© Aliyeh Rasooli Zadeh, 2020
Understanding silicon-mediated disease resistance
through the interaction soybean-Phytophthora sojae
Thèse
Aliyeh Rasooli Zadeh
Doctorat en biologie végétale
Philosophiæ doctor (Ph. D.)
ii
Résumé
Depuis maintenant plusieurs années, il a été démontré que le silicium (Si) protège les plantes dans moult interactions hôte-agent pathogène. Cependant, les mécanismes par lesquels le Si exerce son rôle prophylactique restent flous. Dans les interactions plante-agent pathogène, en particulier dans le cas des agents biotrophes qui reposent fortement sur la formation d’haustoria et la libération d’effecteurs pour leur virulence, l’expression et la localisation des effecteurs dictent souvent le résultat de cette interaction. Il est maintenant connu que le Si s’accumule dans l’apoplaste des tissus végétaux. Étant donné que l'apoplaste est un site clé pour l’interaction entre les effecteurs des agents pathogènes biotrophes et les récepteurs membranaires des cellules végétales, nous avons émis l’hypothèse que le Si interfèrerait avec la reconnaissance effecteur / récepteur, ce qui conduirait à une résistance accrue des végétaux.
Lors de mes travaux de doctorat, nous avons préconisé une approche holistique pour l’étude de l’impact du Si dans l’interaction soya-Phytophthora sojae. Brièvement, nous avons analysé les réponses phénotypiques de l’interaction en présence de Si, nous avons effectué une étude histologique poussée sur les racines de soya infecté par P. sojae, nous avons effectué une analyse transcriptomique complète de la plante et de l’agent pathogène au fil du temps, et nous avons tenté de localiser la présence d’effecteurs au niveau subcellulaire grâce à l’immunocytochimie et le marquage à fluorescence.
Lors de ces travaux, nous avons pu observer une reconnaissance rapide de l'hôte par P. sojae grâce au développement de corps ressemblant à des haustoria, suivie de l'expression et de la libération d'effecteurs dans la région apoplastique et d'une expression élevée des gènes liés à la défense et ce, à un stade précoce. Chez les plantes préalablement traitées au Si, une pathogenèse limitée a été observée, tandis que l’expression des gènes de défense de la plante était limitée et que la présence d’effecteurs tels le Avr6 était à la baisse dans la région apoplastique.
Ces résultats indiquent que le Si interfère avec la reconnaissance de l'hôte par l'agent pathogène, ce qui entraîne une interaction incompatible.
iii
Abstract
Silicon (Si) has been shown to protect plants in a number of host-pathogen interactions, however, the mechanisms by which it exerts its prophylactic role remain elusive. In plant-pathogen interactions, especially a biotroph that relies heavily on the formation of haustorium and release of effectors for its virulence, the expression, and localization of effectors will often dictate the outcome of that interaction. Given that the apoplast is a key site of interaction between effectors and plant defenses receptors, as well as the site of amorphous-Si accumulation, it is not unlikely that Si interferes with effector/receptor recognition, which would lead to an incompatible interaction.
We have conducted a holistic approach by studying the impact of Si in the interaction soybean-Phytophthora sojae through analysis of the phenotypic responses, the histology of P.sojae-infected soybean roots, gene expression analyses for the plant and the pathogen over time, and sub-cellular localization of target effectors through immunolocalization and fluorescence-labeling.
In control plants, we observed a rapid host recognition by P. sojae through the development of the haustorium-like bodies, followed by expression and release of effectors into the apoplastic region and high expression of defense-related genes at early-stage (2-4 dpi). A Si treatment resulted in limited pathogen development, and significantly lower expression and presence of Avr6 in the apoplastic region, as well as a significant reduction in expression of plant defense genes.
These results indicate that the Si interferes with host recognition by the pathogen which translated into an incompatible interaction.
iv
Table of contents
RÉSUMÉ ... II ABSTRACT ... III TABLE OF CONTENTS ... IV LIST OF TABLES ... IX LIST OF FIGURES ... VII ACKNOWLEDGEMENTS ... X FOREWORD/ AVANT -PROPOS ... XIGeneral Introduction ... 1
INTRODUCTION ... 2
PHYTOPHTHORA SOJAE: A NOTORIOUS PATHOGEN OF SOYBEAN ... 2
HOST-PATHOGEN INTERACTION ... 3
EFFECTOR-RECEPTOR EXPRESSION PATTERN ... 3
HOST APOPLASTIC REGION; A MAJOR BATTLEFIELD ... 4
SILICON; AN ALTERNATIVE DISEASE-MANAGING APPROACH ... 5
SILICON ROLE; STRESS ALLEVIATOR ... 6
MODE OF ACTION OF SILICON; INTERFERER? ... 7
RESEARCH PROBLEM... 8
REFERENCES ... 9
CHAPTER 1: Silicon Protects Soybean Plants Against Phytophthora Sojae By Interfering With Effector-Receptor Expression ... 16 RÉSUMÉ... 17 ABSTRACT ... 18 BACKGROUND ... 19 RESULTS ... 20 Phenotypic responses ... 20
Dual RNA-seq analysis of the P. sojae-soybean interaction in the presence of Si ... 21
Soybean root transcriptome ... 21
Defense-related genes ... 22
Secondary metabolism ... 24
Hormone metabolism ... 25
Primary metabolism ... 25
Phytophthora sojae transcriptome ... 25
Annotation ... 26
v
DISCUSSION ... 28
CONCLUSION ... 32
METHODS ... 32
Plant growth conditions ... 33
Phytophthora sojae inoculation ... 33
Microscopic and X-ray analyses ... 34
RNA extraction, library construction and sequencing ... 34
RNA-Seq data analysis ... 34
LIST OF ABBREVIATIONS ... 35
ACKNOWLEDGEMENTS ... 36
REFERENCES ... 37
CHAPTER 2: Silicon Treatment Influence The Localization And Expression Of Phytophthora Sojae Effectors In Interaction With Soybean ... 42
RÉSUMÉ... 43
SUMMARY ... 44
INTRODUCTION ... 45
MATERIALS AND METHODS ... 45
Plant growth conditions and Phytophthora sojae inoculation ... 46
Light microscopy ... 46
Electron microscopy ... 48
Silicon distribution ... 48
Expression analysis... 48
Transformation vectors ... 49
Phytophthora sojae transformation ... 50
Confocal microscopic observations ... 50
Immunolocalization of Avr6 ... 50
RESULTS ... 50
Phenotypic responses ... 51
Infection process ... 51
Subcellular development and localization of Phytophthora sojae using TEM ... 58
Avr6-Rps6 gene expression analysis ... 56
Subcellular localization of Avr6 using immunolabeling assay ... 57
Quantification of subcellular Avr6 release in presence or absence of Si ... 59
DISCUSSION ... 60
ACKNOWLEDGMENTS ... 61
REFERENCES ... 64
Conclusion And Perspectives ... 71
CONCLUSION ... 72
PERSPECTIVES ... 75
vi
Annex I_Supplementary Data For Chapter 1 ... 90 Annex II_Supplementary Data For Chapter 2 ... 96
vii
List of figures
Chapter 1
Figure 1. Effect of silicon (Si) amendments on soybean plants 21 days after inoculation with
Phytophthora sojae. ... 21
Figure 2. Heat map of differentially expressed genes in Phytophthora sojae infecting soybean roots. .... 22
Figure 3. Expression profile of NB-LRRs genes. ... 23
Figure 4. Expression profile of PR genes. ... 24
Figure 5. Expression profile of hormone-related genes. ... 25
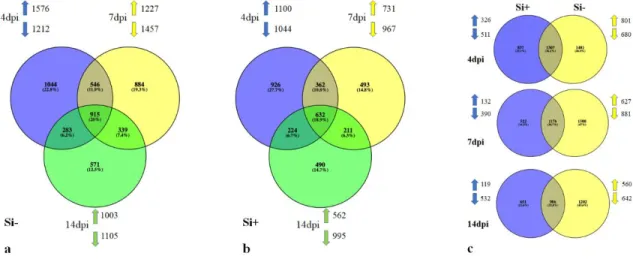
Figure 6. Venn diagram.. ... 26
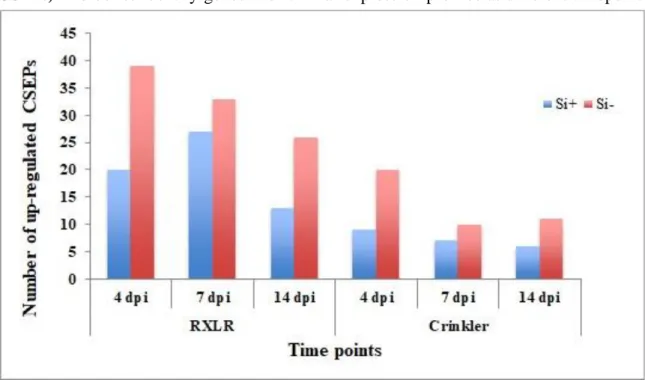
Figure 7. Number of upregulated effector genes in Phytophthora sojae over time. ... 27
Figure 8. Comparison of differentially expressed Phytophthora sojae CSEPs over timepoint. ... 28
Chapter 2 Figure 1. Scanning electron micrographs of encysted zoospores of Phytophthora sojae on inoculated soybean roots ... 52
Figure 2. Phytophthora sojae structures in soybean roots obtained by KOH- aniline blue fluorescence microscopy ... 53
Figure 3. Transmission electron microscopy images of non- infected and Phytophthora sojae-inoculated soybean roots ... 55
Figure 4. Western blot analysis of Phytophthora sojae Avr6 unique antibody. ... 57
Figure 5. Immunolocalization of the effector Avr6 in Phytophthora sojae-inoculated control or silicon-treated soybean plants. ... 58
Figure 6. The expression and subcellular localization of mCherry-tagged Avr6 in stable G418-resistant Phytophthora sojae transformant- inoculated control and silicon-treated soybean roots ... 60
viii Annexes
Annex I-Figure S1. Expression profile of signaling-related genes. ... 91
Annex I-Figure S3. Expression profile of protease inhibitors and polyphenol oxidase. ... 92
Annex I-Figure S2. Expression profile of WRKY transcription factor genes ... 92
Annex I-Figure S5. Heat map of differentially expressed genes involved in primary metabolism. ... 93
Annex I-Figure S4. Expression profile of secondary metabolism-related genes. ... 93
Annex II-Figure S1. The unique peptide sequence for Avr6 through blast result. ... 97
Annex II-Figure S2. Effect of silicon amendments on Phytophthora sojae -inoculatd plants in a compatible interaction. . ... 98
Annex II-Figure S3. SEM-EDS element analyses of soybean root ... 99
Annex II-Figure S4. The non-inoculated soybean roots were used as negative controls in confocal observation. ... 99
ix
List of tables
Chapter 2
Table 1. Relative expression of defense-related genes and Rps-6 gene candidates in silicon-treated and control soybean plants and of Avr6 gene in Phytophthora sojae. ... 60
Annexes
Annex II-Table S1. List of genes and primers sequences used in q-PCR and transformation analyses. ... 100 Annex II-Table S2. Avr6-mCherry sequences. ... 101 Annex II-Table S3. NPTII cassette sequence containing Ham34-HJV promoter, NPTII gene and Ham34
x
Acknowledgements
Undertaking this PhD has been a truly life-changing experience for me, and it would not have been possible without the support and guidance that I received from many people. I would like to take some time to thank all the people without whom this project would never have been possible. Although it is just my name on the cover, many people have contributed to this research in their own particular way and for that I want to give them special thanks. I would like to express my special appreciation and thanks to my supervisor Prof. Richard Bélanger for all the support he gave me during those years. He has been a tremendous mentor for me. His guidance helped me throughout this research project.
My sincere thanks go to Prof. François Belzile, a great teacher with all his difficult questions, which were always meant to improve my work from various perspectives.
But all of these years would not have been passed without the generous support of Ms. Caroline Labbé. I would like to say a very big thank to her. She inspired me during difficult times when I needed words of encouragement. She was always ready to guide me with a big nice smile.
My sincere thanks also go to the rest of my thesis committee: Dr. Mark Gijzen, Prof. Dominique Michaud, and Prof. François Belzile, and Prof. Richard Bélanger for their time and their insightful comments.
I’m glad to have interacted with many unique people in the lab during those years. I thank my fellow lab mates for the stimulating discussions and for all the fun we had.
Nobody has been more important to me in the pursuit of my academic path than the members of my family. I would like to thank my parents and my sister whose love and guidance are with me in whatever I pursue. They are my ultimate role models.
And finally, to Mehdi, who has been by my side throughout this PhD, living every single minute of it, and without whom, I would not have had the courage to embark on this journey in the first place. Mehdi has been a true and great supporter and has unconditionally loved me during my good and bad times. He has been non-judgmental of me and instrumental in instilling confidence. These past several years have not been an easy ride; I truly thank Mehdi for sticking by my side and there are no words to convey how much I love him.
xi
Foreword/ Avant -propos
This thesis includes two primary research articles published over the last two years in peer-reviewed scientific journals. To meet the requirements of Laval U’s Faculté des études supérieures et postdoctorales, those chapters presented in English in the document are preceded by a summary in French. A list of references is presented at the end of each chapter. Co-authors’ contributions to my articles are summarized here below.
CHAPTER 1
Silicon protects soybean plants against Phytophthora sojae by interfering with effector-receptor expression
Aliyeh Rasoolizadeh, Caroline Labbé, Humira Sonah, Rupesh K. Deshmukh, François Belzile, James G. Menzies and Richard R. Bélanger
Les travaux et la rédaction de cet article ont été faits sous la supervision du Prof. Bélanger. Il a été publié en 2018 dans le périodique scientifique BMC Plant Biology. J'ai bénéficié pendant ces travaux de l'aide précieuse de notre professionnelle de recherche, Mme Caroline Labbé et de Mme Humira Sonah, qui m’ont assistée aussi bien pour la réalisation des expériences que pour l’analyse des données.
CHAPTER 2
Localization and expression of effectors during the infection process of Phytophthora
sojae dictate interaction with soybean under silicon treatment
Aliyeh Rasoolizadeh, Parthasarathy Santhanam, Caroline Labbé, Shivaraj Sheelavanta Matha, Hugo Germainand Richard R. Bélanger
Cet article a été rédigé sous la supervision du Professeur Richard Bélanger. Les résultats de ces travaux ont été publié en 2020 dans le périodique Journal of Experimental Botany. Les travaux ont été réalisés en étroite collaboration avec Dr M. Santhanam, un chercheur postdoctoral du laboratoire. Il a travaillé en particulier sur la transformation de Phytophthora sojae et le marquage mCherry. J'ai bénéficié lors de ces travaux de l'aide précieuse de notre professionnelle de recherche, Mme Caroline Labbé, pour la préparation d'échantillons et l'observation par microscopie optique. J'ai bénéficié de l'aide du Professeur Hugo Germain qui m’a assistée aussi bien pour la réalisation de la transformation de P. sojae que pour l’analyse des données.
General Introduction
2
Introduction
Oomycete plant pathogens are destructive against a vast variety of plants important to agriculture, forestry, and natural ecosystems. Among them, Phytophthora sojae (Kauf. & Gerd.), a hemibiotroph, is particularly devastating on its primary host, soybean. Phytophthora sojae causes root and stem rot, and pre- and post-emergence damping-off, causing considerable problems for the agricultural industry. This soil-borne plant pathogen can cause losses of $1 to $2 billion per year worldwide (Tyler et al., 2007). Most Phytophthora species, including P. sojae, attack underground parts of the plant where control methods are often difficult to apply. Once, P. sojae establishes its presence in the field, its eradication seems is nearly impossible with current control methods. Presently, seed-treatment fungicides and resistant varieties are the most commonly used disease management methods. Mefenoxam and metalaxyl, two very similar compounds, can both be used as seed treatments (Dorrance & McClure, 2001). Many oomycete species have developed resistance against those compounds and are no longer sensitive. Several studies have shown no benefit of seed treatment with metalaxyl for some soybean cultivars (Anderson et al., 1982; Guy & Oplinger, 1989; Guy et al., 1989). To date, numerous defense-associated genes have been reported to enhance resistance to P. sojae in soybean. In essence, this pathogen has been controlled successfully with single resistance genes termed Rps (Resistant to Phytophthora sojae) over the years. To date, Rps genes including Rps-1a, Rps1c, Rps-1k, Rps-3a, Rps-6, and Rps-7 are the most commonly deployed commercially (Dorrance & McClure, 2001). While the integration of Rps genes into soybean cultivars is a well-known approach to control the disease, the selection pressure exerted on P. sojae has led to the emergence of new virulence forms or pathotypes. As a result, the population of P. sojae isolates capable of establishing a compatible interaction against soybean lines carrying Rps genes is increasing. This fact forces producers to rely on additional disease management strategies to ensure minimal yield losses. Owing to its economic importance, this species has been widely used as a model species for the study of plant- oomycete pathogen interactions.
Phytophthora sojae: a notorious pathogen of soybean
Phytophthora sojae can disperse by water-borne zoospores and soil-borne oospores. Zoospores are the main means of dispersal, especially when the soil is flooded. The zoospores swim chemotactically toward isoflavone compounds released from the roots of soybean plants. They encyst on the root surface from where the hyphae penetrate the root directly from the cyst (Tyler et al., 2007). Phytophthora sojae can produce specialized hyphal branches called haustoria that invaginate the plant cell and induce the formation of a plant-derived extrahaustorial membrane with the extrahaustorial matrix (Bushnell, 1972; Szabo & Bushnell, 2001). Within this extrahaustorial matrix, water and nutrients are exchanged between the pathogen and the host (Voegele & Mendgen, 2003). The extracellular space is
3
considered important for perception of plant signals by the pathogen and the trafficking of secreted proteins, including effector proteins (Ellis et al., 2006).
Host-Pathogen Interaction
Upon host perception, P. sojae secretes effector proteins into the apoplastic region of plant cells, to promote its development during the biotrophic stage and to induce necrosis during the necrotrophic phase, resulting in effector-triggered susceptibility (Hann et al., 2010; Wang et al., 2011; Dou & Zhou, 2012; Rafiqi et al., 2012; Ma et al., 2017), these can be translocated into the cytoplasm through the cell membrane or the extrahaustorial matrix (EHM, Bozkurt et al., 2012).) These small effector proteins are encoded by Avr genes mostly with RXLR and dEER amino acid motifs (Dong et al., 2011; Bozkurt et al., 2012; Arsenault-Labrecque et al., 2018). In P. sojae more than 14 Avr genes have already been identified (May et al., 2002; Arsenault-Labrecque et al., 2018). In plant-pathogen interactions, effectors are recognized as important virulence factors that are utilized by the pathogen to neutralize plant defense systems namely as PAMPs (Pathogen-Associated Molecular Patterns)-Triggered Immunity (PTI) and Effector-Triggered Immunity (ETI) or/ and change host metabolism so that it can easily colonize in plant tissues (Dou et al., 2008; Bozkurt et al., 2012; Wang et al., 2018). The secretion of specific effector proteins (encoded by specific Avr genes) into the apoplastic area of plant cells, which carry the corresponding R gene, can induce gene-for-gene resistance. However, natural selection often favors the pathogen, which will eventually avoid recognition by R genes through loss or various mutations of the effector and/or by acquisition or activation of additional effectors or decoy proteins that can avoid ETI (Jones & Dangl, 2006; Ma et al., 2017). The coevolutionary dynamics between pathogens and plants have resulted in structurally and functionally diverse families of effectors and immune receptors that are encoded by rapidly evolving genes (Bozkurt et al., 2012; Arsenault-Labrecque et al., 2018). Thus, understanding the pattern of their expression, sub-cellular localization, host-receptors interaction and the mechanisms by which effectors disturb plant processes is essential for developing an alternative or supportive approach for managing and controlling the disease and achieve sustainable resistant plant.
Effector-Receptor expression pattern
Being a hemibiotroph, P. sojae will initiate the infection cycle as a biotroph and switch to a necrotrophic lifestyle at later stages. However, little is known about the molecular mechanisms that promote each phase, or those that regulate the transition between the two stages. Recent advances in effector biology revealed that P. sojae genome contains genes encoding ~ 400 potential RxLR effectors with distinct functional properties and explain their contribution to virulence during different stages of infection by their sequential expression into host plant cells (Koeck et al.,
General Introduction
4
2011; Wang et al., 2011). Notably, the products of a small number of effector repertoire are indispensable for full virulence (Wang et al., 2011). Transcriptomic analysis along with functional interactions among the effectors of P. sojae revealed three temporal secretion patterns of effectors upon host recognition namely as biotrophic, transition and necrotrophic effectors (Wang et al., 2011). The expression of biotrophic effectors is particularly strong at the outset of infection when the pathogen tries to suppress or/and avoid plant defense responses (Mendgen et al., 2002; Panstruga et al., 2003). For instance, previously reported biotrophic effectors, Avr1b, Avh324, PsCRN115, PsXEG1 and its decoy (PsXLP1) are among the highly expressed effectors during the early stage of infection (Dou et al., 2008; Wang et al., 2011; Zhang et al., 2015; Ma et al., 2017). The high activation of defense-related genes in infected plants during the early stage of infection provides evidence of an ongoing battle between plant and pathogen (Moy et al., 2004). The high activation of most of host pattern recognition receptors (PRRs) including; transmembrane receptor kinases and receptor-like kinases (RLKs; Moy et al., 2004; Dodds et al., 2010; Lanubile et al., 2015), Pathogenesis-Related proteins (PRs) and Nucleotide-Binding Leucine-Rich Repeat (NB-LRR; Radwan et al., 2011) have been reported in soybean interaction with pathogen.
Other effectors categorized as necrosis-inducing effectors, which facilitate cell death such as Avh238 and PsCRN63 (Wang et al., 2011; Zhang et al., 2015), showed higher expression during the necrotrophic phase, where the plant usually reacts with an activation of the jasmonic acid (JA) metabolism (Glazebrook, 2005).
Little is known about the function of the weakly expressed effector genes, which comprise about 80% of the effector repertoire. Considering that some of well-known avirulence genes such as Avrla (Qutob et al., 2009), Avr3a (Qutob et al., 2009), and Avrlk (Kale et al., 2010) that can be recognized by specific Rps genes are among this group, further functional analyses are deemed essential.
Host apoplastic region; a major battlefield
Plants are constantly attacked by and exposed to a wide variety of microbes. While most of these interactions will lead to a non-host recognition (Thordal-Christensen, 2003; Kirankumar & Choong, 2004), a sophisticated innate immune system enables plants to circumvent potential pathogens (Jones & Dangl, 2006). When a pathogen successfully overcomes the first physical layer, namely the cuticle and cell wall, it encounters various defense components and receptors in the apoplastic region of plant. The apoplastic space is recognized as a fertile battlefield of host-pathogen exchanges. For instance, to counteract plant hydrolases and protease inhibitors and cell-membrane defense receptors, the pathogen secretes an arsenal of effectors into the apoplastic area, some of which being recognized by plant receptors, leading to the activation of an array of defense responses (Birch et al., 2006; 2009; Jones & Dangl, 2006; Hogenhout et al., 2009). Diverse strategies, including the release
5
of paralogous decoy effectors protect P. sojae apoplastic effectors from host perception and lead to a successful invasion by the pathogen (Ma et al., 2017; Wang & Wang, 2018). In addition of pathogen-driven immune suppression mechanisms, the presence of free water in the apoplastic region favors the establishment of the pathogen (Xin et al., 2016). These lines of evidence emphasize the importance of the apoplastic region in host-pathogen interactions. While it is generally agreed that the collective activity of apoplastic effectors determines the outcome of a given host-pathogen interaction, their subcellular localization after initial release, including the prospect of cytoplasmic translocation, remains elusive.
The mechanisms of RXLR effector entry into plant cells remain under debate. The notion of hypersensitive response (HR) and programmed cell death (PCD) defining an incompatible interaction is interpreted as an evidence to support the cytoplasmic translocation of effectors where they interact with intracellular R gene products. The hypersensitive reaction as an indicator of defense response is questionable, since one should observe numerous lesions on the root surface as a result of PCD, each time a pathogen attempts to infect a resistant plant. The conflicting models for effector translocation, particularly the pathogen-independent manner through binding to phosphorylated lipids (Kale et al., 2010), are debatable based on the lack of reproducibility of the experiments (Yaeno et al., 2011; Bozkurt et al., 2012; Wawraa et al., 2012). Many scientists have tried to identify the sub-cellular localization of effectors by transient expression in N. benthamiana and observed their accumulation around haustoria and extrahaustorial membrane (Bozkurt et al., 2011; Caillaud et al., 2012). Thus, understanding the subcellular localization of P. sojae effectors in their host remains a challenge, while its elucidation could pave the way to develop better approaches for managing and controlling the disease.
Silicon; an alternative disease-managing approach
Silicon (Si) is a ubiquitous element and the second most abundant after oxygen in soil, comprising approximately 28 % of the Earth’s crust (Epstein, 1999; Řezanka & Sigler, 2008; Broadley et al., 2011). Despite this, most sources of Si in soil are present as crystalline aluminosilicates, which are insoluble and not directly available for plants (Richmond & Sussman, 2003). In soil, only a small portion of Si is plant-available in the form of orthosilicic acid (Si(OH)4) at 25°C at concentrations normally ranging from 0.1 to 0.6 mM (Casey et al., 2004; Sommer et al., 2006; Bauer et al., 2011). At physiological pH, the maximum concentration of Si(OH)4 in solution is c. 2mM at 25°C; above this level polymerization into silica (SiO2) gel occurs (Ma et al., 2001). Plants take up Si in the form of monomeric, uncharged orthosilicic acid (Si(OH)4), as the only molecular species likely to cross the root plasma membrane at physiological pH (Raven, 2001). Following Si uptake into the root symplast, Si is translocated upwards to the shoots through the xylem vessels via the transpiration stream. Si in the xylem sap is present in the form of monosilicic acid (Casey et al., 2003; Mitani et al., 2005). In the aerial parts of the plant, silicic acid is further
General Introduction
6
concentrated through loss of water (transpiration) and polymerize to amorphous silica [(SiO2) n × n H2O; known as opal or phytolith] either in the apoplastic area of leaf cells (Iwasaki et al., 2002) and/or in silica-specific cells (Pelega et al., 2010).
Silicon content in plant tissues varies greatly according to plant species, soil properties, Si source and Si amount (Hodson et al., 2005; Deshmukh et al., 2013, Coskun et al., 2019). These differences in Si concentrations in shoots are explained by the different ability of plants to uptake Si from the roots and these can range from 0.1 % to 10 % (on a dry weight basis; Epstein, 1994; Ma et al., 2006). Plants have been classified into three categories as low, intermediate and high Si accumulators based on the Si content found in leaf tissues (Hodson et al., 2005; Wu et al., 2006). Studies show that Si concentration is usually higher in monocot than in dicot species, and monocots are classified as either intermediate or high accumulators. For dicots, many of them are unable to accumulate Si and belong to the low accumulator category with the notable exception of the Cucurbitaceae that are able to absorb more than 1% Si (Epstein, 1999; Hodson et al., 2005; Mitani et al., 2011). The identification of two major genes governing influx and efflux transport of Si in rice by Ma et al. (2006; 2007) has enhanced the general understanding of the molecular mechanisms involved in Si uptake by plants. In the model proposed in rice, Si enters the plant from the external environment in the form of Si(OH)4 through specific influx channels (termed Lsi1) which are localized at the distal side of the plasma membrane of both exodermal and endodermal cells of roots where the Casparian strips are located. For its part, efflux transporters (termed Lsi2), which are localized on the proximal side of the same cells as Lsi1, mediate the efflux of silicic acid out of the cell and load it into the xylem through an ATP-consuming H+ pump (Ma et al., 2007). Inside the xylem, silicic acid [Si(OH)4] will follow the evapotranspiration stream and finally will be deposited in the form of amorphous silica in the apoplast of leaf cells (Ghanmi et al., 2004, Bauer et al., 2011, Coskun et al., 2019).
In soybean, Deshmukh et al. (2013) have identified Si transporters, thus confirming that the species is able to absorb Si. They observed intraspecies variations in terms of Si content in three soybean cultivars ranging from 0.4 to 2.4 %.
Silicon role; stress alleviator
Despite Si ubiquity and abundance in both soils and plants, Si is not considered by physiologists as essential to the life cycle of higher plants (Epstein, 1999), based on the criteria of essentiality described by Arnon and Stout (1939). At the physiological level, transcriptomic analyses suggest that the effects of Si were nil or minimal, in absence of stress, on plant growth and function or metabolic activity (Watanabe et al., 2004; Fauteux et al., 2006; Chain et al., 2009). However, numerous studies have highlighted the prophylactic effects of Si fertilization against plant diseases (Bélanger et al., 2003; Fauteux et al., 2005;
7
Ghanmi et al., 2006; Vivancos et al., 2015; Coskun et al., 2019) and the list of plant– pathogen interactions influenced by Si keeps expanding.
Based on the literature, there is evidence that Si has a better efficiency against fungal pathogens compared to bacteria, virus or nematodes. Among fungal pathogens, those described as biotrophic or hemibiotrophic, such as powdery mildews and Magnaporthe grisea, are arguably the most often cited diseases controlled by Si. In the case of soybean, Arsenault-Labrecque et al. (2012) have shown that a Si treatment was effective against soybean rust caused by the biotrophic fungus Phakopsora pachyrhizi. Lemes et al. (2011) used Si-enriched soil against soybean rust. Results showed that the area under the disease progress curve (AUDPC) in plants treated by Si was lower than that in plants not receiving Si treatments. They also showed that Si applied as a soil fertilizer was more effective than foliar applications, a result attributed to the presence of Si transporters in roots.
Mode of action of silicon; Interferer?
In spite of the major strides explaining the transport of Si in plants, its mode of action remains controversial. Various hypotheses explaining its properties have been proposed over the years starting with the mechanical barrier, which suggests that the deposition of silica polymers (amorphous silica) in intra and inter cellular spaces and accumulation in the cell wall matrix and even inside the cells (Kaufman et al., 1985) can strengthen the cell and produce more rigid cells that can act as a mechanical barrier impeding fungal and pest progress (Wagner et al., 1940, Carver et al., 1994; 1987; Ishiguro, 2001; Moraes et al., 2005). This hypothesis is still sometimes reported today, despite the fact that Okuda & Takahashi (1990) declared it as obsolete based on results showing no sufficient increase in leaf toughness to retard fungal penetration following Si application. A second hypothesis, defining Si as a priming agent able to induce defense responses such as lignin, phenolic compounds and phytoalexins in presence of the pathogen, supplanted the mechanical barrier and was supported in many studies (Cherif et al., 1992; Cherif et al.,1994; Fawe et al., 1998; Fawe et al., 2001; Ghanmi et al., 2004; Rodrigues et al., 2004; Fauteux et al., 2005; Van Bockhaven et al., 2013). However, using transformed Arabidopsis plants with a high ability to absorb Si but unable to mount defense reactions through the SA pathway, Vivancos et al. (2015) concluded that Si did not act as a secondary messenger to protect plants against powdery mildew (Erisyphe cichoracearum). They rather proposed that Si somehow interfered with host recognition. Knowing that biotrophic and hemibiotrophic pathogens select their host range precisely through the release of effectors, it was suggested that Si prevented this recognition and thus subsequent infection. However, conclusive evidence supporting this hypothesis has yet to be reported.
General Introduction
8
Research problem
To better understand the complex interaction between a host and its pathogen, and particularly between receptors and effectors, and to decipher how Si would interfere with this interaction, the complex soybean-P. sojae offers many advantages. First, soybean is now well recognized for its ability to absorb Si (Deshmukh et al., 2013), and the prophylactic effects of Si on the soybean-P. sojae interaction has been established (Guérin et al., 2014). Secondly, soybean and P. sojae have well defined gene-for-gene interactions. Thirdly, both genomes have been reported together with the sequences of specific Rps and Avr genes. Consequently, the exploitation of this knowledge and available tools, offers a unique opportunity to address contemporary challenges that have eluded scientists for many years.
In this doctoral thesis, we report for the first time the detailed comparative infection process of P. sojae between soybean plants treated or not with Si through phenotypic responses, dual transcriptomic analysis and comprehensive microscopy observations over time. We further follow the expression of specific Avr and Rps gene expression in the context of an incompatible interaction and Si amendments. Finally, we document for the first time the sub-cellular localization of an Avr gene through immunolocalization and fluorescence-labelling and how Si interfered with the process. Our results reveal novel observations into the temporal and spatial release of P. sojae effectors furthering our understanding of the complex mechanisms by which Si alters host-pathogen interactions.
9
References
Anderson TR, Buzzell RI. 1982. Efficacy of metalaxyl in controlling Phytophthora root and
stalk rot of soybean cultivars differing in field tolerance. Plant Disease 66:1144-5.
Arnon DID, Stout PRP. 1939. The essentiality of certain elements in minute quantity for
plants with special reference to copper. Plant Physiology 14: 371–5.
Arsenault-Labrecque G, Menzies JG, Bélanger RR. 2012. Effect of silicon absorption on
soybean resistance to Phakopsora pachyrhizi in different cultivars. Plant Disease 96: 37-42.
Arsenault-Labrecque G, Sonah H, Lebreton A, Labbé C, Marchand G, Xue A, Belzile F, Knaus B, Grünwald N, Bélanger RR. 2018. Stable predictive markers for Phytophthora sojae avirulence genes that impair infection of soybean uncovered by whole genome sequencing of 31 isolates. BMC Biology 16.
Bauer P, Elbaum R, Weiss IM. 2011. Calcium and silicon mineralization in land plants:
transport, structure and function. Plant Science 180: 746–56.
Bélanger RR, Benhamou N, Menzies JG. 2003. Cytological evidence of an active role of
silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici). Phytopathology 93: 402–12.
Birch PR, Armstrong M, Bos J et al. 2009. Towards understanding the virulence functions
of RXLR effectors of the oomycete plant pathogen Phytophthora infestans. Journal of Experimental Botany 60: 1133–40.
Birch PRJ, Rehmany AP, Pritchard L, Kamoun S, Beynon JL. 2006. Trafficking arms:
oomycete effectors enter host plant cells. trends in microbiology 14: 8–11.
Bozkurt TO, Schornack S, Banfield MJ, Kamoun S. 2012. Oomycetes, effectors, and the
all that jazz. Current Opinion in Plant Biology 15: 483–92.
Bozkurt TO, Schornack S, Win J, Shindo T, Ilyas M, Oliva R, Cano LM, Jones AM, Huitema E, van der Hoorn RA et al. 2011. Phytophthora infestans effector AVRblb2 prevents secretion of a plant immune protease at the haustorial interface. Proceedings of the
National Academy of Sciences 108: 20832-7.
Broadley M, Brown P, Cakmak I, Feng MJ, Zed R, Zhao F. 2011. 8 Beneficial Elements.
In P. Marschner, ed. Marschner's Mineral Nutrition of Higher Plants. Elsevier Ltd. 249–70.
Bushnell WR. 1972. Physiology of fungal haustoria. Annual Review of Phytopathology 10:
151–76.
Caillaud MC, Piquerez SJ, Fabro G, Steinbrenner J, Ishaque N, Beynon J, Jones JD. 2012. Subcellular localization of the Hpa RxLR effector repertoire identifies a
tonoplast-General Introduction
10
associated protein HaRxL17 that confers enhanced plant susceptibility. Plant Journal 69: 252-65.
Carver TLW, Ingerson-Morris SM, Thomas BJ, Gay AP. 1994. Light-mediated delay of
primary haustorium formation by Erysiphe graminis f sp avenae. Physiological and Molecular Plant Pathology 45: 59–79.
Carver TLW, Zeyen RJ, Ahlstrand GG. 1987. The relationship between insoluble silicon
and success or failure of attempted primary penetration by powdery milde (Erysiphe graminis) germlings on barley. Physiological and Molecular Plant Pathology 31: 133–48.
Casey WH, Kinrade SD, Kinght TG, Rains DW, Epstein E. 2003. Aqueous silicate
complexes in wheat, Triticum aestivum L. Plant Cell and Environment 27: 51–4.
Chain F, Cote-Beaulieu C, Belzile F, Menzies JG, Belanger RR. 2009. A comprehensive
transcriptomic analysis of the effect of silicon on wheat plants under control and pathogen stress conditions. Molecular Plant–Microbe Interactions 22: 1323–30.
Cherif M, Asselin A, Bélanger RR. 1994. Defense responses induced by soluble silicon in
cucumber roots infected by Pythium spp. Phytopathology 84: 236–42.
Chérif M. 1992. Use of potassium silicate amendments in recirculating nutrient solutions to
suppress Pythium ultimum on long English cucumber. Plant Disease 76: 1008–11.
Coskun D, Deshmukh RK, Sonah H, Menzies JG, Reynolds O, Ma JF, Kronzucker HJ, Bélanger RR. 2019. The controversies of silicon’s role in plant biology. New Phytologist 221: 67–85.
Deshmukh RK, Vivancos J, Guérin V, Sonah H, Labbé C, Belzile F, Bélanger RR. 2013.
Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Molecular Biology 83: 303–15.
Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant-pathogen
interactions. Nature Reviews Genetics 11: 539–48.
Dong S, Kong G, Qutob D, Yu X, Tang J, Kang J, et al. 2012. The NLP toxin family in
Phytophthora sojae includes rapidly evolving groups that lack necrosis-inducing activity. Molecular Plant-Microbe Interactions 25: 896–909.
Dou D, Kale SD, Wang X, Jiang RHY, Bruce NA, Arredondo FD, Zhang X, Tyler BM. 2008. RxLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does
not require pathogen-encoded machinery. Plant Cell 20:1 930–47.
Dou D, Zhou JM. 2012. Phytopathogen effectors subverting host immunity: different foes,
11
Dorrance AE, McClure SA. 2001. Beneficial effects of fungicide seed treatments for
soybean cultivars with partial resistance to Phytophthora sojae. Plant disease 85: 1063.
Ellis J, Catanzariti AM, Dodds P. 2006. The problem of how fungal and oomycete
avirulence proteins enter plant cells. Trends in Plant Science 11: 61–3.
Epstein E. 1994. The anomaly of silicon in plant biology. Proceedings of the National
Academy of Sciences, USA 91: 11–7.
Epstein E. 1999. Silicon. Annual Review of Plant Physiology and Plant Molecular Biology 50: 641–4.
Exley C. 2015. A possible mechanism of biological silicification in plants. Frontiers in Plant
Science 6: 853.
Fauteux F, Chain F, Belzile F, Menzies JG, Belanger RR. 2006. The protective role of
silicon in the Arabidopsis–powdery mildew pathosystem. Proceedings of the National Academy of Sciences, USA 103: 17554–9.
Fauteux F, Rémus-Borel W, Menzies JG, and Bélanger RR. 2005. Silicon and plant
disease resistance against pathogenic fungi. FEMS Microbiology Letters 249: 1–6.
Fawe A, Abou-Zaid M, Menzies JG, Bélanger RR. 1998. Silicon-mediated accumulation
of flavonoid phytoalexins in cucumber. Phytopathology 88: 396–401.
Fawe A, Menzies JG, Chérif M, Bélanger RR. 2001. Silicon and disease resistance in
dicotyledons. In: Datnoff LE, Snyder GH, Korndöfer GH, editors. Silicon in agriculture. Amsterdam: Elsevier p. 159–70.
Ghanmi D, McNally DJ, BenhamouN, Menzies JG, B_elanger RR. 2004. Powdery
mildew of Arabidopsis thaliana: a pathosystem for exploring the role of silicon in plant– microbe interactions. Physiological and Molecular Plant Pathology 64: 189–99.
Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic
pathogens. Annual review of phytopathology 43:205–27.
Guérin V, Lebreton A, Cogliati EE, Hartley SE, Belzile F, Menzies JG, Bélanger RR. 2014. A zoospore inoculation method with Phytophthora sojae to assess the prophylactic role
of silicon on soybean cultivars. Plant Disease 98: 1632–8.
Guy SO, Oplinger ES, Grau CR. 1989a. Soybean cultivar response to metalaxyl applied in
furrow and as a seed treatment. Agronomy Journal 81: 529-32.
Guy SO, Oplinger ES. 1989b. Soybean cultivar performance as influenced by tillage system
and seed treatment. Journal of Production Agriculture 2: 57-62.
Hann DR, Gimenez-Ibanez S, Rathjen JP. 2010. Bacterial virulence effectors and their
General Introduction
12
Hodson MJ, White PJ, Mead A, Broadley MR. 2005. Phylogenetic variation in the silicon composition of plants. Annals of Botany 96: 1027–46.
Hogenhout SA, Van der Hoorn RA, Terauchi R, Kamoun S. 2009. Emerging concepts in
effector biology of plant-associated organisms. Molecular Plant- Microbe Interactions 22: 115–22.
Ishiguro K. 2001. Review of research in Japan on the roles of silicon in conferring resistance
against rice blast. Studies in Plant Science 8: 277–91.
Iwasaki K, Maier P, Fecht M, Horst WJ. 2002. Leaf apoplastic silicon enhances
manganese tolerance of cowpea (Vigna unguiculate). Journal of plant physiology 159: 167– 73.
Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444: 323–9.
Kale SD, Gu B, Dou D, Feldman E. 2010. External lipid PI3P mediates entry of eukaryotic
pathogen effectors into plant and animal host cells. Cell 142: 284–95.
Kaufman PB, Dayanandan P, and Franklin CI. 1985a. Structure and function of silica
bodies in the epidermal system of grass shoots. Annals of Botany 55: 487-507.
Kirankumar SM, Choong R. 2004. Non- host resistance: how much do we know? Trends
in Plants Science 9: 97–104.
Koeck M, Hardham AR, Dodds PN. 2011. The role of effectors of biotrophic and
hemibiotrophic fungi in infection. Cell Microbiol 13: 1849–57.
Lanubile A, Muppirala UK, Severin AJ, Marocco A, Munkvold G. 2015. Transcriptome profiling of soybean (Glycine max) roots challenged with pathogenic and non-pathogenic isolates of Fusarium oxysporum. BMC Genomics 16:1089.
Lemes E, Mackowiak CL, Blount A, Marois JJ, Wright DL, Coelho L, and Datnoff LE. 2011. Effects of silicon applications on soybean rust development under greenhouse and field
conditions. Plant Disease 95: 317-24.
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, and Yano M. 2006. A silicon transporter in rice. Nature 440: 688-91.
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, and Yano M. 2007. An efflux transporter of silicon in rice. Nature 448: 209-12.
Ma Z, Lin Z, Tianqiaoc S, Yang W, Qi Z, Yeqiang X, Min Q, Yachun L,Haiyang L, Liang K, Yufeng F et al. 2017. A Paralogous decoy protects Phytophthora sojae apoplastic
13
MaJF, Miyake Y, Takahashi E. 2001. Silicon as a beneficial element for crop plants.In:
Datnoff LE, Snyder GH, Kornd€orfer GH, eds. Silicon in agriculture. New York, NY, USA: Elsevier Science, 17–39.
May KJ, Whisson SC, Zwart RS, Searle IR, Irwin JAG, MacLean DJ, et al. 2002.
Inheritance and mapping of 11 avirulence genes in Phytophthora sojae. Fungal Genetics and Biology 37: 1–12.
Mendgen K, Hahn M. 2002. Plant infection and the establishment of fungal biotrophy.
Trends in Plant Science 7: 352–6.
Mitani N, Ma JF, Iwashita T. 2005. Identification of silicon form in the xylem of rice
(Oryza sativa L.). Plant Cell Physiology 46: 279–83.
Mitani N, Yamaji N, Ago Y, Iwasaki K, and Ma JF. 2011. Isolation and functional
characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. The Plant Journal 66: 231-40.
Moraes JC, Goussain MM, Carvalho GA, Costa RR. 2005. Feeding non-preference of the
corn leaf aphid Rhopalosiphum maidis (Fitch, 1856) (Hemiptera: Aphididae) to corn plants (Zea mays L.) treated with silicon. Ciênciae Agrotecnologia 29: 761-6.
Moy P, Qutob D, Chapman BP, Atkinson I, Gijzen M. 2004. Patterns of gene expression
upon infection of soybean plants by Phytophthora sojae. Molecular plant-microbe interactions 17: 1051–62.
Okuda A, Takahashi E. 1965. The role of silicon. The mineral nutrition of the rice plant:
Symposium, International Rice Research Institute (IRRI). Baltimore, MD, USA: The Johns Hopkins Press, 123–146.
Panstruga R. 2003. Establishing compatibility between plants and obligate biotrophic
pathogens. Current Opinion in Plant Biology 6: 320–6.
Pelega Z, Sarangaa Y, Fahimab T, Aharonic A, Elbauma R. 2010. Genetic control over
silica deposition in wheat awns. Physiologia Plantarum 140: 10-20.
Qutob D, Tedman-Jones J, Dong S, Kuflu K, Pham H, Wang Y, Dou D, Kale SD, Arredondo FD, Tyler BM, Gijzen M. 2009. Copy number variation and transcriptional
polymorphisms of Phytophthora sojae RXLR effector genes Avrla and Avr3a. PLoS ONE 4: e5066
Radwan O, Liu Y, Clough J. 2011. Transcriptional analysis of soybean root response to
Fusarium virguliforme, the causal agent of sudden deathsyndrome. Molecular plant-microbe interactions 24: 958.
General Introduction
14
Rafiqi M, Ellis JG, Ludowici VA, Hardham AR, Dodds PN. 2012. Challenges and
progress towards understanding the role of effectors in plant-fungal interactions. Current Opinion in Plant Biology 15: 477–82.
Řezanka T, Sigler K. 2008. Biologically active compounds of semi-metals. Phytochemistry 69: 585–606.
Richmond KE, Sussman M. 2003. Got silicon? The non-essential beneficial plant nutrient.
Current Opinion in Plant Biology 6: 268–72.
Rodrigues FA, Benhamou N, Datnoff LE, Jones JB, Bélanger RR. 2003. Ultrastructural
and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology 93: 535-46.
Szabo LJ, Bushnell WR. 2001. Hidden robbers: the role of fungal haustoria in parasitism of
plants. Proceedings of the National Academy of Sciences of the United States of America 98: 7654–5.
Thordal-Christensen H. 2003. Fresh insights into processes of non-host resistance. Current
Opinion in Plant Biology 6: 351–7.
Tyler BM. 2007. Phytophthora sojae: root rot pathogen of soybean and model oomycete.
Molecular Plant Pathology 8: 1-8.
Van Bockhaven J, de Vleesschauwer D, Höfte M. 2013. Towards establishing
broad-spectrum disease resistance in plants: silicon leads the way. Journal of Experimental Botany
64: 1281–93.
Vivancos J, Labbé C, Menzies JG, and Bélanger RR. 2015. Silicon‐mediated resistance
of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA) ‐dependent defence pathway. Molecular Plant Pathology 16: 572-82.
Voegele RT, Mendgen K. 2003. Rust haustoria: nutrient uptake and beyond. New
Phytologist 159: 93–100.
Wagner F. 1940. The importance of silicic acid for the growth of some cultivated plants,
their metabolism, and their susceptibility to true mildews. Phytopathologische Zeitschrift 12: 427–79.
Wang Q, Han C, Ferreira AO, Yu W, Tripathy S, Kale SD, Gu B, Sheng Y, Sui Y, et al. 2011. Transcriptional programming and functional interactions within the Phytophthora
sojae RxLR effector repertoire. Plant Cell 23: 2064–86.
Wang Y, Wang Y. 2018. Trick or treat: microbial pathogens evolved apoplastic effectors
15
Watanabe S, Shimoi E, Ohkama N, Hayashi H, Yoneyama T, Yazaki J, Fujii F, Shinbo K, Yamamoto K, Sakata K, Sasaki T, Kishimoto N, Kikuchi S, Fujiwara T. 2004.
Identification of several rice genes regulated by Si nutrition. Soil Science and Plant Nutrition
50:1273–6.
Wawraa S, Bain J, Durward E, Bruijna I, Minora K et al. 2012. Host-targeting protein 1
(SpHtp1) from the oomycete Saprolegnia parasitica translocates specifically into fish cells in a tyrosine-O-sulphate–dependent manner. Proceedings of the National Academy of Sciences 109: 2096.
Wu QS, Wan XY, Su N, Cheng ZJ, Wang JK, Lei CL, Zhang X, Jiang L, Ma JF, and Wan JM. 2006. Genetic dissection of silicon uptake ability in rice (Oryza sativa L.). Plant
Science 171: 441-8.
Xin XF, Nomura K, Aung K, Velásquez AC, Yao J, Boutrot F, Chang JH, Zipfel C, Sheng Yang H. 2016. Bacteria establish an aqueous living space in plants crucial for
virulence. Nature 539: 524–9.
Yaeno T, Hua L, Angela C, Sebastian S, Seizo K, et al. 2011. Phosphatidylinositol
monophosphate-binding interface in the oomycete RXLR effector AVR3a is required for its stability in host cells to modulate plant immunity. Proceedings of the National Academy of Sciences 108:14682–7.
Zhang M, Li Q, Liu T, Liu L, Shen D, Zhu Y, Liu P, Zhou J M and Dou D. 2015. Two
Cytoplasmic Effectors of Phytophthora sojae Regulate Plant Cell Death via Interactions with Plant Catalases. Plant Physiology 167: 164-175.
Chapter 1: Silicon protects soybean plants
against Phytophthora sojae by interfering
with effector-receptor expression
Aliyeh Rasoolizadeh, Caroline Labbé, Humira Sonah, Rupesh K. Deshmukh, François Belzile, James G. Menzies and Richard R. Bélanger. 2018. Silicon protects soybean plants
against Phytophthora sojae by interfering with effector-receptor expression. BMC Plant
17
Résumé
Le silicium (Si) est connu pour protéger les plantes contre les agents pathogènes biotrophes et hémibiotrophes. Cependant, les mécanismes par lesquels il exerce son rôle prophylactique restent inconnus. Pour tenter d'obtenir des informations uniques sur le mode d'action du Si, nous avons mené une analyse transcriptomique comparative complète de l’interaction soya (Glycine max) - Phytophthora sojae, un organisme hémibiotrophique dont la virulence dépend énormément du relargage de ses effecteurs.
Lors de ces travaux, il a été permis de constater que les plantes préalablement traitées au Si étaient fortement protégées de l’infection causée par P. sojae. Nos résultats ont également montré que les plantes exemptes de Si (Si-) montraient une expression élevée des gènes liés à la défense ainsi que des gènes codant pour des récepteurs membranaires suite à l’infection par P. sojae. Cette surexpression était observée très tôt à 4 jours post-infection, et elle diminuait avec le temps pendant que l'agent pathogène progressait dans les racines. Notre analyse transcriptomique a également révélé une forte expression d'effecteurs par P. sojae et dont la nature changeait au fil du temps. En revanche, la réponse transcriptomique des plantes traitées au Si (Si+) était remarquablement non affectée par la présence de P. sojae et l'expression des gènes codant pour les effecteurs par l'agent pathogène était aussi significativement réduite.
Il a été conclu qu’en absence de réponse directe causée par le Si, il devenait patent que l’effet du Si était plutôt passif. Comme le Si s’accumule dans l'apoplaste des tissus végétaux et que ce compartiment est un site d'interaction clé entre les effecteurs de l’agent pathogène et les récepteurs de la plante, l’hypothèse finale serait que le Si interfère probablement avec le réseau de signalisation entre P. sojae et la plante. Ainsi, les effecteurs resteraient prisonniers du réseau créé par l’accumulation du Si dans l’apoplaste et l’agent pathogène ne reconnaîtrait plus la plante comme une source de nourriture. De la même façon, le métabolisme de défense de la plante resterait également muet alors que la présence de signaux d’infection responsables de l’expression accrue des gènes de défense observée chez les plantes témoin (Si-) serait grandement diminuée.
Chapter 1
18
Abstract
Background: Silicon (Si) is known to protect against biotrophic and hemibiotrophic plant pathogens; however, the mechanisms by which it exerts its prophylactic role remain unknown. In an attempt to obtain unique insights into the mode of action of Si, we conducted a full comparative transcriptomic analysis of soybean (Glycine max) plants and Phytophthora sojae, a hemibiotroph that relies heavily on effectors for its virulence.
Results: Supplying Si to inoculated plants provided a strong protection against P. sojae over the course of the experiment (21 d). Our results showed that the response of Si-free (Si-) plants to inoculation was characterized early (4 dpi) by a high expression of defense-related genes, including plant receptors, which receded over time as the pathogen progressed into the roots. The infection was synchronized with a high expression of effectors by P. sojae, the nature of which changed over time. By contrast, the transcriptomic response of Si-fed (Si+) plants was remarkably unaffected by the presence of P. sojae, and the expression of effector-coding genes by the pathogen was significantly reduced.
Conclusion: Given that the apoplast is a key site of interaction between effectors and plant defenses and receptors in the soybean-P. sojae complex, as well as the site of amorphous-Si accumulation, our results indicate that Si likely interferes with the signaling network between P. sojae and the plant, preventing or decreasing the release of effectors reaching plant receptors, thus creating a form of incompatible interaction.
19
Background
Soybean (Glycine max L. Merr.) is economically and agriculturally the most important legume in the world, but its production is compromised by many biotic and abiotic factors. Of primary importance, Phytophthora sojae Kaufm. and Gred. (Kaufman et al., 1985) can cause annual yield losses as high as $200 million in the USA and $1–2 billion worldwide (Lin et al., 2014). Phytophthora sojae is a soil-borne plant pathogen belonging to the oomycetes with a restricted host range, including soybean as its primary host. It causes root and stem rot, and pre- and post-emergence damping-off, particularly in flooded soils where the pathogen can disseminate easily because of its flagellated zoospores (Tyler, 2007). It is described as a hemibiotrophic pathogen and it secretes effector proteins (coded by Avr genes) to manipulate and invade living host cells during the initial biotrophic stage of infection. In plant-pathogen interactions, effectors are recognized as important virulence factors that are utilized by the pathogen to suppress PAMPs (Pathogen-Associated Molecular Patterns)-Triggered Immunity (PTI) and Effector-Patterns)-Triggered Immunity (ETI) in plants or change host metabolism so that it can easily colonize plant tissues (Dou et al., 2008; Wang & Wang, 2018). In response, soybean can carry resistance genes to P. sojae (Rps), that encode, or are predicted to encode, nucleotide-binding leucine-rich repeat (NB-LRR)-type proteins (Gao et al.,2005; Jones et al., 2006 ), which are able to recognize the Avr effector proteins of P. sojae and induce the appropriate defense response (Dodds et al., 2010; Fliegmann et al., 2004). The result of this interaction between Rps genes and Avr genes will often determine compatible or incompatible interactions.
Numerous studies have highlighted the prophylactic effects of silicon (Si) fertilization (Bélanger et al., 2003; Fauteux et al., 2005; Ghanmi et al., 2004) in the search for additional methods to prevent losses in the case of compatible interactions. Interestingly, Si appears to be particularly efficient against biotrophic and hemibiotrophic fungal/oomycete pathogens (Chain et al., 2009; Vivancos et al., 2015). In the case of soybean, Arsenault-Labrecque et al. (2012) have shown that a Si treatment was effective against soybean rust caused by the biotrophic fungus Phakopsora pachyrhizi. In addition, Deshmukh et al. (2013) have identified Si transporters in soybean, thus confirming that the species is receptive to Si and can absorb the element.
The mechanisms inherent to the prophylactic properties of Si have puzzled scientists for many years. Originally, it was suggested that Si deposition along the cell walls created a physical barrier that halted fungal penetration into the plant (Wagner et al., 1940). However, additional studies have linked the presence of Si with diverse plant-defense reactions, thus suggesting that Si may play a role in the induction of acquired resistance (Chérif et al., 1994; Chérif et al., 1992a; Fauteux et al., 2005; Fawe et al., 1998; Ye et al., 2013). In a recent study using Arabidopsis thaliana mutants deficient in salicylic acid (SA) synthesis, Vivancos et al. (2015) showed that Si protected both mutant and wild-type plants against powdery mildew (Erisyphe cichoracearum). This led the authors to suggest that the deposition of Si as
Chapter 1
20
amorphous gel in the apoplast may prevent fungal effectors from reaching their targets, thereby altering the development of the pathogen. This hypothesis becomes particularly relevant in the context of the P. sojae-soybean interaction in light of recent results. Indeed, Ma et al. (2017) recently showed that P. sojae employed an apoplastic decoy strategy with effectors to attack soybean. Xin et al. (2016) further proposed that an aqueous apoplast was required for pathogenicity rather than immunosuppression, a condition that can be altered by silicon’s presence. Finally, Wang et al. (2018), on the basis of recent results with P. sojae, described the apoplastic region as a major battle ground between pathogen effectors and the host apoplastic surveillance system.
Since P. sojae is a hemibiotrophic pathogen that relies heavily on effectors for its virulence, the P. sojae-soybean pathosystem was deemed well-suited to validate and investigate the hypothesis that Si deposition altered the release of virulence factors by P. sojae. In this context, two main objectives were defined: 1) to assess resistance of soybean plants to P. sojae when fertilized with Si, and 2) to analyze the expression of salient genes involved in the virulence of P. sojae and the defense mechanisms of soybean in order to assess if a differential response could be linked to the prophylactic role of Si.
Results
Phenotypic responses
Soybean plants were inoculated with zoospores of P. sojae in a recirculating hydroponic system fed with nutrient solution with and without 1.7 mM Si to compare the phenotypic differences linked to Si. First symptoms of root browning appeared as early as 4 days post inoculation (dpi). Stunting and leaf discoloration followed within a few days, and first cases of mortality were recorded at 15 dpi in the Si- treatment. The differences between Si- and Si+ treatments increased with time, and by 21 dpi, plants in the Si+ treatment were clearly healthier than non-treated plants (Fig. 1a). In terms of dry weight, for non-inoculated plants there was no significant difference between Si- (8.4 ± 0.5) and Si+ plants (8.7 ± 0.4) plants. However, inoculation with P. sojae significantly reduced plant dry weight, but the prophylactic effect of Si was quite apparent as plants were significantly heavier in the Si+ (5.0 g ± 1.9) compared with the Si- (2.0 g ± 1.0) treatment. X-ray microanalysis mapping of
21
soybean confirmed the accumulation of Si throughout the roots in Si+ plants (Fig. 1b), while in the absence of Si amendment, no clear evidence of Si deposition was observed (Fig. 1c).
Figure 1. Effect of silicon (Si) amendments on soybean plants 21 days after inoculation with
Phytophthora sojae. a) Plants in the Si+ treatment was clearly healthier than non-treated plants with
more developed roots, stems and leaves. Comparative X-ray superimposed scanning electron micrographs of soybean root tips in plants treated (b) or not (c) with Si. At least, five plants per treatments were observed. A color scale of Si deposition was used, with blue indicating low Si and red high Si deposition. Black areas indicated no Si deposition.
Dual RNA-seq analysis of the P. sojae-soybean interaction in the presence of Si
A complete comparative transcriptomic analysis of soybean roots and P. sojae was carried out at 0, 4, 7 and 14 dpi to obtain a comprehensive gene-expression profile for both soybean and P. sojae in response to Si application.
Soybean root transcriptome
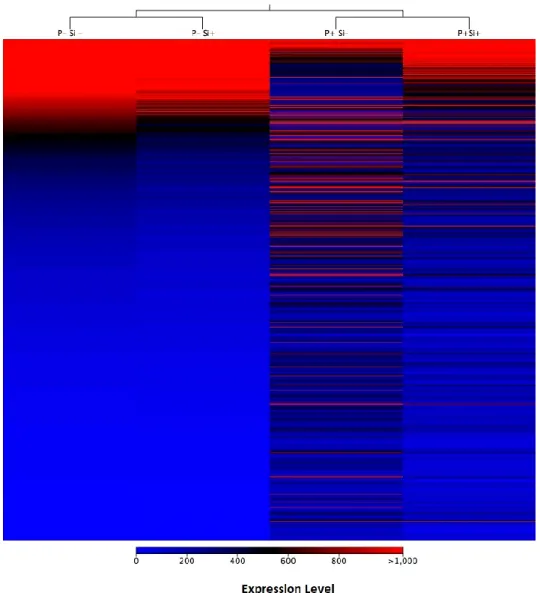
Mapping of the processed reads from roots to the soybean genome showed a very high percentage of mapped reads for non-inoculated samples (control) treated or not with Si. For control plants, 81% and 90% of reads mapped on soybean in Si- and Si+ treatments, respectively. In inoculated plants at 4 dpi, 61% and 76% of reads mapped to soybean in Si -and Si+, respectively (Supporting Information Table S1). Interestingly, the number of differentially expressed genes (DEGs) between control plants treated or not with Si was limited to 50 out of the potential 56,045 genes analyzed, and all were downregulated in the Si+ treatment (Fold-change ≥ 4, FDR p-value ≤ 0.01). On the other hand, plants responded to inoculation of P. sojae (Si-P+ vs. Si-P-) with a differential expression of 3,294 genes (Supporting Information Table S2). Most of genes that were differentially expressed as a result of the infection (Si-P+ vs Si-P-) reverted to a pattern of expression closer to control plants in the Si+ treatment (Si+P+ vs Si-P+) as illustrated on the heat map (Fig. 2).
Functional categorization of the DEGs in P. sojae-infected plants showed that these genes belonged mainly to the following categories: defense-related genes, secondary metabolism,
Chapter 1
22
hormone metabolism, primary metabolism and no-ontology for which no function was annotated.
Defense-related genes
Most known pattern recognition receptors (PRRs) that can activate PTI in plants fall into one of two receptor classes: transmembrane receptor kinases and receptor-like kinases (RLK; Dodds et al., 2010; Lanubile et al., 2015; Liu et al., 2009). In our study, 46 DEGs belonged
Figure 2. Heat map of differentially expressed genes in Phytophthora sojae infecting soybean
roots. In total, 3294 genes were differentially expressed as a result of P. sojae infection at 4 dpi (P+Si-). Heat map shows gene expression pattern in soybean roots inoculated (P+) or not (P-) with P. sojae and treated (Si+) or not (Si-) with silicon. Each gene corresponds to a colored line indicating the normalized mean (n=5) of the differentially expressed transcripts (Fold-change ≥ 4, FDR p-value ≤ 0.01).
23
to the receptor kinase family and 24 RLK showed higher expression at 4 dpi in the Si -treatment (Supporting Information Fig. S1a, b). After PRR activation, the downstream signaling pathway transfers signals from extracellular receptors to cellular responses by mitogen-activated protein kinases (MAPKs) and calcium (Ca2+). MAPKs are ubiquitous signal-transduction components, which have been implicated in both PTI and ETI. Our results showed that out of 9 differentially expressed MAPKs, five had a higher expression at 4 dpi in the Si- treatment (Supporting Information Fig. S1c). Similarly, 33 Ca2+-dependent protein kinases (CDPKs) were highly expressed at 4 dpi (Supporting Information Fig. S1d, Table S3).
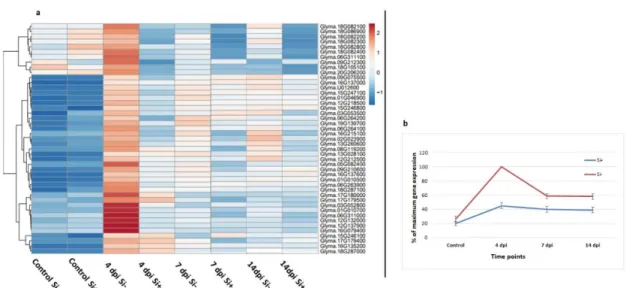
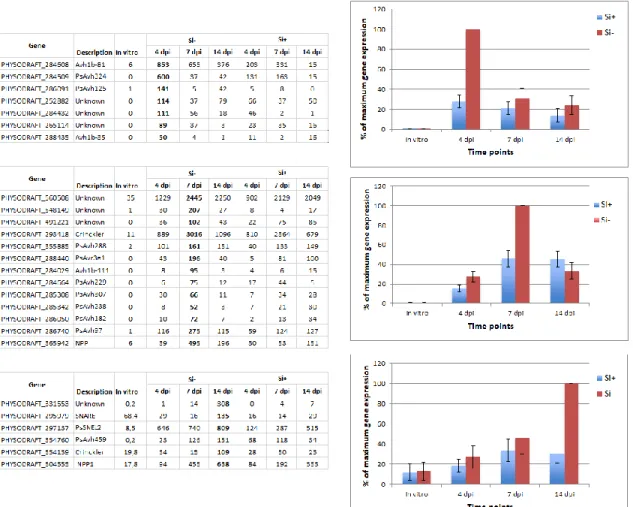
NB-LRR proteins. Out of 80 differentially expressed NB-LRR genes over the experimental period (Fold-change ≥ 4, FDR p-value ≤ 0.01), 45 showed their highest expression at 4 dpi in Si- plants. Heat map results clearly showed a pattern of expression where there was no expression of NB-LRR genes in non-inoculated plants (control) regardless of Si treatment, followed by a sharp increase at 4 dpi in Si- plants. While the expression was reduced at 7 and 14 dpi, it remained significantly higher in Si- plants (Fig. 3, Supporting Information Table S4).
Pathogenesis-related proteins (PRs). Based on cluster analysis, 11 PR genes were found to be differentially expressed in at least one timepoint (Fold-change ≥ 4, FDR p-value ≤ 0.01).
Figure 3. Expression profile of NB-LRRs genes. Heat map (a) and gene expression (b) show a
higher expression of 45 receptor (NB-LRR) genes in Phytophthora sojae-inoculated soybean plants at 4 dpi under Si- compared to Si+ treatment. (b) Graph shows the average relative (%)
expression at each timepoint based on the highest level of expression for each gene as a measure to showcase the trend in expression. Bars represent standard error from the mean (n = 5).