between metal oxide nanoparticles and polymeric ion exchange resin (Adapted from Padungthon, 2013).
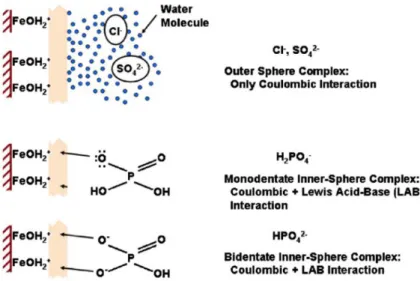
The idea of the synergy is to provide the functional iron oxide particles with a stable host material in which they can react without being leached from the resin matrix. The host resin provides stability and high permeability and surface area. Anions, in this case the mono and bivalent orthophosphate molecules in solution, have been proven to bind with the iron oxide particles dispersed on the nano-enhanced adsorptive resin by forming mono and bidentate inner-sphere complexes with the
23
Figure 4. Orthophosphates form mono and bidentate inner-sphere complexes with the iron oxides on the nano-enhanced adsorptive resin. LAB = Lewis Acid-Base interaction. (Blaney et al., 2007)
Important to note is that, although negatively charged co-ions such as chlorides and sulfates are also attracted to the positively charged iron oxide nanoparticles, they exhibit only outer-sphere coulombic interaction and thus do not compete with phosphates for adsorption sites. Outer-sphere electron transfer does not involve the breaking and formation of bonds while inner-sphere electron transfer does involve the breaking and formation of bonds, and thus creates a stronger bond (Huheey et al., 1993). Blaney et al. (2007) showed that even with high levels of bivalent sulfate molecules in solution, phosphate adsorption patterns on the iron oxide sites remained unaffected, thus confirming the selectivity of the nanoparticles impregnated in the resin.
Very few studies exist that investigate the capacity of a nano-enhanced ion exchange resin to remove phosphorus from contaminated water streams. However, the few existing reports provide evidence for the capability of ferric oxides impregnated on an ion exchange resin to adsorb and desorb phosphorus (Blaney, 2007; Nur, 2014). Blaney et al. (2007) synthesized their own hybrid ion exchange resin by impregnating the commercially available IRA-900 developed by Rohm and Haas Co. anion exchange resin with ferric oxide nanoparticles. They found that their resin was capable of adsorbing and desorbing phosphorus very efficiently, reporting
24
resin saturation after nearly 16000 bed volumes and experienced a 90% recovery rate when regenerated with caustic soda. Moreover, they report that the resin beads used in the study maintained their structural integrity without degradation or iron oxide leaching for over two years, a promising statistic for the longevity and marketability of the resin (Blaney, 2007). Nur et al. (2014) made similar claims when testing the phosphate removal capability of the FerrIX A33e resin. The two studies report strong phosphate binding due to inner sphere complexation with the iron oxide particles (Blaney, 2007; Nur, 2014). Both studies, however, tested the factors in their hypotheses (bed height, flow rate, inlet concentration, pH, etc.) one at a time, and thus cannot provide information as to the effect on resin functionality due to factor interactions.
Regeneration of the resin required a hydroxide wash to remove the phosphorus and then an acid rinse to condition the resin for further adsorption. The acid wash protonates the metal oxide and causes it to behave as a Lewis acid (electron pair acceptor) according to the following general reaction (Padungthon, 2013):
𝐹𝑒𝑂𝐻2+ ̅̅̅̅̅̅̅̅̅̅ ⇔ 𝐹𝑒𝑂𝐻̅̅̅̅̅̅̅̅ + 𝐻+ 𝑝𝐾𝑎 1 𝐹𝑒𝑂𝐻 ̅̅̅̅̅̅̅̅ ⇔ 𝐹𝑒𝑂̅̅̅̅̅̅̅ + 𝐻− + 𝑝𝐾𝑎 2
The protonation and deprotonation of the metal oxide, in this case ferric oxide, occurs due to shifts in the solution pH around the point of zero charge (Padungthon, 2013). The point of zero charge (PZC) is the pH at which the net total particle charge is zero and at which the metal oxide will be effectively neutral (Sposito, 1998). For hydrous ferric oxide, the PZC was determined experimentally to be around 7.9 (Kosmulski, 2009). Driving the pH above this PZC with a hydroxide solution will deprotonate the iron oxides on the FerrIX A33e resin and cause them to release the bound orthophosphates into the solution and take on the form FeO-. Conversely, decreasing the pH following the deprotonation using an acid rinse will protonate the
iron oxides two-fold, converting them to FeOH2+ and allowing them to act as a Lewis
25
regeneration process that will help determine the chemistries and quantities of the acidic and basic washes used for this resin during the regeneration step.
Each of the studies found during the literature review proposed its own "recipe" of alkaline and acidic rinsing steps in order to regenerate the resin. However, one study in particular, that of Nur et al. (2015), provided the most solid frame of reference for the scope of this project. The same resin as used in this project (FerrIX A33e) was studied and regenerated using a combination of a hydroxide solution (sodium hydroxide in this case) and a salt (sodium chloride). This is because, although the active sites on the iron oxide nanoparticles are strongly selective towards phosphates, the anion exchange resin into which the nanoparticles are impregnated also contributes towards total phosphate capture. The hydroxide solution is used to raise the solution pH, changing the surface chemistry of the metal oxide nanoparticles and causing a shift in charge that releases the adsorbed orthophosphate ions. However, this process does not affect the active sites on the ion exchange resin. It is thus pertinent to introduce a concentrated salt solution, in this case potassium chloride, in order to release the phosphate that is bound to the anionic exchange resin. The salt, once dissociated in solution, will introduce a high concentration of chloride ions into the system. The particular resin used in this study is more selective to chloride ions than to phosphates, so the high concentration will drive the desorption of phosphate into the effluent solution. The resin will not lose efficiency in this case if the waste stream being treated has a higher concentration in phosphate than in chloride ions. The concentration differential will be enough to release the chloride ions from the resin and allow phosphates to bind once more. However, should the wastewater solution have a higher concentration of chlorine than phosphate, the ion exchange sites will likely be inhibited by the high presence of chloride ions, and the nanoparticles will do the majority of the work adsorbing phosphates from the wastewater solution.
Nevertheless, recovering the phosphorus from the resin in a useable form was not the focus of the studies conducted by Nur et al. (2014) and Blaney et al. (2007). They
26
both regenerated the column with caustic soda: 2% NaOH (Blaney et al., 2007) and 1M NaOH (Nur, 2014). Although operating costs for regeneration present a key limitation for implementation of the technology, neither study mentioned the use of a low-cost residual product to desorb the phosphorus from the resin. While Blaney et al. (2007) mention following the hydroxide rinse with an acid wash to prime the column for further experiments, Nur et al. (2014) make no mention anywhere of an acid wash step during their regeneration process. This could perhaps be the reason why they report a severe reduction in adsorption capacity in the FerrIX A33e resin after a mere 3 cycles of adsorption and regeneration (Nur et al., 2014). Nur et al. (2014) precipitated calcium phosphate with fertilizer potential by adding calcium chloride to the effluent solution, and Blaney et al. (2007) make reference to the effluent being used for struvite precipitation in a separate process, but do not integrate this as part of their study. While both projects mentioned potential recovery of the desorbed phosphorus, the recovery efforts were all downstream of the adsorption and desorption processes. After the phosphate was desorbed in both cases, further chemicals had to be added in order to recover it in a useable form. The studies did not perform a cost analysis, but the requirement of additional chemical products limits the feasibility of this technology at a large scale, as it is desired to be competitive with traditional phosphorus removal strategies.
The above studies and work paved the way for this master’s project. By strategically selecting the regeneration chemistries, it may be possible to recover a nutrient-rich product of interest directly from the column and as such reduce the chemical costs. The desorption and valorization focus of this study provides a step further towards closing the nutrient loop and seeks to eliminate common problems experienced by current removal and recovery technologies.
27
2. Description of master’s project
2.1 Objectives and originality
The primary objective of this master’s project was to design, build, and perform
experiments using an adsorption microcolumn to study the effect of different innovative and sustainable regeneration chemistries on the efficiency of nutrient removal and recovery as a fertilizer product using a commercially available nano-enhanced adsorptive resin. The studied regeneration chemistries involve chemicals chosen to yield an N-P-K (nitrogen-phosphorus-potassium) hydroponic fertilizer post-desorption as well as low-cost byproducts obtained from other nutrient recovery processes upstream in WWTPs. With the growing demand for innovative and green processing of nutrients in industry and the environment, the need for technology focused on remediating nutrient pollution problems in the most simple and cost-effective way appears. In an effort to develop such a technology, this project covered much ground in the way of bench-scale laboratory experiments, testing the resin and innovative regeneration solutions themselves, as well as theoretical modeling and scale-up feasibility forecasting. Along the way, the following specific objectives were set to ensure the success of this project:
1. Design and construct a bench-scale microcolumn system capable of providing the necessary flow rates and an experimental plan for nutrient recovery experiments.
2. Develop different (novel) low-cost regeneration washes with varying strengths, chemistries, volumes, and flow rates for phosphate recovery tests.
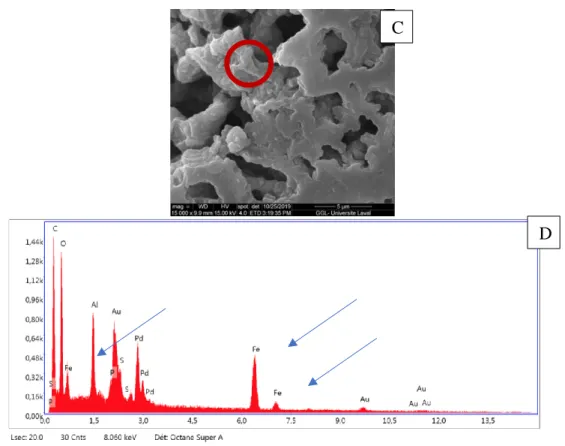
3. Characterize the FerrIX A33e resin using scanning electron microscopy (SEM) and continuous and batch phosphate adsorption tests. 4. Perform desorption experiments for each selected regeneration chemistry.
28
5. Test the resin for longevity by repeating the adsorption/desorption cycle five times with the different regeneration chemistries.
6. Analyze the composition and valorization potential of the recovered nutrient solutions.
7. Develop and validate a regression model for the resin function with respect to the different regeneration chemistries and regeneration parameters.
8. Perform a preliminary scale-up and economic feasibility analysis to evaluate the potential for this technology in industry.
These objectives were put in place to ensure the development of a thorough and innovative solution to the nutrient pollution problem. As demonstrated in the review of relevant literature, no other studies have explored direct post-adsorption nutrient recovery and the use of low-cost byproducts. Doing so in this case will provide industry and the water resource community with cutting-edge, meaningful information as to a potential nutrient recycling strategy going forward and will open the door to further study in this area. After conversations with professionals in the biogas and wastewater treatment industries in Canada, it is apparent that phosphorus recovery is an important aspect of their current work. Moreover, many of them working with farm bioreactors and other small-scale applications emphasized the need for simplicity, low cost, and robustness. Hence, this technology may potentially comprise an important part in anaerobic digestion treatment trains, a rapidly growing industry across Canada.
This study took a different frame of reference to previous work done by Padungthon et al. (2013) in that it places a higher emphasis on the desorption and industrial implementation aspect of the project. Studies in the past regarding this technology, and even this particular resin, showed capability of phosphate adsorption on the iron oxide sites impregnated into the resin matrix (Nur et al, 2015). However, there is a lack of understanding in the desorption process for these resins, due to the majority
29
of the focus being on the adsorption and removal aspect. Therefore, results generated in this study will provide clarity to industry as to the best way to desorb the captured phosphate and regenerate the resin to best suit their needs. Nano-enhanced adsorptive media can be implemented as an alternative to common nutrient recovery methods (e.g. at small WWTPs where struvite recovery is not feasible) as well as a complement to processes, such as struvite precipitation, that generally leave trace amounts of phosphorus in effluent streams. Furthermore, with the end goal of a hydroponic fertilizer in mind, and considering the varying phosphorus needs of different species, a wide range of phosphate concentrations in the regenerant effluent are interesting to be studied. Should this system be capable of producing multiple different combinations of N-P-K fertilizers, the marketability of the technology and accompanying regression model would increase greatly. The ultimate goal of this project (beyond this MSc thesis) is the pilot-scale implementation into current wastewater treatment and biogas production systems in order to recover and reuse phosphorus currently being lost in the environment. Larger-scale data on phosphorus adsorption and desorption in a real wastewater treatment process will strengthen the results generated by this study and make strides towards the further implementation of this technology at full-scale. Thus, this study will leave a profound impact on the agriculture, wastewater, and water resource communities and provide a potential path for imperative remediation of many crucial environmental problems that plague the global community.