Implication of NuA4 histone acetyltransferase complex
in transcription regulation and genome stability
MÉMOIRE
Xue Cheng
Maîtrise en biologie cellulaire et moléculaire
Maître ès sciences (M.Sc.)
Québec, Canada
iii
RÉSUMÉ
Le génome est organisé sous forme de chromatine afin de contourner la problématique d’espace limité dans le noyau. De plus, cette structure hautement condensé est une barrière physique aux processus cellulaires qui nécessite l’accès à l’information génétique. Les dernières années d'études ont dévoilé des complexes modificateurs de la chromatine comme des acteurs clés dans plusieurs mécanismes de modulation de la chromatine. L'un de ces modificateurs est NuA4, un complexe conservé au cours de l’évolution qui acétyle les histones H2A, H2A.Z et H4. Dans cette thèse, en utilisant Saccharomyces cerevisiae comme organisme modèle, nous avons identifié l'implication de NuA4 dans l'incorporation de H2A.Z et la biosynthèse des voies purines. Dans une seconde partie, nous étudions la participation de NuA4 dans la réponse aux dommages de l'ADN. Plus précisément, nous avons caractérisé la phosphorylation des sous-unités NuA4 dépendante de Mec1/Tel1. L’ensemble de ces travaux, comment NuA4 coordonne différentes activités cellulaires.
v
Abstract
Cell genome is packaged into chromatin in order to compensate the limited space within the nucleus. However, this highly condensed structure also presents strong physical barriers for cellular processes using DNA as templates. Recent years of studies have unveiled chromatin modifying complexes as key players in several mechanisms of chromatin modulation. One of these modifiers is NuA4, an evolutionary conserved large multi-subunit histone acetyltransferase complex that acetylates histone H2A, H2A.Z and H4. In this thesis, using Saccharomyces cerevisiae as model system, we identified the implication of NuA4 in global histone variant H2A.Z incorporation and purine biosynthesis pathways. Moreover, we also show previously uncharacterized involvement of NuA4 in DNA damage response pathways through Mec1/ Tel1-dependent phosphorylation events on NuA4 subunits. Further analysis will shed light on detailed mechanisms about how NuA4, as a multifunctional complex, coordinates various cellular activities.
vii
Table of Contents
RÉSUMÉ ... iii
Abstract ... v
Table of Contents ... vii
List of Tables ... xi
List of Figures ... xiii
List of Abbreviations ... xv
Acknowledgements ... xxiii
Chapter 1Introduction ... 1
1.1 Chromatin structure and function ... 2
1.1.1 Chromatin ... 2
1.1.2 Histone posttranslational modifications ... 3
1.1.2.1 Acetylation ... 6
1.1.2.2 Methylation ... 6
1.1.2.3 Phosphorylation ... 7
1.1.2.4 Other histone posttranslational modifications ... 7
1.1.3 Incorporation of histone variants ... 7
1.1.3.1 H2A.Z ... 8
1.1.3.2 H2A.X ... 9
1.1.3.3 Other histone variants ... 10
1.1.4 ATP-dependent chromatin remodeling ... 10
1.2 NuA4 histone acetyltransferase complex ... 10
1.2.1 Piccolo NuA4... 11
1.2.1 TINTIN... 11
1.2.3 Eaf2-Yaf9-Arp4-Act1 sub-module ... 11
1.2.4 Other NuA4 subunits: Eaf1 and Tra1 ... 12
1.3 NuA4 function in gene regulation... 13
1.4 NuA4 function in genome stability ... 15
1.5 Project Rationale ... 16
Chapter 2 Eaf1 links the NuA4 histone acetyltransferase complex to Htz1 incorporation and regulation of purine biosynthesis ... 19
2.1 Foreword ... 20
2.2 Résumé ... 20
2.3 Abstract ... 20
2.4 Introduction ... 22
viii
2.5.1 Yeast strains and reagents. ... 24
2.5.2 ChIP-on-chip. ... 24
2.5.3 Northern Blot and Microarray analysis. ... 24
2.5.4 GST-pull-down, and HAT assay. ... 25
2.5.5 Chromatin ImmunoPrecipitation (ChIP). ... 25
2.6 Results ... 27
2.6.1 Loss of Eaf1 affects genome-wide Htz1-enrichment ... 27
2.6.2 NuA4 is implicated in regulation of gene network for purine biosynthesis ... 28
2.6.3 Loss of Eaf1 cripples induction of a number of ADE genes ... 30
2.6.4 NuA4 interacts with Pho2 and Bas1 in vitro ... 31
2.6.5 Enrichment of Htz1 and acetylated H4 at inactive ADE promoters is dependent on NuA4 ... 33
2.6.6 Loss of nucleosomes upon ADE gene induction ... 34
2.6.7 Htz1 K14 acetylation at ADE gene promoter is dependent on NuA4... 37
2.7 Discussion ... 38
Chapter 3 Implication of Mec1/Tel1-dependent phosphorylation of NuA4 histone acetyltransferase complex in DNA damage response pathways ... 43
3.1 Foreword ... 44
3.2 Résumé ... 44
3.3 Abstract ... 45
3.4 Introduction ... 46
3.5 Materials and Methods ... 48
3.5.1 Yeast Strains and Manipulation ... 48
3.5.2 Tandem-Affinity Purification (TAP) ... 50
3.5.3 Two-dimensional gel electrophoresis ... 51
3.5.4 Western Blot ... 51
3.5.5 Histone AcetylTransferase (HAT) Assay ... 52
3.5.6 Silver Stain ... 52
3.5.7 Spot Assay ... 52
3.5.8 Rad53 checkpoint activation/recovery assay ... 53
3.5.9 TCA Protein extraction ... 53
3.6 Results ... 54
3.6.1 NuA4 subunits are phosphorylated upon DNA damage ... 54
3.6.2 Eaf1 is phosphorylated in vivo upon DNA damage ... 55
3.6.2 Eaf1 phospho-mutants do not alter complex integrity or activity ... 56
3.6.3 Eaf1 and Eaf3 phospho-mutants do not affect Rad53 checkpoint activation or recovery in slx4Δ background ... 58
ix 3.6.4 Eaf1 phospho-mutants show diverse functional links with DNA-damage
response effectors ... 59
3.6.5 Verification of SGA data ... 60
3.6.6 Involvement of phosphorylation events on other NuA4 subunits upon DNA damage ... 61
3.7 Discussion and perspectives ... 62
Chapter 4 Discussion and Perspectives ... 65
xi
List of Tables
Table 1 - Histone Variants- Type, Function and Distribution. ... 8
Table 2 - List of NuA4 Subunits ... 16
Table 3 - Strains Used In This Study. ... 24
Table 4 - Primers used in ChIP-qPCR ... 26
Table 5 - Yeast Genes Down-regulated in eaf1Δ Cells Grown in Rich Media ... 29
Table 6 - S. cerevisiae Strains Used. ... 49
Table 7 - Primers Used In This Study. ... 49
xiii
List of Figures
Figure 1 - Cell organizational network of chromatin structure. ... 2
Figure 2 - Schematic of the structure of histones in nucleosomes. ... 4
Figure 3 - X-ray crystal structure of the nucleosome core particle of chromatin. ... 5
Figure 4 - Example of recruitment of mediators through reader modules that recognize certain histone PTMs. ... 5
Figure 5 - Schematic representation of NuA4 structure organization and functions of submodules. ... 13
Figure 6 - Model of NuA4 involvement in PHO5 transcription regulation. ... 14
Figure 7 - Model of NuA4 implication in DNA damage response. ... 15
Figure 8 - NuA4 affects Htz1 incorporation independently of transcription rate. ... 28
Figure 9 - NuA4 is important for ADE gene induction. ... 30
Figure 10 - Bas1 and Pho2 directly interact with NuA4 complex in vitro. ... 32
Figure 11 - The presence of H4 acetylation and Htz1 at repressed ADE17 promoter is NuA4-dependent. ... 34
Figure 12 - Loss of both H4 acetylation and Htz1 variant rapidly upon induction at ADE17 promoter along with nucleosome disassembly. ... 36
Figure 13 - Htz1 is acetylated at K14 by NuA4 at ADE17 promoter. ... 37
Figure 14 - Model of step-wise ADE promoter architecture for gene activation. ... 38
Figure 15 - The DNA damage response cascades and Mec1/Tel1 kinases. ... 47
Figure 16 - Mec1/Tel1 targeting sites mapped in Eaf1. ... 55
Figure 17 - Eaf1 is phosphorylated in vivo upon DNA damage. ... 56
Figure 18 - Eaf1 SQ-mutants do not alter complex integrity or activity. ... 57
Figure 19 - Eaf1 and Eaf3 SQ-mutants do not alter Rad53 checkpoint activation or recovery. ... 59
Figure 20 - Eaf1 phosphorylation mutants show genetic interactions with DNA damage response effectors. ... 60
Figure 21 - Mutants containing phospho-mimic Eaf1 do not show significant phenotype. 61 Figure 22 - Tra1 is phosphorylated at SQ site upon DNA damage independent of Eaf1-SQ phosphorylation status. ... 62
Figure 23 - Model for the implication of NuA4 and histone modification crosstalks in DNA repair events. ... 68
xv
List of Abbreviations
2D-gel Two-dimensional gel electrophoresis 53BP1 p53 binding protein 1
Act1 actin 1
ADE ADEnine requiring Akt/PKB Protein Kinase B Arp Actin-Related Protein
ATM ataxia telangiectasia mutated ATP adenosine triphosphate
ATR ataxia telangiectasia and Rad3 related BAF53 BRG1-associated factors 53
Bas1 BASal 1
Bdf1 bromodomain factor 1 BRCT BRCA1 C Terminus CH core histones
CHD1 chromodomain helicase DNA binding 1 ChIP chromatin immunoprécipitation
Chk1 CHeckpoint Kinase 1 Chk2 CHeckpoint Kinase 2 DDR DNA damage response
DMAP1 DNA methyltransferase 1 associated protein 1 DNA Deoxyribonucleic acid
DNA-PK DNA-dependent protein kinase Dot1 disruptor of telomeric silencing 1 DSB double strand break
Eaf Esa1p-Associated Factor
EPC1 enhancer of polycomb homolog 1 Epl1 Enhancer of Polycomb Like 1
Esa1 essential SAS2-related acetyltransferase 1 GAS41 Glioma-Amplified Sequence 41
Gcn5 general control nonderepressible 5 GPS Group-based Prediction System GST Glutathione S-transferase HAT histone acetyltransferase HDAC histone deacetylase HDM histone demethylase
xvi
HMT histone methyltransferase
HO HOmothallic switching endonuclease HR homologous recombination
HSA helicase SANT associated Htz1 Histone Two A Z1
HU hydroxyurea
Ies4 Ino Eighty Subunit 4 ING3 inhibitor of growth 3 INO80 INOsitol 80 complex IR ionizing radiation ISWI imitation SWI/SNF MBT Malignant Brain Tumor
MDC1 Mediator of DNA damage checkpoint protein 1 Mec1 mitosis entry checkpoint 1
MMS methyl methanesulfonate
Mrc1 Mediator of the Replication Checkpoint 1 MRG15 MORF4-Related Gene on chromosome 15 MRGBP MRG-binding protein
MRN MRE11–RAD50–NBS1
mRNA message RNA MS mass spectrometry NFR nucleosome-free region NHEJ non-homologous end-joining NuA4 nucleosome acetyltransferase of H4 OD Optical Density
ORF open reading frame PCR polymerase chain reaction PHD plant homeodomain PHO PHOsphate metabolism PI3K phosphatidylinositol-3 kinase
pl point isoelectric Pol II RNA polymerase II
PTM post-traductional modification
qPCR quantitative polymerase chain reaction Rad RADiation sensitive
RNA Ribonucleic acid
S/T-Q serine or threonine followed by glutamine SAGA Spt, Ada, Gcn5 acetyltransferase
xvii SC synthetic complete
SCD S/T-Q cluster domain
SRCAP snf2-related CBP activator protein SCSM Synthetic Complete Supplement Mixture SDS3 Suppressor of Defective Silencing 3 SDS-PAGE SDS-Polyacrylamide gel electrophoresis SGA synthetic genetic array
Slx4 Synthetic Lethal of unknown (X) function 4 SON short oligonucleosomes
SWI/SNF mating type switching/sucrose non fermentation Swr1 Swi2/Snf2-related ATPase 1
SWR-C Swi2/Snf2-related ATPase Complex Taf1 TBP associated factor 1
TAP tandem affinity purification Tel1 telomere maintenance 1 TEV tobacco etch virus TFIID transcription factor II D
Tip60 Tat interacting protein (60kDa) Tra1 yeast homolog of TRRAP1
TRRAP transformation/transactivation domain associated protein TSS Transcription Start Site
UAS Upstream Activating Sequence UV Ultraviolet light
WT wild type Yaf9 yeast AF-9
Yng yeast homolog of mammalian ING YPD Yeast-Peptone-Dextrose
xix
To my loving families.
xxi Three treasures I pursue in life: The courage to change the things I can; The serenity to accept the things I cannot change; And the wisdom to know the difference.
xxiii
Acknowledgements
Firstly, I would like to sincerely thank my supervisor Dr. Jacques Cote. Thank you for accepting me to your lab, giving me time to adjust and adapt to the new environment, guiding and inspiring me throughout my master study. Your excellent mentoring and admirable personalities have been nurturing me in the pursuit of science.
Secondly, I want to thank all the current lab members and former lab members, especially Marie-eve, Anne-lise and Dorine. It is through the everyday discussions that I found myself constantly reflecting and learning. I truly enjoy being in this environment. “Everybody in the lab can talk science.”
Thirdly, I want to thank my family for their endless love and support. Thanks to my parents for being open-minded, letting me pursuit what I want in life, even if that means their only child will not be always around.
Last but not least, I want to thank my boyfriend Francois Boulanger and his family. Being far away from one’s family and familiar culture is not always easy, and it is you and your family’s care and support that warmed me all through the ups and downs in life, sentimental family-gathering festivals and harsh Quebec winters.
Thanks to you all.
1
Chapter 1
Introduction
2
1.1 Chromatin structure and function 1.1.1 Chromatin
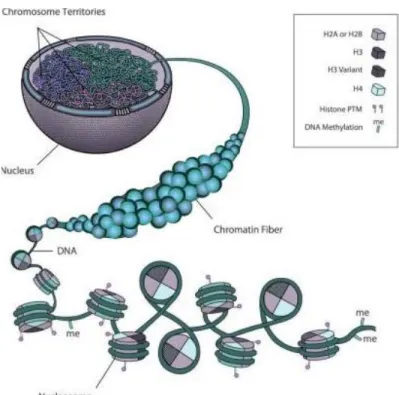
Evolution has shaped eukaryotic cells to be capable of facing and solving two vital tasks related to its genetic information carrier, the genome. On one hand, the genome needs to be highly condensed in order to encompass the nearly 2-meter of DNA into the nucleus (Peterson and Laniel, 2004), the diameter of which is only ~6 μm (Bruce Alberts, 2002) (Figure 1). On the other hand, the underlying DNA within this condensed structure needs to be accessible at specific times and situations. Cells coordinate these two seemingly conflicting needs through chromatin structure. By modulating the different properties of chromatin, cells are able to adjust according to the environment and carry out different cellular activities such as transcription, replication and DNA damage repair.
Figure 1 - Cell organizational network of chromatin structure. Modified from (Rosa and Shaw, 2013).
3 The basic unit of chromatin is the nucleosome, which consists of about 147bp of DNA wrapping around a protein octamer with two copies of four types of histones, H2A, H2B, H3 and H4 (Kornberg, 1974). There are several mechanisms that cells have adopted to modulate the chromatin structure in order to achieve precise temporal and spatial regulation of cellular activities. These mechanisms include histone posttranslational modifications, incorporation of histone variants, and ATP-dependent chromatin remodeling.
1.1.2 Histone posttranslational modifications
While DNA is wrapped around the histone core to form nucleosomes, the amino-terminal tails of the histones extend out from the nucleosomes, providing a platform for various types of modification including acetylation, methylation, phosphorylation and ubiquitination (Figure 2). Different modifications, either alone or in different combinations, can regulate chromatin structure in different manners. As the interaction between DNA and histones are mostly based on the positive charge on histones and negative charge on DNA, some histone modifications can directly affect this DNA-histone interaction, increasing the accessibility for transcriptional regulatory proteins to DNA templates and thus altering chromatin structure and gene expression (Figure 3) (Struhl, 1998). For example, multiple histone acetylation events on H4 can attenuate the positive charge on H4 N-terminus tail, resulting in less stable nucleosomes that are susceptible to loss and genomic regions that are more accessible for transcriptional factors. Therefore, the presence of this modification pattern at gene promoter regions often associates with active transcription (Steunou et al., 2014).
4
Figure 2 - Schematic of the structure of histones in nucleosomes.
a) Histone N-terminus tails stretch out from nucleosomes. b) Examples of different modifications occurring on histones. A, acetyl; C, carboxyl terminus; E, glutamic acid; M, methyl; N, amino terminus; P, phosphate; S, serine; Ub, ubiquitin. Adapted from (Marks et al., 2001)
A major second mechanism is termed “effector-mediated” mechanism. In this case, the modifications on histones can serve as docking sites for the binding of effector proteins through specific reader domains (Figure 4). By this recruitment, the modified histone tails can act as integrating platforms, permitting local chromatin structure to receive and interpret information from upstream signaling cascades (Musselman et al., 2012).
5 Figure 3 - X-ray crystal structure of the nucleosome core particle of chromatin.
A) Histone protein octamer is surrounded by 147 base pairs of DNA. B, C) Histones are highly positively charged at DNA contact points and histone tails. Blue color represents positive charge and red color represents negative charge. All figures were displayed by PyMol software. B, C electric potentials are generated by vacuum electrostatics (PDB: 1AOI).
Figure 4 - Example of recruitment of mediators through reader modules that recognize certain histone PTMs.
Me, methylation; ac, acetylation; ph, phosphorylation. Modified from (Musselman et al., 2012)
6
1.1.2.1 Acetylation
Since first identified in 1964 as a potential regulator of RNA synthesis (Allfrey et al., 1964), histone acetylation has been one of the histone posttranslational modifications (PTMs) that are most characterized. The presence of acetylation is catalysed by histone acetyltransferases (HATs) through transferring an acetyl group from acetyl-CoA to the lysine-amino groups on the N-terminal tails of histones (Carrozza et al., 2003). On the contrary, the removal of acetyl-group is carried out by histone deacetylases (HDACs). Disturbance of histone acetylation homeostasis and deregulation of HATs and HDACs activities have been tightly associated with human diseases including cancers (Avvakumov and Cote, 2007; Steunou et al., 2014). Histone acetylation has been implicated in multiple cellular functions, such as transcription, replication and DNA repair through both direct mechanism and effector-mediated mechanism (Carrozza et al., 2003; Lee and Workman, 2007; Li et al., 2007; Steunou et al., 2014; Unnikrishnan et al., 2010). As mentioned above, certain lysine acetylation events can directly alter physical properties of histone-DNA interaction or disrupt nucleosome higher-order associations (Shogren-Knaak et al., 2006), resulting in a more open chromatin structure. Alternatively, histone acetylation can also recruit effectors that contain bromodomain(s) (Figure 4), such as Gcn5, Bdf1, Taf1 and Brd4. These proteins are components of specific chromatin remodelling complexes or transcriptional machineries (e.g. Gcn5 for SAGA, Bdf1 for SWC1-C, Taf1 for TFIID). This recruitment can indirectly interpret histone acetylation marks into specific downstream biological responses.
1.1.2.2 Methylation
Methylation is an abundant type of histone PTM as it can occur on both lysine and arginine residues with multiple methylation states. Lysine methylation can have three methylation states, namely mono- (me1), di- (me2) and tri- (me3), while arginine can undergo mono- and symmetrical or asymmetrical di-methylation (Latham and Dent, 2007). Histone methylations are catalysed by histone methyltransferases (HMTs) from either de novo status or lower methylation states, and removed by histone demethylases (HDMs).
Unlike acetylation, methylation does not remove the positive charge on lysines. Distinct physicochemical properties among different states of methylation allow the specific binding
7 of diverse effectors through reader modules (Figure 4), including chromodomain, Tudor, MBT and PHD finger domains (Musselman and Kutateladze, 2011; Musselman et al., 2012; Taverna et al., 2007). Studies have shown that recruitment of these reader modules by different methylation states of specific residues correspond to specialized biological functions. For example, H3K4me3 is generally linked to active transcription sites, while H3K9me3 has been defined as a mark for heterochromatin (Taverna et al., 2007).
1.1.2.3 Phosphorylation
Histone phosphorylation is another modification that has become a topic of vigorous investigation. This modification is catalysed by specific kinases through addition of a negatively charged phosphate moiety to the hydroxyl group of serine or threonine residues which can be removed by phosphatases (Taverna et al., 2007). With sites detected on all four core histones, histone phosphorylation events have been implicated in a variety of cellular processes, such as transcription regulation, apoptosis, cell cycle progression, DNA repair, and chromosome condensation (Banerjee and Chakravarti, 2011; Cheung et al., 2005; Hurd et al., 2009; Krishnamoorthy et al., 2006; Utley et al., 2005). Again, this functions through direct interference of DNA-histone interactions, recruitment of effector proteins, or crosstalk with other types of modifications.
1.1.2.4 Other histone posttranslational modifications
With the recent combination of mass spectrometry, metabolic-labelling and antibody-based detection, at least eight types of PTMs on more than 60 histone residues have been reported in vivo (Tan et al., 2011). Future works will meet the challenges of defining the properties of these PTMs in different contexts and translating them into specific biological outputs. 1.1.3 Incorporation of histone variants
Besides genes encoding canonical core histones, cells also possess genes that express other histones termed as histone variants. Generally, histone variants differ from canonical histones on primary amino acid sequence and structural level (Zlatanova et al., 2009). Moreover, unlike canonical histones whose genes are clustered in the genome and expressed during early-S phase of the cell cycle, histone variant genes tend to have scattered genomic
8
distribution and are constitutively expressed across the cell cycle (Rosa and Shaw, 2013; Talbert and Henikoff, 2010).
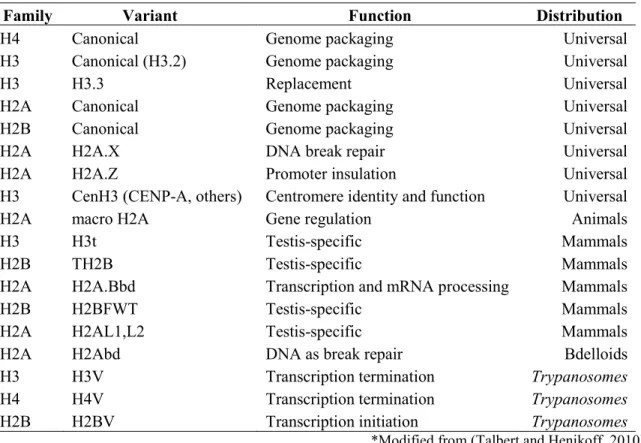
Histone variant incorporation, similar to histone posttranslational modifications, contributes to the regulation of diverse cellular activities, including DNA repair, meiotic recombination, chromosome segregation, transcription initiation and termination, sex chromosome condensation and sperm chromatin packaging etc.(Talbert and Henikoff, 2010) (Table 1).
Table 1 - Histone Variants- Type, Function and Distribution.
Family Variant Function Distribution
H4 Canonical Genome packaging Universal
H3 Canonical (H3.2) Genome packaging Universal
H3 H3.3 Replacement Universal
H2A Canonical Genome packaging Universal
H2B Canonical Genome packaging Universal
H2A H2A.X DNA break repair Universal
H2A H2A.Z Promoter insulation Universal
H3 CenH3 (CENP-A, others) Centromere identity and function Universal
H2A macro H2A Gene regulation Animals
H3 H3t Testis-specific Mammals
H2B TH2B Testis-specific Mammals
H2A H2A.Bbd Transcription and mRNA processing Mammals
H2B H2BFWT Testis-specific Mammals
H2A H2AL1,L2 Testis-specific Mammals
H2A H2Abd DNA as break repair Bdelloids
H3 H3V Transcription termination Trypanosomes
H4 H4V Transcription termination Trypanosomes
H2B H2BV Transcription initiation Trypanosomes
*Modified from (Talbert and Henikoff, 2010) 1.1.3.1 H2A.Z
H2A.Z is an evolutionary conserved histone variant that constitutes about 5 to 10% of the total H2A population (West and Bonner, 1980). H2A.Z shares about 60% amino acid identity with canonical H2A (Jackson et al., 1996) and distributes non-randomly across the genome (Li et al., 2005; Zhang et al., 2005). Although debate remains regarding to the structure and stability aspect of H2A.Z-containing nucleosomes, some structural features which
9 distinguish H2A.Z from H2A have been established, including a unique C-terminal tail that specifies H2A.Z deposition and an extended surface charge patch that assist regulation of chromatin compaction (Billon and Cote, 2013).
H2A.Z incorporation is catalyzed by ATP-dependent SWR1-complex (SWR-C) in yeast (yeast H2A.Z is termed as Htz1) and SRCAP and p400 complexes in higher eukaryotes through the exchange of H2A-H2B dimer with H2A.Z-H2B dimers within nucleosomes (Altaf et al., 2009; Kobor et al., 2004; Mizuguchi et al., 2004). Genome-wide studies show that H2A.Z localizes around nucleosome-free regions (NFRs), which correspond to the transcription initiation sites. Enrichment of H2A.Z is found over the promoter regions of transcriptionally inactive genes in yeast (Guillemette et al., 2005; Li et al., 2005; Zhang et al., 2005), and this presence has been shown to be involved in presetting mechanism for favoring nucleosomes disassembly upon gene activation (Altaf et al., 2010; Auger et al., 2008; Zhang et al., 2005). Moreover, Htz1 is also found to be acetylated in vivo, and this acetylation event is associated with genome-wide gene activity in yeast (Millar et al., 2006). Besides transcription, H2A.Z incorporation has also been involved in cellular activities such as chromosome segregation, telomere silencing and genome integrity (Billon and Cote, 2013; Krogan et al., 2004; Lu et al., 2009).
1.1.3.2 H2A.X
H2A.X is another histone variant of H2A that constitutes about 10% of the H2A population in mammals and about 90% in S. cerevisiae. The most striking feature of this histone variant is that H2A.X can be phosphorylated on a large chromatin domain upon DNA damage. The phospho-event occurs at Serine 139 in mammals and Serine 129 in yeast, and phosphorylated H2A.X is termed as γH2A.X. During repair of DNA double strand breaks (DSBs), γH2A.X is one of the first mark to appear at the lesions and spread rapidly to ~50kb on each side of DSB in yeast, even up to megabases in mammalian cells.
γH2A.X is catalyzed by members of phosphatidylinositol-3 kinase-like kinase (PI3KK) family (Mec1/Tel1 in yeast, ATR/ATM/DNA-PKcs in mammals). γH2A.X possesses a crucial position in DNA damage response pathway by transducing and amplifying the local damage signal through recruitment/ interaction with DNA damage repair effectors such as the MRN complex, Mdc1 and 53BP1 (Banerjee and Chakravarti, 2011). After repairing the
10
damaged DNA, phosphorylation on S139 is removed by chromatin-associated phosphatases, which allows cells to re-enter the cell cycle (Macurek et al., 2010).
1.1.3.3 Other histone variants
Recent years have also witnessed the characterization of new histone variants and their functions (Montellier et al., 2013; Morillo Prado et al., 2013) (Table 1). Many newly identified variants show tissue-specific or developmental-stage-specific expression and incorporation, highlighting the concept of histone variant as an essential factor of chromatin specialization within the spatial and temporal complexity of biology.
1.1.4 ATP-dependent chromatin remodeling
As the accessibility of the DNA-template is generally hampered by the presence of nucleosomes, nucleosomes at certain genomic regions need to be moved, ejected or restructured. This need of chromatin modulation relies on ATP-dependent chromatin remodeling complexes (remodelers). There are four classes of remodelers, including SWI/SNF, imitation switch (ISWI), INO80 and Mi-2/CHD groups (Erdel et al., 2011). Current thinking suggests that each type of remodeler acts in distinct manners and carries out unique biological functions. For example, as mentioned above, SWR-C is responsible for histone dimer exchange with H2A.Z-H2B, an activity important for modulation of promoter architecture (Kobor et al., 2004; Mizuguchi et al., 2004; Wu et al., 2005). Besides the involvement in dimer exchange, ATP-dependent remodelers are also implicated in transcriptional activation and repression, sister chromatid cohesion and DNA repair (Cairns, 2005; Morrison and Shen, 2009).
1.2 NuA4 histone acetyltransferase complex
NuA4 histone acetyltransferase is a large chromatin modifying complex that consists of 13 subunits. The main function of NuA4 is to acetylate lysines on histone H2A, H2A.Z and H4 N-terminus tails (Steunou et al., 2014). It is performed by Esa1, the only HAT essential for viability in Saccharomyces cerevisiae (Allard et al., 1999; Utley et al., 1998). The involvement of NuA4 in different cellular activity regulations such as transcription, DNA
11 repair, cell cycle progression, chromosome segregation and chromatin boundary maintenance (Lu et al., 2009; Steunou et al., 2014).
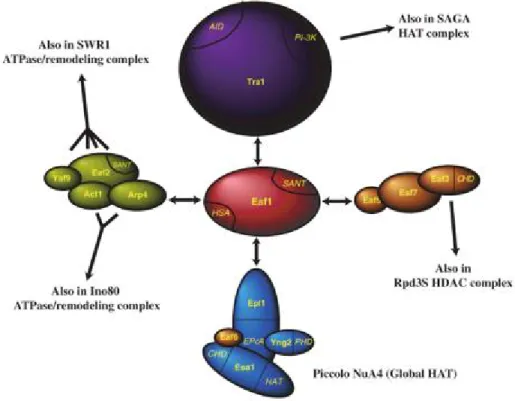
The multifunctional nature of the NuA4 complex benefits from the context of its multi-subunit assembly. Several functional sub-complexes/sub-modules have been identified with Eaf1 as main scaffold subunit (Auger et al., 2008) (Figure 5). These sub-modules include Piccolo NuA4 (Epl1, Yng2, Eaf6, Esa1), TINTIN (Eaf5/7/3), Eaf2-Yaf9-Arp4-Act1 sub-module and Tra1 recruitment sub-module.
1.2.1 Piccolo NuA4
Piccolo NuA4 is a sub-complex of NuA4 that contains Epl1, Yng2, Eaf6 and catalytic subunit Esa1 (Auger et al., 2008; Boudreault et al., 2003). Piccolo NuA4, unlike its parental complex NuA4, acts mainly on nucleosome substrate compared to naked histones through its Yng2 and Epl1 subunits (Ranjan et al., 2013; Selleck et al., 2005). Since Piccolo is smaller in size yet functionally active on chromatin, it has been proposed that Piccolo NuA4 is responsible for global histone acetylation while NuA4 carries out targeted acetylation events in a more specific fashion through transcription activator-directed recruitment (Boudreault et al., 2003). 1.2.1 TINTIN
NuA4 subunits Eaf5, Eaf7 and Eaf3 can exist outside of NuA4 and form an independent trimeric sub-complex referred as “TINTIN” (Trimer Independent of NuA4 involved in Transcription Interactions with Nucleosomes) (Cheng and Cote, in press). Unlike NuA4, the enrichment of which is mainly around promoter regions, TINTIN enriches over the coding region of active genes (Rossetto et al., 2014). In agreement with this localization, it is found to interact with elongating RNA polymerase II, H3K36me3 and proposed to assist the disruption of nucleosomes, pol II progression and nucleosome refolding in its wake (Rossetto et al., 2014).
1.2.3 Eaf2-Yaf9-Arp4-Act1 sub-module
NuA4 shares four subunits (Eaf2, Yaf9, Arp4 and Act1) with ATP-dependent chromatin remodeler SWR-C, the main function of which is to incorporation histone variant H2A.Z. As these two chromatin regulatory complexes also show strong genetic interactions and functional cooperation (Altaf et al., 2010; Auger et al., 2008; Lu et al., 2009), it has been
12
proposed that the shared sub-module may play a critical role in facilitating the actions of NuA4 and SWR-C on chromatin, such as in regulation of histone variant H2A.Z (Altaf et al., 2009; Lu et al., 2009).
1.2.4 Other NuA4 subunits: Eaf1 and Tra1
NuA4 subunits Eaf1 and Tra1 also possess crucial functions. Eaf1 is the scaffold protein of NuA4 and is essential for complex integrity (Figure 5). It is also the only subunit unique to NuA4 (Auger et al., 2008), providing a valuable tool to study NuA4 without the involvement of other chromatin complexes (e.g. INO80, SWR-C) or sub-complexes (e.g. Piccolo NuA4, TINTIN). As NuA4 and SWR-C show genetic interactions and Eaf1 displays structural similarities with human p400 ATPase, a subunit of NuA4 human homologous complex- TIP60, it has been proposed that the human complex is actually a physical and functional merge of NuA4 and SWR-C through scaffold proteins Eaf1 and Swr1 (Auger et al., 2008), highlighting the complexity of chromatin regulation cross-talks that contribute to diverse chromatin landscapes.
Tra1 is a large ATM-related protein that is shared by HAT complexes (Allard et al., 1999; Grant et al., 1998). One of the functions of Tra1 is to recruit histone HAT complexes to gene promoter regions by interacting with transcription activators (Allard et al., 1999; Brown et al., 2001). Consistent with this, Tra1 human homolog TRRAP has been identified as an essential cofactor for c-Myc- and E2F-mediated oncogenic transformation (McMahon et al., 1998).
13 Figure 5 - Schematic representation of NuA4 structure organization and functions of submodules.
Eaf1 acts as scaffold protein for complex assembly (Auger et al., 2008).
1.3 NuA4 function in gene regulation
It has been well documented that chromatin modifying complexes such as HATs can be recruited to gene promoter regions by specific transcriptional factors and function as transcription coactivators (Narlikar et al., 2002). NuA4 can be recruited by transcription factors through its Tra1 subunit, and the activity of NuA4 has been implicated in transcriptional regulation (e.g. ribosomal genes (Brown et al., 2001; Reid et al., 2000)). Moreover, studies from our group demonstrated that the NuA4 complex is recruited to the PHO5 gene promoter by the direct interaction with Pho2 DNA binding factor. This targeting is part of a pre-setting mechanism of the PHO5 gene for rapid transcriptional activation in response to phosphate starvation (Nourani et al., 2004). Additional studies also showed that the crosstalk between NuA4 and ATP-dependent chromatin remodeling complex SWR-C
14
presets PHO5 promoters with enrichment of both H2A.Z and acetylated histones (Altaf et al., 2010; Auger et al., 2008) (Figure 6).
Figure 6 - Model of NuA4 involvement in PHO5 transcription regulation. Modified from (Fuda et al., 2009).
a) When phosphate (Pi) is sufficient, PHO5 gene is repressed. Transcription factor Pho2 recruits NuA4 to the upstream activating sequences (UAS). H4 acetylation and Htz1 variant preset nucleosomes prior to induction. b) Pi starvation induces translocation and recruitment of Pho4 and results in local chromatin hyperacetylation. c) Additional recruitment and contributions of chromatin remodelers assist the nucleosome eviction and gene activation.
Htz1
Htz1 Htz1 Htz1
15 1.4 NuA4 function in genome stability
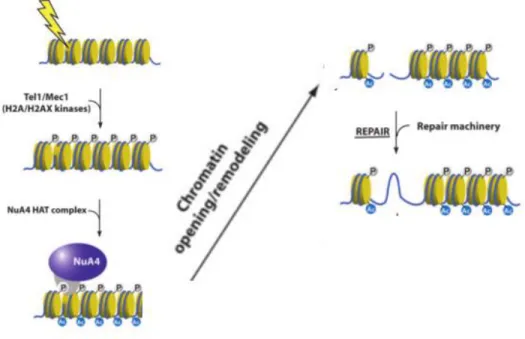
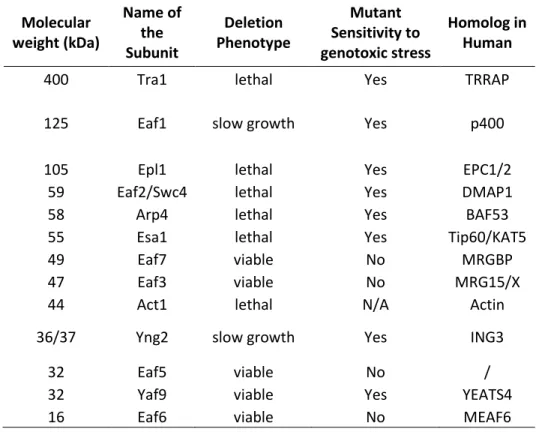
Intriguing links have been made between NuA4 and genome stability. First of all, many mutants of NuA4 subunits are sensitive to DNA-damaging agents, suggesting the involvement of these subunits in DNA repair pathways (Table 2). In addition, NuA4 activity near damage site is also important for DNA repair events. Local chromatin requires modification/relaxation in order to allow DNA repair. NuA4 has been shown to be one of the earliest factors that are recruited around DNA DSBs in vivo (Downs et al., 2004). A functional model has been proposed for this recruitment in yeast. DNA damage activates sensory kinases Mec1/Tel1 (ATR/ATM), which phosphorylate H2A at S129, generating histone mark γH2A. NuA4 is recruited to DSB sites and through the interaction between γH2A and Arp4, NuA4 is retained at the break sites and acetylates the chromatin around the break (Altaf et al., 2009; Downs et al., 2004) (Figure 7). In agreement with this model, it has been shown that the presence of NuA4 is directly required for non-homologous end joining repair of DSBs, homologous recombination and replication-coupled repair (Bird et al., 2002; Choy and Kron, 2002) (Robitaille et al, unpublished data).
Figure 7 - Model of NuA4 implication in DNA damage response. Modified from (Utley et al., 2005)
16
Table 2 - List of NuA4 Subunits
Molecular weight (kDa) Name of the Subunit Deletion Phenotype Mutant Sensitivity to genotoxic stress Homolog in Human
400 Tra1 lethal Yes TRRAP
125 Eaf1 slow growth Yes p400
105 Epl1 lethal Yes EPC1/2
59 Eaf2/Swc4 lethal Yes DMAP1
58 Arp4 lethal Yes BAF53
55 Esa1 lethal Yes Tip60/KAT5
49 Eaf7 viable No MRGBP
47 Eaf3 viable No MRG15/X
44 Act1 lethal N/A Actin
36/37 Yng2 slow growth Yes ING3
32 Eaf5 viable No /
32 Yaf9 viable Yes YEATS4
16 Eaf6 viable No MEAF6
1.5 Project Rationale
As described above, chromatin structure/modification modulates critical cellular activities and the NuA4 histone acetyltransferase complex functions in multiple chromatin transactions. Since first purified from yeast in 1999, additional data have highlighted key functions of this large complex, yet detailed mechanisms remain to be delved. This thesis presents data on two aspects of NuA4 functions: its role in gene activation, and its regulation during DNA damage response.
1. The crosstalk between chromatin modifying complexes provides an additional level of epigenetic regulation. It has been shown that NuA4 activity stimulates SWR-C exchange activity in vitro (Altaf et al., 2010) and NuA4 affects H2A.Z deposition at PHO5 promoters in vivo (Auger et al., 2008). Chapter 2 is the follow-up study through analyzing the genome-wide effect of NuA4 on H2A.Z incorporation, and the effect of NuA4 on global transcription regulation using a NuA4-specific mutant eaf1Δ.
17 2. The presence of NuA4 at DSB sites has been shown, yet a series of subsequent questions remain unanswered, for example, does NuA4 affects the choice of repair pathways? Is NuA4 activity/substrate specificity regulated at the DNA breaks? Is NuA4 physically/functionally cooperate with other proteins during DNA repair? Chapter 3 presents an attempt to shed light on these questions through the analysis of posttranslational modifications on NuA4.
Using Saccharomyces cerevisiae as model system, several biochemical and genetic/genomic techniques were adopted (such as ChIP, Tandem-Affinity-Purification (TAP), Histone acetyltransferase (HAT) assay, synthetic genetic assay (SGA)) in order to improve our understanding of NuA4 function in different cellular processes.
19
Chapter 2
Eaf1 links the NuA4 histone acetyltransferase complex to Htz1 incorporation
and regulation of purine biosynthesis
Xue Cheng*1, Andréanne Auger*1, Mohammed Altaf1, Simon Drouin2, Rhea T. Utley1,
FrançoisRobert2and Jacques Côté #1
1 St-Patrick Research Group in Basic Oncology, Laval University Cancer Research Center, Centre de Recherche du CHU de Québec-Axe Oncologie, Hôtel-Dieu de Québec, Quebec City, Quebec G1R 2J6, Canada.
2 Chromatin and Genomic Expression research unit, Institut de recherches cliniques de Montréal (IRCM), Montréal, Québec H2W 1R7, Canada.
* These authors contributed equally to this work.
# Corresponding author: (418) 525-4444 ext. 15545; Jacques.Cote@crhdq.ulaval.ca
Running title: NuA4 regulates Htz1 incorporation and purine biosynthesis Key words: NuA4; Eaf1; H2A.Z; Htz1; purine biosynthesis; ADE; acetylation; transcription.
20
2.1 Foreword
This manuscript will be submitted to the journal “Eukaryotic Cell”. I performed the ChIP and qPCR experiment regarding to Figure 11 and 12, and the qPCR experiment in Figure 13. Andréanne Auger performed experiment in Figure 8 and 9. Rhea T. Utley performed experiment in Figure 10. Mohammed Altaf performed ChIP in Figure 13. Simon Drouin and FrançoisRobert performed ChIP-on-chip labeling, hybridization, and reading. We thank Eric Paquet for bioinformatics analysis. I wrote the manuscript.
2.2 Résumé
L'architecture de la chromatine aux promoteurs des gènes est cruciale pour leur régulation afin d'orchestrer efficacement et avec précision les différentes fonctions cellulaires. Des études précédentes ont identifié l'effet stimulateur du complexe acétyl-transferase d’histone NuA4 sur l'incorporation du variant d'histone H2A.Z (Htz1) au promoteur de PHO5. Des expériences in vitro indiquent également une interrelation intrigante entre NuA4 et le complexe d’incorporation de H2A.Z, SWR-C. Au cours de ce travail, nous avons étudié le rôle d’Eaf1, sous-unité d'échafaudage de NuA4, dans l'expression globale des gènes et l'incorporation de Htz1 à l’échelle du génome entier. Nous avons constaté que la perte d’Eaf1 affecte les niveaux de Htz1 principalement aux promoteurs qui sont normalement hautement enrichis en ce variant d'histone. L'analyse du profile d’expression des cellules mutantes pour Eaf1 a dévoilé que la suppression d’Eaf1 paralyse l'induction de plusieurs gènes d’ADE, suggérant une relation entre NuA4 et les gènes impliqués dans la voie de biosynthèse de la purine. De plus, NuA4 interagit directement avec le domaine d'activation de Bas1, un facteur de transcription clé des gènes de la voie de synthèse de l’adénine. Finalement des expériences d'immunoprécipitation de la chromatine (ChIP) démontrent que les nucléosomes sur le promoteur inactif d’ADE17 sont déjà acétylés par NuA4 et enrichis en Htz1. Lors de l'induction d’ADE17, ces nucléosomes préparés pour l’activation induisent rapidement l'expression de ce gène, dans un mécanisme similaire à celui de PHO5 et conduisant au désassemblage des nucléosomes. Ces différents événements moléculaires représentent un cas particulier de l’interrelation entre l’acétylation dépendante de NuA4 et l'incorporation du variant d'histone Htz1, préparant la structure de la chromatine sur les promoteurs ADE pour son remodelage et l’activation de la transcription subséquente.
21 2.3 Abstract
Proper modulation of promoter chromatin architecture is crucial for gene regulation in order to precisely and efficiently orchestrate various cellular activities. Previous studies have identified the stimulatory effect of the histone modifying complex NuA4 on the incorporation of the histone variant H2A.Z (Htz1) at the PHO5 promoter. In vitro studies with a reconstituted system also indicated an intriguing crosstalk between NuA4 and the H2A.Z-loading complex, SWR-C. In this work, we investigated the role of NuA4 scaffold subunit Eaf1 in global gene expression and genome-wide Htz1 incorporation. We found that loss of Eaf1 affects Htz1 levels mostly at the promoters that are normally highly enriched in the histone variant. Analysis of eaf1 mutant cells by expression array unveiled a relationship between NuA4 and the gene network implicated in the purine biosynthesis pathway, as EAF1 deletion cripples induction of several ADE genes. NuA4 directly interacts with Bas1 activation domain, a key transcription factor of adenine genes. Chromatin immunoprecipitation (ChIP) experiments demonstrate that nucleosomes on the inactive ADE17 promoter are already acetylated by NuA4 and enriched in Htz1. Upon induction, these poised nucleosomes respond rapidly in inducing conditions to activate adenine gene expression, in a mechanism likely reminiscent of the PHO5 promoter, leading to nucleosome disassembly. These detailed molecular events depict a specific case of crosstalk between NuA4-dependent acetylation and incorporation of histone variant Htz1, presetting chromatin structure over ADE promoters for subsequent chromatin remodeling and activated transcription.
22
2.4 Introduction
Transcriptional activation is frequently accompanied by local remodeling of chromatin structure over promoter regions. Three major mechanisms have been implicated in this process, namely histone posttranslational modifications, ATP-dependent chromatin remodeling and incorporation of histone variants (see review (Li et al., 2007) and references therein). These mechanisms facilitate transcription by either sliding promoter nucleosomes or assisting nucleosome loss, resulting in an increased accessibility of DNA to transcription activators. Collaboration between multi-subunit complexes responsible for these activities leads to a precise and specific control over gene expression. Studies from recent years suggest that different sets of genes, constitutive and inducible genes for example, adopt rather distinct strategies in utilizing these mechanisms to modulate promoter architecture (Cairns, 2009; Tirosh and Barkai, 2008).
Recent work has shown the tight yet complex functional link between two yeast chromatin modifying complexes, histone acetyltransferase complex NuA4 and chromatin remodeling complex SWR-C, converging at the understanding of histone variant H2A.Z. SWR-C is a 14-subunit complex responsible for histone variant H2A.Z incorporation (Kobor et al., 2004; Krogan et al., 2003; Mizuguchi et al., 2004). It shares 4 subunits with NuA4 (Altaf et al., 2010; Galarneau et al., 2000; Kobor et al., 2004; Krogan et al., 2003; Le Masson et al., 2003; Zhang et al., 2004), the main function of which is to acetylate histone H2A and H4 (Allard et al., 1999; Utley et al., 1998). These acetylation events are important for SWR-C recruitment and activity on chromatin, likely through its bromodomain containing subunit Bdf1 (Altaf et al., 2010; Durant and Pugh, 2007). One of the shared subunit, Yaf9, is implicated in helping antagonize silencing near telomeres (Zhang et al., 2004; Zhou et al., 2010). Moreover, NuA4 is also found to acetylate H2A.Z both in vitro and in vivo (Auger et al., 2008; Keogh et al., 2006; Millar et al., 2006). The acetylated H2A.Z is implicated in regulation of Htz1 dynamics at promoters (Millar et al., 2006) and Htz1 function in proper heterochromatin maintenance (Babiarz et al., 2006). Consistent with the intimate relationship between SWR-C and NuA4, it was proposed that human TIP60 complex is a physical and functional merge of the two complexes (Altaf et al., 2009; Auger et al., 2008; Doyon et al.,
23 2004), resulting in a single multifunctional complex that comprises all aforementioned mechanisms of chromatin regulation.
The functional crosstalk between histone modifications by NuA4 and histone variant incorporation by SWR-C has been carefully analyzed at PHO5. In Saccharomyces cerevisiae, the PHO system modulates phosphate metabolism and allows cells to adapt to inorganic phosphate environment. When phosphate is present, the PHO5 gene is maintained in a transcriptionally repressed state by a particular chromatin structure on promoter region. Upon gene activation in phosphate starvation conditions, the promoter nucleosome structure undergoes a remodeling process, generating nuclease hypersensitive regions (Almer and Horz, 1986; Almer et al., 1986). The hypersensitivity to nucleases is caused by loss of histone-DNA contacts, leading to the removal of local nucleosomes (Boeger et al., 2003; Brown et al., 2011; Reinke and Horz, 2003). Studies have shown that NuA4 is required in this opening of chromatin structure through its histone acetylation activity and effect on Htz1 incorporation, presetting the promoters for proper gene induction (Auger et al., 2008; Li et al., 2007; Nourani et al., 2004).
Although the interplay between NuA4 activity and Htz1 incorporation has been reported both in vitro and at certain genes in vivo, the global-scale of NuA4 effect on Htz1 incorporation remains poorly studied. To this end, in this study we investigated the effect of NuA4 on global Htz1 variant incorporation in yeast. We show that eaf1 deletion mutant affects genome-wide Htz1 incorporation over several hundred of promoters. Moreover, we found that eaf1Δ also decreases the expression level of genes implicated in purine biosynthesis pathway. Eaf1 directly interacts with ADE gene transcription factor Bas1 and Pho2, and EAF1 deletion cripples ADE gene activation. We demonstrate that inactive adenine promoters are enriched in Htz1 and acetylated H4 in a NuA4-dependent manner. Induction of adenine genes results in efficient nucleosome loss, whereas the levels of Htz1 and H4 acetylation enrichment remain unchanged, suggesting a presetting mechanism for inducible adenine gene transcription. Finally, we show that NuA4 also acetylates Htz1 at ADE promoter, which is likely to favor nucleosome disassembly. These detailed molecular events indicate that adenine promoters, similar to PHO5, are preset by NuA4-dependent events for subsequent chromatin remodeling and induced transcription, highlighting the crosstalk
24
between histone modifications and histone variant incorporation in the process of transcription modulation.
2.5 Materials and methods 2.5.1 Yeast strains and reagents.
All strains used in this study are based on S288C BY4741 background and listed in Table 1. Wild type, htz1 deletion mutant strain was purchased from Open Biosystems. All yeast manipulations were performed according to standard protocols unless specifically indicated.
Table 3 - Strains Used In This Study.
Strain Genotype Reference
BY4741 MATa his3∆1 leu2∆0 met15∆0 ura3∆0 (Brachmann et al., 1998) htz1∆ (1703) MATa his3∆1 leu2∆0 met15∆0 ura3∆0 htz1∆::KanMX4 Open biosystems QY1158 MATa his3∆1 leu2∆0 met15∆0 ura3∆0 eaf1∆::KanMX4 This study
2.5.2 ChIP-on-chip.
Chromatin immunoprecipitation and genome-wide location analysis with microarrays carrying 2–4 probes for each gene were performed as described previously (Guillemette et al., 2005; Rossetto et al., 2014; Utley et al., 2005). Htz1 enrichment level was normalized on H3 level for nucleosome occupancy. Genes defined as “High-Htz1-occupancy” were isolated with a cut-off of 3 and then related to transcription frequencies using the data of (Holstege et al., 1998).
2.5.3 Northern Blot and Microarray analysis.
Yeast cells were grown overnight in YPD, diluted to OD600 0.25 in SC+adenine or SC-adenine media and grown to OD600 around 1.0. Cells were then washed and RNA was isolated using the hot phenol method as described (Schmitt et al., 1990). An amount of 15 to 20 µg of RNA was analyzed by Northern blotting as described (Galarneau et al., 2000). The used probes were ORFs from ADE1, ADE2, ADE5,7, ADE13 and ACT1 obtained by PCR and radiolabelled by random primer (Amersham Biosciences). For microarray analysis, total RNA was isolated using the Qiaquick RNeasy Miniprep kit (Qiagen) and mRNA was isolated
25 using PolyA tail (Promega) and analysed by Deming Xu at the Best Yeast Microarray Center in Toronto.
2.5.4 GST-pull-down, and HAT assay.
Specific regions of Bas1 and Pho2 proteins were fused to GST (pGEX-4T3) (Fig. 10a) and purified from Escherichia coli on glutathione sepharose following standard procedures. Protein concentrations were normalized by coomassie staining on gels (Fig. 10b) and equivalent amounts were used in GST pull-down assays with purified NuA4 followed by nucleosomal HAT reactions essentially as described (Utley et al., 1998).
2.5.5 Chromatin ImmunoPrecipitation (ChIP).
ChIP experiment was performed essentially as described previously (Auger et al., 2008; Nourani et al., 2004; Utley et al., 2005). Briefly, overnight cultures were diluted and grown to OD600 0.5-0.8 in 200ml media. Cell cultures were cross-linked with 1% formaldehyde for 20 min at room temperature. Crosslinking was quenched by the addition of glycine to a final concentration of 125 mM for 5 min. Cells were centrifuged 5 min 5000 g at 4°C, washed twice with ice cold water, once with 1 ml of TBS and resuspended in 0.5 ml of FY lysis buffer (HEPEs 50 mM pH 7.5, NaCl 140 mM, Triton X-100 1%, EDTA 1 mM, Na deoxycholate 0.1%, 1mM PMSF). Cell lysis was carried out using mini-BeadsBeater until around 70% of the cells were lysed. Cells were then centrifuged and pellet was washed with 1 ml FA lysis buffer until the supernatant was clear. Pellet was resuspended in 1 ml of the FY lysis buffer for sonication. Sonication was carried-out using the Bioruptor apparatus from Diagenode (30 sec sonication followed by 60 sec rest, repeated 10- 12 times), which was then centrifuged for 30 min at 4°C 20 000 g. The supernatant containing the chromatin was placed in a new tube. For ADE induction, cells were grown in SCSM-Ade media supplemented with adenine to OD600 0.5-1.0. For induction, cells were centrifuged, washed in water and resuspended in SCSM-Ade media supplemented with adenine for time 0 or resuspended in SCSM-Ade and allowed growing for the induction time period before cross-linking.
Sonication of chromatin led to DNA fragments range between ~200-500 bp. Immunoprecipitation was carried out using 100 µg chromatin supplemented with FA lysis
26
buffer to a final volume of 450 ul. 22.5ul of the mix was taken as input. Corresponding antibodies were then added: 0.5 µl of anti-Htz1 (Upstate 07-718), 0.5 µl anti-hyperH4ac (Upstate Penta), 0.5 µl anti-H3 C-terminus (Abcam Ab1791), 2ul anti-Pol II (8WG16) and anti-AcHtz1K14 (kindly provided by Michael Grunstein). After an overnight incubation at 4°C on a wheel, 12.5 µl of protein A sepharose (GE Healthcare) was added and incubation was continued for 4 h at 4°C on wheel. The beads were washed twice with 1 ml FA lysis buffer, once with FA lysis buffer 500 mM NaCl, once with 1 ml Wash Buffer #2 (Tris/HCl 10 mM pH 8.0, LiCl 250 mM, NP-40 0.5%, sodium deoxycholate 0.5%, EDTA 1mM) and once with TE. Beads were eluted with 100 µl T50E10 1% SDS for 15 min at 65°C. The eluted
material was placed in a new tube and beads were eluted once more with 150 µl TE 1% SDS. The second elution was pooled with the first one. DNA was de-crosslinked overnight at 65°C. After de-cross-linking, 240 µl of TE and 5ug of RNase were added and incubated at 37°C for 15-minute, after which 15 mg of proteinase K and 200 µg of glycogen were added and tubes were placed at 37°C for 2 hours. 25 µl of NaCl 5M was added before the extraction with phenol chloroform and precipitation with ethanol. After centrifuging at 20 000 g, 4°C for 30 min, pellets were air dried and resuspended in 100 µl NTE. PCR primers used were analyzed for linearity range, efficiency, specificity, melting curves and products using a LightCycler (Roche). Primer sequences are listed in Table 4.
Table 4 - Primers used in ChIP-qPCR
Gene/Primer Direction Sequence
ADE17 -237bp /forward ATCATTTATAAAGAAGATCCTACCC
ADE17 -122bp /reverse ATAGATCCGAACGTGATATG
ADE17 +717bp/ forward TTTGTTGGATGCTCTAAATTCC
ADE17 +843bp/ reverse ATCAGACAATGGGATACCCA
ADE17 +1485bp /forward CGGCCAAATTCCAACAGAAG
27 2.6 Results
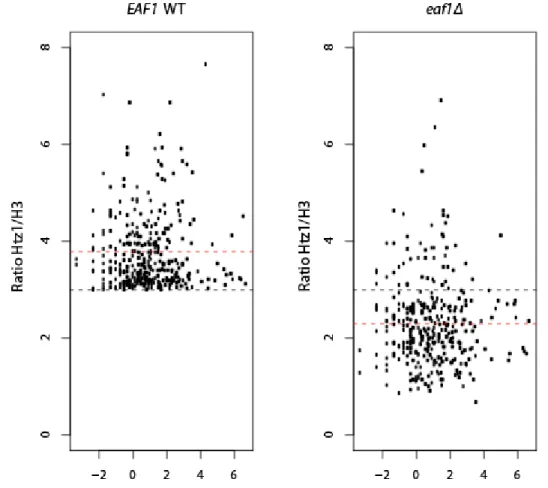
2.6.1 Loss of Eaf1 affects genome-wide Htz1-enrichment
Previous studies have shown that NuA4 stimulates SWR1-dependent Htz1 incorporation and depletion of NuA4 activity decreases Htz1 occupancy at PHO5 promoter regions (Altaf et al., 2010; Auger et al., 2008; Nourani et al., 2004), suggesting a tight crosstalk between the two chromatin modifying complexes. Nevertheless, as these studies have been limited to the observations at specific loci, to what extent this crosstalk would take place globally remains unknown. In order to further address the question of how NuA4 affects Htz1 global incorporation, we performed chromatin immunoprecipitation assay (ChIP) of Htz1 and histone H3 in both wild-type (WT) and eaf1Δ strains and hybridized the recovered DNA to microarrays with 2-4 probes for each gene (ChIP-on-chip). Deletion of NuA4 subunit Eaf1 was chosen since it is the scaffold platform for NuA4 complex assembly and also the only subunit unique to NuA4 (Auger et al., 2008; Mitchell et al., 2008). Htz1 occupancy was measured by relative Htz1 enrichment compared to corresponding H3 signal.
In the WT strain, Htz1 is preferentially enriched at repressed/basal promoter regions compared to gene bodies (data not shown) and do not correlate with level of transcription, consistent with the previous reports (Li et al., 2005; Zhang et al., 2005). While Htz1 presence does not require NuA4, this enrichment level is significantly decreased in eaf1Δ strain for a large number of genes, in agreement with what has been observed at PHO5 promoter. Notably, promoters which harbor a high-occupancy of Htz1 (Htz1/H3>3; p<0.05; n=429) are most profoundly affected by EAF1 deletion (Fig.8; gene list is available upon request). These results expand the reported observation and identify NuA4 as a crucial factor in Htz1 level maintenance at the most enriched promoters.
28
Figure 8 - NuA4 affects Htz1 incorporation independently of transcription rate.
Scatter plot of loci identified as high Htz1 occupancy loci (Ratio Htz1/H3 ≥ 3) by transcription rate (log2 (Transcription rate mRNA/hour)) in WT (left) and eaf1Δ strain (right). Red line shows the mean value and the black line represents the cut-off. Transcription frequencies in (Holstege et al., 1998) was used in the analysis with transcription rate. 2.6.2 NuA4 is implicated in regulation of gene network for purine biosynthesis
Both NuA4 activity and Htz1 incorporation are implicated in transcription regulation. NuA4 has been shown to acetylate promoter nucleosomes, an event considered to assist nucleosome loss to favor transcription activation (Nourani et al., 2001; Nourani et al., 2004; Reid et al., 2000; Utley et al., 1998). Htz1 is enriched in basal/repressed gene promoters, and involved in activation of certain genes (Li et al., 2005; Zhang et al., 2005). As eaf1Δ mutant decreases Htz1 occupancy of a subset of genes, whether the transcription level of these genes are also
29 disturbed by EAF1 deletion remains to be answered. To this end, we examined the expression profile in both WT and eaf1Δ strains by microarray. The genes with the most affected Htz1 level (Figure 8) are not significantly overlapped with those most decreased expression levels (Table 5). As expected, RP gene and PHO gene expression are affected by Eaf1/NuA4, as reported previously (Boudreault et al., 2003; Galarneau et al., 2000; Nourani et al., 2004; Reid et al., 2000). Surprisingly, we also found a series of genes implicated in purine biosynthesis pathways among these most affected by EAF1 deletion (Table 5), suggesting a role of NuA4 in regulation of purine biosynthesis.
Table 5 - Yeast Genes Down-regulated in eaf1Δ Cells Grown in Rich Media eaf1Δ /WT Gene
0.274 RPL15B ribosomal protein
0.33 ADE13 Bas1/Pho2-regulated, purine biosynthesis
0.362 ADE1 Bas1/Pho2-regulated, purine biosynthesis
0.391 PHO12 Pho2-regulated
0.393 SHM2 purine biosynthesis, Bas1/Pho2-regulated 0.423 ADE5,7 Bas1/Pho2-regulated, purine biosynthesis 0.437 MTD1 purine biosynthesis, Bas1/Pho2-regulated
0.444 BAT2
0.452 ADE2 Bas1/Pho2-regulated, purine biosynthesis
0.474 GCV2 Bas1 site, purine pathway
0.479 SER1(ADE9) Bas1 site, purine biosynthesis
0.485 YHB1
0.494 PHO11 Pho2-regulated
0.51 GCV1 Bas1 site, purine pathway
0.519 ADE17 Bas1/Pho2-regulated, purine biosynthesis
0.52 LEU1
0.526 YLR345w
0.53 LAP4
0.535 BNA1
0.545 DDR48
0.547 ADE3 Bas1/Pho2-regulated, purine biosynthesis
0.553 YKL100C
0.556 ECM9
0.556 ADE12 Bas1/Pho2-regulated, purine biosynthesis
0.556 YOR239w
0.569 SDS3 HDAC subunit
0.576 YML005w
0.576 PHO5 Pho2-regulated
Mi croa rra y a na l ys i s performed on RNA i s ol a ted from eaf1 Δ cel l s compa red to RNA i s ol a ted from wi l d type (WT) cel l s . Here l i s ts the 28 genes tha t a re the mos t a ffected by the a bs ence of EAF1 (from 0.274 for RPL15B to 0.576 for PHO5 , 1.0 repres ents wi l d type expres s i on l evel ). eaf1 Δ cel l s s how a ma rked decrea s e i n the
expres s i on of 12 genes i nvol ved i n puri ne bi os ynthes i s (Ba l d) i ncl udi ng 7 ADE genes . PHO5 expres s i on l evel i s a l s o a ffected by EAF1 del etion.
30
2.6.3 Loss of Eaf1 cripples induction of a number of ADE genes
In adenine sufficient environment, the transcription of ADE genes are basal/repressed. The deprivation of available adenine will trigger a series of ADE gene expression in order to adapt to the environment (Daignan-Fornier and Fink, 1992; Gedvilaite and Sasnauskas, 1994; Som et al., 2005). To investigate further the role of Eaf1/NuA4 in ADE gene activation and purine biosynthesis, activation of ADE genes was analysed by Northern blot. RNAs were extracted from both WT and eaf1Δ strains and several ADE gene expression level were examined using specific probes. In the WT strain, when adenine is present in the media, the ADE genes show basal level of transcription (Fig. 9, left lanes), which may be due to the gradual depletion of adenine in the media, loss of chromatin architecture or transcription noise (see discussion). ADE starvation condition induces the ADE expression in WT cells, yet this effect is lost in the eaf1Δ strain (Fig. 9, right lanes). Results from microarray and Northern blot analysis demonstrate that activation of ADE expression is dependent on Eaf1/NuA4.
Figure 9 - NuA4 is important for ADE gene induction.
Northern blot analysis performed with RNA isolated from wild type (WT) and eaf1Δ cells grown in the presence or absence of adenine. ADE1, ADE2, ADE5,7, ADE13 gene expression was compared to the expression level of ACT1 control.
31 2.6.4 NuA4 interacts with Pho2 and Bas1 in vitro
Two transcription factors Pho2 and Bas1 have been shown to regulate adenine gene induction (Koehler et al., 2007; Rolfes et al., 1997; Som et al., 2005). Previous studies have shown that Bas1 is constantly present at ADE promoters in an inactivated state. Upon induction, Bas1 undergoes a conformation change and recruits Pho2 (Som et al., 2005). Interestingly, previous works also show that Pho2 directly interacts with NuA4 in vitro and in vivo, a step considered crucial to preset the promoter of PHO5 gene for induction (Nourani et al., 2004). Collectively, these bring us to the question whether NuA4 is recruited by Pho2 and/or Bas1 in the case of ADE gene regulation. To address this question, GST fusion proteins were constructed (Fig. 10a) and purified (Fig. 10b). Equal amount of GST-fusion proteins (Fig. 10b) were used to perform pull down assay with purified NuA4 complex, where both flow-through and beads are subject to HAT assay. NuA4 shows direct interaction with N-terminal homeodomain of Pho2 as reported (Nourani et al., 2004), and interestingly, it also interacts with Bas1 activation domain, with an even stronger affinity (Fig. 10c). These results indicate that NuA4 could regulate ADE gene expression through direct recruitment by promoter-bound transcription factors Pho2 and Bas1.
32
Figure 10 - Bas1 and Pho2 directly interact with NuA4 complex in vitro.
A) Schematic representation of the GST fusion proteins used in this study. B) Indicated fusion proteins were run in 12% SDS–PAGE and coomassie stained. C) Equal amount of input, flow-though, and beads from GST-pull-down with purified NuA4 complex were assayed for histone acetyltransferase (HAT) with chromatin. HAT reactions were visualized on 18% SDS-PAGE. Pho2N serves as a positive control (Nourani et al., 2004).
33 2.6.5 Enrichment of Htz1 and acetylated H4 at inactive ADE promoters is dependent on NuA4
The histone acetyltransferase activity provided by NuA4 is required for the presetting of inducible PHO5 promoter during the transition from a transcriptionally inactive to an active state in absence of phosphate (Nourani et al., 2004). Having identified the aforementioned importance of Eaf1 subunit in regulation of ADE gene expression, we set out to investigate the chromatin dynamics over the promoters of ADE genes, and the implication of NuA4 during this process. Using chromatin immunoprecipitation (ChIP), we first look at H4 acetylation level (a major activity of NuA4), Htz1 incorporation level at ADE17 promoter region under repression and the effect of Eaf1 therein. ADE17 locus was chosen since its expression is affected by EAF1 deletion in microarray and it was also shown that Htz1 is enriched at its transcription start site (TSS) (Mizuguchi et al., 2004). Both wild-type and eaf1Δ strains are grown in synthetic complete media, in which the adenine supplement is sufficient. Under this repressed condition, ADE17 promoter region shows enriched H4 acetylation and Htz1 compared to control locus, and this enrichment is significantly decreased in eaf1Δ strain (Fig. 11 a-b). These results suggest the involvement of NuA4 in chromatin structure at basal ADE promoters.
34
Figure 11 - The presence of H4 acetylation and Htz1 at repressed ADE17 promoter is NuA4-dependent.
H4ac enrichment A) and Htz1 enrichment B) at ADE17 promoter region (-237bp to -122bp) in WT and eaf1Δ is measured by chromatin immunoprecipitation (ChIP). htz1Δ serves as negative control for Htz1 IP. Potential nucleosome occupancy variation was corrected by H3 signal at the same locus. Standard deviation is shown based on two biological repeats. 2.6.6 Loss of nucleosomes upon ADE gene induction
Since uninduced ADE17 promoter shows an enrichment in both H4 acetylation and Htz1, we wonder whether this enrichment merely represents a basal chromatin status (in this case, the enrichment of H4 acetylation and Htz1 could be expected to increase during induction), or
35 stands for a preset mechanism to poise promoters for subsequent induction (in this case, both levels would be expected decrease along with nucleosome loss). In order to test this, we performed ChIP time course experiment by exposing cells to adenine starvation condition for 0 (no starvation), 5 or 20 minutes. RNA polymerase II ChIP across the ADE17 gene suggests short transient pol II recruitment/enrichment with an expression peak around time-point 5-min (Fig. 12a; see discussion). This expression correlates with the rapid loss of nucleosomes measured by the decrease of H3 signal (Fig. 12b). After confirming an efficient induction, we then examined the H4 acetylation level and Htz1 dynamics during gene induction. Both H4 acetylation and Htz1 levels decrease significantly within 5 minutes (Fig. 12c, e), consistent with the observed disassembly of nucleosomes (Fig. 12b), while both levels stay unchanged on the remaining nucleosomes after correction for nucleosome signals (Fig. 12d, f). These results suggest a mechanism that ADE17 promoter is preset by H4 acetylation and Htz1, resulting in susceptible nucleosomes to favor rapid induction.
As shown previously, eaf1Δ affects both H4 acetylation and Htz1 level at silenced ADE17 promoters (Fig. 11). If this chromatin state at ADE17 promoter indeed represents a preset mechanism, one could expect eaf1 mutant would disrupt this poised status and alter nucleosome stability. Indeed, eaf1 mutant shows a higher nucleosome occupancy at ADE17 promoter in repressed condition (Fig. 12g).