HAL Id: hal-01466485
https://hal.archives-ouvertes.fr/hal-01466485
Submitted on 13 Feb 2017
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Graphene-Based Supercapacitors Using Eutectic Ionic
Liquid Mixture Electrolyte
Zifeng Lin, Pierre-Louis Taberna, patrice Simon
To cite this version:
Zifeng Lin, Pierre-Louis Taberna, patrice Simon. Graphene-Based Supercapacitors Using
Eutec-tic Ionic Liquid Mixture Electrolyte. Electrochimica Acta, Elsevier, 2016, vol. 206, pp. 446-451.
�10.1016/j.electacta.2015.12.097�. �hal-01466485�
O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 16653
To link to this article : DOI:10.1016/j.electacta.2015.12.097
URL :
http://dx.doi.org/10.1016/j.electacta.2015.12.097
To cite this version :
Lin, Zifeng and Taberna, Pierre-Louis and
Simon, Patrice Graphene-Based Supercapacitors Using Eutectic
Ionic Liquid Mixture Electrolyte. (2016) Electrochimica Acta, vol.
206. pp. 446-451. ISSN 0013-4686
Any correspondence concerning this service should be sent to the repository
Graphene-Based
Supercapacitors
Using
Eutectic
Ionic
Liquid
Mixture
Electrolyte
Zifeng
Lin
a,b,
Pierre-Louis
Taberna
a,b,*
,
Patrice
Simon
a,baUniversitePaulSabatierToulouseIII,CIRIMATUMRCNRS5085,118routedeNarbonne,31062Toulouse,France b
ReseausurleStockageElectrochimiquedel’Energie(RS2E),FRCNRS3459,France
Keywords:
supercapacitors graphenefilm ionicliquideutectic workingtemperaturewindow
ABSTRACT
Compact graphene filmswerepreparedandelectrochemically testedatvarioustemperatures ina eutectic of ionic liquid mixture (1:1 by weight or mole N-methyl-N-propylpiperidinium bis (fluorosulfonyl)imideandN-butyl-N-methylpyrrolidiniumbis(fluorosulfonyl)imide)electrolyte.Alarge temperaturewindowfrom-30!Cto80!Cwasachievedtogetherwithalargepotentialwindowof3.5Vat
room temperature and below. A maximum gravimetric capacitance of 175F.g"1 (85mAh.g"1) was
obtainedat80!C.130F.g"1(63mAh.g"1)and100F.g"1(49mAh.g"1)werestilldeliveredat-20!Cand
-30!Crespectively.Besides,avolumetriccapacitanceof50F.cm"3wasachievedwithathickgraphene
film(60mm).Theoutstandingperformanceofsuchcompactgraphenefilmintheeutecticionicliquid mixtureelectrolytemakesitapromisingalternativetoactivatedcarbonforsupercapacitorapplications, especiallyunderextremetemperatureconditions.
1.Introduction
Thehightheoreticalspecificsurfacearea(upto2630m2g"1)
combined with low resistivity (10"6
V
.cm) as well as highmechanical strength and chemical stability make graphene a
promising active material for supercapacitors [1,2].As a result,
intensive research efforts have been made to characterize
graphene and graphene-based materials in supercapacitors
applications [1,3–5]. Specific capacitance beyond 200F.g"1 has
beenachievedinbothaqueousand organicelectrolytes[4,6–9], which is similaror even betterto many carbon materials like
porous activated carbon [10]. However, the low density of
graphene often comes with a low volumetric capacitance (F.
cm"3),whichisaconcerninmostofapplications,especiallyfor
micro devices [11]. Besides, restacking of graphene film still remainsanissueandblocksthewaytohighercapacitance[12,13].
Accordingly, efforts have been devoted to synthesize more
compactbutun-restackedgraphenefilms.In2013,D.Li'sgroup
[7] proposed a method to develop a dense graphene film by
capillary compressionofgraphenegelfilminthepresenceofa nonvolatile liquid electrolyte,wherethepresence ofelectrolyte betweenthelayerspreventstherestackingofgraphenelayers.
Theenergy densitybeingproportional tothevoltagesquare (E¼1=2$CV2where E is the energy density, C is the
specific capacitanceandVistheworkingpotentialwindow),maximizing thecellvoltageisofhighimportancefordesigning highenergy
devices. Today, most of the graphene and graphene-based
materials have been characterized in aqueous or conventional
organicelectrolytes[11,12].Asaresult,maximumvoltageupto3V werereported[2,14].
Ionic liquid electrolytes provide a large working potential window(morethan4Vinsomecases)[15,16].Inaddition,high chemicalstability,negligiblevaporpressureandnon-flammable propertiesmake ionicliquidsverypromisingaselectrolytesfor bothsupercapacitorsandbatteries[15,17].Oneofthefirstreport
about the use of ionic liquid electrolytes in graphene-based
supercapacitorswasdonebyVivekchandandetal[18],whofound aspecificcapacitanceof75F.g"1withina3.5Vpotentialwindow.
Samegroupreportedareducedgraphenewithenhanced
capaci-tanceof258F.g"1(measuredat5mV/s) bynitrogen-doped[19].
Ruoff's group synthesized activated graphene materials with
extremelyhighspecificsurface area–upto3100m2g"1–able
delivering 200F.g"1 (0.7 Ag"1) in EMIMTFSI ionic liquid [20].
Despite efforts were devoted to improve the performance of
graphene-basedsupercapacitorsin ionicliquidelectrolytes, less attentionwas paid to theoperation temperaturerange
perfor-mance of supercapacitors. Limited by high viscosity and low
meltingpointnearroomtemperature,neationicliquidsarestill
*Correspondingauthor.
E-mailaddress:taberna@chimie.ups-tlse.fr(P.-L.Taberna).
unusableatsub-zerotemperatures[15–22].Therefore,aeutecticof ionicliquidmixturewithlowviscositywasdesignedbyLinetal.as anelectrolyteforsupercapacitors,whichturnedouttopossessa largeworkingtemperaturerangefrom-50!Cto80!Calongsidea widepotential window(up to3.7V)[21,23]. Thiseutecticionic liquidmixtureiscomposedofthesameanion(bis(fluorosulfonyl) imide (FSI)) but two different cations (pyrrolidium (PYR) and
piperidinium (PIP)) with the same molecular formula. The
differenceofmolecularstructure andtheassociatedasymmetry amongthecationswerethoughttohinderlatticeformation,thus
lowers the melting point while maintaining good miscibility
withinalargeworkingtemperaturewindow.Conductivityvalues
of28.9mS/cmand4.9mS/cmweremeasuredat100and20!C,
respectively,asreportedearlier[23].
Inthispaper,weproposetouseadensifiedgrapheneelectrode suchasproposedbyLi’sgroup[7].incombinationwithanionic liquideutecticmixtureaselectrolytetoimproveboththevoltage
window – that is the energy density – and the operating
temperaturerange. 2.Experimental 2.1.Electrodespreparation
Commerciallyavailablegraphitepowder(KS44graphite, pur-chasedfromIMERYSGraphite&CarbonCorporation)wasusedas
the raw material. Graphite oxide was prepared by a modified
Hummersmethod[24].Briefly,amixtureofgraphiteflakes(1.0g,
1wt equiv) and KMnO4 (6.0g, 6wt equiv) was added to a
9:1 mixture of concentrated H2SO4/H3PO4 (120:13mL). The
reaction was then heated to 50!C and stirred for 12hours,
followingbyanaddition of200mLicewith1mL 30wt%H2O2.
Afterwashingbywater,30%HClandethanol,thegraphiteoxide dispersionwasobtained.Grapheneoxidedispersionwasachieved by1hoursonicationand20minutescentrifugationat10,000rpm ofgraphiteoxidewaterdispersion.
Inafirststep,graphenedispersionwasobtainedthroughthe reduction of graphene oxide dispersion by hydrazine solution. 0.2mLhydrazine(35wt%inwater)and0.35mLammonia(28wt%
in water) solution was added into graphene oxide dispersion
(0.5mgmL"1, 100mL)inaglassvialforstirringover1hour(100!C). 60mL 0.5 mgmL"1 reduced graphene dispersion was used for
vacuum filtration, and an Anodiscã membrane (Whatman
company)withan averagepore sizeof 0.1
m
mwas used. Aftervacuum filtration, graphene film was carefully peeled off and
immersedindeionizedwaterovernighttoremovetheremaining hydrazineandammonia[7].
Inasecond step,thegraphenediscfilmswerepunched out
(7mmdiameter)andtransferredintoacetonitrileformorethan 48htototallyreplacethewaterwithacetonitrile.Filmswerethen immersedin10wt%(0.27M)ionicliquidmixtureinacetonitrile formorethan72hours,toexchangetheacetonitrileingraphene filmwiththeionicliquidsolution.Sampleswerethencollected,
clippedbetweentwoglassplatesandmovedtoavacuumoven
under 80!C. After 48hours, the volatile acetonitrile was
completely evaporated and nonvolatile ionic liquids remained
insidethegraphenefilm,whichisexpectedtolimittherestacking ofgraphenelayers.Asaresultofthedensification,thethickness ofthegraphenefilmwasgreatlydecreased(fromaround180
m
mto60
m
m), whilethediameter wasmeasured closeto5.4mm.Herein, the graphene film was well prepared and ready for
electrochemicaltests.
Theweightoftheelectrodeswasmeasuredafter electrochemi-caltests.Specifically,theelectrodeswerewashedbyacetonitrile and ethanol to eliminate the trapped electrolyte and then the
weightwasmeasuredaftervacuumdrying.
2.2.Electrochemicaltests
Graphenefilmswereusedasworkingelectrodesdirectlyafter vacuumdryingwithoutanybinders.Aeutecticionicliquidmixture
composed of (1:1 by weight or molar ratio)
N-methyl-N-propylpiperidinium bis(fluorosulfonyl) imide (PIP13-FSI) and
N-butyl-N-methylpyrrolidinium bis(fluorosulfonyl) imide (PYR14
-FSI) was prepared and used as the electrolyte [21]. Bothionic liquidswereboughtfromSolvionicSA(France).Two25mm-thick porousAl2O3separatorswereusedtogetherwithgold(forRHD
cell)orplatinum(forSwagelokcell)asthecurrentcollector. Forhighandlowtemperaturetests,aRHDcell(Figure.S1)was used,whichenablesfinelyprecisecontrolofthecelltemperature from"40upto100!C[25].Afterreachingeachtesttemperature, thetemperaturewasheldatleasttwohoursbeforemeasurements toensurethecellhasreachedthedesiredtemperature.Testswere
performed by using an Autolab PGSTAT128N (Metrohm,
Switzerland).Besides,three-electrodeSwagelokcellwere assem-bledandtestedatroomtemperature,usingasilverwireas
quasi-reference electrode and tested by using a VMP3 potentiostat
(Biologic, S.A.).All supercapacitorscells mentioned abovewere
assembled in a glovebox under argon atmosphere (waterand
oxygencontentslessthan1ppm).
Electrochemicalimpedancespectroscopy(EIS)measurements
werecarriedoutatopencircuitvoltage(OCV)withanamplitudeof 10mVatthefrequencyrangefrom0.01Hzto200kHz.Capacitance wascalculatedfromcyclicvoltammogramsbymeasuringtheslope oftheintegrateddischargechargeQversusdischargetime(s)plot
and divide by scan rate (V/s). Gravimetric capacitance was
calculated by dividingthe electrode capacitance bythe weight ofgrapheneononeelectrode,accordingtoequation(1)
C¼
D
QD
t$#$m ð1ÞWhereCisthespecificcapacitance(F.g"1);DQ
Dt istheslopeofthe
integrateddischargechargeQversusdischargetimeplot(A);#is thescanrate(V/s)andmisthemassofgrapheneononeelectrode (g).
2.3.Characterization
X-raydiffractionpatternswererecordedbyanX-ray diffrac-tometer(D4ENDEAVOR,Bruker,Germany)usingCuK
a
radiation (l
=0.154nm)withanoperationvoltageof40kVand currentof 40mA.SEMobservationsofthegraphenefilmsweremadeusinga
JSM-6510LV Scanning Electron Microscope after completion of
Fig.1.X-Raydiffractionpatternsofgraphite(GP),graphiteoxide(GO),graphene film(GF)andgraphenefilmfilledwithelectrolyte(GF-IL).Inset:zoominthe8!-35!
electrochemicaltests,forthesakeofaccuracyinthefilmthickness measurement.
3.Resultsanddiscussion
X-Ray diffraction patterns of graphite, graphite oxide and graphenefilmswithorwithoutelectrolytearepresentedinFig.1.
GF stands for graphene films without electrolyte, obtained by
vacuum filtrationthat is containingwater inside. GF-IL arethe graphenefilmsobtainedafterelectrolyteimmersionthatcontain ionic liquid mixture electrolyte after acetonitrile removal. The (002)peakaround26.0!ofpristinegraphite(GP)correspondsto an expected interlayer spacing of 0.336nm. For graphite oxide (GO),thepeakshiftsto9.0!asaresultoftheincreasedinterlayer spacingto0.976nmafteroxidation[26].Nopeakswereobserved for graphenefilmfilledornotwithelectrolyte.It wasexpected sinceforgraphenefilm/GF(withoutelectrolyte),whichcontains morethan90wt%percentofwaterinside,theexfoliatedgraphene
flakes were separated from each other by water, therefore no
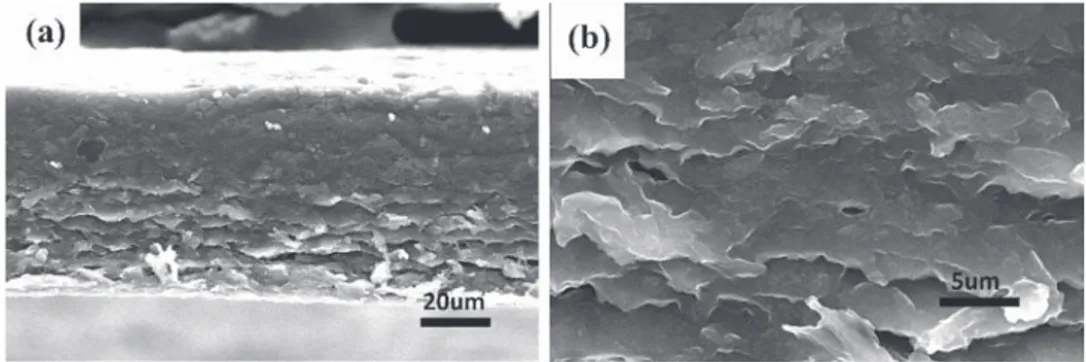
restackinghappens[13].Ontheotherhand,whenelectrolytefilled graphenefilms/GF-IL(waterreplacedbynonvolatileelectrolyte), thepresenceoftheelectrolyteisassumedtopreventrestacking. SEMobservationsofthefilmsareshowninFigs.2.Ascanbe
seen in Fig. 2a graphene films/GF present a homogeneous
thicknessof60
m
mwhichenablesaccuratedeterminationofthe volume(1.374' 10"3cm3)toobtainthevolumetriccapacitance.Alayer-by-layerstructurewas showninFig.2b,asaresultofthe filtrationprocess.Suchalayeredstructureisassumedtobeatthe originofthedensificationofthefilmsascomparedtoconventional
three-dimensional disordered structure, resulting in a high
volumetricenergydensity[7].
Fig.3showsCyclicVoltammograms(CVs)ofanasymmetric 3-electrode Swagelok cell at roomtemperature,ata scan rate of 20mV/s,intheionicliquidmixtureelectrolyte.Therectangular shape of theCVreveals acapacitive behavior. Highgravimetric capacitance (175F.g"1) was achieved, superior to most of the
reducedgrapheneoxidesreportedsofarinionicliquidelectrolytes orconventionalorganicelectrolytes(Table.S1)[7–28].Inaddition, alargepotentialwindowof3.5V(from-2Vto1.5V(vsAgwire)) makethesematerialsmorepromisingfordesigninghighenergy supercapacitors.
Asymmetricaltwo-electrodeSwagelokcellwasassembledand testedatroomtemperature.Thehighfrequencyloopobservedon theNyquistplot(Fig.4a)isassumedtooriginatefromthecontact resistancebetweenthecurrentcollectorsandthefilm[29].The highfrequencyseriesresistancewasmeasuredat40
V
.cm2,whichisinthesamerangeaspreviouslyreportedforthiselectrolyteat roomtemperature[23].Whengoingdowntolowfrequencies,the fastincreaseoftheimaginarypartoftheimpedancerevealsthe capacitive behavior of the electrode. Fig.4b shows theCVs at differentscanrates.Highcapacitanceof170F.g"1wasobtainedata
scanrateof5mV/swithina3.5Vpotentialrange,whichisbeyond mostof theconventional carbons reported sofar [10–31]. The changeofthecapacitancewiththepotentialscanrateisshownin
Fig. 4c. More than 75% of the maximum capacitance was still
preserved at 50mV/s, thus highlighting the decent power
performanceofthecelldespiteusinganeationicliquidelectrolyte.
The associated volumetric capacitance, calculated from the
electrode thickness, was 50F.cm"3 at 5mV.s"1, which is also
comparabletoconventionalactivatedcarbonpowders[10].
Fig. 5 shows the electrochemical characterization at higher temperature,namely40!C,60!Cand80!C.Asexpected,thehigh frequencyseriesresistancedecreaseswithincreasingtemperature (Fig. 5a), due tothe decrease of the electrolyte viscosity. The improvedcapacitivebehavioratelevatedtemperatureascanbe
seen by the sharp increase in the imaginary part at lower
resistance; it is associated withthe increase of theelectrolyte conductivitywiththetemperature[21,23].Theimproved capaci-tive behavior with the temperature is also visible on the CVs (Fig.5b),withcapacitiveelectrochemicalsignatures.However,a limitationinthepotentialwindowfrom3.5Vdownto3.2Vwas observedwhenthetemperaturewasincreasedfrom40!Cto80!C. Such a behavior is associated withtheelectrochemical activity (oxidation) of the ionic liquid electrolyte at high potential, as already reported elsewhere [21]. Specific capacitance was in-creasedfrom160F.g"1upto175F.g"1withincreasingtemperature
becauseoftheviscosityfalloff.
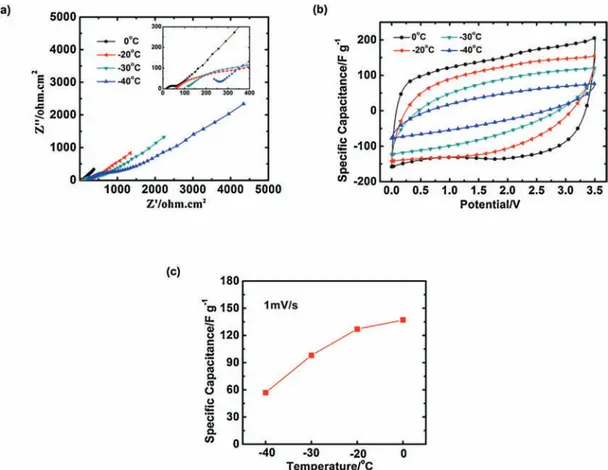
Fig.6showstheelectrochemicalcharacterizationsatlowand
negative (sub-zero) temperature. Owing to the high viscosity
underlowtemperature,aslowscanrateof1mV/swasapplied.The Nyquistplot(Fig.6a)showsanimportantincreaseoftheseries
resistance at negative temperature, in agreement with the
decreaseoftheelectrolyteconductivity[23].Theelectrochemical
Fig.2. SEMimagesofcross-sectionmorphologyofgraphenefilmafterelectrochemicaltests.Scalebar:20mm(a),5mm(b).
Fig.3.CyclicVoltammogramsatroomtemperaturein N-Methyl-N-propylpiper-idiniumbis(fluorosulfonyl)imide(PIP13-FSI):N-Butyl-N-methylpyrrolidiniumbis (fluorosulfonyl)-imide(PYR14-FSI)ionicliquidmixture(1:1byweightormolar ratio).Thepotentialscanratewas20mV/s,withapotentialwindowfrom-2Vto 1.5V/Reference(usingasilverwireaspseudo-referenceelectrode).
Fig.4.Electrochemicalcharacterizationatroomtemperatureofa2-electrodecellassembledwithdensegraphenefilms,ineutecticmixtureelectrolyte:EISNyquistplot(a), CVsatvariousscanrates(b),changeofthespecificcapacitancewiththepotentialscanrate(c).
Fig.5.Electrochemicalcharacterizationat40!C,60!Cand80!Cofa2-electrodecellassembledwithgraphenefilms,ineutecticmixtureelectrolyte.Nyquistplots(a),CVs(b)
signature(Fig.6b)stillshowsacapacitivebehaviordownto-30!C whileitbecomestooresistiveat-40!C.Acapacitanceof135F.g"1
(63mAh.g"1)wasobtainedat0!Cand100F.g"1(49mAh.g"1)at
-30!C.Whenthetemperaturegoesdownto-40!C, the electro-chemicalbehaviorisgovernedbytheohmicdropsinsidethebulk oftheelectrolyte.
These results showthat thecombination ofdensegraphene
filmswithaeutecticionicliquidmixturecanbepromisingasan alternative to conventional porous carbons for supercapacitor applications.Beyondthehighgravimetricandvolumetric capaci-tance obtained at room temperature (165F.g"1 and 50F.cm"3,
respectively),thelargepotentialwindow(3.5V)andtemperature operation range (from -30!C to 80!C) make them suitable for designinghighenergydensitysupercapacitors.
4.Conclusions
Using vacuum filtration, electrolyte immersionand selective evaporation,compactgraphenefilmscharacterizedbya layer-by-layerstructurewaspreparedandusedaselectrodematerialsfor supercapacitorswithoutbinders.Thankstotheuseofaeutectic ionic liquidmixtureaselectrolyte,a largeworkingtemperature
window from -40 to 80!C could be explored. A maximum
gravimetric capacitance of 175F.g"1 (85mAh.g"1) was reached
at80!C.Despitethecapacitanceat-40!Cwaslimitedat60F.g"1
(30mAh.g"1),130F.g"1(63mAh.g"1)and100F.g"1(49mAh.g"1)
werestillachievedat-20!Cand-30!Crespectively.Thepotential windowwasincreasedupto3.5Vatroomtemperatureand3.2Vat 80!C,thusleadingtosubstantialimprovementinthecellenergy density.Additionally, avolumetriccapacitanceof50F.cm"3was
alsoachievedwithathickgraphenefilmof60
m
m.Thesematerials couldbeapromisingalternativetoactivatedcarbonoperatinginconventional electrolytes for supercapacitorapplications, espe-ciallyunderextremetemperatureconditions.
Acknowledgements
ZifengLINissupportedbyChinaScholarshipCouncil(CSC).P.S. acknowledgesthesupportfromEuropeanResearchCouncil(ERC, AdvancedGrant,ERC-2011-AdG,Projectno.291543-IONACES). References
[1]R.Raccichini,A.Varzi,S.Passerini,B.Scrosati,Theroleofgraphenefor electrochemicalenergystorage,NatMater14(2015)271–279. [2]M.D.Stoller,S.J.Park,Y.W.Zhu,J.H.An,R.S.Ruoff,Graphene-Based
Ultracapacitors,NanoLett8(2008)3498–3502.
[3]J.X.Zhu,D.Yang,Z.Y.Yin,Q.Y.Yan,H.Zhang,GrapheneandGraphene-Based MaterialsforEnergyStorageApplications,Small10(2014)3480–3498. [4]Y.Xu,Z.Lin,X.Zhong,X.Huang,N.O.Weiss,Y.Huang,X.Duan,Holeygraphene
frameworksforhighlyefficientcapacitiveenergystorage,NatCommun5 (2014).
[5]R.Raccichini,A.Varzi,S.Passerini,B.Scrosati,Theroleofgraphenefor electrochemicalenergystorage,NatMater(2014)advanceonlinepublication. [6]M.F.El-Kady,V.Strong,S.Dubin,R.B.Kaner,LaserScribingof
High-PerformanceandFlexibleGraphene-BasedElectrochemicalCapacitors, Science335(2012)1326–1330.
[7]X.W.Yang,C.Cheng,Y.F.Wang,L.Qiu,D.Li,Liquid-MediatedDenseIntegration ofGrapheneMaterialsforCompactCapacitiveEnergyStorage,Science341 (2013)534–537.
[8]R.R.Salunkhe,Y.-H.Lee,K.-H.Chang,J.-M.Li,P.Simon,J.Tang,N.L.Torad,C.-C. Hu,Y.Yamauchi,Nanoarchitecturedgraphene-basedsupercapacitorsfor next-generationenergy-storageapplications,Chemistry(Weinheimander Bergstrasse,Germany)20(2014)13838–13852.
[9]C.N.R.Rao,K.Gopalakrishnan,U.Maitra,ComparativeStudyofPotential ApplicationsofGraphene,MoS2,andOtherTwo-DimensionalMaterialsin EnergyDevices,Sensors,andRelatedAreas,ACSAppl.Mater.Interfaces7 (2015)7809–7832.
[10]A.Burke,R&Dconsiderationsfortheperformanceandapplicationof electrochemicalcapacitors,Electrochim.Acta53(2007)1083–1091. Fig.6. Electrochemicalcharacterizationat0!C,-20!C,-30!Cand-40!Cofa2-electrodecellassembledwithgraphenefilms,ineutecticmixtureelectrolyte.Nyquistplots(a),
CVsat1mV.s"1
(b)andchangeofthespecificcapacitancewiththetemperatureatascanrateof1mV.s"1
[11]P.Simon,Y.Gogotsi,CapacitiveEnergyStorageinNanostructured Carbon-ElectrolyteSystems,AccountsChem.Res.46(2013)1094–1103.
[12]J.H.Lee,N.Park,B.G.Kim,D.S.Jung,K.Im,J.Hur,J.W.Choi,Restacking-Inhibited 3DReducedGrapheneOxideforHighPerformanceSupercapacitorElectrodes, AcsNano7(2013)9366–9374.
[13]X.W.Yang,J.W.Zhu,L.Qiu,D.Li,BioinspiredEffectivePreventionofRestacking inMultilayeredGrapheneFilms:TowardstheNextGenerationof High-PerformanceSupercapacitors,AdvancedMaterials23(2011) 2833-+. [14]M.Gali!nski,A.Lewandowski,I.Ste˛pniak,Ionicliquidsaselectrolytes,
Electrochim.Acta51(2006)5567–5580.
[15]M.Armand,F.Endres,D.R.MacFarlane,H.Ohno,B.Scrosati,Ionic-liquid materialsfortheelectrochemicalchallengesofthefuture,NatureMaterials8 (2009)621–629.
[16]M.Galinski,A.Lewandowski,I.Stepniak,Ionicliquidsaselectrolytes, Electrochim.Acta51(2006)5567–5580.
[17]R.D.Rogers,K.R.Seddon,Ionicliquids—Solventsofthefuture?Science302 (2003)792–793.
[18]S.R.C.Vivekchand,C.S.Rout,K.S.Subrahmanyam,A.Govindaraj,C.N.R.Rao, Graphene-basedelectrochemicalsupercapacitors,J.Chem.Sci.120(2008)9– 13.
[19]K.Gopalakrishnan,K.Moses,A.Govindaraj,C.N.R.Rao,Supercapacitorsbased onnitrogen-dopedreducedgrapheneoxideandborocarbonitrides,SolidState Communications175–176(2013)43–50.
[20]Y.W.Zhu,S.Murali,M.D.Stoller,K.J.Ganesh,W.W.Cai,P.J.Ferreira,A.Pirkle,R. M.Wallace,K.A.Cychosz,M.Thommes,D.Su,E.A.Stach,R.S.Ruoff, Carbon-BasedSupercapacitorsProducedbyActivationofGraphene,Science332 (2011)1537–1541.
[21]W.Y.Tsai,R.Y.Lin,S.Murali,L.L.Zhang,J.K.McDonough,R.S.Ruoff,P.L.Taberna, Y.Gogotsi,P.Simon,Outstandingperformanceofactivatedgraphenebased supercapacitorsinionicliquidelectrolytefrom-50to80degreesC,Nano Energy2(2013)403–411.
[22]K.Hayamizu,Y.Aihara,H.Nakagawa,T.Nukuda,W.S.Price,Ionicconduction andiondiffusioninbinaryroom-temperatureionicliquidscomposedofemim BF4andLiBF4,J.Phys.Chem.B108(2004)19527–19532.
[23]R.Y.Lin,P.L.Taberna,S.Fantini,V.Presser,C.R.Perez,F.Malbosc,N.L. Rupesinghe,K.B.K.Teo,Y.Gogotsi,P.Simon,CapacitiveEnergyStoragefrom -50to100degreesCUsinganIonicLiquidElectrolyte,JournalofPhysical ChemistryLetters2(2011)2396–2401.
[24]D.C.Marcano,D.V.Kosynkin,J.M.Berlin,A.Sinitskii,Z.Z.Sun,A.Slesarev,L.B. Alemany,W.Lu,J.M.Tour,ImprovedSynthesisofGrapheneOxide,AcsNano4 (2010)4806–4814.
[25]L.Negre,B.Daffos,P.L.Taberna,P.Simon,Solvent-FreeElectrolytesfor ElectricalDoubleLayerCapacitors,JournaloftheElectrochemicalSociety162 (2015)A5037–A5040.
[26]A.M.Dimiev,J.M.Tour,MechanismofGrapheneOxideFormation,ACSNano (2014).
[27]Y.Chen,X.O.Zhang,D.C.Zhang,P.Yu,Y.W.Ma,Highperformance supercapacitorsbasedonreducedgrapheneoxideinaqueousandionicliquid electrolytes,Carbon49(2011)573–580.
[28]T.Kim,G.Jung,S.Yoo,K.S.Suh,R.S.Ruoff,ActivatedGraphene-BasedCarbons asSupercapacitorElectrodeswithMacro-andMesopores,AcsNano7(2013) 6899–6905.
[29]C.Portet,P.L.Taberna,P.Simon,C.Laberty-Robert,ModificationofAlcurrent collectorsurfacebysol–geldepositforcarbon–carbonsupercapacitor applications,Electrochim.Acta49(2004)905–912.
[30]G.P.Wang,L.Zhang,J.J.Zhang,Areviewofelectrodematerialsfor electrochemicalsupercapacitors,Chem.Soc.Rev.41(2012)797–828. [31]C.Largeot,C.Portet,J.Chmiola,P.-L.Taberna,Y.Gogotsi,P.Simon,Relation
betweentheIonSizeandPoreSizeforanElectricDouble-LayerCapacitor, JournaloftheAmericanChemicalSociety130(2008)2730–2731.