Acyclovir
vs
isoprinosine
(immunovir) for
suppression
of
recurrent
genital herpes simplex
infection
GR Kinghorn, P DWoolley, R NTThin, J De Maubeuge, J M Foidart, R Engst
Abstract
Objective-To compare the efficacy and
safety of oral acyclovir (400 mg twice
daily) with oral isoprinosine (500mg
twice daily) in the suppression of recur-rentgenital herpes.
Design-Double-blind, double-dummy,
randomised, controlled, parallel group
trial.
Setting-13 centres in UK, Belgium and Germany.
Subjects-127 immunocompetent
patients
with
frequently
recurring genital herpes.
Mainoutcomemeasures-Proportions
ofpatients reporting recurrences,
recur-rence frequency, and mean duration of lesions during breakthrough recurrences ineachtreatmentgroupduringa6month treatmentperiod; timetofirstrecurrence
during treatment and follow-up after
treatmentcessation.
Results-During treatment, acyclovir
recipients showed significant differences (p < 0.05) whencompared with isoprino-sinerecipientsin termsofalower propor-tionreportingrecurrences
(31%
vs96%),
a reduced mean number of reported
recurrences per patient (0.6 vs 3.6), a
shorter mean duration of
breakthrough
lesions(6.4daysvs8.2days), andalonger meantime
(standard
error)tofirstrecur-rence (143.7 (9.1) daysvs 40.5 (5.4) days. The mean time to first recurrence after treatmentcessation didnotdiffer between the twogroups. Ascomparedwithplacebo recipients, isoprinosine treated patients
had an increased recurrence frequency
(3.6 vs 2.5)
during
treatment, and ashorter time to first recurrence after
treatment cessation. All treatments were
well tolerated without serious adverse events ortoxicity.
Conclusions-Acyclovir
is veryeffective
in suppressing recurrent genital herpes and is clearly superior to isoprinosine whichis notclinicallyuseful
inthedosage studied.Introduction
Recurrentgenital herpes (RGH) infections are becoming increasingly common and are often
a source of considerable physical discomfort and emotional disturbance inaffected patients. Numerousdifferenttreatmentshave been sug-gested for the management of patients with genital herpes; these include antiviral drugs
and
immuno-modulators.'
2 The majority ofthese
preparations
havebeen shown in clinical trialstobeineffective. However, bothacyclovir
and inosinepranobex/isoprinosine
have been reported to reduce the frequency of recur-rences in separate controlledtrials.3"'l
The aim of this study was to compare the efficacy and tolerance of these oral agents in thesuppression of RGH infections in compar-ison with placebo.
Patientsand methods
Studydesign
Thiswasamulti-centre,blind, double-dummy, randomised,
controlled,
parallel
group trialinpatients with ahistory of RGH.The study was approved by the Ethical
Committee of each
hospital prior
topatient
entry.Patientselection and exclusion
Patients aged 16years or more with a known history offrequentattacks ofrecurrentherpes genitalis (sixormoreperyear)wereinvited to
participate.
Aculturepositive
recurrence dur-ing the observation period was necessary for inclusion in the study. Women who were not adequately protected against pregnancy, andpatients
of either sexwith known HIV infec-tion, other causes of immunosuppression, a history ofgastrointestinal malabsorption, renal disease with a creatinine clearance below 50ml/min, gout orhyperuricaemia, or severe atopiceczemawereexcluded. Allpatients
were informed of the experimental nature of the treatmentandwereaskedtogive theirconsent toparticipate, priortoentering the study. Patient managementEligible patients were initially observed with-out treatmentfor 8 weeks. During this obser-vationperiod, theywere requiredto complete daily diary cards detailingsymptoms andsigns ofrecurrences. Theywere asked to return to the clinic either at the first signs of a sub-sequent recurrence,whenseparateswabswere taken from external and internallesions pres-ent, or otherwise every 4 weeks when diary cardswere checked andreplaced.
They then progressed to the treatment period which continued for 24 weeks. Treat-mentwas commenced 7 days after the startof thefirst recurrencesubsequent to the observa-tionperiod.Eachpatientwas given two bottles of tablets and instructed to take one tablet from each twice daily. They were randomly assigned to treatment with one of the follow-ing: DepartmentofGU Medicine, Royal HallamshireHospital, SheffieldS102JF,UK GRKinghorn PDWoolley Department of GU Medicine, St. Thomas' Hospital, London, UK R NT Thin Hopital Universitaire, St.Pierre, Dermatologie, Brussels, Belgium J DeMaubeuge Baviere-Gynaecologie, Liege J MFoidart Dermatologische KlinikundPoliklinik derTechnischen
Universitaet, Munchen,Germany
REngst
Addresscorrespondenceto:
Dr GRKinghorn Accepted for publication
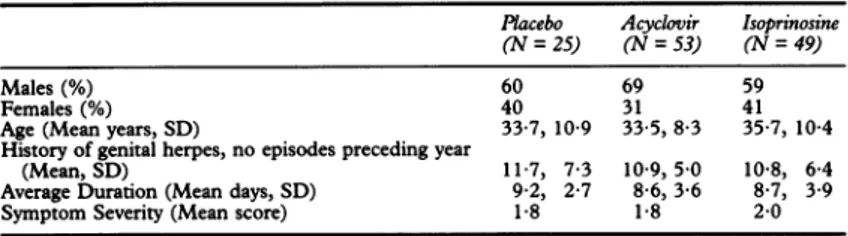
Table 1 Demographicdata atstudy entry
Placebo Acyclovir Isoprinosine (N=25) (N=53) (N =49)
Males (%) 60 69 59
Females (%) 40 31 41
Age (Mean years,SD) 33.7, 10-9 33-5, 8-3 35-7,10-4 History of genital herpes,noepisodespreceding year
(Mean, SD) 11-7, 7-3 10-9,5.0 10-8, 6-4
AverageDuration(Mean days,SD) 9-2, 2-7 8-6, 3-6 8-7, 3-9
SymptomSeverity (Mean score) 1-8 1-8 2-0
(1) active acyclovir* (400mgtwice daily) and isoprinosine placebo; (2) active isoprinosinet (500mg twice daily) and acyclovir placebo;
(3) both isoprinosine placebo and acyclovir placebo.
Theprotocoled ratio of patients assignedto
theacyclovir, isoprinosine, andplacebo treat-mentgroups were 2:2:1 respectively. The code forallocating patientstoeach of thetreatment groups was prepared using a computer
gen-erated randomisationtablebytheDepartment ofClinical Statistics andDataHandling, Well-come Research Laboratories, Beckenham, Kent.
Patients were followed-up for a further 24
weeks after cessationoftreatment ("followup
period") to assess theirpost-treatment
recur-rence pattern. The first tworecurrences were nottreated, thereafter the patientswereissued
withacyclovircream.
Measurements and evaluations
Throughouttreatment andsubsequent follow
up, patients reported compliance and symp-toms on diary cards. They were observed
monthly and during recurrences of infection
for clinical and safety assessments. Whenever
possible, the patient suspicion ofrecurrence was clinically confirmed. Concomitant
medi-cationwasnotrestricted,butwas recorded in
eachpatient's caserecordform.
Blood samples were taken for routine
hae-matological and biochemical measurements
immediately before treatment started, once everyeight weeks duringthetreatmentperiod, and at the first visit after treatment was
discontinued. Haematological parameters
measured includedhaemoglobin, haematocrit, red blood cell count, white blood cell count,
neutrophil, lymphocyte and platelet counts.
Biochemical parameters measured included
serumcreatinineconcentration, ureaanduric
acid concentrations and aspartate
transami-nase asanindicator of liver function.
All adverse events were recorded and rated
by the investigator for severity and for the possibility of an association with the study
drug(s) ateach visit.
Data analysis
For all analyses, the data were pooled over centresbecause of the low patient numbers in
some centres.
Summary statistics to describe theage, sex,
*Acyclovirtablets(400 mg) with matching placebo were made, packed andlabelledby theWellcome Foundation Limitedat Dartford,UK.
tIsoprinosinetablets (500 mg) were obtained from a whole-saler.The tabletsweremade, packed and labelled byEdwin BurgessLimited,Aylesbury, UK. Matching inerts were made by the WellcomeFoundation at Dartford, UK.
number and severity of HSV episodes were calculated for the patientsin each of the three treatment groups.The efficacy and laboratory data were dual entered and edited into a computerdatabasesystemfor
analysis.
All the summary statistics were produced using the SAS statistical package. The Kaplan-Meier curves were calculated using the SASproce-dure PROC LIFETEST.
Results
Number of patients
A total of 127 patients were entered into the study. Ten patients didnot
experience
a recur-rence during the observation period. One withdrew from thestudy and nine continued in the treatmentandfollowupperiods.
Of these five receivedacyclovir,
two received isoprino-sineandtworeceivedplacebo.Thus,
atotal of 126 patients were included for theefficacy
analysis.Demographic details and baseline characteristics Demographic details and baseline character-istics of patients at entry are summarised in table 1. The three treatment groups were
comparable for mean age, sex ratio, and preceding historyof recurrentgenital herpes. Number
of patients reporting
recurrentlesions(table
2)During two months observation, the percen-tagesofpatients
reporting
one or morerecur-rences were91% in the acyclovirgroup, 96% in the isoprinosine group and 92% in the placebogroup. During the6monthtreatment period, however, the percentage of
patients
reportingone or more recurrences was31%
in theacyclovir
group, 96% in theisoprinosine
group and 79% in the placebo group. The percentage ofpatients in the acyclovir group was significantly lower (p <0.05) than the percentagesin either theplacebo group ortheisoprinosine
group.During followup,thepercentageofpatients reportingone or morerecurrences was82%in the acyclovir group, 69% in the isoprinosine group and 50% in the placebo group. The difference between the percentages in the
acyclovir and isoprinosine
groups were not statistically significant, however there weresignificantly
fewer recurrences amongst placebo treated patients (p <005).Numberof reported recurrent episodes
Themeannumber ofreported recurrent infec-tionsperpatient during the2months observa-tion period was similar in all three treatment groups (table 2).
During treatment, the mean number of
reportedrecurrences perpatient was 0.6 in the acyclovirgroup, 3.6 inthe isoprinosine group and 2.5 in the
placebo
group.
These mean values were significantly different (p<0.05) between all pairsoftreatments. In the acyclovir group only 3.6% of patients had three or more reportedrecurrencescompared with 59.2% in the isoprinosine group and 41.7% in the placebogroup.Table2 Frequency ofrecurrences
Treatment group 95%confidence intervals fordifferences
Placebo(N = 24) Acyclovir (N=53) Isoprinosine(N=49) A-P I-P A-I No.(%) patients reporting a recurrence
Observation 22-0(92%) 50-0 (91%) 47-0 (96%)
Treatment 19-0 (79%) 17-0 (31%) 47.0(96%) (-68-4%, -27.6%)* ( 19%, 34 2%) (-78-4%, -51-6%)* Follow-up 12-0(50%) 45.0(82%) 34 0(69%) ( 9-6%, 54.4%)* (-4-8%,428%)* ( -3-5%, 29.5%) Mean no. recurrencesperpatients
Observation 2-13 2-29 2-76
Treatment 2-54 0-64 3-57 (-2-90, -0-91)* ( 001, 2.04)* (-373, -2-14)*
Follow-up 1-67 2-82 2-92 (-011, 2-41) (-004,253) (-111,092)
Meantime(days)tofirstrecurrence
Treatmentt 56.2(10-9) 143-7 (9.1) 40.5(5.4) ( 597,1152)* (-39.5, 8.0) ( 82-5, 123-9)*
Follow-up* 244-7 (10-6) 239-5 (8.0) 240-4(9.8) (-31-3, 20.8) (-32-7, 240) (-25-7, 23.9) Treatmenttostudyends 88-1 (22.6) 188-2(14-3) 40.5(5.4) ( 47.7,152-5)* (-93-1,-2-1)* ( 117-7, 177-6)*
*Significantat5%level.
tTofirstrecurrenceduring treatment.
*Tofirstrecurrenceinfollow-up(fromstartoftreatmentperiod). STofirstrecurrenceatanytimeduringtreatmentorfollow-up.
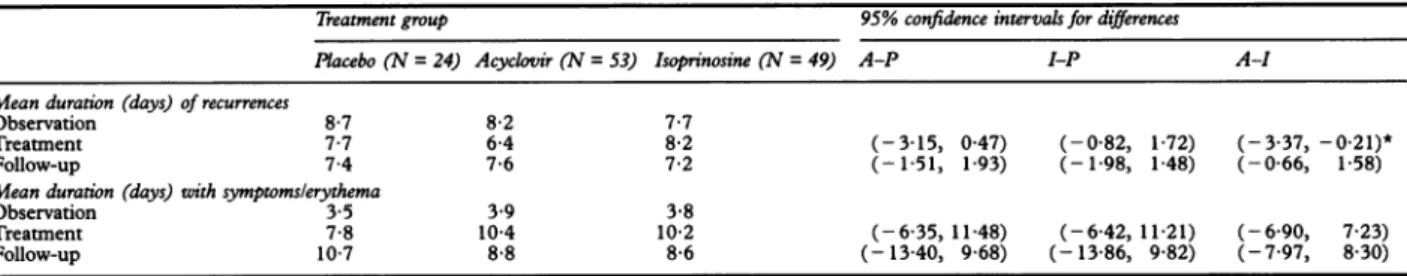
Table 3 Durationofrecurrencesand symptoms
Treatmentgroup 95%confidenceintervalsfor differences Placebo (N=24) Acyclovir (N = 53) Isoprinosine (N =49) A-P I-P A-I Meanduration(days) of recurrences
Observation 8-7 8-2 7-7
Treatment 7-7 6 4 8-2 (-315, 0.47) (-0-82, 1-72) (-3-37, -0-21)*
Follow-up 7-4 7-6 7-2 (-151, 193) (-198, 1-48) (-0-66, 1-58)
Mean duration (days)with symptomslerythema
Observation 3-5 3.9 3-8
Treatment 7-8 10-4 10-2 (-635, 11-48) (-6-42, 11-21) (-6-90, 723)
Follow-up 10-7 8-8 8-6 (-13-40, 9.68) (-13-86, 982) (-7.97, 830)
*Significantat5%level.
During follow up, the mean number of
reportedrecurrencesperpatientwas2.8inthe acyclovirgroup, 2-9 in the
isoprinosine
group and 1.7 in the placebo group.There were no statisticallysignificant differences betweenany pairs oftreatmentgroups.Numberof days fromtreatmentonset tofirst recurrence
Themeantimetothe firstrecurrencefrom the start of treatment was estimated for each treatment group.This was
significantly longer
(p <0.05) foracyclovir recipients
(143.7days),
than forisoprinosine
recipients
(40.5 days) or for placebo recipients(56'2
days)(table
2).Themeantimetothefirstrecurrence in thepost-treatment
period, measured from the startof thetreatmentperiod
wasvirtually
the same in all three treatmentgroups.The distributions ofthe Kaplan-Meier esti-mates of proportions of recurrence-free patients showed there were significant
differ-p-0.0001 -Pb-A bo --bore~ 02 . 0.0 L ._ . . 0 W0 100 150 200
DAYFROM START OF TREATMENT Figure1 Timeto 1st recurrentepisode duringtreatmentandj
study.
ences between the acyclovir and
placebo
groups(p =0.0001) and between theacyclovir and isoprinosine groups (p=0.0001) (fig 1). There was no significant difference between the placebo and isoprinosine groups. During followup,there was no statistical evidence to suggestthat the distributions ofKaplan-Meier estimates of proportions of recurrence free patients differed betweentreatment groups (fig 2).Meandurationofrecurrences
During both the observation and post-treat-ment periods, there were no differences between thetreatment groupswithrespect to the mean duration of recurrences (table 3). However,duringtreatment,themeanduration for the acyclovir group (6.4 days) was sig-nificantly shorter (p<
0'05),
than for the isoprinosine group (8.2 days).Mean durationof symptomslerythema
The mean duration of symptoms/erythema
(that
is,
the totalnumber ofdaysperpatient, withsymptomsand/or erythema, but without lesions) was calculated for eachtreatment
group.The 95% confidence intervals for differ-ences between the means included zero and gave no evidenceforanystatistically significant differences between any pairs oftreatment
during thetreatment orfollow-upperiods. AdverseeventsIngeneral, all treatmentswere extremely well
tolerated with only six patients reporting 11
. adverse events, six in the acyclovir group (in
2Wo 300 3so threepatients),four in theisoprinosinegroup
(intwopatients) andoneinthe placebogroup.
follow-upforallpatients in These wereinvariably mild, self-limiting, and
ofdoubtful attributabilityto drug therapy.
1.0 o0 1 a# m W ma W m mU' ma 0 cc 0.6
h
OAh10r W M z "I a. 0ol--APo
0olh
0AMF
021P 160 180 200 220 240 260 280 300 320 DAYFROM START OF TREATMENTFigure2 Timetolst recurrentepisodeduring follow-up for all patients in thestudy.
Clinicallaboratoryevaluations
Themajority of patients did notsufferabi mal biochemical or haematological chan The few detected abnormal values were g
erally lower than the expected values, tl weremild,sporadic,did not follow apartic patternandusually returned tonormal.
Discussion
The results of thisstudyaresimilar to thos previous placebo controlled trials3 wi have confirmed the efficacy of acyclovii suppressingrecurrentgenital
herpes.
Isopr sine (Immunovir),animmunomodulator%lowtoxicity,has in somestudiesbeenshow reduce the severityand speed of resolutioi mucocutaneous herpes simplex react
tions.'2-17
Although it has been claimec reduce the duration of viralsheddingandtto healing in patients with primary
gei
herpes, a recent comparative study sho that acyclovir treated patients had a she duration ofsymptoms and viralshedding, healed more quickly than those treatedisoprinosine.`
However, neither prepara appearedtohave anyeffectonthe timetorecurrence or the frequency of
subseqi
recurrences.
The efficacy of a high dose isoprino regimen (1 g four times daily) has also E compared with acyclovir800mg daily for suppression of frequently recurring ger
herpes.`9
In thistrial, 100% ofpatientsrec ing isoprinosine experiencedrecurrences c paredwithonly 36% of those receivingacy virexperienced recurrencesduringtreatmo The results of the present study, usin lower comparative dose of isoprinosine, similar.Acyclovir treatedpatients experien asignificantlylower mean number ofrepo.
recurrencesduring treatment and significa longermean times tofirstrecurrence as c pared to those receiving isoprinosine or cebo.When
breakthrough recurrences du treatmentoccurred,
acyclovir treated pati had a significantly shorter mean duratior clinically diagnosed lesions than those tre. with isoprinosine.Thus,
treatment with; clovir wasmarkedly superior to treatmentiisoprinosine which did not show any dem strableadvantages overplacebo treatment. treatments were well tolerated with no
dence ofclinically significant effects on haema-tological and biochemical parameters.
The Kaplan-Meier curves for time to first recurrence in the post-treatment period were not significantly different among treatment
groups. However, a significantly higher pro-portion of patients who had received acyclovir suffered recurrences during follow up com-pared withthose treated withplacebobutnot
isoprinosine. Whilst the number of recurrences in theacyclovirtreatmentgroupduringfollow up was greater than in the placebo treatment group, the frequency of recurrences in the
30 placebogroup decreased from the observation to the treatment period, and again from the
treatment to the follow up period.This lower than
expected
ratein theplacebo
group in both the treatment and follow up periods (perhaps nor- due to small patient numbers and/or too short iges. an observation period), confounds the resultsgen-
somewhat. Nevertheless,recentresults (Baten-hese horst et al, personal communication) have ular suggestedthat inpatients receivingoral acyclo-virforsuppression ofrecurrentgenital herpes infection for periods of 4 years, when treat-mentisterminated,the time tofirstrecurrence is considerably longer than for patients who ,eof received shorter courses oftherapy.hich The results of this study demonstrate that
r in acyclovir therapy issubstantiallymoreeffective ino- than isoprinosine therapy for the suppression with of recurrent genital herpes. Similar results ,nto indicating the superiorityofacyclovirfor sup-n of pression ofrecurrent attacks have now been tiva- shown for studies of both low (1 g) andhigh 1 to (4g) daily doses of isoprinosine.
Acyclovir
is time clearlythe treatment ofchoice forthe manage-nital ment of patients with both first episode and wed recurrent genital herpes. In the latter, the rter choice between episodic treatment with self-and initiated short treatment coursesoforal acyclo-withvir``
ortopical
5%acyclovir
cream,23
24tion (both of which areofprovenclinical
efficacy)
first and continuous oral suppression, willdepend
aent uponindividualpatient
assessment notonlyof their frequencyofepisodes but also the other Isine physical, psychological and social effects of )een theirrecurrentdisease.the
nital The authorsgratefully acknowledge the participation of the
ei- following:
'om- DrJ Nabarro,Central MiddlesexHospital, London,UK;Dr0 Arya, Royal Liverpool Hospital, Liverpool, UK; Dr D C
dCIo- McDonald-Burns, Royal Free Hospital, London, UK; Dr J ent. Morias, Brussels, Belgium; Dr D Parent and Dr Lenniguin, Erasme, Brussels,Belgium; Prof Ch. Lapierre and Dr Gexeau, ig a Baviere-Dernmatologie,Liege, Belgium; Prof EVan Hecke and
are ProfKint,KlinikVoosHurdziekten,Ghent,Belgium.
iced We also acknowlege the support ofTheDepartment of Clinical
rted Statistics and DataHandling,WellcomeResearchLaboratories,
Lntly
Beckenham,
Kent,
UK.om-pla- 1 Belsey EM, Adler MW Current approaches to the diagnosis ofherpes genitalis. Br J Venereal Dis 1978;54:115-20.
ring 2 Corey L, Holmes KK, Benedetti J, Critchlow C. Clinical ents course of genital herpes. Implications for therapeutic
trials. In: NahmiasAJ, DowdleWR, Schinazi RF, eds. The
n of Hurman Herpes Virus. New York: Elsevier, 1981:
ated 496-502.
3 Douglas JM, Critchlow C, Benedetti J, et al. A double blind acy- study of oral acyclovir for suppression of recurrence of
with genital herpes simplex virus infection. N EngiJ Med
1984;310:155-6.
ion- 4Straus SE, Takiff HE, Seidlin M, etal. Suppression of All frequentlyrecurring genitalherpes.Aplacebocontrolled
double blind trial of oral acyclovir. N Engl J Med evi- 1984;310:1545-50.
5 MindelA, Weller IVD, Faherty A,etal. Prophylactic oral
acyclovir in recurrent genital herpes. Lancet 1984;ii: 57-9.
6 MindelA, Faherty A, Carney 0, Patou G, Freris M,
Williams P. Dosage and safety of longtermsuppressive acyclovir therapy for recurrent genital herpes. Lancet 1988;i:926-8.
7 Kinghorn GR, Jeavons M, Rowlands M,etal. Acyclovir
prophylaxis ofrecurrent genital herpes: a randomised
placebo controlled crossover study. Genitourin Med 1985;61:387-90.
8 Halsos AM, Salo OP, Lassus A, et al. Oral acyclovir suppression ofrecurrentgenital herpes: adouble blind
placebo controlled crossover study. Acta Derm Venerol [Stockh] 1985;65:59-63.
9 SaloOP, Lassus A. Treatment ofrecurrentgenital herpes withisoprinosine.EurJSex TransmDis 1983;i: 101-5. 10Galli M, Lazzarin A, Moroni M, Zanussi C. Inosiplex in
recurrent herpes simplex infections. Lancet 1982;ii: 331-2.
11 Editorial. Immunovirtested ingenital herpes and AIDS. Pharmaceutical,Journal1984;233:385.
12 Editorial. Inosine pranobex and mucocutaneous, herpes. Lancet1985;i:200-1.
13 Corey L, Chiang WT, Reeves WC, Starum WE, BrewerL,
Holmes KK. Effect of isoprinosine on the cellular immuneresponseininitial genital herpes infection. Clin Res1979;27:41A.
14TalbotDJ, Menday AP. Inosinepranobex and
mucocuta-neousherpes. Lancet 1985;i:877.
15 Galli M, Lazzarin A,Moroni M,Zanussi C. Inosiplex in
recurrent herpes simplex infection. Lancet 1 982;ii: 331-2.
16 Salo 0,Lassus A.Treatment ofrecurrent genital herpes with isoprinosine. Eur_J Sex Transm Dis 1983;1: 101-5.
17 Bouffat P, Saurat JH. Isoprinosineasatherapeuticagentin
recurrentmucocutaneousinfections duetoherpesvirus [Abstr].IntJr Immunopharmacol 1980;2:193.
18 Mindel A,Kinghorn GR, Allason-Jones E,etal.Treatment
of first attack genital herpes: acyclovir versus inosine pranobex.Lancet1987-i:1 171-3.
19 Mindel A, Carney0, Sonnex C, etal. Suppression of frequently recurring genital herpes: acyclovirv inosine
pranobex.Genitourin Med 1989;65:103-5.
20 NilsenAE, AssenT, Halsos AM, Kinge BR, Tjotta EAL,et
al.Efficacy of oralacyclovir in thetreatmentofinitial and
recurrentgenital herpes. Lancet 1982;ii:571-3. 21 Salo OP, Lassus A, HoviT. Double-blind
placebo-con-trolled trialof oralacyclovir inrecurrentgenital herpes. EurJSex Transm Dis1983;1:95-8.
22 ReichmanRC, Badger GJ, Mertz GJ, etal.Treatment of
recurrent genital herpes simplex infections with oral acyclovir.JAMA 1984;251:2103-7.
23 Fiddian AP, Kinghorn GR, Goldmeier D, etal. Topical acyclovir in thetreatmentof genital herpes:acomparison
with systemic therapy. _J Antimicrob Chemother 1983;12
(Suppl B):67-77.
24KinghornGR. Topical acyclovircreaminthetreatmentof
recurrentherpes simplex virus infections. Scand Infect Dis 1985;47(Suppl):58-62.