Gene-environment interaction in Paget’s disease of bone
Mémoire
Mohamed Numan
Maîtrise en médecine moléculaire
Maître ès sciences (M.Sc.)
Québec, Canada
Résumé
La maladie osseuse de Paget (MP) est une maladie métabolique de l’os. Bien que les facteurs génétiques jouent un rôle important dans la pathogénie de la MP, les facteurs environnementaux tels que la résidence rurale et l’exposition au chauffage au bois ont été associés avec la MP. Afin d’étudier le rôle des polluants de l'air extérieur et intérieur sur la MP, nous avons administré un questionnaire chez 140 patients canadiens-français avec la MP et 113 témoins sains. Ce questionnaire portait sur la pollution de l'air extérieur, comme la résidence près d'une autoroute, d’une station de bus, de train ou d’un aéroport, d’une station d'essence, et sur les polluants de l'air intérieur en mettant l'accent sur les combustibles de chauffage (charbon, bois, huile) et l'exposition au tabac. Dans un sous-groupe de patients, la concentration urinaire de 17 métaux lourds et de 11 hydrocarbures aromatiques polycycliques a été mesurée par spectrométrie de masse. À la lumière de ce que nous savions dès le questionnaire et les dosages urinaires, nous avons identifié certains toxiques pouvant être des facteurs de risque pour la MP. Pour explorer les effets in vitro de ces toxiques sur les ostéoclastes dans la MP, nous avons réalisé une différentiation in vitro de monocytes du sang périphérique provenant de plus de 40 participants, patients, porteurs sains de mutation dans le gène SQSTM1, et des témoins sains, en ostéoclastes traités avec ou sans les toxiques identifiés. La morphologie des ostéoclastes, le pourcentage de résorption osseuse, les niveaux d'expression génique, et les niveaux de stress oxydatif cellulaire ont été analysés. Les résultats ont montré un effet inhibiteur du condensé de la fumée de cigarette et des métaux lourds sur la morphologie et la fonction des ostéoclastes. De plus, des taux élevés de stress oxydatif chez les ostéoclastes des patients ont été observés, et un profil hétérogène des effets de métaux lourds sur l'expression des gènes a été identifié.
Abstract
Paget's disease of bone (PDB) is a metabolic bone disease. Although genetic factors play an important role in the pathogenesis of PDB, environmental factors such as rural residence and the exposure to wood heating was associated with PDB. In order to study the role of outdoor and indoor air pollutants on PDB, we performed a survey in 140 French-Canadian patients with PDB and 113 healthy controls. The survey covered the outdoor air pollution such as the residence near a highway, a bus station, a train or an airport or a gas station, and indoor air pollutants by focusing on heating fuels (carbon, wood, oil) and exposure to tobacco smoke. In a subgroup of patients, urinary concentration of 17 heavy metals and 11 polycyclic aromatic hydrocarbons was measured by mass spectrometry. In light of what we knew from the survey and urinary assays, we identified certain toxics that could be risk factors for PDB. To explore the in vitro effects of these toxics on osteoclasts in PDB, we conducted in vitro monocytes differentiation from peripheral blood of more than 40 participants, patients, healthy carriers of p.Pro392Leu mutation, and healthy controls, which osteoclasts were treated with or without the identified toxic. The morphology of osteoclasts, the percentage of bone resorption, gene expression level, and cellular oxidative stress levels were assayed. The results showed an inhibitory effect of cigarette smoke condensate and heavy metals on morphology and function of patients’ osteoclasts. Further, high levels of oxidative stress in patients’ osteoclasts were observed, and a heterogenic profile of heavy metals effect on gene expression was identified.
Table of Contents
Résumé ... iii Abstract...v List of Tables ... ix List of Figures ... xi Abbreviations ... xiii Acknowledgments ... xv Chapter 1: Introduction ... 1 1.1 Bone physiology ... 1 1.1.1 Introduction ... 1 1.1.2 Bone cells ... 21.1.3 Bone modeling and remodeling at a glance ... 9
1.1.4 Regulation of bone remodeling ... 11
1.2 Paget’s disease of bone (PDB) ... 15
1.2.1 Introduction ... 15
1.2.2 Epidemiology of PDB ... 15
1.2.3 Etiology of PDB ... 17
1.2.4 Symptoms, diagnosis, and management of PDB ... 19
1.2.5 Complications ... 22
1.3 Pathophysiology of Paget’s disease of bone ... 23
1.3.1 Cellular phenotype of osteoclast in PDB ... 23
1.3.2 Genetic factor as a main cause ... 23
1.3.3 SQSTM1 gene ... 25
1.4 Air pollutants and musculoskeletal diseases ... 33
1.4.1 Introduction ... 33
1.4.2 Outdoor and indoor air pollutants ... 34
1.4.3 Harmful effects of air pollution on musculoskeletal diseases ... 34
1.4.4. Physiological reactions against heavy metals ... 36
Chapter 3: Materials and methods ... 41
3.1 Recruitment of participants and Survey ... 41
3.2 Urinary dosages ... 41
3.3 Toxics selection ... 42
3.4 Cell cultures ... 49
3.5 Quantitative real-time PCR ... 49
3.6 Oxidative stress levels measurement ... 52
3.7 Statistical analyses ... 53
Chapter 4: Results ... 55
4.1 Association of indoor and outdoor air pollutants with PDB ... 55
4.2 Urinary measurements of heavy metals and polycyclic aromatic hydrocarbons... 61
4.3 Osteoclast morphology and bone resorption abilities ... 67
4.3.1 Lead (Pb) ... 67
4.3.2 Tobacco smoke condensate ... 68
4.3.3 Cadmium (Cd) ... 69
4.3.4 Mercury (Hg)... 70
4.3.5 Bismuth (Bi) ... 71
4.4 Gene expression analyses in osteoclasts... 72
4.5 Impact of toxic on oxidative stress in osteoclasts ... 74
Chapter 5: Discussion ... 75
5.1 Main results found in this study ... 75
5.2 Interpretation of results ... 75
5.3 Comparison with the literature ... 77
List of Tables
Table 1. Osteoclasts and osteoblasts coupling factors. ... 7
Table 2. Essential systemic regulators for bone remodeling. ... 12
Table 3. Essential local regulators for bone remodeling ... 14
Table 4. Bisphosphonates drugs and their doses... 21
Table 5. Disease-causing genes and main genes containing common genetic variants associated with PDB (74) ... 24
Table 6. SNPs found to be associated with PDB according to GWAS (75) ... 29
Table 7. Environmental risk factors that predispose to PDB ... 31
Table 8. Heavy metals involvement in musculoskeletal diseases ... 34
Table 9. Investigated genes and their role in detoxification and autophagy ... 37
Table 10. Human body accumulation of heavy metals, and effects on human bone diseases... 43
Table 11. Selected toxics and choice of the dose for in vitro cell cultures ... 47
Table 12. Sequence primers and gene description ... 50
Table 13. Environmental factors associated with PDB, results of multivariate analysis ... 55
Table 14. Environmental factors associated with familial PDB and non-familial form of PDB, results of multivariate analysis ... 58
Table 15. Urinary dosage of heavy metals and polycyclic aromatic hydrocarbons (global analysis) ... 61
List of Figures
Figure 1. Anatomical classification of bone types (3) ... 2
Figure 2. Hematopoiesis lineages: origin of different types of blood and bone tissue cells (1)... 3
Figure 3. Osteoclast morphology and mechanism of its function (6) ... 4
Figure 4. Osteoclast life cycle (5) ... 5
Figure 5. Illustration of the bone remodeling process and the cell types (13)... 8
Figure 6. Endochondral ossification steps ... 9
Figure 7. Bone remodeling cycle: the five phases of the bone remodeling cycle(15) ... 11
Figure 8. Prevalence of PDB for the current survey (2005–06) and an earlier survey in Auckland (1996–98) according to age for both genders (30) ... 16
Figure 9. Comparison in prevalence of radiographic PDB in Dunedin city/New Zealand between 2001 and 1983 surveys according to age and gender (36) ... 17
Figure 10. Limb bone curvature as a sign of PDB (51, 52) ... 20
Figure 11. Increased skull size, venous dilatation, and skull bone deformation IN PDB patients(51, 52) .... 20
Figure 12. The multi-domain structure of p62/SQSTM1 protein (78) ... 26
Figure 13. Different functions of p62/ SQSTM1 protein (78) ... 28
Figure 14. Osteoclasts formation and bone resorption activities in vitro in presence and absence of administration of lead. ... 67
Figure 15. Osteoclasts formation and bone resorption activities in vitro in presence and absence of administration of tobacco smoke condensates. ... 68
Figure 16. Osteoclasts formation and bone resorption activities in vitro in presence and absence of cadmium. ... 69
Figure 17. Osteoclasts formation and bone resorption activities in vitro in presence and absence of mercury.. ... 70
Figure 18. Osteoclasts formation and bone resorption activities in vitro in presence and absence of bismuth.. ... 71
Figure 19. Osteoclast gene expression analyses of candidate genes over housekeeping genes standardized for the exposure to different toxics ... 73
Abbreviations
118S · Homo sapiens 18S ribosomal RNA A
ADNg · Homo sapiens 3-beta-hydroxysteroid
dehydrogenase/delta-5-delta-4-isomerase (3-beta-HSD) gene
B
Bi · Bismuth
BMP-2 · bone morphogenetic protein 2 BMP-6 · Bone morphogenetic protein 6 bp · Base Pair
C
Cd or cd2 · Cadmium CI · confidence Interval
CSC · Cigarette Smoke Condensate
CTHRC1 · Collagen triple helix repeat containing 1 D
DAPI Fluorescent stain
(4',6-diamidino-2-phenylindole)
F
FTC · Federal Trade Commission G
G6PD · Glucose-6-phosphate dehydrogenase GM-CSF · Granulocyte-macrophage
colony-stimulating factor
GSTM3 · Homo sapiens glutathione S-transferase
mu 3 · 35
GSTM4 · Homo sapiens glutathione S-transferase mu
4
GWAS · Genome-wide association studies
H
Hg · Mercury I
IGFs · Insulin-like growth factors IL-3 · Interleukin-3
M
MAP1LC3B · Homo sapiens microtubule associated
protein 1 light chain 3 beta
MAP1LC3B2 · Homo sapiens microtubule associated
protein 1 light chain 3 beta 2
M-CSF · Macrophage colony-stimulating factor MT3 · Homo sapiens metallothionein 3 (MT3) MVNP · Measles virus nucleocapsid protein N
Nrf2 · The nuclear factor erythroid 2-related factor O OCL Osteoclasts OPG · Osteoprotegerin OR · Odds ratio P P · P-Value P392L · Pro392Leu mutation
PAHs Polycyclic aromatic hydrocarbons Pb · Lead
PDB · Paget’s disease of bone PDGF · Platelet-derived growth factor
PPIB · peptidylprolyl isomerase B (cyclophilin B) R
RANKL · Receptor activator of nuclear factor
kappa-B ligand
REF · Reference
RUNX2 · Runt-related transcription factor 2 S
SQSTM1 · Sequestosome 1 T
TGF-β · Transforming growth factor β TNF · Tumor necrosis factor
TNFSF11 · Tumor necrosis factor ligand super-family,
member 11
TRAP · Tartrate-resistant acid phosphatase U
Acknowledgments
It was a great period of intensive learning and fruitful work. I would like first to thank my supervisor Dr Laëtitia Michou for all the support I was given to accomplish my research.
I can never forget his great inspiring words giving me confidence and enthusiasm in every conference we attended and every paper we published. Thanks, Dr Jacques Brown.
Thanks to my colleagues Edith Gagnon, Nathalie Amiable, Sonia Jean, Nathalie Paquet, Nicole Alméras, Lucie Ratelle, and Iris Silva for their great collaboration throughout this time. You helped me greatly and I would like to emphasize your excellent cooperation in my research project.
Thanks to Suzanne Picard, the first professor who taught me how to write, how to speak in French at the great Laval University in the beautiful Québec City.
Thanks to all committees who decided to grant me prizes, awards, and scholarships; Louise Côté scholarship’s committee, The Network for Oral and Bone Health Research’s (RSBO) committee, Fondation du Centre de recherche du CHU de Québec’s committee, and the perseverance award of faculty of literature - school of languages’ (at Laval university) committee.
I would like to reflect on all people who have encouraged me and helped me during this period. I would also like to thank my parents for their wise advices and their support all my life.
Thank you very much, everyone! Merci beaucoup et au plaisir!
Mohamed Numan
Chapter 1: Introduction
1.1 Bone physiology
1.1.1 Introduction
Bone is the most essential tissue in the human body which gives strength and support. Furthermore, it is a renewable source for blood cells (hematopoiesis) and a reservoir for fat and mineral homeostasis. Moreover, bone also has a protective role for sensitive non-regenerable neurons and a physical mechanical role as a support for the fleshy body mass, muscles, and tendons attachment. In addition to its protective, storage, and renewable supplementary roles, bone and its joints allow a flexible body movement and maintain its strength and appearance (1, 2).
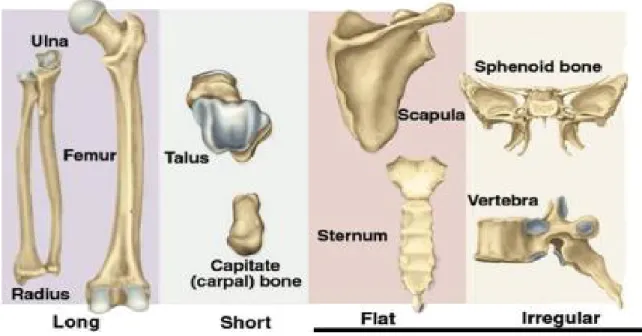
Anatomically, bone can be classified into long bones, short bones, sesamoid bones, irregular bones, and flat bones. Fig. 1 (3) illustrates examples of long bones such as femur, tibia, humerus and forearm; short bones such as talus and capitate; flat bones such as skull, sternum, scapula and pelvis; and irregular bones such as vertebra and sphenoid bone. Bones can be further classified according to their structure into cortical (compact) and trabecular (spongy or cancellous) bone (4). Cortical bone structure represents 80% of bone mass and is characterized by a slower bone turnover (bone remodeling) in comparison with trabecular bone. Cortical bone consists of small units of cylindrical structures called osteons (Haversian system). These osteons are connected by Haversian canals, and the gaps between these canals are filled with interstitial lamella. Trabecular bone is porous, loosely structured, and characterized by a higher bone remodeling. Histologically, bone can be classified into woven or lamellar bone (4).
Figure 1. Anatomical classification of bone types (3) 1.1.2 Bone cells
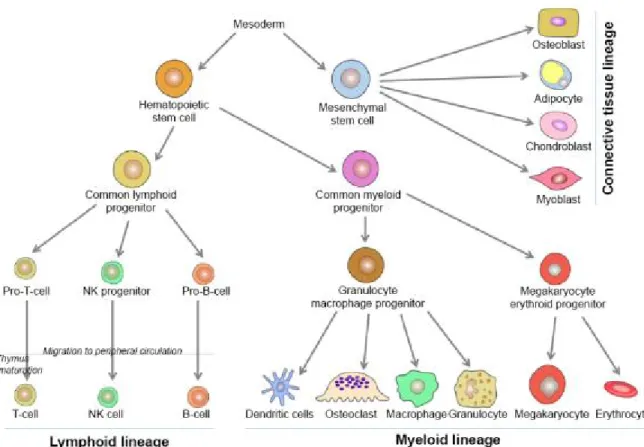
Embryologically, stem cell lineages originate in the yolk sac during the third week of development. Subsequently, these cells differentiate into more specialized cells. For example, bone marrow pluripotent stem cells are divided into two types of multipotent progenitor stem cells: common lymphoid cells and common myeloid cells. Common lymphoid progenitor cells are divided into the following three cell types: pro-T-cells, which differentiate into T-cells; natural killer progenitors, which differentiate into natural killer cells; and pro-B cells, which differentiate into B-cells. However, common myeloid progenitor cells differentiate into granulocyte/macrophage progenitor and megakaryocyte erythroid progenitor cells. Moreover, granulocyte/macrophage progenitor cells are divided into monocytes and granulocytes. In turn, monocytes, which migrate to and reside in bone
Figure 2. Hematopoiesis lineages: origin of different types of blood and bone tissue cells (1) The most important bone cells consist of three different cell types called osteoclasts, osteoblasts, and osteocytes.
1.1.2.a Osteoclasts
Osteoclasts have a hematopoietic origin. Mature osteoclasts are large multinucleated cells, and they are capable of removing organic and mineral components of bone. Furthermore, osteoclasts are involved in the bone turnover cycle, mainly in the bone resorption activity (Fig. 4) (1).
There are many functional and structural features which characterize mature osteoclasts. The most important osteoclast characters are multinucleation and ruffled border. Multinucleation results from
aggregation and fusion of mononuclear precursor cells, while ruffled border consists of fusion between the targeted transport vesicles and the apical membrane of the osteoclast. Ruffled border plays an important role in acidification and resorption of bone surface. In brief, acidification process occurs by formation of carbonic anhydrase, followed by secretion of protons (H+) (5); see (Fig. 3)
1. Mature osteoclasts contain three or more nuclei, and they can contain up to 20 nuclei. 2. TRAP enzyme (TRAPase) expression up-regulation is performed by mature osteoclasts. 3. Mature osteoclast has bone resorption abilities.
Figure 4. Osteoclast life cycle: aggregation and fusion of mononuclear precursor cells to form mature and multinucleated osteoclasts (5)
Immunologically, proliferation of osteoclast precursors occurs as a response to macrophage colony-stimulating factor (M-CSF), interleukin-3 (IL-3), and granulocyte-macrophage colony-colony-stimulating factor (GM-CSF). As soon as proliferation occurs, osteoclast precursors are differentiated to become committed mononucleated preosteoclasts. These committed mononucleated preosteoclasts aggregate and fuse together forming multinucleated osteoclasts in response to RANKL (Receptor activator of nuclear factor kappa-B ligand) (5), see (Fig. 4)
1.1.2.b Osteoblasts:
Osteoblasts originally derive from mesenchymal stems cells. They play essential roles in hematopoietic cell growth and differentiation, osteoclasts differentiation, bone formation, and mineralization of the secreted bone matrix (Fig. 2, 4) (1, 7).
Furthermore, osteoblasts contribute in the osteoclastogenesis via two important cytokines of tumor necrosis factor (TNF) members’ super-family proteins: RANKL and OPG. RANKL is a protein produced by TNFSF11 gene, while Osteoprotegerin (OPG) is a decoy receptor of RANKL that inhibits osteoclasts maturation, differentiation, and activation (1, 2).
Interaction process between the two main bone lineages, i.e., osteoclasts and osteoblasts, includes many other proteins that have crucial functions in bone modeling and remodeling (8-10); as demonstrated in Table 1.
Table 1. Osteoclasts and osteoblasts coupling factors: interaction between the two main bone lineages, osteoclasts and osteoblasts includes many other proteins that have crucial function in bone modeling and remodeling.
Coupling Factors Proteins Function References
Matrix-derived factors
Transforming growth factor β (TGF-β)
bone morphogenetic protein 2 (BMP-2) platelet-derived growth factor (PDGF), and the insulin-like growth factors (IGFs) Induce recruitment, migration, and differentiation of osteoblast progenitors (8-10) Osteoclast-secreted factors Cardiotrophin-1, sphingosine-1-phosphate, Wnt 10b, BMP-6 (Bone morphogenetic protein 6), CTHRC1 (collagen triple helix repeat containing 1) and complement factor 3a (C3a)
Induce bone formation by stimulating recruitment and differentiation of osteoblast precursors (8-10) Osteoclast membrane-bound factors EphrinB2 Semaphorin D
Osteoclasts use these proteins to induce osteoblasts activation
(8-10)
1.1.2.c Osteocytes:
Osteocytes are undividable star-shaped long-lived bone cells, commonly found in mature bone (11). Both osteocytes and osteoblasts have the same mesenchymal stem cells origin. Some osteoblasts
may remain in the mineral matrix and are subsequently called osteocytes. There are approximately 42 billion osteocytes in the adult human skeleton (Fig. 5) (12).
Figure 5. Illustration of the bone remodeling process and the cell types (13)
Bone formation or ossification consists of intramembranous ossification and endochondral ossification. These two steps begin in the embryonic stage and continue in the postnatal period. Intramembranous ossification is the step in which important skeleton bone structures are formed such as skull, scapula, and clavicle. In intramembranous ossification, osteoblasts begin to appear as a consequence of mesenchymal cells condensation and solidification. In turn, initial osteoblasts
Figure 6. Endochondral ossification steps: the cartilage template developed over time to become bone (14)
1.1.3 Bone modeling and remodeling at a glance
Bone is considered as a dynamic connective tissue that constantly changes. As such, bone modeling is a key mechanism that contributes to the ossification process in which bone shape is changed and bone mass is increased. This process is an active process during childhood and adolescence, yet becomes less frequent in adults except in some pathological cases such as hypoparathyroidism, renal osteodystrophy, or administration of anabolic agents (5). Yet, bone remodeling contributes to the renewal of bone cells and continues during life in contrast to bone modeling, which is not
considered a lifelong process. Bone remodeling is an essential mechanism for the following main reasons (5, 15):
1. It prevents accumulation of old bone; 2. It keeps integrity of the skeleton; and 3. It keeps plasma calcium homeostasis.
A normal complete duration of bone remodeling cycle is about 4-6 months, while resorption by osteoclasts occurs in about 3-6 weeks. The remodeling cycle duration may be changed under pathological factors. To illustrate, the bone remodeling cycle can be summarized in the following five phases (Fig. 7), (5, 15):
- Activation: positioning of osteoclasts on bone surface and start of differentiation, maturation, and activation.
- Resorption: mature osteoclasts come in direct contact with the mineralized matrix after peeling the bone lining cells. In consequence, mature osteoclasts begin to resorb the mineralized matrix, leading to liberation of the collagen fragments.
- Reversal: stopping osteoclasts resorption process and beginning of bone formation.
- Formation: osteoblasts form the bone by secretion of a non-mineralized substance called osteoid (organic matrix).
Figure 7. Bone remodeling cycle: the five phases of the bone remodeling cycle(15) 1.1.4 Regulation of bone remodeling
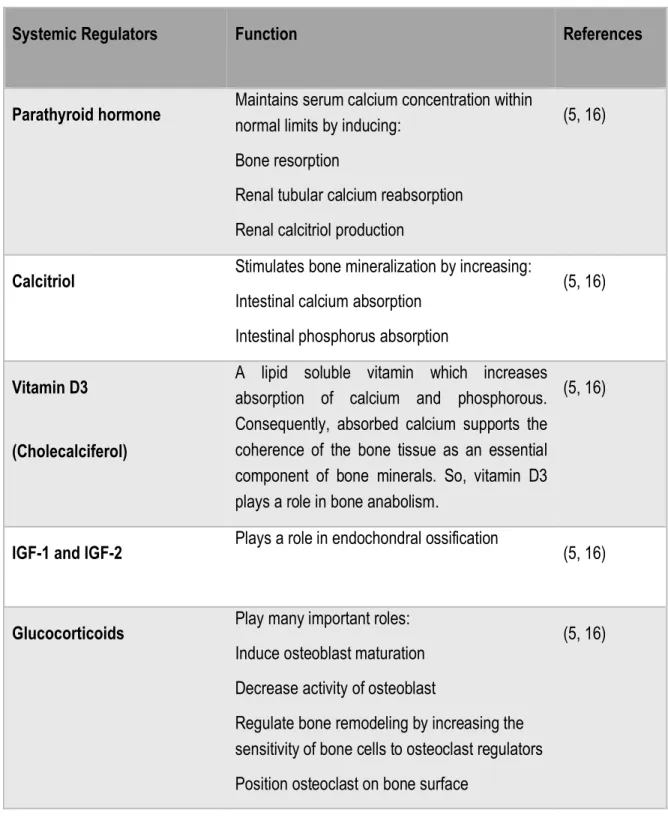
The process of bone remodeling is a highly cooperative osteoimmunological mechanism. In addition, several hormones, cytokines, and proteins are systematically and locally involved in bone remodeling. Table 2 and Table 3 demonstrate the most essential regulators of the bone remodeling process (16):
Table 2. Essential systemic regulators of bone remodeling.
SystemicRegulators Function References
Parathyroid hormone Maintains serum calcium concentration within normal limits by inducing:
Bone resorption
Renal tubular calcium reabsorption Renal calcitriol production
(5, 16)
Calcitriol Stimulates bone mineralization by increasing: Intestinal calcium absorption
Intestinal phosphorus absorption
(5, 16)
Vitamin D3
(Cholecalciferol)
A lipid soluble vitamin which increases absorption of calcium and phosphorous. Consequently, absorbed calcium supports the coherence of the bone tissue as an essential component of bone minerals. So, vitamin D3 plays a role in bone anabolism.
(5, 16)
IGF-1 and IGF-2 Plays a role in endochondral ossification (5, 16)
Glucocorticoids Play many important roles: Induce osteoblast maturation Decrease activity of osteoblast
Systemic Regulators Function References
Thyroid hormones Hyperthyroidism increases bone turnover (5, 16)
Estrogens Play many important roles in reducing bone resorption and increasing bone formation by the following effects:
Prevent osteoclasts formation Induce osteoblasts proliferation Decrease osteoblasts apoptosis
Locally increase the production of IGF-I, IGF-2, TGF-β, and OPG
(5, 16)
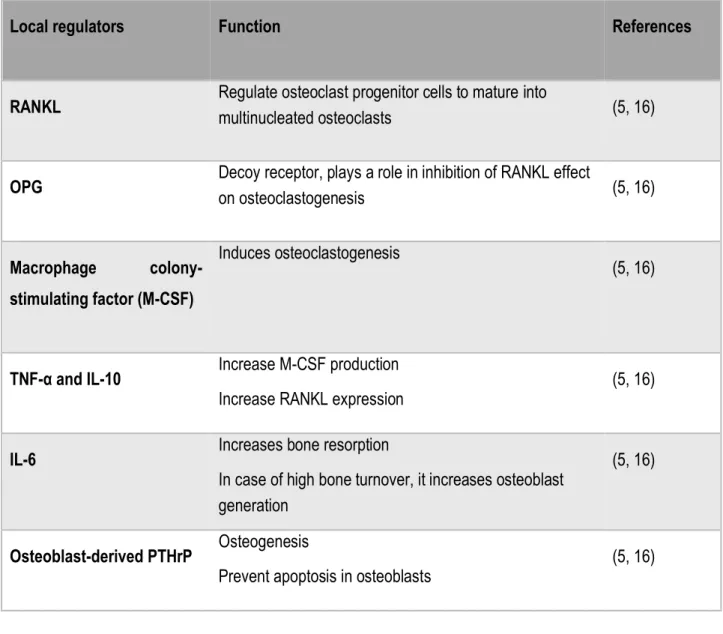
Table 3. Essential local regulators of bone remodeling
Local regulators Function References
RANKL Regulate osteoclast progenitor cells to mature into multinucleated osteoclasts (5, 16)
OPG Decoy receptor, plays a role in inhibition of RANKL effect on osteoclastogenesis (5, 16)
Macrophage
colony-stimulating factor (M-CSF)
Induces osteoclastogenesis
(5, 16)
TNF-α and IL-10 Increase M-CSF production
Increase RANKL expression (5, 16)
IL-6 Increases bone resorption
In case of high bone turnover, it increases osteoblast generation
(5, 16)
Osteoblast-derived PTHrP Osteogenesis
1.2 Paget’s disease of bone (PDB)
1.2.1 IntroductionPaget’s disease of bone (PDB), previously called osteitis deformans, is regarded as the second most frequent metabolic bone disorder after osteoporosis. An increased bone resorption by osteoclasts accompanied by aberrant osteoblastic bone formation is the main pathologic characteristic of PDB. Consequently, increased bone remodeling leads to deformation and weakened bone structures.
1.2.2 Epidemiology of PDB
PDB is a model of focal bone metabolic disorders that mostly affect aging people. Studies have confirmed that the prevalence of PDB proportionally increases with age (17, 18). In addition to age, gender is also considered an important factor that contributes to prevalence of PDB, as men are more affected (17). Concerning race, slight or no difference has been reported in prevalence of PDB in individuals with different skin colors (17, 19). Difference in prevalence has been reported between Arabs (0%) and Jews (1%) (20); however, prevalence in Asians is rare in comparison to European descents (21). Furthermore, only one-third of patients with PDB have a familial form (autosomal dominant pattern of inheritance with incomplete penetrance) (7, 22-24). In recent years, the severity and prevalence of PDB has decreased (25-33). In addition, Italy (rural areas of Campania and Tuscany) (34) and Spain (rural areas of Cabrera fields and Vitigudino) reported an association of PDB with rural residence versus urban inhabitants; however, in the past, the most affected urban area was Lancashire in the United Kingdom (UK) (17, 35-38).
Furthermore, Caucasians older than 55 years old have a prevalence of more than 3% (1). The UK has the highest prevalence, which is 4.6% among hospital patients older than age 55 (21), and Japan has the lowest one, which is 0.00028% (17). Moreover, there is a high prevalence in France (2.7% in Bordeaux, 2.4% in Rennes, and 2% in Nancy), Dublin in Ireland (1.7%), Valencia in Spain (1.3%), Essen in Germany (1.3%), Palermo in Italy (1.0%), Porto in Portugal (0.9%), Galloway in Ireland (0.7%), Athens in Greece (0.5%), and Malmo in Sweden (0.4%) (17, 21, 33). Recently, a decline in severity and prevalence of PDB in former high prevalence countries has been observed (see Fig. 8, 9) (25-33).
Figure 8. Prevalence of PDB in the most recent survey (2005–06) and an earlier survey (1996-98) in Auckland according to age for both genders (26)
Figure 9. Comparison in prevalence of radiographic PDB in Dunedin city/New Zealand between 2001 and 1983 surveys according to age and gender (32)
1.2.3 Etiology of PDB
1.2.3.a Role of genetic factors in the etiology of PDB
Genetic factors are strongly involved in the etiology of PDB such as mutations in SQSTM1 gene. 30 mutations in SQSTM1 gene were reported in familial forms and unrelated affected patients with PDB. Furthermore, two genome-wide association studies (GWAS) have found common single nucleotide polymorphisms (SNPs) associated with PDB in CSF1, OPTN, TNFRSF11A, PML, RIN3, and NUP205 genes (1, 39, 40). Common and rare coding variants of RIN3 gene were found to associated with PDB, the encoded protein being involved in the regulation of osteoclasts functions (41). In addition, other studies established that OPTN protein can negatively influence osteoclasts differentiation (1, 42).
Mutations in SQSTM1 and other variants in seven genetic loci may influence the severity and susceptibility to PDB. These loci contain some genes that have many important functions in osteoclastic pathways such as CSF1, DC-STAMP, TNFRSF11A, and OPTN genes. These loci have been proposed as a predicting tool for bone extent and severity in PDB (42), but it has not yet been validated.
However, the genetic factor does not provide answers to other questions such as why a higher prevalence of PDB exists in rural areas than in urban areas, (17, 35-38) why a remarkable decline in the prevalence and severity of PDB has occurred during recent years (25-33), and why there is no difference in prevalence of PDB between black African descents and white European descents persons in Atlanta (17, 19, 43, 44).
1.2.3.b Environmental factors
• Hypothesis of a viral infection
For many years, the involvement of environmental factors as an important cause of PDB has been suspected. To illustrate this fact, many studies discussed the relation between the viral infections and the abnormal excess of activity of osteoclasts(45). Although controversial in the literature, Measles virus, respiratory syncytial virus, and canine distemper virus are assumed to play a causal role in PDB. Furthermore, several studies discussed the involvement of viral infection in inducing PDB; inclusion bodies contained in osteoclasts are similar to Paramyxoviral nucleocapsids (1, 46). It has also been established that Measles virus nucleocapsid protein (MVNP) increases the production of IL-6; consequently, this increases the production of TAFII-17 and increases the sensitivity of osteoclasts to 1,25-(OH)2 vitamin D3. Moreover, MVNP expression in osteoclasts was reported to increase its activity excessively (1, 39).
• Hypothesis of contact with animals and rural residence
Studies in Italy investigated the role of the animal contact in rural areas in PDB pathogenesis. According to Gennari et al. (47), Campania (Italy) has the highest prevalence of animal contact in PDB patients whether they have a mutation in SQSTM1 gene or not. Moreover, 90% of the families, which have no mutations and from which the familial cases of PDB are considered, were in direct animal contact. Inversely, proportional relationship between age and prevalence of animal exposure is observed in PDB patients; however, there is a direct proportional relationship between age and SQSTM1 mutation in patients of most Italian regions (47-49).
1.2.4 Symptoms, diagnosis, and management of PDB
At least 70% of pagetic patients are asymptomatic and are usually diagnosed fortuitously by radiographs or by biochemical markers such as total serum alkaline phosphatase (50). There are many pathological symptoms as well as skeletal, neurological, cardiovascular, nephrological, and neoplastic complications; these symptoms are described in detail below (50, 51):
- Skeletal disorders: increased head size (Fig. 11), headache, bone pain, limbs curvature. (Fig. 10)
- Neurological disorders: pain due to the continuous compression of deformed bones on nerves of spinal cord and/or brain, loss of hearing or vision. (Fig. 11)
- Cardiovascular disorders: complications may lead to heart failure. - Nephrological disorders: kidney stones.
Management of PDB relies on pain relief, which may arise from bone deformity, and prevention of any future complication. Pharmacological treatments by anti-resorptive agents, such as bisphosphonates (ex. alendronate, etidronate, pamidronate, risedronate, tiludronate, and zoledronic acid), suppress the excessive aberrant bone resorption. Hence, non-steroidal anti-inflammatory drugs and other analgesics may be needed to relief pain in some cases.
Bisphosphonates are divided in two categories. The less potent group such as clodronate are characterized by the absence of nitrogen in its chemical structure. However, the nitrogen containing group such as alendronate, ibandronate, pamidronate, risedronate, and zolendronate are more potent (54). Generally, bisphosphonates are not well absorbed from the gut and can easily chelate with calcium due to its high affinity with minerals. Moreover, they should be used with high caution in patients with chronic renal insufficiency (55).
Table 4 Bisphosphonates drugs used in Canada and their route of administration and doses
Bisphosphonates drugs Administration route Dose
(for treatment of PDB)
References
Zoledronic acid (Reclast, Aclasta®)
IV infusion Single 5mg (56)
Alendronate (Fosamax®) Orally 40 mg once daily
for six months
(57)
Risedronate (Actonel®) Orally Daily 30 mg for 2
months
Recently, denosumab, a heterotetrameric IgG2 monoclonal antibody created to suppress RANKL, has been developed for osteoporosis treatment. It consists of two heavy chains containing 447 amino acids for every chain and two light chains comprising 215 amino acids for every chain. Moreover, it has a high affinity and specificity for RANKL, as denosumab binds tightly with RANKL, preventing its normal pathway to its receptors RANK; this also prevents RANKL role in osteoclastogenesis. This mechanism of action can play a crucial role in decreasing pathogenic osteoclastogenesis. Clinical trials revealed a significant reduction in bone resorption marker C-telopeptide (CTX) after the administration of 60 mg of denosumab subcutaneously; approximately 70% reduction within 6 hours and 80% within 3 days (7, 58, 59).
In the future, denosumab may represent a paradigm shift in the treatment of PDB in comparison with the classical treatment (bisphosphonate) (59), yet no randomized controlled trial was performed for this indication. Moreover, denosumab is also mentioned in the treatment of metastasis from solid tumors. Recent studies confirmed that denosumab can reduce risk of fractures in patients with cancer better than placebo and other drugs such as zoledronic acid and pamidronate (7, 60-65).
1.2.5 Complications
Although PDB can be managed in most patients at an earlier stage of the disease, many possible complications can occur. Most important complications are deafness, blindness, fractures, spine or limb curvature, and cartilage joints damage, excessive bleeding during bone repair surgery, osteoarthritis, kidney stones, heart failure, or bone cancer (39, 50, 51).
1.3 Pathophysiology of Paget’s disease of bone
1.3.1 Cellular phenotype of osteoclast in PDBOsteoclasts are the main cells involved in the bone resorption process. They have an essential role in bone growth in infants and in maintaining bone integrity in adults. Normal osteoclasts participate in bone modeling and remodeling mechanisms through removing organic bone matrix and minerals. However, osteoclasts dysfunctions may lead to weaker and deformed bone. Decreased bone resorption is observed in case of osteopetrosis, while increased bone resorption characterizes postmenopausal osteoporosis and PDB.
Morphologically, pagetic osteoclasts are characterized by an excessive number of nuclei per cell, reaching up to 20 or more nuclei/cell, an increase in osteoclastogenesis, a greater size than non-pagetic osteoclasts, and their excessive bone resorption abilities.
1.3.2 Genetic factor as a main cause
As a common skeletal disorder, PDB has an autosomal dominant pattern of inheritance with incomplete penetrance (42). Moreover, approximately 40% of patients have a positive family history that confirms the involvement of genetic factor in its pathogenesis (31). In addition, several mutations and common genetic variants are associated with PDB (see Table 4). The most frequent mutation is a germline mutation (p.Pro392Leu) (68), which harbored on the ubiquitin domain of the p62 protein. This protein is encoded by the Sequestosome 1 (SQSTM1) gene (2) and the mutation is linked to classical late onset PDB. Recently, post-zygotic p.Pro392Leu mutations were also detected in a subset of pagetic patients non-carriers of any germline mutation in SQSTM1 gene (69). Other disease-causing germline mutations contribute to the pathogenesis of PDB-related disorders such as TNFRSF11B gene mutations that encode osteoprotegerin protein (OPG), which leads to juvenile PDB. Valosin Containing Protein (VCP) gene mutations encode p97-mutated protein and lead to inclusion body myopathy with PBD with frontotemporal dementia (IBMPFD syndrome). Further, a number of common genetic variants were found to be associated with PDB and occur in genes that encode essential proteins for osteoclastogenesis and autophagy machinery such as OPTN, RANK, M-CSF, Rab, Ras, NUP205, and SLC13A4 (42).
However, some SQSTM1 germline mutation carriers do not develop the disease, as there are patients without a family history. In these cases, the contributing or modifying factors are largely unknown. Some hypotheses suggest a likely pathogenic involvement of viral infection, such as Measles virus (70), or other Paramyxoviruses-like (71) respiratory syncytial virus (RSV) (72), animal contact in rural areas (34, 47), or insufficient exposure to calcium and vitamin D treatment during childhood (73).
Table 5. Disease-causing genes and main genes containing common genetic variants associated with PDB (74)
1.3.3 SQSTM1 gene
In 2001, first evidence of linkage to chromosome 5 was found in 11 of 24 French-Canadian families with PDB and 18 of 112 unrelated patients with PDB (UPDB). Patients were further found to have a p.Pro392Leu mutation (substitution of Proline into Leucine amino acid at codon 392) of SQSTM1 protein that encodes a ubiquitin binding protein called p62 which corresponding gene is located in chromosome 5 (49, 75). Although the p.Pro392Leu mutation of SQSTM1 gene occurs in 20-50% of familial PDB, several studies reported 27 other mutations in the same gene in families from the UK, Australia, New Zealand, the US, the Netherlands, Italy, France, and China (49). Moreover, 25-50% of familial PDB patients have mutations in SQSTM1 gene (76). SQSTM1 gene harbors 28 missense or truncating mutations that encode a set of dysfunctional proteins, which functional effect on ubiquitin-associated domain and on inducing nuclear factor- kB (NF-kB) were reported (49, 77).
1.3.3.a Structure of p62/SQSTM1 protein
P62/SQSTM1 is a stress-inducible protein that has three crucial cellular functions in autophagy, oxidative stress, and amino acid sensing (78). P62/SQSTM1 has many essential domains such as Phox1 and Bem1p (PB1) domain, a zinc finger (ZZ), two nuclear localization signals, a TNF receptor-associated factor 6 (TRAF6) binding domain, a nuclear export signal, an LC3-interacting region (LIR), a Keap1-interacting region (KIR), and a ubiquitin-associated (UBA) domain (see Fig. 12) (1, 78).
Figure 12 The multi-domain structure of P62/SQSTM1 protein (78) 1.3.3.b Main functions of p62/SQSTM1 protein
1- Inducing and suppressing autophagy
Several studies on PDB have assessed the role of p62/SQSTM1 in autophagy. Polyubiquinated non-functional proteins aggregation should be directed to the autophagosome by p62/SQSTM1 protein after binding to Atg8/LC3 that is present at the surface of the autophagosome. The non-functional proteins are subsequently degraded by lysosomal action. In PDB, change in p62/SQSTM1 may also alter the normal autophagy pathway, resulting in accumulation of aggregated ubiquitinated proteins. Consequently, these aggregations may induce osteoclastogenesis by activation of the NF-kB pathway (2).
For the regulation of autophagy, p62/SQSTM1 binds to the Raptor, RagC, and TRAF6 on lysosomal membrane that leads to mTORC1 activation. Activated mTORC1 phosphorylates S6K and ULK1/2, induces protein synthesis, and suppresses autophagy (see Fig 13a) (78).
2- Anti-oxidant activity by inducing Nrf2 activation
Deactivation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway occurs as a result of ubiquitination of Nrf2 protein through binding of Keap1 homodimer to Nrf2-DLGex and Nrf2-ETGE motifs on Nrf2 protein. Thus, Keap1 has an imperative role in suppressing Nrf2 protein. On the other hand, the role of p62/SQSTM1 protein in autophagy can be induced in response to stressors. To
1.3.3.c SQSTM1 gene in animal models
Similar to the human mutation, p.Pro392Leu, a mouse model with a p.Pro394Leu mutation, has been generated. Mice that carry the mutation exhibited increased bone loss due to excessive osteoclasts activity and hyper-responsivity to tumor necrosis factor ligand super-family member 11 (also known as RANKL) and to TNF. Unlike the pathogenic mechanism in human PDB, there is no increase in number of nuclei in mature osteoclasts, no increased osteoclastogenesis, no excessive responsivity to 1,25-dihydroxy vitamin D3, and there are no inclusion bodies in cytoplasm or in nucleus (49). 1.3.3.d Single nucleotide polymorphisms (SNPs) in other genes associated with PDB
Thanks to GWAS, many SNPs have been found to be associated with PDB in genes such as CSF1, TM7SF4, TNFRSF11A, CNOT4, NUP205, SLC13A4, OPTN, RIN3, PML, and GOLGA6A.
Table 6. SNPs found to be associated with PDB according to GWAS (75)
Locus Nearest Gene(s)
SNP Encoded
protein(s)
Function(s) of the encoded protein
1p13 CSF1 rs10494112 M-CSF Cytokine inducing osteoclast and macrophage differentiation 8q22 TM7SF4 rs2458413 DC-STAMP Formation of multinucleated
osteoclasts and macrophage polykaryons
18q21 TNFRSF11A rs3018362 RANK An essential receptor for osteoclast differentiation
7q33 CNOT4, NUP205, and SLC13A4
rs4294134 Nucleoporin Essential for nuclear pore complex
10p13 OPTN rs1561570 Optineurin NF- kB signaling and autophagy regulation
14q32 RIN3 rs10498635 Ras and Rab interactor 3 protein
Role in vesicular trafficking Affects osteoclasts function 15q24 PML rs5742915 PML protein - Cell growth, apoptosis, and
senescence regulation
- TGF beta- signaling regulation 15q24 GOLGA6A rs5742915 A member of
the golgin family of proteins
- Membrane fusion role
- Supportive structural role in the Golgi cisternae
1.3.3.d Environmental factors
Over the past 25 years, epidemiological studies have established a significant decline in the prevalence and severity of PDB in the UK, New Zealand, and a number of European countries, yet not in Italy (75). Moreover, there is a delayed onset of PDB in offspring inheriting SQSTM1 mutations (75, 76).
Table 7. Environmental risk factors that predispose to PDB
Environmental Risk Factors for PDB
Triggers and Mechanisms References
Viral infection hypothesis Paramyxoviruses, such as Measles Virus, Canine Distemper Virus, and Respiratory Syncytial Virus, are candidates that may be involved in the pathogenesis.
MVNP decreases Forkhead box O3 /Sirtuine1 (FoxO3/Sirt1) signaling and increases interleukine 6 (IL-6); in turn, this triggers excessive osteoclasts activity. In vivo experiments demonstrated formation of pagetic osteoclasts in transgenic mice models following transfection of MVNP gene. Moreover, Measles Virus Nucleocapsid Transcripts are detected in patients with PDB blood cells. However, until now, the mechanism of MVNP-induced formation of pagetic osteoclasts remains unclear.
(80-84)
Animal Contact hypothesis López-Abente et al. studies in Spain and Gennari et al. studies in Italy (85, 86) support the hypothesis that rural lifestyle with domestic animals’ contact, such as dogs and cattle, is associated with PDB pathogenesis. They, moreover, suggest that infectious agents in domestic animal may have a causative role.
(48, 87-89)
Air pollutants Tobacco and wood-heating smoke that contain heavy metals may cause autophagy impairment and induce disorders due to their high productivity of free radicals and, consequently, oxidative stress.
1.4 Air pollutants and musculoskeletal diseases
1.4.1 IntroductionAir pollution is one of the most important inducers of harmful formation of free radicals and cellular oxidative stress (2). Furthermore, it is associated with many systemic, cellular specific inflammatory reactions (91) and neurodegenerative and musculoskeletal diseases (2). It also increases morbidity and mortality of cardiovascular (92, 93) and respiratory diseases (92, 93). Bone mineral density (BMD) is reduced in chronic immune and inflammatory disorders such as rheumatoid arthritis and chronic obstructive pulmonary disease and their treatments (91). Studies reported a correlation between chronic exposure to tobacco smoke (94, 95), particulate matter (96), wood-heating smoke (90), and low BMD. Moreover, a difference in cytokines levels following chronic exposure to air pollution was reported such as an increase in IL-6 levels in children exposed to tobacco smoke versus non-exposed children (97).
The source of 44.1% of the fine particles, Particulate Matter (PM2.5), emissions in the province of Québec is residential wood-heating systems. To illustrate, PM2.5 is a suspended tiny particle in the volatile gas phase of tobacco smoke or residential wood heating. The harmful effect of these particles is due to their contents of heavy metals, polycyclic aromatic hydrocarbons (PAHs), sulfates, nitrates, carbon, and organic substances. As an “air-borne pollutant”, there is another harmful effect because of their ability to move over a wide geographical area during long periods and extended spaces which, consequently, can infect new non-polluted highly populated regions or food farms (2).
Cadmium as an example of heavy metals interferes with the metabolism of calcium and increases its excretion (98). Moreover, cadmium was found to be associated with stimulation of serum alkaline phosphatase activity (98). Moreover, cadmium contaminated rice was found to be associated with bone diseases such as Itai-Itai in Japan (99-103). Other studies have confirmed the role of cadmium as a trigger for some significant bone disorders such as osteomalacia and osteoporosis (104), severe bone damage (105), and fractures (105) and non-bone diseases such as renal tubular cell dysfunction (104). Mercury and Cadmium were associated with calcium homeostasis in some studies (106).
1.4.2 Outdoor and indoor air pollutants
Residential wood heating and tobacco smoking are important sources of indoor pollutants, while transportation and industry emissions are examples of outdoor air pollutants (2). Increased level of heavy metals or Polycyclic aromatic hydrocarbons (PAHs) can result from exposure to outdoor or indoor air pollutants (2). Due to their ability to accumulate in the human body, including the skeleton, heavy metals are deemed to be an environmental factor that predisposes to some musculoskeletal disorders (96).
1.4.3 Harmful effects of air pollution on musculoskeletal diseases
Bone is a vital reservoir for minerals which are actively exchanged with blood. Many harmful chemical substances, such as heavy metals, are highly and readily transferred from blood to bones and then very slowly released from bone to blood, and they contribute to many serious inflammatory reactions in skeletal system.
To illustrate, Table 8 presents examples of heavy metals known to be components of air pollutants in various musculoskeletal diseases.
Table 8. Heavy metals and their potential involvement in musculoskeletal diseases
Heavy metal name
Source of the indicated heavy metal
Involvement in musculoskeletal diseases
References
Antimony Transport air pollutions, cigarette smoke,
Bismuth (Bi) Medications Changes in children’s skeleton growth after Bismuth in anti-syphilis treatment
(110, 111)
Cadmium(Cd) Transport air pollutions, cigarette smoke, wood-heating smokes
Yes (ex. Itai-Itai disease in the Cd-polluted Jinzu River basin in
Toyama, cigarette smoke and wood-heating smoke which contain many heavy metals; particularly cadmium can induce rheumatoid arthritis)
(99-103)
Cesium Transport air pollutions, cigarette smoke, wood-heating smokes
Severe bone marrow depression (112)
Chromium Industry pollutants, transport air pollutions, cigarette smoke, wood-heating smokes
Incomplete ossification of skull bone in fetus
(113)
Mercury (Hg) Mining industry pollutants, waste incineration, rocks, soils, air, water, polluted rivers and lakes, polluted fish, polluted plants
- (114, 115)
Nickel Industry pollutants, transport air pollutions, cigarette smoke, wood-heating smokes
No histological alterations were observed in bone of rats and mice exposed to nickel sulfate
(116)
Lead (Pb) Industry pollutants, transport air pollutions, cigarette smoke,
wood-two studies have suggested a role for lead in PDB
heating smokes Selenium Industry pollutants,
transport air pollutions, food supplement
Necessary for bone health (120)
1.4.4. Physiological reactions against heavy metals
Metallothionein (MT) is an important group of enzymes that has antioxidant effects (121) and acts as a negative regulator on apoptosis (122). Moreover, they play an important role in carcinogenesis and in drug resistance (122). Mammalian MT-1 and MT-2 isoforms have a protective effect against heavy metals toxicity such as cadmium, mercury, silver, platinum and zinc, and consequently against heavy metals induced oxidative stress (122). Another important family of enzymes group encoded by Glutathione S-transferase mu genes (GSTMs) consists of GSTM1, GSTM2, GSTM3, GSTM4, and GSTM5 genes and located on chromosome 1 (123). GTSTM1 protein is encoded in muscles, however, GSTM3 protein was found in brain (123). GSTM family enzymes have a general regulator and protective role against heavy metals such as lead, cadmium, copper, and zinc (124, 125).
Table 9. Investigated genes and their role in detoxification and autophagy
Investigated Genes Expressed protein Function References
MT3 MT3 1- Binds to heavy metals
and drugs.
2- Antioxidant activity. 3- Growth Inhibitory Factor.
(126, 127)
SQSTM1 SQSTM1 Crucial role in autophagy (2, 128-130)
MAP1LC3B (LC3) LC3 Autophagosome marker
that plays a role with p62/ SQSTM1 protein in autophagy.
(131)
GSTM3 and GSTM4 GSTM3 and GSTM4 Essential role in
detoxification and neutralization of free radicals in oxidative stress.
Chapter 2: Hypothesis and objectives
Hypothesis
Several publications raised many questions over the main causal factors, other than genetics, that may trigger the pathogenesis of PDB. The most important dilemma that has not yet been resolved relates to the environmental factors, other than viral or animal contacts, that may be involved in PDB pathogenesis. A significant decline in the prevalence and severity of symptoms of PDB over time has been observed (25-33). Furthermore, there are middle-aged or older p.Pro392Leu mutation carriers who did not develop a clinical phenotype of PDB (135-138). Considering all the aspects cited above, we have formulated the following hypothesis:
Environmental factors may modulate the expression of p.Pro392Leu mutation in PDB patients
In order to address this hypothesis, the present project has two specific objectives:
1- To evaluate the exposure to indoor and outdoor air pollutants in our French-Canadian cohort. 2- To evaluate the effect of environmental factors on the pagetic cellular phenotype, and on genes and proteins expression.
Chapter 3: Materials and methods
3.1 Recruitment of participants and survey
This study was approved by the CHU de Québec-Université Laval Ethics Committee and all participants signed a consent form before inclusion in the study. We studied 140 patients with PDB (familial and non-familial forms) and 113 healthy controls from the French-Canadian cohort, who previously answered a general survey on environmental factors and who agreed to participate to this new study (90). An affected participant was diagnosed with PDB if at least two of the following criteria were satisfied: 1) an increase in total serum alkaline phosphatase level and/or 2) a typical aspect of PDB on bone radiographs and/or 3) an abnormal whole-body bone scan. Familial forms are defined by the presence of at least one relative affected with PDB. Controls were unrelated healthy adults without personal or familial history of PDB and with normal total serum alkaline phosphatase levels at inclusion. None of the controls carried any mutation in SQSTM1 gene. Controls were not matched for age and sex with PDB patients. All participants lived in the same geographic area within a 120 km radius of Quebec City. In the new survey, we focused on the history of residence and proximity to sources of indoor and outdoor air pollutants during childhood and adulthood. Outdoor pollution sources were represented by residence close to a highway, an airport, a train, a bus, or a gas station, while indoor pollution sources were represented by frequent exposure to heating combustibles (carbon, wood, and oil) and cigarette smoke exposure at home.
3.2 Urinary dosages
We measured by mass spectrometry concentrations of 17 heavy metals (antimony, arsenic, beryllium, bismuth, cadmium, cesium, chromium, cobalt, copper, tin, manganese, mercury, molybdenum, nickel, lead, selenium, and zinc) and 11 PAHs such as hydroxyphenanthrene, 1-hydroxypyrene, 1-naphthol, 2-hydroxyfluorene, 2-hydroxyphenanthrene, 2-naphthol, 3-hydroxyfluorene, 3-hydroxyphenanthrene, 4-hydroxyphenanthrene, hydroxyfluorene, 9-hydroxyphenanthrene) in urinary samples of a subgroup of patients (n=15 to 46) and controls (n=12 to 48).
3.3 Toxics selection
After collecting literature data and results of urinary dosages, we selected toxics that can accumulate in the human body. Subsequently, we performed in vitro experiments using monocytes from peripheral blood differentiated in vitro into mature osteoclast to examine the impact of these toxics on osteoclast phenotype in patients with PDB and healthy controls. Four heavy metals (lead, cadmium, bismuth, and mercury) and cigarette smoke condensate were selected for in vitro experiments (Supplementary tables 8 and 9). All heavy metals powders which are lead nitrate, cadmium chloride, mercury chloride, and bismuth trinitrate pentahydrate were purchased from Sigma Aldrich co. The Cigarette Smoke Condensate (CSC) was purchased from Murty Pharmaceuticals; Lexington, KY, which is prepared using Federal Trade Commission’s (FTC) smoke machine and University of Kentucky's 3R4F Standard Research Cigarettes (139). In order to determine the optimal dose of toxic for our in vitro experiments, we prepared a gradient of diluted heavy metals from 20 µM to 500 µM and CSC solutions from 5 µg/ml to 100 µg/ml applied on lymphoblast immortalized by Epstein-Barr virus cultures. Next, we selected the highest dose of toxic that has no effect on lymphoblast’s viability and proliferation.
Table 10. Human body accumulation of heavy metals, and effects on human bone diseases Heavy metal name Human body accumulation Yes/No? (reference) If Yes, accumulates in which organ/cell line (reference)? Associated with known bone disease (reference) Association with GSTM3? (reference) Association with GSTM4? (reference) Association with SQSTM1/p 62? (reference) Protein transporters or other markers (reference) Presence in wood heating component s (reference)
Antimony Yes (140) accumulation in the liver and
gastrointestinal tract after oral exposure (107, 141, 142)
No (107) Yes (143) - - - No (144)
Arsenic Total Yes (145-149)
brain, bladder, heart, lung, liver, kidney, pancreas, spleen, muscles, skin, hair, also in fluids such as bile, blood, and stomach juices (145-149) PDB, Bone marrow toxicity (108, 109) - - Yes (150, 151) - No (144) Bismuth Yes (152, 153) potentially impairs neonatal bone growth? (154), Effects in
Gastrointestinal tract (152, 153)
Cadmium Yes (99, 155) - kidneys and liver together represent 50% of the body’s accumulation - Chronic cadmium exposure affects secondarily the bones. (99) Yes, such as Itai-itai disease: (99, 101) Yes (156, 157)
Yes (158) Yes (151) weakly genotoxic (157) No (144) Cesium age dependent (159)
does not accumulate in any particular part of the body (112) Cesium retention is -severe bone marrow depression (112) - - - - No (144)
tract (160) Copper No (162) Not accumulated
except in case of (Wilson’s disease) due to a genetic
defect(162), it accumulates in liver (162)
- Yes (163) - Yes (164) No studies
were found regarding genotoxicity in humans after inhalation, oral, or dermal exposure to copper or its compounds .(162) Yes (144)
Tin Yes (165) Lung, bone, brain, kidney, heart, liver, spleen, gastrointestinal tract, muscle (165)
- - - No (144)
Manganese Yes (166) Brain (166) - -
with
GSTM1(167)
Mercury Yes (155, 167)
kidneys, brain. (168) - Yes (169) and with GSTM1(170) - - - No (144) Nickel No (116) No (116) No histological alterations were observed in bone of rats and mice exposed to nickel sulfate.(116)
Yes (157) Yes (157) Yes (157) - nickel, cadmium, and chromium are weakly genotoxic (157) Yes(144)
Lead Yes (171) Bone (171) PDB
(117-119)
- - - - Yes(144)
Table 11. Selected toxic and choice of the dose for in vitro cell cultures
Toxic Cell type Dose that simulates the chronic
exposure to toxic or tested dose that keeps cell’s viability
(according to literature)
References Selected doses
after in vitro cell viability test (during 21 days)
Cadmium Human B lymphoblast
and human osteoblasts from the knees
100 nM (0.1 µM) (175-177) 500 nM
Mercury Human monocytes 100 nM (0.1µM) (178, 179) 300 nM
Lead Mice bone marrow and human
lymphocytes
200 nM (0.4 µM) (180-182) 300 nM
Bismuth Macrophages, vascular endothelial cells derived from bovine aorta, rat alveolar macrophage cell line
100 µM (152, 153, 183-192) 100 µM
exposure Molybdenum
Dose-dependent (174)
a little in bone with increased doses (174)
Cigarette Smoke Condensate (CSC)
Immortalized human bronchial epithelial cells (BEAS-2B)
3.4 Cell cultures
We collected 50 ml of peripheral blood from participants in order to harvest monocytes from healthy controls, non-familial PDB patients, and p.Pro392Leu mutation healthy carriers. Mononuclear cells from human peripheral blood (PBMCs) were isolated by density gradient centrifugation using the Ficoll-Paque method. Furthermore, cells were counted using the Bio-Rad TC20 automatic cell counter. Next, the cell number concentration was adjusted to 3x106 cells/mL. Cell cultures were
exposed to one of the heavy metals or CSC and differentiated in vitro by the use of RANKL (Peprotech, Rocky Hill) and hMCSF (eBioscience, San Diego) during 21 days. For RNA extraction and protein lysate, the cells were seeded in a 6-well plate: 3 mL of suspended cells/well (9x106 cells).
For immunofluorescence assays (Lab-Tek, Fisher; purchased from Scientific, Ottawa), the cells were seeded in 4 wells. Sterilized, demineralized, and distilled water was added to 2 wells and toxic was added to 2 other wells of 8 well-slide Lab-Tek: 400 µl of suspended cells/well (1,2x106 cells). Additionally, in order to examine the bone resorption abilities of mature osteoclasts, cells were seeded in 4 wells of Osteoassay. Sterilized, demineralized, and distilled water was added to 2 wells; one contained 40 ng/ml RANKLcalled ctrl 40, toxics (concentrations in Table 9 were added to 2 other wells; one contained 40 ng/ml RANKL. All wells were using a 36-well osteoassay plate (purchased from Fisher Scientific, Ottawa): 300 µl of suspended cells/well (1 x106 cells). Cells were left overnight
at 37ºC with 5% CO2 in order to facilitate monocytes to attach to the plastic surface of the culture plate, while lymphocytes usually remain in suspension and can be removed when changing the medium. Medium were changed every 3-4 days. Alpha-MEM medium, which contains 10% FBS + 1% Penicillin-Streptomycin was purchased from Wisent, St-Bruno. . Fluorescence-based staining for TRAP relying on ELF97 phosphatase substrate (Thermo Fisher Scientific) was performed as well as DAPI (4',6-diamidino-2-phenylindole) Fluorescent stain (Thermo Fisher Scientific) for nuclei and phalloidin (Thermo Fisher Scientific) for cell membrane.
3.5 Quantitative real-time PCR
Cell lysates homogenization is firstly performed using Qiazol buffer (Qiagen, Germantown), followed by a total RNA extraction using the RNeasy micro kit on-column DNase treatment (Qiagen, Hilden). The extracted amount was quantified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington), and subsequently qualified using an Agilent BioAnalyzer 2100 (Agilent
Technologies, Santa Clara). PCR reaction was performed using 0.5-5 ug of isolated RNA, 200 U of Superscript III RNase H-RT (Invitrogen Life Technologies, Burlington), 50 mM Tris-HCl pH 8.3, 300 ng of oligo-dT18, 50 ng of random hexamers, 75 mM KCl, 5 mM dithiothreitol, 3 mM MgCl2, 500 uM deoxynucleotides triphosphate, and 40 U of Protector RNase inhibitor (Roche Diagnostics, Indianapolis). To enhance the reaction productivity, incubation for 10 min was performed at 25°C, followed by longer incubation for 1 hour at 50°C. Additionally, cDNA was purified using a purification kit (Qiagen, Hilden). Designation of the Oligoprimer pairs was performed using GeneTool 2.0 software (Biotools Inc, Edmonton); next, the GenBank database performed the verification. Integrated DNA Technology (Coralville) was used in synthesis. Furthermore, LightCycler 480 SYBRGreen I Master Reagent (Roche Diagnostics, Indianapolis) with 2% DMSO and LightCycler 480 (Roche Diagnostics, Mannheim, DE) were used in the reaction. Following Luu-The et al. methods (195); the mRNA copies numbers were calculated. In addition, Glucose-6-phosphate dehydrogenase (G6PD), peptidylprolyl isomerase B (cyclophilin B) (PPIB), and 18S ribosomal RNA (18S) were used as a reference gene (196). Consequently, Quantitative Real-Time PCR reactions occurred in the following conditions: 45 cycles, denaturation at 98°C for 10 seconds, annealing at 60-62°C for 10 seconds, elongation at 72°C for 14 seconds and then 74°C for 5 seconds (reading). Experiments were performed by the CHU de Québec Research Center (CHUL) Gene Expression Platform, and were compliant with MIQE guidelines (197-199).
Table 12. Sequence primers and gene description
Gene Symbol
(GSTM3) GSTM4 Homo sapiens glutathione S-transferase mu 4 (GSTM4), 4 transcripts NM_0008 50 98 CCCCAGAGGAGGTCGCAGTTC/G CGGATGTCCCAGTACCCCAGT MAP1LC3 B Homo sapiens microtubule associated protein 1 light chain 3 beta (MAP1LC3B) NM_0228 18 211 CGGGCTGAGGAGATACAAGGGA/ TGGATGCTGCTCTCGAATAAGTC MAP1LC3 B2 Homo sapiens microtubule associated protein 1 light chain 3 beta 2 (MAP1LC3B2) NM_0010 85481 218 TCGGGAGTGCAGGGCCAGATC/T GGATGCTGCTCTCGAATAAGTC SQSTM1 Homo sapiens sequestosome 1 (SQSTM1), common region to 3 transcripts NM_0039 00 157 GGCGGAGCAGATGAGGAAGAT/T GGCATCTGTAGGGACTGGAG G6PD Homo sapiens glucose-6-phosphate dehydrogenas e (G6PD), nuclear gene encoding mitochondrial protein NM_0004 02 121 GATGTCCCCTGTCCCACCAACTC TG/GCAGGGCATTGAGGTTGGGA G
PPIB Homo sapiens peptidylprolyl isomerase B (cyclophilin B) (PPIB) NM_0009 42 179 GAAGAAGGGGCCCAAAGTCAC/C ACGATGGAATTTGCTGTTTTTGTA G 18S Homo sapiens 18S ribosomal RNA NR_0032 86 226 ACGGACCAGAGCGAAAGCATT/T CCGTCAATTCCTTTAAGTTTCAGC T
ADNg Homo sapiens
3-beta-hydroxysteroid dehydrogenas e/delta-5- delta-4-isomerase (3-beta-HSD) gene (intron) M38180 260 GAAGGGCAGAGGTGGAACTAGA A/AACAAAGACCAAAGACCAGTGA GA
3.6 Oxidative stress levels measurement
Quantitative measurement of 8-OHG (8-hydroxyguanosine), which indicates the oxidative damage on RNA, was measured using a competitive ELISA (OxiSelect™ Oxidative RNA Damage ELISA Kit, CELL BIOLABS, INC) on RNA extracted from osteoclast lysates. 8-OHG/BSA (Bovine serum albumin) conjugate preabsorbed EIA plate was added to 8-OHG samples and controls followed by