Corrélation entre l'expression de HIF tronc cérébral
et la réponse ventilatoire à l'hypoxie chez les rats et les
souris
Mémoire
Manju Shahare
Maîtrise en Neurobiologie
Maître ès sciences (M.Sc.)
Québec, Canada
© Manju Shahare, 2016
Corrélation entre l'expression de HIF tronc cérébral
et la réponse ventilatoire à l'hypoxie chez les rats et les
souris
Mémoire
Manju Shahare
Sous la direction de
Dr. Jorge Soliz
Département de Médecine,
Université Laval,
Québec, Canada.
iii
RÉSUMÉ
Compte tenu de la faible disponibilité de l’oxygène (hypoxie) en haute altitude, l’adaptation à ce milieu constitue un vrai défi pour les espèces adaptées au niveau de la mer. Aussi, le rat et la souris constituent un modèle pertinent pour la compréhension des facteurs qui contribuent à une bonne adaptation en haute altitude. En effet, les rats et les souris de laboratoire élevées à haute altitude durant plusieurs générations possèdent un phénotype différent: les souris présentent une plus importante ventilation, des valeurs d’hématocrite/ hémoglobine diminuées et une hypertension pulmonaire réduite. Ces différences indiquent une mauvaise adaptation des rats qui montrent également une importante mortalité en haute altitude. Néanmoins, les mécanismes impliqués dans cette différence entre ces deux espèces ne sont pas connus.
Nous avons donc recherché dans un premier temps si les différences observées entre rats et souris sont également présentent au niveau de la mer, puis dans un second temps si cela avait un lien avec l'expression du senseur moléculaire d'oxygène HIF (Hypoxia Inducible Factor).
Nous avons mené une étude au niveau de la mer (Québec, Canada - 98m) pour comparer les réponses ventilatoire et moléculaire entre les rats et les souris. Pour se faire, les animaux sont exposés pendant 6 heures à différents gradients d’oxygène :
21%, 15%, et 12% O2. La ventilation est mesurée par pléthysmographie à corps entier. La consommation d’oxygène (VO2) et la production de CO2 (VCO2) sont évaluées durant la même période d’exposition. Après 6 heures d’exposition, les animaux sont anesthésiés et le tronc cérébral rapidement prélevé pour effectuer une mesure de l’expression de HIF-1α à l’aide de la technique ELISA (Enzyme LinkImmunosorbent Assay).
Comparé aux rats, les souris présentent une élévation du débit ventilatoire, une diminution de la VO2 et de la VCO2 et une augmentation de l’équivalant ventilatoire à l’O2 (Ve/VO2) et au CO2 (Ve/VCO2) durant l’exposition à 15 et 12% d’O2. De plus, l’expression de HIF-1α au niveau du tronc cérébral est plus élevée chez les souris en comparaison à celui des rats.
En conclusion, la différence de la réponse ventilatoire à l’hypoxie peut être liée à la différence d’expression de HIF-1α au niveau du tronc cérébral. Ces resultantssuggèrent que les souris possèdent une prédisposition génétique permettant une réponse adaptée en milieu hypoxique et pouvant aisément expliquer la facilité que possède cette espèce à survivre et à établir sa colonie en haute altitude.
iv
ABSTRACT
Successful adaptation at high altitude is very challenging for sea level natives due to the low level of available oxygen (hypoxia). Rats and mice offer an interesting model to understand the factors that contribute to efficient adaptation to high altitude. Indeed, laboratory rats and mice that have been raised at high altitude for several generations have a different phenotype with mice showing higher ventilation, lower hematocrit/hemoglobin values, and lower pulmonary hypertension. These differences are clearly a failure of adaptation to high altitude in rats, as underlined by data showing high mortality in the colony of high altitude rats. However the underlying mechanisms behind these differences are poorly understood. We sought to address whether these differences are also apparent in mice and rats living at Sea level, and if they are related to different responses of the O2 molecular sensor HIF (Hypoxia Inducible Factor).
To test these hypotheses, we chose to perform the study at sea level i.e. at Quebec City, Canada (98m) to compare the ventilatory and molecular responses in male rats and mice. The animals were exposed to different oxygen gradients 21%O2, 15 % O2 and 12% O2 for 6 hours. Ventilation was measured by whole bodyplethysmography, oxygen consumption (VO2) and CO2 production rate (VCO2) were also measured during the exposure. After the 6 hour’s exposure, the animals were anesthetised, and the brainstem quickly dissected, Brainstem HIF-1α expression was measured by Enzyme Link Immunosorbent Assay (ELISA).
Compared to rats, mice had higher minute ventilation, lower VO2, VCO2, and higher ventilatory equivalent to oxygen and carbon dioxide, (Ve/VO2,Ve/VCO2) at 15% and 12% O2. In addition, mice also had higher brainstem HIF-1α expression compared to rats.
We conclude that the differences in ventilatory responses to hypoxia at sea level might be due to differences in expression of HIF-1α in the brainstem. This suggests that mice have a genetic pre-disposition that ensure adequate response to hypoxia. This trait helps to explain that mice are able to survive and successfully establish natural colonies at high altitude.
v TABLE OF CONTENTS CONTENTS Pg. No. Résumé iii Abstract iv Table of Contents v
List of tables viii
List of figures ix
Abbreviations x
Acknowledgement xii
Presentation and Participations xiii
I- INTRODUCTION 1
1. Hypoxia 2
1. 1 High altitude Physiology 3
1.2 The ventilatory response to hypoxia 6
1.2.1 The peripheral chemoreceptors: the carotid bodies 7
1.2.2 Time domains of the hypoxic ventilatory response 9
1.3 The metabolic response to hypoxia 11
1.4 Molecular response to hypoxia 12
1.4.1 Structure and composition of HIF 12
1.4.2 Regulation of oxygen homeostasis by HIF-1 α 13
1.4.3 HIF-1α target genes 15
1.4.3.a Epo is HIF-1 target genes involved in erythropoiesis and ventilatory response to hypoxia
18 1.4.3.b HIF-1 target genes involved in glucose metabolism 18
1.4.3.c HIF-1 target genes involved in Angiogenesis 19
vi
1.5 Induction of Brainstem HIF-1α expression in response to hypoxia 21
II- OBJECTIVE AND HYPOTHESIS OF THE STUDY 22
III- MATERIALS AND METHODS 24
3.1Animals 25
3.2 Experimental Groups 25
3.3 Measurement of Respiratory and metabolic parameters 25
3.3.1 Whole body Plethysmography 25
i) Experimental setup 25
ii) Plethysmoraphy recordings 26
3.3.2 Analysis of respiratory and metabolic parameters 28
3.4 Allometric scaling 29
3.5 Tissue Sampling 30
3.6 Measurement of molecular parameters 30
3.6.1 Nuclear Protein extraction (HIF-1 α from brainstem) 30
3.6.2 Enzyme Link Immunosorbent Assay 31
3.7 Statistical Analysis 32
IV- RESULTS 33
4.1.Adult mice had had higher minute ventilation than rats during hypoxic exposure 34
4.1.1 Mass-specific values 35
4.1.2 Mass-corrected values 36
4.2. Adult mice had lower O2 consumption (V.O2), CO2 production rate (V.CO2), (V.CO2/V.O2) and higher ventilatory equivalent for oxygen (V.e/ V.O2) and for carbon dioxide exchange ( V.e/V.CO2) than rats
38
4.3 Adult mice had higher brainstem HIF-1 alpha expression than rats with decreasing O2 concentration/hypoxic exposure
40
V- DISCUSSION 41
5.1 Limitation of the experimental approach 42
5.2 Higher ventilation and reduced metabolic rate allows hyperventilation in mice exposed to hypoxia
43 5.3 Higher Brainstem HIF-1 alpha expression link to better ventilatory acclimatization in mice
45 5.4 Contribution of peripheral chemoreceptor to modulate pulmonary ventilation in mice
vii
VI- CONCLUSION AND FUTURE PROSPECTIVE 48
viii
LIST OF TABLES
TABLES Pg. No.
Table.1: Selected HIF-1 target genes 16
Table. 2: Mass specific values for respiratory parameter in rats and mice 34 Table. 3: Mass specific data for metabolic variables in rats and mice 35
ix
LIST OF FIGURES
FIGURES Pg.No.
Fig.1: The "cascade of oxygen" at sea level and high altitude 4 Fig.2: Comparative physiology in rats and mice at high altitude 6
Fig.3: Control of breathing 8
Fig.4: Time domains of hypoxic ventilatory response 9
Fig.5: Structure and composition of HIF and their isomers 13
Fig.6: Schematic illustration of HIF-1α regulation 15
Fig.7: Schematic representation of whole-body plethysmography 26 Fig.8: Ventilatory variables at different oxygen gradients in rats and mice 37 Fig. 9: Metabolic variables in adult rats and mice (2-3 months old) 39 Fig.10: Expression of brainstem HIF-1 alpha in adult rats and mice 40 Fig.11: Bidimensional plots for ˙Ve vs. ˙VO2 showing the effect of hypoxia in rats and mice
44
Fig.12: Bidimensional plots for ˙Ve vs. ˙VCO2 showing the effect of hypoxia in mice and rats
x
ABBREVIATIONS
O2= Dioxygen
CO2= Carbon dioxide
PO2= Partial pressure of oxygen
PaO2= Arterial partial pressure of oxygen
PaCO2= Arterial partial pressure of Carbon dioxide pH2O= Vapor pressure of water
V.e = Minute Ventilation Vt= Tidal volume Fr= Respiratory frequency V.O2= Volume of O2Consumed V.CO2= Volume of CO2 produced V.CO2/V.O2=Respiratoryexchangeratio V.e/V.O2= Ventilatory equivalent to O2 V.e/V.CO2= Ventilatory equivalent to CO2 HIF= Hypoxia Inducible Factor
EPO= Erythropoietin
VEGF= Vascular endothelial growth factor GLUT-1= Glucose transporter -1
CRLR= Calcitonin Receptor Like Receptor ANGPT2= Angiopoietin 2
NOS2a= Inducible nitric-oxyde synthase pfkfb3= 6 phosphofructo-2-kinase
PDK1= Pyruvate dehydrogenase kinase 1
PRKAA1= AMP-activated alpha 1 catalytic subunit. IGF-1= Insulin-like growth factor 1
xi ATP= Adenosine triphosphate
PHD= Prolyl hydroxylases bHLH= basic helix–loop–helix
ODDD= O2-dependent degradation domain NAD= N-terminal activation domain CAD= C-terminal activation domain HRE= Hypoxia-responsive element 2OG= 2-oxoglutarate
xii
ACKNOWLEDGEMENT
I would never have been able to finish my dissertation without the guidance of my Supervisor, committee members, friends, and my family.
I would like to first thank my family for all their love and support. I owe the fact that their faith and confidence allows me this far to pursue my studies. They were always encouraging me with their best wishes.
I would like to express my sincere gratitude to my advisor, Dr. Jorge Soliz, for his encouraging guidance, persistence, patience, and providing me with an excellent atmosphere for doing research. Without your motivation, enthusiasm, immense knowledge and financial support during my research I wouldn’t have been able to successfully take this step towards finishing my education.
In addition, I owe deepest gratitude to Dr. Vincent Joseph for providing excellent hands on training, advices and useful suggestions during study, without which I could not have succeeded. I would like to also thank Dr. Richard Kinkead and Dr. Aida Bairam for providing the beautiful educational environment during my stay in the lab.
Last but not the least my friends Sandeep, Praveena, Shahid, Louana, Alexandra, who help me professionally and emotionally by cheering me up and stood by me through the good times and bad.
xiii
PARTICIPATIONS AND PRESENTATIONS
This research thesis was performed as part of my ‘Masters in Neurobiology’. This offered me the pinnacle of experiences and training reward. In addition to learning in the wonderful world of research and to provide a solid theoretical and practical training, my masters also allowed me to participate in various presentations, includes:
HIF: Molecular determinants of adaptation at high altitude in rats and mice. ManjuShahare, Jorge Soliz and Vincent Joseph at DIXIEME COLLOQUE SCIENTIFICETUDIANT DE LA SOCIETE LEGALLOIS POUR L’ETUDE DE CONTROLERESPIRATOIRE (SLECR). La Jouvence, Orford, Quebec, Canada. 6-9th February 2014.
Correlation between brainstem HIF expression and ventilatory response to hypoxia in rats and mice.Manju Shahare, Jorge Soliz and Vincent Joseph at ONZIEME COLLOQUE SCIENTIFIC ETUDIANT DE LA SOCIETE LEGALLOIS POURL’ETUDE DE CONTROLE RESPIRATOIRE (SLECR). La Jouvence, Orford, Quebec, Canada. 7-9th February 2015.
1
2
1. Hypoxia
Oxygen (O2) is essential for the survival of almost all forms of life on earth. A balanced oxygen environment is required for normal cellular and metabolic functions since either an increase or a decrease in the oxygen levels can be detrimental to the cells by disrupting the oxygen homeostasis. (Semenza, 2012a; Semenza, 2012b). Hypoxia may be defined as a relative deficiency in oxygen availability/delivery for maintaining adequate physiological oxygen tensions, and thus resulting in an imbalance between demand and supply of oxygen (Hopfl et al., 2004; Lu and Kang 2010). A change in the oxygen environment will trigger a cascade of physiologic and biochemical events to compensate the reduced O2 pressure. If these events are not able to adequately compensate the reduced O2 pressure, pathological processes may develop and affect enzyme activities, mitochondrial function, cytoskeletal structure, membrane transport, and antioxidant defenses. In all cases, limited oxygen availability decreases oxidative phosphorylation resulting in a decreased synthesis of energy-rich phosphates eg. Adenosine triphosphate (ATP), and limitation of physical and mental activities (Maltepe and Saugstad, 2009).
In mammals, many factors can contribute to this oxygen imbalance, such as a decreased concentration of functional hemoglobin or a reduced number of erythrocytes that impaired the ability of blood to carry oxygen to the tissue (anemic hypoxia), an inability of cells to take up or utilize oxygen from the blood stream (histotoxic hypoxia), reduced tissue perfusion (ischemic hypoxia) and an insufficient oxygen availability to the lungs (hypoxemia/hypoxic hypoxia). (Hockel and Vaupel 2001). Hypoxic hypoxia occurs when the partial pressure of O2 (PO2) in arterial blood falls. This could be caused by several factors such as respiratory problems e.g., hypoventilation, diffusion impairment, caused by pulmonary edema, ventilation– perfusion mismatch or anatomic shunt of blood past the gas exchange region, blocked airways, drowning or reduction of the oxygen partial pressure in the environment, as occurring at high altitude.
3
In response to hypoxia, most animal species are able to alter their physiology to increase the uptake of oxygen from the lungs (by increasing their ventilation), and to optimize its cellular utilization through the activation of specialized cellular and molecular O2 sensors distributed throughout the body.
In the present study we compared the respiratory and molecular responses to acute exposure to hypoxic hypoxia in rats and mice. Interestingly, these two species have a different tolerance to high altitude (see below) and we sought to address whether these differences in responses are also apparent in mice and rats living at sea level, and if they are related to different responses of the O2 molecular sensor. Accordingly, we will first describe how the central and peripheral chemoreceptors in nervous system control ventilation under hypoxic condition, and how the Hypoxia-Inducible-Factor 1 (HIF-1 – the main molecular O2 sensor) is regulated by O2 level.
1. 1 High altitude Physiology
Altitude refers to the terrestrial elevation over 1500 m and is commonly divided into high altitude: 1500 to 3500 m, very high altitude: 3500 to 5500 m and extreme altitude: 5500 to 8850 m (Gallagher, 2004). At altitude, the fraction of O2 in the air remains the same, but as barometric pressure decreases, the partial pressure of O2 decreases with altitude. Consequently, there is less O2 available to breath at high altitude. Because the atmospheric PO2 is lower at high altitude, the gradient driving O2 transport from the atmosphere to the cells is considerably less than at sea level, and the fall of partial pressure of O2 at each consecutive step in the O2 cascade is less at high altitude than at sea level (Fig.1).
4
(Torrance et al., 1970) Fig.1: The "cascade of oxygen" at sea level and high altitude.
The y-axis represents the partial pressure of oxygen in mm Hg (left) or kilopascal (‘Kpa’ right) in different compartments of the respiratory system (ambient air, alveolar gas, arterial blood, mixed venous blood). The blue line indicates the drop in partial pressure of oxygen at sea level and at high altitude (4500m).
Exposure to hypoxia induces different responses such as increased pulmonary ventilation and hematocrit values, or induction of pulmonary hypertension among others. In species living at high altitude, a good adaptation is generally indicated by high hemoglobin–oxygen affinity, high pulmonary ventilation, low hematocrit and low pulmonary hypertension. In human, Tibetan have been living in the Tibetan plateau (~4000m) for at least 25,000 years and Andeans (3000-4000 m) for 12,000 years, also showing successful adaptation at high altitude. These two populations are adapted to life in a hypoxic environment to support their maintenance, growth, development and reproduction with different traits (Petousi et al., 2013). Tibetans have lived on the Qinghai– Tibetan plateau for many years and have developed unique phenotypes, such as protection from polycythemia which has been linked further to prolyl hydroxylases 2 (PHD2) mutation, resulting in the down regulation of the hypoxia inducible factor (HIF) pathway. (Tashi et al.,2014). Different species of animals have been living at high altitude under hypoxic condition for an extended period of time such as pika from the Tibetan plateau, Ilama in the Andes and Himalayas‟ yak. These animals are endemic to the high altitude regions, and are
5
thereforeconsidered adapted to high altitude. It is believed that high altitude exerts selective evolutionary pressure primarily due to its hypoxic environment, resulting in multiple adaptive responses over the course of generations. Among animals that are not endemic to high altitude, but have been brought by human migrations mice and rats are an interesting case. According to ecological reports of South America, mice can be found at 4000-5000m of altitude but rats are noticeably absent above 2000m-2500m (Sydney, 1997).
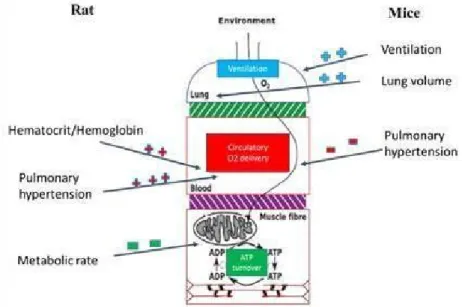
A recent comparative study at high altitude using rats and mice at the Bolivian institute for altitude biology (IBBA - La Paz, altitude 3600 m), which have been originally imported from France (IFFA-CREDO) in 1992 (23 years ago), and constantly bred at 3600 m from almost 30 generations, reported lower hemoglobin and hematocrit values, higher ventilation and lung volume with lower pulmonary hypertension in mice compared to rats (Lemoine et al., 2015). In rats, poor adaptation to altitude leads to high hematocrit and hemoglobin values, higher pulmonary hypertension, lower metabolic rate and high mortality (Fig.2) (Lumbroso et al., 2012; Lemoine etal., 2015).
Mice also had a higher ventilation and tidal volume compared to rats,which are likely linked to differences in the oxygen sensing abilities of the respiratory control system to maintain pulmonary ventilation under hypoxic condition. One of the intriguing questions that emerged from these studies was to know whether this “resistance” to hypoxia is a pre-determined trait characterizing rats and mice. Given that these 2 species are widely used worldwide to study hypoxic responses for bio-medical research, this question does not appear trivial. As a first approach, we sought to determine whether rats and mice have different responses to hypoxia at the physiological (ventilatory response to hypoxia), and molecular level (expression of HIF-1).
6
(Lemoine et al., 2015)
Fig. 2: Comparative physiology in rats and mice at high altitude.
This figure describes the physiological differences in rats and mice living at high altitude. Compared to mice, rats had high level of hematocrit/hemoglobin values, very high pulmonary hypertension and lower metabolic rate. Mice had higher ventilation and higher lung volume than rats.
1.2 The ventilatory response to hypoxia
The mammalian lungs have an impressing capacity to increase ventilation about 20 times higher than the resting ventilation, therefore being able to fulfill O2 needs under extreme conditions (Ganong, 1997). Hyperventilation is the first response to the reduction of environmental oxygen, and is the most important feature of acclimatization to high altitude (Joseph and Pequignot, 2009). The hypoxic ventilatory response (HVR) is important in respiratory physiology since it reflects the global output of a neurological system that integrates the hypoxic stimulation of peripheral sensors (carotid bodies), the central translation of peripheral inputs to the phrenic nerve and the metabolic response of the organism.
7
1.2.1 The peripheral chemoreceptors: the carotid bodies
The main peripheral chemoreceptors identified in mammals are the aortic and carotid bodies. However, only carotid bodies have a significant influence in small mammals such as mice and rats (Gonzalez et al., 1994). Carotid bodies are small bilateral organs situated near the carotid bifurcation that permanently measure oxygen arterial pressure. While the main stimulus of carotid bodies is the decline of PaO2, carotid bodies are also stimulated by other physical or chemical components in blood such as PaCO2, pH, temperature, arterial flow and pressure or osmolarity (Kumar and Prabhakaran, 2012; Gonzalez et al., 1994).
The carotid bodies are the most vascularized organs in the body (five times higher than brain (Gonzalez et al. 1994), and are highly innervated by efferent sensory fibers and afferent fibers from the autonomic nervous system. The main innervation comes from the carotid sinus nerve, a branch of the glossopharyngeal nerve that contains fibers proceeding from the petrosal ganglion. The carotid sinus nerve conveys the sensory information generated by the activation of the carotid body, and sends this information to the nucleus tractus solitarius (NTS), located in the dorsomedial medulla (in the brainstem) (Fig. 3). The NTS is the entry point of a variety of visceral sensory afferent inputs, including pulmonary mechanoreceptors, peripheral chemoreceptors, and others (Finley et al., 1992). The NTS is connected to the major respiratory centers of the ventrolateral medulla, also in the brainstem, and the activation of NTS neurons by carotid sinus nerve afferents contributes to increase the activity of the neuronal network that control ventilation, also named the central respiratory command (CRC). The carotid bodies also receive parasympathetic and sympathetic efferent innervation, the last one through small ganglio-glomerular nerves coming from the superior cervical ganglion (Kumar and Prabhakaran, 2012; Gonzalez et al., 1994).
8
(Campbell and Reece, 2005) Fig.3: Control of breathing.
This figure describes the role of peripheralchemoreceptors in control of breathing. The sensors in the aorta and carotid arteries detect changes in oxygen level in the blood and signal the pons and medulla of the brainstem to increase the breathing rate when oxygen level becomes low.
Structurally, carotid bodies contain two types of cells, type I and type II, surrounded by fenestrated sinusoidal capillaries and conjunctive tissue (McDonald etal., 1981; Gonzalez et al., 1994). The type I or glomus cells are chemosensitive andhave a neural origin (Kondo et al., 1982). These cells look like classical neurosecretory cells and their morphology resembles that of the chromaffine adrenal medullar cells. The glomus cells have dense-core granules containing neurotransmitters and neuromodulators that are released upon exposure to hypoxia. The principal transmitters between chemosensitive cells and nerve endings in the carotid bodies appear to be acetylcholine and ATP (Zhang et al., 2000; Rong et al., 2003), which depolarize the Hering‟s nerve ending though post-synaptic purinergic and nicotinic receptors. Dopamine appears to be a potent modulator, mainly inhibitor, of the response of the chemosensitive cells to hypoxic exposure ( Gonzalezet al., 1995). Surrounding the glomus cells are the type II cells. These cells do not have vesicles and morphologically resemble glial cells of theperipheral nervous system. These cells are also called sustentacular cells (Kondo et al., 1982).
9
1.2.2 Time domains of the hypoxic ventilatory response
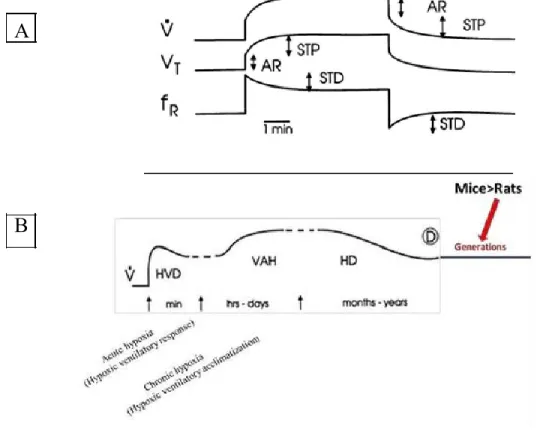
HVR involves complex interplay between several physiological distinct mechanisms which differ by various features, there are: (1) the specific stimuli that elicit them (e.g. pattern and intensity of hypoxic exposure); (2) the time course ofthe response (seconds to years); (Fig.4). (3) The effects on the components of ventilation (tidal volume vs. frequency); (4) the direction of their effects (facilitation vs. depression); and (5) the neurochemicals necessary for their manifestation. (Powell et al., 1998).
A
B
(Powell et al., 1998) Fig.4: Time domains of hypoxic ventilatory response.
Figure A describes ventilatory responses after a brief (seceonds to minutes) hypoxic exposure (Acute response (AR), short-term potentiation (STP), short-term depression (STD)).Figure B describes the ventilatory responses during prolonged hypoxic exposures include hypoxic ventilatory decline (HVD), ventilatory acclimatization to hypoxia (VAH) and hypoxic desensitization (HD) followed by extended hypoxic response across the generations. Of note, after several generations of life at high altitude, mice had higher ventilationthan mice.
10
Following acute hypoxia, the organism reacts by a reflex loop initiated in the carotid body that produces a quick augmentation of ventilation in order to compensate the drop of oxygen supply. Hypoxic hyperventilation is necessary to limit the drop of arterial oxygenation and is a vital response during severe hypoxic exposure. During this period short-term potentiation (STP) is initiated (Eldridge and Millhorn et al., 1986). STP is characterized by a second augmentation phase of ventilation that appears after some minutes upon hypoxic exposure. This mechanism of respiratory neuron hyperexcitation can be explained in part by the presynaptic calcium accumulation that increases the release of several neurotransmitters (Wagner et al., 1991). The electric stimulation of the carotid body fibers is able to induce STP, which indicate that glomus cells are directly implicated in the initiation of this phenomenon (Hayashi et al., 1993). When the hypoxic stimulation persists (some minutes), a ventilatory depression appears which is characterized by a decrease of the respiratory rate. This phenomenon is termed short-term depression and it is thought to depend on the noradrenergic A5 group located in the rostral part of the ventrolateral medulla. In fact, it was demonstrated that a lesion of A5 abolishes the short-term depression (Coles and Dick, 1996).
In the case of sustained hypoxic stimulation (mins to hrs) a new phase of ventilatory response appears which is termed hypoxic ventilatory depression (HVD) or “roll off” (Vizek et
al., 1987) and principally involves the decrease of the tidal volume. It was hypothesized for a
long time that respiratory alkalosis which follows sustained hypoxia was mainly responsible for the HVD. In fact, central chemoreceptors sensitive to CO2 are able to induce an augmentation of the ventilation (Nattie, 1999). During alkalosis, the lower CO2 concentration decreases the activation of these receptors producing a diminution of the ventilation. However it was demonstrated that the HVD is also produced under isocapnic conditions (Bisgard, 1995). Thus, the HVD seems to be a reflex of a central inhibition, or due to decreased activity of the peripheral chemoreceptors (Neubauer et al., 1990).
Ventilatory acclimatization to hypoxia (VAH) appears days or weeks after hypoxic exposure. VAH is characterized by a progressive augmentation of ventilation until reaching a plateau. The time at which VAH is completed for a given hypoxic stimulus is species dependent.
11
While in humans and rats it is established after 10 days (Bisgard, 1995), in mice it occurs after three days (Malik et al., 2005). VAH is absolutely dependent on the peripheral chemoreceptors (Busch et al., 1985; Smith etal., 1986), and it was demonstrated that mice with a partial knockout for Hypoxia inducible factor -1 α (HIF-1α) exhibited reduced carotid body sensitivity to hypoxia and consequent impaired VAH (Bisgard, 1995; Kline et al., 2002). Finally, exposition to hypoxia across several generations as occurring in animal species or human population permanently living at high altitude might be accompanied by a blunting of hypoxic sensitivity, resulting in relative hypoventilation and a phenotype of mal-adaptation to altitude. As an example, an important fraction of high altitude natives develop a high altitude disease (Chronic mountain sickness) mainly characterized by a pathological erythrocytosis and alveolar hypoventilation (Monge et al., 1992). Interestingly, chronic mountain sickness occurs almost exclusively in adult male subjects and postmenopausal women living in the Andes (Leon-Velarde et al., 1997).
1.3 The metabolic response to hypoxia
To cope with hypoxic hypoxia, mammals are provided by two physiological strategies: Increasing the rate of O2 uptake by increasing ventilation, or decreasing the rate of O2 consumption. The decrease of the O2 consumption rate leads to a decrease of the production of energy (ATP), and a decreased body temperature. This response is highly efficient, is conserved across evolution, and under extreme conditions of hypoxia it ensures survival in species that show stunningly long-lasting resistance to anoxia such as aquatic turtles (Hochachka et al., 1996; Hochachka and Lutz, 2001). While in mammals this extreme response is not possible, a certain degree of metabolic rate reduction is observed upon exposure to acute hypoxia, particularly in newborn or in small adults, in which metabolic rate is high relatively to their body mass (Mortola, 1999; Singer, 2004). The decrease of metabolic rate might be accompanied by a reduction of core body temperature, due to a reduction of the thermoregulatory set point. Current models are consistent with the hypothesis that hypoxia activates cAMP dependent pathways in the hypothalamic pre-optic area, causing an elevation of the thermal sensitivity of pre-optic warm-sensitive neurons, in turn leading to an inhibition of thermogenesis and activation of heat loss (Steiner and Branco 2002; Branco et al., 2006; Bicego et al., 2007): in other words, this response is a decrease of the thermoregulatory set point initiated in the pre-optic hypothalamic area. Gasotramsmitters such as Nitric Oxide, Carbon monoxide and hydrogen sulfide appear as
12
critical mediators of this response (Branco et al., 1987; Paro et al., 2001; Steiner and Branco 2002; Branco et al., 2014). Interestingly, blockade of NMDA receptors amplifies the hypoxic-induced fall of body temperature and this effect is present in developing rats between the 4th and the 20th post-natal day (Baig and Joseph 2006). These data clearly illustrates that this process is tightly regulated by the nervous system rather than a passive response to O2 limitation (Gordon and Fogelson 1991; Barros et al., 2001; Steiner and Branco 2002; Branco et al., 2014).
To take into account the ventilatory and metabolic responses to hypoxia, it is possible to report the ratio of ventilation to O2 consumption or to CO2 production rate (Ve/VO2 or Ve/VCO2 - Morgan, 2014).
1.4 Molecular response to hypoxia
Mammalian cells are able to sense a decrease in oxygen tension and exposure to a low oxygen environment triggers several immediate and long-term adaptive mechanisms both at physiological and molecular level. Hypoxia induces various systemic cardiorespiratory responses. However, these compensatory mechanisms are not sufficient to meet oxygen demand of the central nervous system, especially during prolonged exposure to hypoxia. Therefore at molecular level these responses during hypoxia are mediated by expression of HIF.
1.4.1 Structure and composition of HIF
HIF is a heterodimer that consists of the inducible HIF-α subunit and the constitutively expressed HIF-1β. Both α and β subunits belong to the family of the basic helix–loop–helix (bHLH) and PER-ARNT-SIM (PAS) domain-containing transcription factors (Fig.5 - Wang et al., 1995). bHLH and PAS domains mediate
DNA binding and dimerization; the other domains in the α subunit include a unique O2 -dependent degradation domain (ODDD) and two transactivation domains: the N-terminal activation domain (NAD) and C-terminal activation domain (CAD). Three structurally closely related α subunits (HIF-1α, HIF-2α, and HIF-3α) have been identified to date (Huang and Bunn, 2003; Masoud and Lin, 2015). Among this HIF- 1α and HIF-2α are the best characterized. HIF-
13
1α is expressed ubiquitously, whereas HIF-2α displays tissue-specific expression (Wiesener et
al., 2003). HIF-3α has multiple splice variants, including the best known, inhibitory PAS domain
protein, which is a short protein that functions as a dominant-negative inhibitor of HIF-1 (Makino et al., 2002). The HIF-1 heterodimer binds to a conserved HIF-binding sequence within the hypoxia-responsive element (HRE) in the promoter or enhancer regions of target genes, thereby eliciting their transactivation and an adaptive hypoxic response (Semenza, 2003).
(Masoud and Lin, 2015).
Fig.5: Structure and composition of HIF and their isomers.
This figure describes the functional domains (bHLH, PAS and TAD) for proteins related to bHLH-PAS family. HIF-1α and HIF-2α share highdegree of amino acid sequence similarities and both of them have two distinct TADs (C-TAD and ODDD N-TAD). In contrast, HIF-3α only has N-TAD.
1.4.2 Regulation of oxygen homeostasis by HIF-1 α
Regulation of HIF-1 alpha is oxygen dependent. Under normoxic conditions, HIF-1α is hydroxylated primarily by specific prolyl hydroxylases (PHD1, PHD2 and PHD3) at two conserved proline residues (Pro 402 and Pro564) situated within its ODDD. PHD1, PHD2, and PHD3 have closely related catalytic domains and belong to the superfamily of 2-oxoglutarate (2OG)-dependent oxygenases.
14
In order to be active, PHDs require O2, the citric acid cycle intermediate 2-oxoglutarate 2OG as a co-substrate, plus Fe (II) and ascorbate as cofactors. (Bruick and McKnight, 2001; Epstein et al., 2001; Jaakkola et al., 2001). HIF-1α hydroxylation facilitates binding of tumor suppressor gene: von Hippel–Lindau protein (pVHL) to the HIF-1a ODD (Ohh et al., 2000; Ivan
et al., 2001). pVHL forms the substrate-recognition module of an E3 ubiquitin ligase complex,
(Kondo and Kaelin, 2001) which directs HIF-1α poly-ubiquitylation and in the final step, polyubiquitylated HIF- α is degraded in the 26S proteasome (Maxwell, 1999). Moreover an additional hydroxylation event in the CAD domain ensures that any HIF-1α that escapes degradation is rendered inactive. This process involves the hydroxylation of an asparagine at Asn803 residue instead of a proline by asparaginyl hydroxylase and suppresses the recruitment of CBP/p300 co-activators (Lando et al., 2002a). This activates FIH-1 (factor inhibiting HIF-1), leads to steric hindrance of the interaction between α subunits and the coactivator proteins p300/CBP. This hindrance prohibits the transactivation of target genes under high pO2 (Lando et
al., 2002b).
Upon exposure to hypoxia, hydroxylation of prolyl and asparaginyl residues is inhibited. This inhibition enables the α subunit to escape proteolytic degradation and allow efficient translocation into the nucleus where the phosphorylated form of HIF-1 alpha dimerizes with HIF-1 β through intermolecular interactions between the HLH and PAS domains. The α:β heterodimer associated with the transcriptional coactivators p300/CBP to regulate transcription of various target gene via HRE binding sites (Fig.6).(Brahimi-Horn et al., 2005; Shimoda, 2012).
15
(Shimoda, 2012). Fig.6: Schematic illustration of HIF-1α regulation.
Under normoxic conditions, prolyl hydroxylase domain (PHD) proteins use molecular oxygen as a substrate to hydroxylate HIF-1α. Once hydroxylated, HIF-1α binds von Hippel-Lindau (VHL) protein and becomes polyubiquitylated (Ub) and targeted for proteosomal degradation. Under hypoxic conditions, PHD activity is reduced and HIF-1α escapes hydroxylation, accumulating and translocating to the nucleus where it binds with HIF-1β and CBP/p300 at the hypoxia response element (HRE).
1.4.3 HIF-1α target genes
Activated HIF-1α plays a crucial role in adaptive responses to changes in O2 in organisms through transcriptional activation of over 100 downstream genes which regulate vital biological processes required for survival under hypoxia. HIF-1 activity leads to the upregulation of genes that are involved in erythropoiesis,iron metabolism, apoptosis, glucose metabolism, angiogenesis/vascular tone and cell proliferation/survival etc. (Semenza, 2001a; Semenza, 2003b).The following table summarizes the HIF-1α target genes information.
16 Table.1: Selected HIF-1 target genes.
Gene Product Functions References
α1B-adrenergic receptor Vascular tone (Eckhart et al., 1997) Adenylate cyclase Nucleotide metabolism (Wood et al., 1998) Adrenomudulin Vascular tone, cell survival (Cormier-Regard et
al.,1998)
Aldolase A Glucose metabolism (Iyer et al., 1998; Ryan etal., 1998)
Aldolase C Glucose metabolism (Iyer et al., 1998)
Carbonic anhydrase 9 pH regulation (Wykoff et al., 2000)
Ceruloplasm Iron metabolism (Mukhopadhay et al., 2000)
Endothelin-1 Vascular tone (Hu et al., 1998)
Enolase 1 Glucose metabolism (Iyer et al., 1998)
Erythropoietin Erythropoiesis, cell survival (Jiang et al., 1996) Glucose transporter 1 Glucose metabolism (Iyer et al., 1998; Ryan
etal., 1998)
Glucose transporter 3 Glucose metabolism (Hogenesch et al.,1998) Glyceraldehyde-3-P-
dehydrogenase Glucose metabolism (Iyer et al., 1998; Ryan etal., 1998)
Heme oxygenase Vascular tone, cell survival (Lee et al., 1997)
Hexokinase 1 Glucose metabolism (Iyer et al., 1998)
Hexokinase 2 Glucose metabolism (Iyer et al., 1998)
IGF-binding protein 1 Cell proliferation and survival (Tazuke et al., 1998) IGF-binding protein 2 Cell proliferation and survival (Feldser et al., 1999) IGF-binding protein 3 Cell proliferation and survival (Feldser et al., 1999) Insulin-like growth factor
(IGF-2) Cell proliferation and survival (Feldser et al., 1999) Lactate dehydrogenase A
NIP3 Cell proliferation and survival
(Iyer et al., 1998; Ryan
17
Nitric oxide synthase 2 Cell proliferation and survival (Bruick, 2000)
p21 p35srj Cell proliferation and survival (Palmer et al., 1998Carmeliet et
al.,1998)
Phosphofructokinase L Glucose metabolism (Bhattacharya et al.,1999) 2-kinase/fructose-2,6-
biphosphatase Apoptosis (Iyer et al., 1998)
Phosphoglycerate kinase 1
Vascular tone, cell survival, Cell
proliferation (Minchenko et al., 2002)
Plasminogen activator
inhibitor 1 Regulation of HIF-1 activity
(Iyer et al., 1998; Ryan etal., 1998)
Prolyl-4-hydroxilase α (I) Glucose metabolism (Kietzmann et al., 1999) Pyruvate kinase M Glucose metabolism (Takahashi et al., 2000)
RTP801 Glucose metabolism (Shoshani et al., 2002)
Transferin Glucose metabolism (Rolfs et al., 1997)
Transferin receptor Angiogenesis, (Lok et al., 1999)
Transforming growth factor β3
Collegen metabolism, Glucose
metabolism (Tacchini et al., 1999)
Triosephosphate
isomerase Apoptosis, cell survival (Iyer et al., 1998)
Vascular endothelial
growth factor Iron metabolism (Iyer et al., 1998; Ryan et
VEGF receptor 1 Angiogenesis, cell proliferation, Glucose metabolism, Angiogenesis/cell survival
angiogenesis (Gerber et al., 1998)
18
1.4.3.a Epo is HIF-1 target genes involved in erythropoiesis and ventilatory response to hypoxia.
In response to hypoxia, the capacity of red blood cells to transport oxygen isup-regulated by the expression of EPO gene involved in erythropoiesis. HIF-1α beingan important transcription factor for EPO, releases from the kidneys and increases the O2 carrying capacity of blood by stimulating erythropoiesis in bone marrow (Semenza, 1999).
Studies on transgenic mouse (Tg6), which over-expresses EPO in the brain and in the blood suggested that EPO exerts effects on the hypoxic ventilatory response both centrally and peripherally. In Tg6 mice, there is an increased level of norepinepherine in the A5 cell group of the pons, which could contribute to increase the respiratory frequency (Soliz et al., 2007). However, systemic EPO also increases frequency by acting on the carotid body (Soliz et al., 2005). In addition, the Tg21 line of transgenic mice reported 3-fold higher EPO levels in the brainstem than wild type mice. With chronic hypoxia, Tg21 mice increase ventilation more than wild type mice (Soliz et al., 2005). Although HIF-1α is widely expressed, HIF-2α has now emerged as the main regulator of the hypoxic induction of EPO in vivo. Recently histological studies, demonstrated that the location of HIF-2α expressing renal cells coincided with the location of EPO-producing renal interstitial fibroblast-like cells (Paliege et al., 2010). Moreover, genetic studies in mice have demonstrated that renal and liver EPO synthesis is HIF-2 dependent and not HIF-1 dependent (Chavez et al., 2006; Rankin et al., 2007).
1.4.3.b HIF-1 target genes involved in glucose metabolism
Depletion of O2 changes the energy metabolism of cells, the cells don't use the oxygen
dependent metabolic pathway such as the tri carboxylic acid (TCA) cycle to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of ATP. Instead of the TCA cycle, the cells switch to the O2-independent metabolic pathway, and they start using glycolysis as the primary mechanism of ATP production (Dang and Semenza, 1999; Seagroves et al., 2001). The TCA cycle provides 38 ATPs from glucose, but glycolysis provides only two. Therefore, the hypoxic condition requires more glycolysis than normoxic condition. HIF-1 regulates the expression of all enzymes
19
in the glycolytic pathway as well as expression of the glucose transporters gene GLUT1 and GLUT3 that mediate cellular glucose uptake (Chen et al., 2001). Studies on glucose transport and metabolism in hypoxia-ischemic rat brain causes upregulation of GLUT1 and GLUT3 gene expression via HIF-1α to increase glucose transport and glycolytic rate (Vannucci et al., 1998). Enhanced lactate production and lower intracellular pH results from the increase in anaerobic glycolysis, potentially limiting this source of ATP despite sufficient glucose supply (Swietach et
al., 2000). Regulation of pH has its own importance in cell death under hypoxia, thus
mechanisms of pH regulation via carbonic unhydrase is likely to be vital pathways for survival. Thus, transmembrane carbonic anhydrases were reported to regulate the pH by converting protons and bicarbonate to carbon dioxide, so that it could taken up by erythrocytes for transportation to the lung (Wykoff et al., 2000). Whereas increased levels of GLUT1 could satisfy the elevated glucose requirement for anaerobic glycolysis under hypoxia. In addition, GLUT1 may support the adaptive response of the brain during hypoxia which facilitates glucose transport from the blood into brain parenchyma through the blood–brain barrier (Wenger, 2002).
1.4.3.c HIF-1 target genes involved in Angiogenesis
Angiogenesis is the development of new blood vessels and is subjected to a complex control system with proangiogenic and antiangiogenic factors. One of the most important proangiogenic factors is the vascular endothelial growth factor (VEGF). It is one of the major target genes of HIF-1 and participates in the angiogenesis by recruiting endothelial cells into hypoxic and a vascular area and stimulates their proliferation (Neufeld et al., 1999; Josko et al., 2000; Conway etal., 2001). It has also been shown that hypoxia induces the expression of VEGFmRNA and protein, suggesting that hypoxia stimulates angiogenesis through the up-regulation of VEGF expression (Neufeld et al., 1999; Harris, 2000).
Among seven members of VEGF family, VEGF-A plays a central role in angiogenesis and neovascularization, by increasing delivery of both oxygen and energy substrates. VEGF-A expression can be induced when cells are subjected to hypoxia or hypoglycemia. This response seems to depend on Hypoxia Regulated/ Responsive Element/Enhancer sequences in the 5’ and 3’ regions of the VEGF-A gene (Tsuzuki et al., 2000). HIF-1 directly activates the expression of
20
vascular endothelial growth factor which promotes the formation of new blood vessels, thus restoring the supply of O2 and nutrients during hypoxia.
VEGF interacts with its receptor, VEGFR, which is specifically expressed in endothelial cells, and this stimulates endothelial cell proliferation (Josko et al., 2000; Conway et al., 2001). Disruption of the genes in Flk-1-deficient mice encoding the VEGF tyrosine-kinase receptors VEGFR-2 (Shalaby et al., 1995) and VEGFR- 1 (Fong et al., 1995) results in severe abnormalities of blood vessel formation in homozygous animals. In addition, mice lacking one of the two VEGF alleles die before birth because of defects in the development of the cardiovascular system indicating that the development of the cardiovascular system depends on the generation of precise VEGF concentration gradients, and decrease in the amounts of the VEGF produced during the development of the embryo may lead to decreased angiogenesis with fatal consequences (Ferrara et al., 1996).
1.4.3.d HIF-1 target genes involved in Cell Proliferation/Survival
Hypoxia-induced growth factors most notably insulin-like growth factor-2 (IGF2) and transforming growth factor-alpha (TGF-α) are known to promote cell proliferation and survival. (Feldser et al., 1999; Krishnamachary et al., 2003). Binding of these factors to their cognate receptors the insulin-like growth factor 1 receptor (IGFIR) and epidermal growth factor receptor (EGFR) respectively, activates signal transduction pathways mitogen-activated protein kinase (MAPK) and Phosphatidylinositol 3-OH kinase (PI3K), that lead to cell proliferation and survival by activating HIF-1 activity. Activation of HIF-1 system leads to increased HIF-1 transcriptional activity of target genes, encoding IGF2 and TGF-α, thereby contributing to autocrine-signaling pathways that are crucial for cancer progression (Semenza, 2003). In addition PI3K activity is also increased in hypoxic conditions (Chen, 2001). PI3K is one of the key downstream mediators of many tyrosine kinase signaling pathways, and is involved in regulating cell proliferation and suppression of apoptosis. The PI3K pathway is inhibited by the phosphoinositide phosphatase (PTEN), and mutations in PTEN enhance HIF-1 activated responses (Zundel et al., 2000).
21
1.5 Induction of Brainstem HIF-1α expression in response to hypoxia
Physiological responses to hypoxia occur at the systemic and cellular level. The systemic response is mediated by chemoreceptor stimulation and activation of the central and peripheral nervous system whereas, the cellular response is mediated by HIF. Understanding the adaptive capacity of the brain to deal with oxygen deficiency is important because the molecular mechanisms responsible for this appear to be activated under hypoxia and other pathophysiological conditions. (Bergeron et al., 1999; Jin et al., 2000).
Immunohistochemical study revealed that HIF-1α displays an organ-specific expression in mice under normoxic condition and increases in response to systemic hypoxia. The maximum HIF-1α expression was found after 4–5 hrs of hypoxic exposure in brain and other organs. The achievement of maximal HIF-1α expression depends on the degree and duration of the hypoxic exposure and is different between the different organs (Stroka et al., 2001).
Interestingly, some studies suggested that HIF-1α induction is the primary regulatory response that triggers the cascade of different events leading to physiological acclimatization. Several study suggested that HIF deletion in knock out (KO) mice impairs normal responses and ventilatory acclimatization to hypoxia (Klein et al., 2002; Powell et al., 2008). HIF-1α increases in the Central Nervous Systen (CNS) and brainstem is probably a major central structure for HIF-1α expression during hypoxia as respiratory nuclei of brainstem are responsible to modulate ventilation under hypoxic condition (Pascual et al., 2001; Lindsey et al., 2013). Therefore, it might be predicted that an understanding of the patterns of HIF expression in the brainstem would provide important insights into processes mediated by these molecules during hypoxia. While the literature is rich describing the ventilatory response to hypoxia in mice and rats separately, literature directly comparing ventilatory responses between these species is poor. Moreover, the interspecies comparison of hypoxic ventilation from the perspective of the HIF expression in the brainstem was not yet performed. Based on the in line evidences we hypothesized that the differences in ventilatory response in adult rats and mice are linked to differences in the brainstem HIF expression in response to hypoxia.
22
II- OBJECTIVE AND HYPOTHESIS OF THE
STUDY
23
The fact that rats and mice raised at high altitude have different resistance to hypoxia is intriguing. Because these animals are not endemic at high altitude, but have been imported by humans, we postulated that the higher “resistance” to hypoxia in mice compared to rats is a pre-determined trait characterizing these species. As a first approach, we sought to determine whether rats and mice have different responses to hypoxia at the physiological (ventilatory response to hypoxia), followed by molecular level (expression of HIF-1).
24
25
3.1 Animals
We used 20 adult male Sprague-Dawley rats and 22 male FVB mice of age 2-3 months old from Charles River, St. Constant, Quebec, Canada. The animals had access to water and food ad libitum and are maintained under a 12:12-h light-dark cycle, with controlled humidity and temperature. The animals were allowed to adapt for one week at animal house prior to experiment. All the experimental protocol is approved by local animal care committee of ‘University Laval’ in accordance with guidelines of the Canadian Council of Animal Care (CCAC).
3.2 Experimental Groups
Adult rats and mice were divided in three groups. First, normoxic group of 20.9% O2 and remaining two were hypoxic group of 15% O2 and 12% O2 obtained by mixing a predetermined flow of nitrogen gas to room air. Each group has minimum 6 and maximum 8 animals which were exposed for 6 hrs.
3.3 Measurement of Respiratory and Metabolic Parameters 3.3.1 Whole body Plethysmography
i) Experimental setup
Whole body Plethysmography (Emka Technologies, Paris, France) was used to measure the ventilatory and metabolic parameters in adult rats and mice (Seaborn et al., 2013).The respiratory flow trace was recorded using a differential pressure transducer. The flow of air through the chamber was set and continuously monitored at 0.35 l/min for mice and 1.5 l/min for rat using a pump and gas flow restrictor/monitor (Emka Technologies, France). Inlet and outlet gases were alternatively subsampled, directed toward a water pressure analyzer (RH-300), and then the air was dried and directed to an oxygen/carbon dioxide analyzer (AEI technology, USA) for respiratory gases analysis. All signals (plethysmograph, gas analyzers, and flow meter) were directed towards computer running the software (Spike2-7.06, CED-Cambridge Electronic Designs.UK) for online storage and calculation of respiratory and metabolic values (Fig.7).
26
Fig.7: Schematic representation of whole-body plethysmography (Seaborn.et al.,2013) ii) Plethysmoraphy recordings
First, calibration of oxygen and CO2 analyser (AEI Technologies) was done using 20.9 % O2 and 5.09% CO2 gas followed by calibration of chamber by using known volume of air (0.5ml for mice/3ml for rat). Later level of O2 is maintained to 20.9% for normoxic and 12%, 15 % for hypoxic condition using nitrogen gas. Once the O2level get stabilized, animal was placed inside the chamber for 6 hours to measure the respiratory and metabolic recordings. Respiratory traces were recorded to determine the frequency (fR, breaths/min), tidal volume (VT, ml), and minute ventilation (VE = fR x VT). During each measurement, a small, accuratelymeasured volume of air was injected rapidly into the animal chamber for calibration purposes. The pressure change caused by injection was used in the calculation of tidal volume by the equation (Drorbauh and Fenn, 1955). VT was obtained via integration of the negative downward deviations of the flow trace and corrected using a standard equation expressed as BTPS (Bartlett, 1970).
27
VT (BTPS) = PT/PK x VK x [TR (PB-PC)/ (TR [PB-PC] – TC [PB-PR])] 1
The symbols in the equation 1 are defined as follows:
VT = tidal volume
BTPS = volume expressed in terms of vapor pressure of water and corrected for body temperature of the animal/organism
PT = amplitude of the respiration pressure change
PK = amplitude of the calibration of pressure variation
VK = calibration volume (ml)
TR = internal temperature of the animal (K)
TC = temperature in the plethysmography chamber (K)
PB = barometric pressure (mmHg)
PC = saturated vapor pressure in the plethysmography chamber (mmHg)
PR = saturated vapor pressure at the internal temperature of the animal (mmHg)
Oxygen consumption (VO2) and CO2 production (VCO2) rates were calculated using the following equations (Lighton, 2008).
28
O2 consumption = Flow x [(O2in –O2out) – O2out x (CO2 out – CO2in)]
(1–O2out)
2
CO2 production = Flow x [(CO2out –CO2in)-CO2out x (O2 in – O2out)]
(1–CO2out)
3
Where, ‘Flow’ is the flow of air measured before entry into the chamber, ‘O2, in’ and ‘CO2, in’ are the gas fractions in the inflowing air (considered at 20.9% and 0.038%, respectively), and O2, out and CO2, out are the gas fractions measured in the outflowing line. The respiratory exchange ratio was calculated as CO2 production/O2 consumption.
In these equations, O2 and CO2 concentrations were corrected with the following term: PB/ (PB- PH2O), where, PB is barometric pressure, and PH2O is the partial pressure of water in the inflowing or outflowing air. This correction compensates for the diluting effect of water pressure on measured O2 and CO2 levels (Melanson et al., 2010).
3.3.2 Analysis of respiratory and metabolic parameters
Ventilatory and metabolic recordings were averaged every hour over the 6 hours after the onset of normoxic and hypoxic exposure by selecting periods of stable breathing patterns without movements. Periods of recording showing error due to body movements were excluded.
Analysis of respiratory parameters, respiratory frequency (fR), tidal volume (VT),VO2 and VCO2 were analyzed from the plethysmograph. All metabolic volumes are expressed in conditions of BTPS and expressed per 100 grams of body weight (Bartlett, 1970).
29
The respiratory quotient (RQ) or respiratory coefficient, is a dimensionless number obtained by dividing the volume of carbon dioxide (VCO2) produced by an organism to the volume of oxygen consumed (VO2). This quotient is useful because the volumes of CO2 and O2 produced depends on which fuel source is being metabolized for the production of ATP. Measuring RQ is a convenient way to gain information about the source of energy an animal is using. Eg.Glucose oxidation or fatty acid oxidation. We can then compare the metabolism of animals under different environmental conditions. The respiratory quotient (RQ) was calculated as CO2 production/O2 consumption (VCO2/VO2). The ventilatory equivalent for oxygen and carbon dioxide exchange, was calculated as (VE/ VO2: ‘ml’ of air need to ventilate to consume ‘ml’ of oxygen (O2) and VE/ VCO2: ‘ml’ of air need to ventilate to produce ‘ml’ of carbon dioxide (CO2) (Lemoine et al., 2015).
3.4 Allometric scaling
Allometry, is also called biological scaling, which describes the change in organisms in relation to proportional changes in body size. Allometric scaling is the standard approach to compare animals of the different sizes. (Maina et al., 1989; Stahl, 1967).
Allometric equations in general represented as ‘X = aMb ’
Where, X is biological variable, M is a measure of body size, and b is scaling exponent. The most common example of allometry is geometric scaling, in which surface area is a function of body mass. The scaling exponent ‘b’ were obtained by calculating the slope of a regression line fitted through a log–log plot of a parameter (X) as a function of body mass (M).
In general, for organisms that preserve their basic shape as they vary in size, the organisms linear dimensions vary as the 1/3 and their surface area as the 2/3 powers of their body mass. Another important example of scaling is based on Kleibers law. Which describes the relationship of energy consumption (or metabolic rate) and body mass in mammals: metabolic rate scales as the 3/4 power of body mass.
30
We used allometric scaling to compare physiological and morphological values between rats and mice, from the above equation,we reported mass-specific variables with Mb. For the respiratory variables, we used the scaling variable calculated by Stahl (1967), which are: fR, b=−0.25; VT, b=1.04; V˙E, b=0.8; VO2 consumption and VCO2 production, b=0.75. Data corrected for the allometric scaling variables are referred as mass corrected values in the text description (Lemoine et al., 2015).
3.5 Tissue Sampling
Once the plethysmography recordings were finished, anesthesia with 2-3 % isoflurane was circulated inside the plethysmography chamber under hypoxic condition. Body temperature was evaluated immediately after the animals were perturbed by the anesthesia, in the way that body temperature was not altered by the isoflurane (Albrecht M. et al., 2014), followed by cardiac puncture to collect the blood sample under hypoxic condition. Then immediately animal was sacrificed and brainstem was collected.
3.6 Measurement of molecular parameter
3.6.1 Nuclear Protein extraction (HIF-1 α from brainstem)
We used nuclear protein extraction kit (Item No: 10009277) from Cayman Chemical Company USA. Rat and mice brainstems were used for extraction of transcription factor i.e. HIF-1 alpha. The summarised protocol was as follows:
Whole Brainstem was taken into prechilled vial containing known volume of ice- cold 1x hypotonic buffer supplemented with DTT and Nonidet P-40 per gram of tissue. The sample vial was homogenized with homogeniser on ice for 15 min. Then prechilled micro centrifuge tubes were centrifuged at 300 x g for 10 min at 4 O C to separate first cytosolic fraction. Pellet obtained from centrifugation was gently resuspended in additional volume of 1x hypotonic buffer into each vial for complete lysis of cells followed by additional 15 min incubation on ice. After
31
incubation, Nonidet P-40 was added, mixed well and centrifuged at 14,000 x g for 30 seconds (pulse spin) at 4O C in a micro centrifuge to separate second fraction of cytosolic fraction. The pellet was re-suspended in known volume of ice –cold 1x Extraction buffer (with protease and phosphatase inhibitors). Each vial was vortexed for 15 seconds at the highest setting and then rocked gently on ice for 15 minutes using shaking platform. Sample was vortexed for additional 30 seconds at the highest setting and rocked gently for an additional 15 minutes. The sample was then centrifuged at 14,000 x g for 10 minutes at 4 O C to obtained nuclear fraction as a supernatant. Aliquoted fraction were stored at -80 O C and used further for transcription factor assay.
3.6.2 Enzyme Linked Immunosorbent Assay (ELISA)
We used HIF-1 α transcription factor assay kit (Item No: 10006910) fromCayman Chemical Company, USA. Rat and mice brainstem nuclear fraction were used to detect HIF-1 α. The summarised protocol was as follows:
Ready to use 96 well ELISA plate was used. Known volume of complete transcription factor binding assay buffer (CFTB), - competitor dsDNA, positive control and sample containing HIF-1 α was added in appropriate wells. The ELISA plate was incubated at 4 OC for overnight. After incubation all the wells were washed five times with known volume of 1x wash buffer and then known volume of HIF-1 α antibody was added except blank well followed by 1 hr incubation at room temperature. After incubation wells were washed properly with 1x wash buffer and secondary antibody was added in each well except blank well. Again incubated was done for additional 1 hr at room temperature. Each well were washed again with 1x wash buffer, then developing solution was added in each well and incubated for another 15 – 45 min. After final incubation, stop solution was added in each well and absorbance was measured at 450 nm.
32
3.7 Statistical Analysis
We used Graph Pad Prism software (version 6.04 for windows) for all analyses. All values are reported as the means ± SEM and the significant P value was set as 0.05. P values are reported in the figures with following general pattern: *, **, *** and **** for P, 0.05, 0.01, 0.001 and 0.001, respectively. We used two way ANOVA to compare hypoxic ventilatory response (moderate hypoxia; 15% O2 and severe hypoxia; 12 % O2) and expression of HIF-1 alpha between rats and mice.
33
34
4.1. Adult mice had higher minute ventilation than rats during hypoxic exposure 4.1.1 Mass-specific values
Mass-specific values for respiratory and metabolic parameters are presented in tables 2 and 3. In rats and mice, respiratory frequency (Fr) and minute ventilation (V.e) recorded in hypoxia were higher than in normoxia, and values for metabolic rate decreased. Significant interaction between species and hypoxia appeared for respiratory frequency, minute ventilation, V.O2, V.CO2, and V.e/V.O2, showing different responses to hypoxia in mice compared to rats. The mass-specific values indicate that compared to rats, mice have a higher respiratory frequency, tidal volume, and minute ventilation under hypoxia, and higher values of V.e/V.O2 and V.e/V.CO2, indicating effective hyperventilation.
Table. 2: Mass-specific values for respiratory parameters in rats and mice
Parameters O2 level Rats Mice P values
(%) No. of 21% 6 6 - animals (n) 15% 5 7 - 12% 6 7 - Body 21% 333 ± 21 26.7 ± 0.6 O2 = 0.1 Weight (g) 15% 367 ± 13 29.1 ± 0.7 Species < 0.0001 12% 320 ± 17 26.9 ± 0.2 x =0.15 Rectal 21% 36.8 ± 0.2 35.4 ± 0.3 O2 = 0.004 temperature 15% 36.3 ± 0.2 35.5 ± 0.3 Species < 0.0001 at end of experiment 12% 35.6 ± 0.3 34.6 ± 0.1 x = 0.4 (Tr OC)
35 Fr (bpm/bw) 21% 103±3 136±9 O2< 0.0001 15% 134±6** 217±6**** Species < 0.0001 12% 162±6**** 269±7**** x < 0.0001 Vt (ml/bw) 21% 0.48±0.05 0.62±0.05 O2 = 0.002 15% 0.43±0.03 0.61±0.06 Species = 0.002 12% 0.63±0.060.058 0.89±0.11** x = 0.65 21% 49.5 ± 4.3 86.2 ± 8.3 O2< 0.0001 V.e 15% 57.7 ± 6.6 131± 14* Species < 0.0001 (ml/min/bw) 12% 102 ± 10* 236± 29**** x = 0.02
This table represents mass specific values for respiratory variables in rats and mice. n- no. of animals; O2 level (%)– oxygen level in percentage; species- Rat vs mice; x- significant interaction between species and hypoxia; All values are reported as the means ± SEM; *, **, *** and **** for P, 0.05, 0.01, 0.001 and 0.001, respectively.
Table. 3: Mass specific data for metabolic variables in rats and mice
O2 level
Parameters (%) Rat Mice P values
V.O2
(ml/min/bw0.75) 21% 3.38 ± 0.07 7.03 ± 0.58 O2 < 0.0001 15% 1.90 ± 0.3* 3.86 ± 0.61**** Species < 0.0001 12% 1.93 ± 0.08* 2.56 ± 0.15**** x = 0.02