Pour l'obtention du grade de
DOCTEUR DE L'UNIVERSITÉ DE POITIERS UFR des sciences fondamentales et appliquées
Laboratoire de neurosciences expérimentales et cliniques - LNEC (Poitiers) (Diplôme National - Arrêté du 7 août 2006)
École doctorale : Biologie-santé - Bio-santé (Limoges) Secteur de recherche : Neurosciences
Cotutelle : Université libanaise
Présentée par :
Joanna Kalaani
Molecular guidance of dopaminergic cells transplanted in a mouse model of Parkinson's disease
Directeur(s) de Thèse :
Laetitia Prestoz, Bassam Badran, Mohamed Jaber Soutenue le 22 janvier 2016 devant le jury Jury :
Président Afsaneh Gaillard Professeur des Universités, Université de Poitiers
Rapporteur Fatiha Nothias Directeur de recherche CNRS, Université Pierre et Marie Curie
Rapporteur Benjamin Dehay Chargé de recherche INSERM, Université de Bordeaux
Membre Laetitia Prestoz Maître de conférences, Université de Poitiers
Membre Bassam Badran Professeur, Université libanaise, Beyrouth
Membre Mohamed Jaber Professeur des Universités, Université de Poitiers
Membre Kazem Zibara Professeur, Université libanaise, Beyrouth
Pour citer cette thèse :
Joanna Kalaani. Molecular guidance of dopaminergic cells transplanted in a mouse model of Parkinson's disease [En ligne]. Thèse Neurosciences. Poitiers : Université de Poitiers, 2016. Disponible sur l'Intranet de l'Université de Poitiers <http://theses.univ-poitiers.fr>
THESE EN COTUTELLE
Pour obtenir le grade de Docteur délivré par L’Université de Poitiers
Faculté des Sciences Fondamentales et Appliquées Ecole Doctorale: Bio santé n°524
Et
L’Université Libanaise
Ecole Doctorale des Sciences et Technologie Spécialité: Neurosciences
Présentée et soutenue publiquement par Kalaani Joanna
Le 22 Janvier 2016 **********
Molecular Guidance of Dopaminergic Cells Transplanted in a Mouse
Model of
Parkinson’s disease
**********
Directeurs de thèse: Dr. PRESTOZ Laetitia et Pr. BADRAN Bassam Co-directeurs de thèse: Pr. JABER Mohamed et Pr. HAMADE Eva
Membres du Jury:
Mme Fatiha Nothias, Directrice de Recherche, CNRS UMR 8246……….………Rapporteur M. Benjamin Dehay, Chargé de Recherche, CNRS UMR 5293………..Rapporteur M. Kazem Zibara, Professeur, Université Libanaise……….…...…..Examinateur Mme Afsaneh Gaillard, Professeur des Universités, INSERM U-1084…………....…..…..Examinateur M. Mohamed Jaber, Professeur des Universités, INSERM U-1084………... Co-directeur de thèse M. Bassam Badran, Professeur, Université Libanaise……….………..Directeur de thèse Mme Laetitia Prestoz, Maître de conférence, INSERM U-1084……….……..Directeur de thèse
Acknowledgment
I would like to express special appreciations to my supervisor Dr. Laetitia Prestoz who accompanied me throughout my thesis work. I wouldn’t be able to finish my thesis without her supportive guidance.
I would like to thank equally Pr. Mohamed Jaber my co-director and the director of the Experimental and Clinical Neurosciences Laboratory (LNEC) for his enthusiasm and irreplaceable support and for providing the best environment and facilities to complete our work.
I would like to thank also my co-directors in Lebanon Pr. Bassam Badran and Pr. Eva Hamade. Their valuable support and constructive critiques allowed me to expand my experience and knowledge.
I am very grateful to Pr. Afsaneh Gaillard, leader of the Cell Therapy in Brain Pathologies group. Not only she helped me throughout my research project technically and theoretically but also she was a source of inspiration and motivation.
I would like to express special appreciation to Pr. Joelle Roche for her valuable advice and contribution to the project.
I would like to also thank the chemistry research group laboratory (IC2MP) headed by Dr Philippe Bertrand for giving me the opportunity to participate in Nanoparticle project. Deepest gratitude is also due to the committee members Pr. Fatiha Nothias, Pr. Benjamin Dehay and Pr. Kazem Zibara for their direction and dedication.
I would like to thank all members of LNEC, researchers, past and present PhD students Sandie, Audrey Lafragette, Audrey Leguen, Obelia, Marine, Nissrine, Mejda and Celine, as well as Post-docs Tristan, Sophie and Bhaskar. I also thank all the staff members, Maurine, Marie-Laure, Sebestian, Anais, Virginie, Pauline and Emilie for their help and support. I would like also to thank past and present secretary staff Mme. Françoise Couturier, Mme. Valerie Ouvrard and especially Mme. Aurélie Leger for her support. Finally, you only know that you succeeded in a place when you make life lasting memories! I need to thank my family in Poitiers: Haydar Awada, Tareq Al-Sagheer, Christelle Makhlouf, Zeinab Tarhini, Sami Hamade, Nahla Araji, Sara Basbous, Hussein Samoury, Mira Raad, Rana Fneich, Josette Al-Sebaaly, Joseph Bassil, Zeinab Ezzedine, Odissa abu Mihrez, Josiane Kaddisse, Sami Hachem, Toni Abi Nahem, Ahmed Moussa, Charbel Khoury, Ahmed Takash. I would like to thank my special friends in Limoges Nathalie Sleiman, Josiane Simaan and Maha Krayem, and in Lebanon Catherine El Khoury, Fadwa Arab, Khalil Makhoul, Munther Fatfat and George Fares.
Finally I would like to offer my special love and gratitude to my beloved family for their endless love, throughout the duration of my studies.
To my first teacher
“Because you say so, I will let down the nets”
To my family
Nada, Elias, Jony, Juliana and Rafka I am proud of you
Abstract
Parkinson’s disease (PD) is characterised by the degeneration of the dopaminergic nigrostriatal pathway. Cell therapy using intranigral transplantation of foetal ventral mesencephalon (VM) cells in a mouse model of PD results in anatomical and functional reconstruction of the pathway. This suggests a role for axon guidance molecules (GMs) in reconnecting transplanted cells to their striatal target. To test this hypothesis, we studied the expression of axon GMs in the intact adult brain, on cells used for transplantation and in a mouse model of PD after cell therapy. In the intact brain, we showed that GMs as semaphorin7A (Sema7A) and Sema3A and their corresponding receptors, plexinC1 and neuropilin1, retain an expression at the protein level, therefore showing a possible role for these guidance cues in the adult brain. Moreover, using microarray, we studied GM receptor expression profiles in two types of cells used for transplantation and exhibiting different functional ameliorations. Robo2, neuropilin1, neuropilin2, EphA5 and DCC receptors showed differential expression between the two cellular populations, indicating their possible contribution to the different functional outcomes observed. In the lesioned mouse brain, we observed, using RT-qPCR, variations of mRNA expression of these axon GMs after intranigral transplantation of foetal VM derived cells, thus suggesting the implication of Sema3A, Sema3F, and Sema7A in the reconstruction of the pathway. Overall, this work highlights particular importance of semaphorins in the nigrostriatal pathway reconstruction. Integrating these cues in transplantation procedures can possibly optimize cell therapy for PD patients.
Keywords: Parkinson’s disease, cell therapy, axon guidance molecules, nigrostriatal dopaminergic pathway, quantitative PCR, immunohistochemistry, gene chip microarray, organotypic tissue culture.
Résumé
La maladie de Parkinson (MP) est caractérisée par une dégénérescence des neurones dopaminergiques de la voie nigrostriée. La thérapie cellulaire, par transplantation intranigrale de cellules fœtales issues de mésencéphale ventral (MV), assure un rétablissement anatomique et fonctionnel de cette voie. Des molécules de guidage axonal (MGA) joueraient ainsi un rôle dans la reconnexion axonale des cellules transplantées. Pour tester cette hypothèse, nous avons étudié l'expression de MGA dans le cerveau adulte intact et dans des cellules destinées à la transplantation, ainsi que dans le cerveau adulte d’un modèle murin de la MP après transplantation. Dans le tissu intact, nous avons montré que semaphorin7A (Sema7A) et Sema3A et leurs récepteurs, plexinC1 et neuropilin1, conservent leur expression protéique. De plus, grâce à l’utilisation de puces à ADN, nous avons montré que les récepteurs Robo2, neuropilin1, neuropilin2, EphA5 et DCC sont exprimés de manière différentielle dans les deux populations cellulaires utilisées pour la transplantation. Ceci suggère que ces molécules seraient impliquées dans la restauration fonctionnelle observée. Enfin, dans le tissu lésé, nous avons observé, par RT-qPCR, des variations d'expression de l'ARNm de ces MGA après transplantation intranigrale des cellules fœtales du MV, suggérant plus particulièrement l’implication de Sema3A, Sema3F et Sema7A dans la reconstruction de la voie. Ce travail met en lumière l'action de sémaphorines dans le guidage axonal des cellules transplantées. L'intégration de ces MGA dans les procédures de transplantation pourrait aider à optimiser les procédures de thérapie cellulaire dans la MP.
Mots clefs: Maladie de Parkinson, thérapie cellulaire, molécules de guidage axonal, voie dopaminergique nigro-striée, PCR quantitative, immunohistochimie, puces à ADN, culture organotypique.
Acknowledgements List of abbreviations List of Figures
Introduction………1
I. Parkinson’s disease……….………. 3
II. Lessons from the developing nigrostriatal dopaminergic pathway……..…..….24
II. Axon guidance molecules in adult brain………...38
IV. Aim of the project………...…45
Chapter 1: Axon Guidance Molecule Expression in the Mouse Intact Adult Nigrostriatal Pathway and in Cells Used for Transplantation………... 47
I. Introduction………...……49
II. Experimental approach ………...50
III. Results………...54
IV. Discussion and future considerations……….…...58
Chapter 2 : Axon Guidance Molecule Expression After Cell Therapy in a Mouse Model of Parkinson’s Disease……….….65
I. Summary……….………...68
II. Article in revision in the journal of Restorative Neurology and Neuroscience….71 III. Complementary experiments………..………….… 94
General Discussion………..…104
I. Overview of the variations of expression of axon guidance molecules in a mouse model of Parkinson’s disease after cell therapy using feotal ventral mesencephalon cells ………106
II. Semaphorins may be essential for the reconstruction of the degenerated pathway after transplantation ………110
III. May axon guidance molecules be helpful in future cell therapy procedures?....114
IV. Conclusion and perspectives……….…..117
Supplementary Study: Cellular uptake of Biocompatible Gold Nanoparticles ……….…119
I. Summary …..………..……..121
II. Article submitted to the journal Nanoparticle Research………123
List of abbreviations
CAPIT: core assessment program for intracerebral transplantations CNS: central nervous system
CPu: caudate putamen DBS: Deep brain stimulation DCC: deleted in colorectal cancer ESC: embryonic stem cells
GDNF: glial cell-derived neurotrophic factor GMs: guidance molecules
GPe: external segment of the globus pallidus GPi: internal segment of the globus pallidus GPI: glycosylphosphatidylinositol
iPS cells: induced pluripotent stem cells MFB: medial forebrain bundle
MPP: 1-methyl-4-phenylpyridinium MPTP: 1-methyl-1,2,3,6 tetrahydropiridine NAcc: Nucleus accumbens
Nrp: neuropilins
6-OHDA: 6-hydroxydopamine PD: Parkinson’s disease
PET: positron emission tomography Plxn: Plexins
Sema: semaphorin
SNpc: substantia nigra pars compacta SNr: substantia nigra pars reticulate STN: subthalamic nucleus
TH: tyrosine hydroxylase Thal: thalamus
VM: ventral mesencephalon VTA: ventral tegmental area
List of figures
Figure 1. Depigmentation of the substantia nigra in PD. ... 3
Box 1. UK Parkinson’s Disease Society Brain Bank’s clinical criteria for the diagnosis of probable Parkinson’s disease ... 5
Figure 2. Dopaminergic pathway degeneration in PD... 5
Figure 3. Light microscopy images showing Lewy bodies and Lewy neurites ... 5
Figure 4. Aetiology of PD ... 6
Figure 5. Direct and indirect basal ganglia circuitry in normal state and in PD ... 12
Figure 6. The Progress of cell replacement therapy in the treatment of PD ... 13
Figure 7. Cell replacement therapy in a rodent model of PD ... 16
Figure 8. Schematic representation of different dopaminergic cell sources for transplantation in PD ... 18
Figure 9. Mechanisms of axon guidance during development... 24
Figure 10. Four conserved families of axon guidance molecules. ... 25
Figure 11. Family of Semaphorins and their receptor complexes. ... 28
Figure 12. Dopaminergic neuron cell groups in the adult rodent brain in a sagittal view ... 30
Figure 13. Axon guidance in the midbrain between E11.5 and E13.5. ... 31
Figure 14. Axon guidance through the medial forebrain bundle E13.5... 33
Figure 15. Axon guidance in the telencephalon between E14.5-E18.5. ... 36
Figure 16. Factors contributing to axon regeneration failure in mature CNS……..……….41
Figure 17. Reconstruction of the nigrostriatal pathway after intranigral transplantation of ESC-derived dopaminergic neurons and Sema3C-expressing cells in rat model of PD……...………..44
Figure 18. Immunohistochemistry localization of receptors in the VM……….….………..……54
Figure 19. Immunohistochemistry localization of Sema3A ... 55
Figure 20. Immunohistochemistry localization of Sema7A ... 55
Figure 21. Microarray and RT-qPCR analysis of Robo receptor expression in foetal VM cells and in ESC-derived dopaminergic neurons. ... 56
Figure 22. Microarray and RT-qPCR analysis of semaphorin receptor expression in foetal VM cells and in ESC-derived dopaminergic neurons. ... 57
Figure 23. Microarray and qRT-PCR analysis of DCC receptor expression levels in foetal VM cells and in ESC-derived dopaminergic neurons. ... 58
Figure 24. Microarray and RT-qPCR analysis of EphA5 receptor expression in foetal VM cells and in ESC-derived dopaminergic neurons. ... 58
Figure 25. Explant co-culture of embryonic VM and newborn derived forebrain ... 95
Figure 26. Mid sagittal whole mounted brain slices of E13.5 embryos cultured for different periods in vitro. ... 97
Figure 27. Forebrain and VM explants co-cultured for different periods in vitro... 97
Figure 28. Forebrain and VM explants co-cultured for different periods in vitro... 98
Figure 29. Effects of blocking Nrp receptors on dopaminergic axonal growth. ... 99
Figure 30. Blocking effects of anti-Nrp1 and anti-Nrp2 antibodies on dopaminergic fibres growth from E13.5 VM explants towards P5 forebrain explants ... 99
Figure 31. Variations of axon guidance molecule mRNA expression in five regions along the nigrostriatal pathway 1 day and 7 days after substantia nigra lesion. ... 106
2
Figure 32. Variations of axon guidance molecule mRNA expression in five regions along the nigrostriatal pathway 1 day and 7 days after transplantation of foetal VM cells ... 106 Figure 33. Axon guidance molecules related to the reconstruction of the degenerated pathway109
1
2
I.Parkinson’s disease ... 3
1. General background and historical overview ... 3
1.1. Epidemiology ... 4
1.2. Symptomatic features and diagnosis... 4
1.3. Neuropathology ... 5
1.4. Aetiology and pathogenesis ... 6
1.5. Experimental models of Parkinson’s disease ... 8
2. Available therapeutic options ... 10
2.1. Pharmaceutical treatment ... 10
2.2. Deep brain stimulation of the Basal Ganglia ... 11
3. Restorative cell therapy: Historical view and current status ... 13
3.1. Early studies: transplantation of Adrenal Chromaffin Cell ... 14
3.2. Ectopic Transplantation of Foetal Tissue: achievements and challenges ... 15
3.3. Alternative sources of dopaminergic cells ... 18
3.4. Rewiring the pathway through Intranigral transplantation ... 20
3.5. Bridge transplantation procedures ... 21
II. Lessons from the developing nigrostriatal dopaminergic pathway ... 24
1. Different families of axon guidance molecules drive axonal navigation ... 24
1.1. Family of Netrins ... 25
1.2. Family of Ephrins ... 26
1.3. Family of Semaphorins... 28
1.4. Family of Slits ... 29
2. Role of axon guidance molecules in the development of the nigrostriatal dopaminergic pathway ... 30
2.1. Axon guidance in the midbrain ... 31
2.2. Axon guidance through the medial forebrain bundle ... 32
2.3. Innervating striatal targets ... 34
III.Axon Guidance Molecules in Adult Brain... 38
1. Expression and Function of Axon Guidance Molecules in Intact Adult Brain ... 38
2. Axon Guidance Molecules defects and Disease Susceptibility ... 39
3. Axon Guidance Molecules in Lesioned Adult Brain ... 41
4. Axon Guidance Molecules in Transplantation Procedures ... 43
3
I.
Parkinson’s disease
1. General background and historical overview
Parkinson’s disease (PD) is an age related progressive neurodegenerative movement disorder with an average onset age of 60 years (de Rijk et al., 1995). It was first described clinically by James Parkinson in 1817 in his famous monograph ‘An Essay on the
Shaking Palsy’. In this report Parkinson detailed the cases of six patients and defined
what he called the shaking palsy or ‘paralysis agitans’ disease which is characterized by “Involuntary tremulous motion, with lessened muscular power, in parts not in action and even when supported; with a propensity to bend the trunk forward, and to pass from a walking to a running pa ce: the senses and intellects being uninjured” (Lees et al., 2009). Jean Martin Charcot later provided further thorough description of the disease and of its cardinal symptomatic features, and proposed the modern nomenclature of the syndrome as ‘maladie de Parkinson’ (Charcot and Vulpian, 1862).
Regarding the Pathological aspects of the disease, Meynert in 1871 suggested that PD is related to defects in the basal ganglia function (Meynert, 1871). Then, Tretiakoff (1919) emphasized the importance of the substantia nigra through his studies on nine cases of PD where he found depigmentation and lesions in this nucleus in all studied cases (Figure 1). Tretiakoff also confirmed the previous work of Lewy (1914) who reported the presence of spherical cytoplasmic inclusions in PD, referred to as Lewy bodies (Fahn, 2003). In 1959 Carlsson suggested that dopamine could be involved in motor control in basal ganglia and its depletion in the striatum could be at the base of PD symptoms. This was later supported by postmortem studies that showed a reduced level of dopamine in the striatum and substantia nigra of parkinsonian patients (Ehringer and Hornykiewicz,
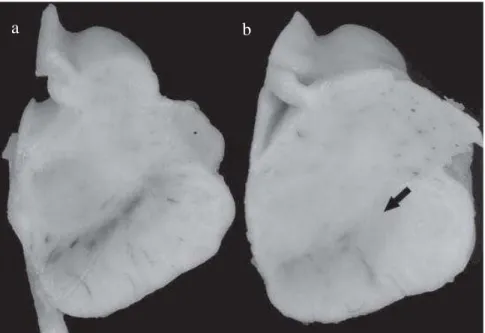
Figure 1. Depigmentation of the SN in PD. A transverse section of the midbrain at the level of the third nerve in (a) a normal elderly individual and (b) a patient with PD. The neurodegeneration of neuromelanin containing cells results in the depigmentation of SN, most significantly in the lateral region as indicated by the arrow. PD: Parkinson’s disease; SN:substantia nigra (Modified from Nass and Przedborski, 2011).
b a
4
1960). Therefore it was suggested in 1966 that PD is possibly caused by alterations in the dopamine-containing nigrostriatal pathway (Hornykiewicz, 1966).
1.1.Epidemiology
PD is the second most common neurodegenerative disorder (de Lau and Breteler, 2006). In industrialised countries it affects approximately 0.3% of the total population and 1% of people older than 60 years (Samii et al., 2004). The estimated total number of people with PD around the world is 10 million (Hegarty et al., 2014). Some studies indicated a higher prevalence of PD in men than in women, while others did not find any difference in prevalence between different genders (reviewed in de Lau and Breteler, 2006).
1.2.Symptomatic features and diagnosis
Parkinson’s disease is manifested in several motor and non-motor features that tend to evolve from mild unilateral symptoms through to a severe disabling state. The cardinal motor symptoms of PD include resting tremor, bradykinesia, rigidity, and postural instability. Non-motor complaints are also associated with PD and may appear earlier than the classic motor features. These symptoms include autonomic dysfunction such as constipation and postural hypotension, in addition to cognitive and neurobehavioral abnormalities, sleep disorders and sensory abnormalities such as anosmia, paresthesias and pain (Jankovic, 2008). There is no available diagnostic test that can reliably predict the disease. Diagnosis is usually done clinically based on physical examination, although several other atypical Parkinsonian syndromes share similar clinical motor symptoms and may be confused with PD, such as vascular parkinsonism, progressive supranuclear palsy, and multiple system atrophy (Gazewood et al, 2013). However diagnostic criteria
5
have been developed by the UK Parkinson’s Disease Society Brain Bank (Box 1) and the National Institute of Neurological Disorders and Stroke, and significantly improve the accuracy of the clinical diagnosis. Generally correct diagnosis of PD is predicted when patients exhibit a combination of the cardinal features of the disease, excluding other possible causes of parkinsonism such as the exposure to some drugs like calcium channel blockers, atypical antipsychotics, gastrointestinal prokinetics, and antiepileptic drugs (Jankovic, 2008; Shin and Chung, 2012). Gradual symptom progression and a response to PD pharmaceutical treatment are important features that would further support the diagnosis, while post-mortem neuropathologic confirmation remains the ultimate approach for the definitive diagnosis (Savitt et al., 2006; Olanow et al., 2009).
1.3.Neuropathology
The major hallmark of the disease is the neurodegeneration of the neuromelanin containing dopaminergic neurons of the substantia nigra pa rs compacta (SNpc) and the subsequent depletion of dopamine neurotransmitter in their projection site: the dorsolateral striatum (Figure 2) (Bernheimer et al., 1973; Marsden, 1983). However degeneration also affects other extranigral neuronal systems including the dorsal motor nuclei of the hypoglossal and vagal nerves, the raphe nuclei, isthmic nuclei, and the anterior olfactory nucleus as well as the olfactory bulb (Braak et al., 2003).
Another histological hallmark of PD is the accumulation of inclusions located in neuronal perikarya called Lewy bodies, and neuronal processes that are referred to as Lewy neurites (Braak et al., 1999; Spillantini et al., 1998). At the ultrastructural level, Lewy bodies consist of dense granular material and filamentous structures that are 10 to 15 nm in diameter (Nass and Przedborski, 2011) (Figure 3). These structures are mainly
(Modified from Jankovic, 2008)
Box 1. UK Parkinson’s Disease Society Brain Bank’s clinical
criteria for the diagnosis of probable Parkinson’s disease
Step 1 Bradykinesia
At least one of the following criteria: Rigidity
4–6 Hz rest tremor
Postural instability not caused by primary visual, vestibular, cerebellar or proprioceptive dysfunction
Step 2
Exclude other causes of parkinsonism Step 3
At least three of the following supportive (prospective) criteria: Unilateral onset
Rest tremor
Progressive disorder
Persistent asymmetry primarily affecting side of onset Excellent response (70–100%) to levodopa
Severe levodopa induced chorea (dyskinesia) Levodopa response for 5 years or more
Figure 3. Light microscopy images showing Lewy bodies and Lewy neurites that are usually expressed in the substantia nigra and other brain regions in Parkinson’s disease patients. (a) Two neurons containing α-synuclein-positive Lewy bodies indicated by red arrows, Lewy neurites are also present and are indicated by black arrows. Scale bar, 20 µ m. (b) Pigmented nerve cell with two α-synuclein-positive Lewy bodies. Scale bar, 8 µ m. (c) α-Synuclein-positive extracellular Lewy body. Scale bar, 4 µm (Modified from Spillantini et al., 1997).
Figure 2: Dopaminergic pathway degeneration in PD. (A) sagittal view cartoon of the normal nigrostriatal pathway (blue) that consists of dopaminergic neurons located in the substantia nigra in the ventral midbrain that extend axonal projections to the striatum. (B) In PD, the progressive degeneration of these neurons leads to a loss of dopaminergic innervation of the striatum and consequently a reduction in striatal dopamine levels. PD: Parkinson’s disease (Modified from O′Keeffe et al., 2014).
6
composed of α-synuclein (Spillantini et al., 1997), a presynaptic protein of unknown function which is mutated in some familial cases of the disease. In addition to the substantia nigra, lewy bodies are also found in the locus coeruleus, nucleus basalis, hypothalamus, cerebral cortex, cranial nerve motor nuclei, and central and peripheral components of the autonomic nervous system (Olanow and Tatton, 1999). Therefore PD involves different brain regions and is not confined to the nigral region.
PD can be considered to have a threshold like onset whereby pathological features may develop up to 20 years before any motor symptom manifestations (Hawkes et al., 2010). Therefore by the time of diagnosis 50 to 70 % of the SNpc neurons would be already lost (Postuma et al., 2010).
1.4.Aetiology and pathogenesis
There are two types of PD first, the familial PD which is usually characterized by an early-onset, it contributes to less than 10% of the cases and is related to genetic predisposition second, the late-onset or idiopathic form which comprises more than 85% of all cases. Therefore PD is multifactorial, with a combination of both environmental and genetic factors that are likely to contribute to the disease onset (Figure 4). In addition, aging is a major cause that strongly relates to the disease incidence (Simunovic et al., 2009) however, little is known about how the aging process contributes to the pathogenesis.
PD appears to have an X-linked pattern of inheritance. The first gene identified as being implicated in familial Parkinsonism was SNCA coding for alpha-synuclein. Mutations in this gene lead to misfolding and aggregation of this protein into a neurotoxic species causing autosomal-dominant PD (Polymeropoulos et al., 1996). In addition to
alpha-Figure 4. Aetiology of PD. Genetic risk factors and environmental factors that provoke oxidative stress and excitotoxicity lead to mitochondrial dysfunction in the brain and cause the degeneration of the dopaminergic neurons of the SNpc, resulting in PD. DJ-1: Protein deglycase; LRRK2: Leucine-rich repeat kinase 2; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; mtDNA: mitochondrial DNA; PD: Parkinson’s disease; PINK1: PTEN-induced putative kinase1; ROS: rea ctive oxygen species; SNpc: substantia nigra pars compacta (Modified from Barreto et al., 2015).
7
synuclein several genes are now recognized as causative genes associated with mendelian forms of the disease, either with autosomal dominant (SNCA, LRRK2) or recessive inheritance (PARK2/Parkin, PINK1, DJ-1/PARK7) (Verstraeten et al., 2015). LRRK2 encodes leucine-rich repeat kinase 2, a large multifunctional protein with two enzymatic domains (GTPase and kinase), mutations in this gene are associated with autosomal-dominant, late-onset parkinsonism (Zimprich et al., 2004). Parkin is known to act as an E3-ubiquitin ligase and play a functional role in proteosomal degradation and receptor trafficking. Mutations in parkin are the most common cause of autosomal recessive PD (Dawson and Dawson, 2010; Kitada et al., 1998). PINK1 encodes PTEN-induced putative kinase 1, which is a mitochondrial serine/threonine-protein kinase. It is involved in protecting the cells from stress-induced mitochondrial dysfunction. Mutations in this gene are associated with early-onset autosomal recessive form of the disease (Valente et al., 2004). DJ-1 gene which is also an oncogene, has various functions including transcriptional regulation, antioxidative stress reaction, and mitochondrial regulation (Bonifati et al., 2003; Ariga et al., 2013).
On the other hand, the environmental hypothesis suggests that PD-related neurodegeneration results from exposure to dopaminergic neurotoxins. MPTP (1-methyl-1,2,3,6 tetrahydropiridine) is an environmental toxin that has been directly linked to development of acute Parkinsonism (Langston et al., 1983). It is a contaminant of a synthetic opiate that can cross the blood-brain barrier and cause Parkinsonism through its toxic metabolite 1-methyl-4-phenylpyridinium (MPP+). MPP+ is selectively up taken by dopaminergic cells and inhibits mitochondrial complex 1 in the respiratory chain. Additional environmental toxins have been identified including pesticides, particularly
8
paraquat and rotenone (Tanner, 1992). The discovery of the neurotoxic effects of MPTP provided substantial evidence implicating mitochondrial dysfunction in the pathogenesis of PD (Langston et al., 1983). It is now widely recognized that reduced bioenergetic capacity, increased oxidative stress and reduced resistance to stress are major pathogenic factors contributing to the neurodegeneration in PD. In addition to mitochondrial dysfunction other factors such as protein aggregation, proteasomal stress, lysosomal dysfunction and aberrant autophagy were observed in different studies of both genetic and sporadic forms of the disease and thus were associated with the pathogenesis process (reviewed in Dexter and Jenner, 2013).
1.5.Experimental models of Parkinson’s disease
Parkinson’s disease is, to a large extent, specific to the human species. However studies conducted on induced experimental animal models of PD have largely contributed to the understanding of the pathology and pathogenesis of the disease, and allowed better assessment of potential efficacy of treatments in reversing its motor defects. There are two types of animal models that replicate most of the pathological features of this disease. First, the transgenic models that are based on genetic mutations in the recently identified genes that cause familial cases of PD (Blesa et al., 2012). Second, the neurotoxic models that are developed through local or systemic administration of toxins, that selectively affect the catecholaminergic system including 6-hydroxydopamine (6-OHDA), MPTP, rotenone and paraquat, ubiquitin proteasome system inhibitors, and bacterial endotoxins as neuroinflammatory triggers (Le et al., 2014; Blandini and Armentero, 2012). These neurotoxic models cause degeneration of the neuronal populations in a process similar to that of PD, involving oxidative stress which leads to
9
the production of reactive oxidative species (Blesa et al., 2012). A drawback of this kind of models is its inability to replicate the progressive development of PD, thus the phenotype produced in this model represents a late-stage of PD (Le et al., 2014). The first animal model of PD was developed by Ungerstedt who was the first to use 6-OHDA to cause specific lesion of the SNpc dopaminergic neurons (Ungerstedt, 1968). Since then, 6-OHDA is considered the classical and the most used toxin induced animal model. It could be used in mice, cats, dogs, and monkeys, but it is extensively used to generate rodent models of the disease (Blesa et al., 2012). Since 6-OHDA does not cross the blood-brain barrier it is administered by a direct stereotaxic injection into the regions where the dopaminergic neurons are localized: in SN, median forebrain bundle (MFB), or striatum. 6-OHDA toxicity affects the catecholaminergic neurons due to the selective affinity for the dopamine and norepinephrine transporters, through which this hydroxylated toxic form of dopamine is transported to the cytoplasm of the dopaminergic neurons (Luthman et al., 1989). Therefore, to prevent damage of the noradrenergic neurons OHDA is often combined with a selective noradrenaline reuptake inhibitor. 6-OHDA is most commonly injected in the medial forebrain bundle (MFB) region causing a marked anterograde degeneration of dopaminergic neurons of the nigrostriatal pathway within 12 hours following the administration, and lesion of striatal dopaminergic innervations starting after 48 hours (Blandini and Armentero, 2012). Bilateral injections of 6-OHDA results in severe complications such as aphagia and adipsia and could possibly lead to death, this is why it is preferably administered unilaterally with the contralateral side serving as control (Ungerstedt, 1971). The limb asymmetry produced by the unilateral lesion and the subsequent contralateral motor impairment could be
10
assessed quantitatively by behavioural tests and drug induced rotational tests, which is important for the evaluation of functional repair of nigrostriatal pathway after transplantation. Although Lewy bodies were not detected in histological sections of models induced by 6-OHDA, which does not have any effect on other brain regions as observed in PD, however this neurotoxin succeed in inducing dopaminergic neuronal degeneration, dopamine reduction, and neurobehavioral deficits (Tieu, 2011).
Both genetic and neurotoxic models of PD have limitations and fail to reproduce all the pathological and clinical features of the disease. Thus, an appropriate model must be chosen carefully based on the investigated aspect of the disease (Jackson-Lewis et al., 2012). While genetic models provide information about the aetiology and the pathogenic mechanisms of the disease, neurotoxin models are more suitable for pathological and therapeutic studies (Dauer and Przedborski, 2003).
2. Available therapeutic options
2.1.Pharmaceutical treatment
The most prominent symptom of PD is the motor disability which is linked to the depletion of dopamine in the striatal region. Therefore therapeutic options are targeted for the repair of these motor complications through pharmacological dopamine replacement strategies. The dopamine precursor levodopa (L-3, 4-dihydroxyphenylalanine) is the gold standard medication that can eliminate most of these symptoms at early stages of the disease such as tremors and muscle stiffness. It is administered with dopa decarboxylase inhibitor such as carbidopa to inhibit the peripheral metabolism of levodopa, therefore allowing higher concentrations of levodopa to enter the brain. However, additional motor and nonmotor complications appear within several years of treatment and worsen in the
11
late stages of illness, some being related to the treatment (short half-life) and others caused by the disease progression (Brichta et al., 2013). Motor fluctuations that reflect rises and falls of levodopa plasma levels following individual doses (wearing-off effect) and dyskinesia (hyperkinetic involuntary movements) are the major complications that appears secondary to the levodopa treatment (Marsden and Parkes, 1976). This has led to the appearance of alternative and complimentary treatments such as dopamine agonists, catechol-O-methyl transferase inhibitors (COMTIs) or monoamine oxidase type B inhibitors (MAOBIs) that are administered as monotherapy or in combination with levodopa (Maranis et al., 2011).
2.2.Deep brain stimulation of the Basal Ganglia
The basal ganglia circuit is involved in the planning, initiation and execution of correct voluntary movements through both direct and indirect pathways (G E Alexander et al., 1986). It consists of multiple subcortical nuclei including the striatum: caudate nucleus, putamen and nucleus accumbens (NAcc), the external (GPe) and internal (GPi) segments of the globus pallidus, the subthalamic nucleus (STN) and the SNpc and the substantia nigra pars reticulate (SNr). The main task of the circuit is to process the signals that flow from the cortex to produce an output signal that returns to the cortex through the Thal (Blandini et al., 2000). The direct pathway connects the striatum to the internal segment of the GPi and SNr (GPi/SNr) that constitutes the output nuclei of the basal ganglia projecting inhibitory connections to the brainstem and the Thal which in turn project back to the cerebral cortex. On the other hand, the indirect pathway connects the striatum to the output nuclei through synaptic connections with the GPe and then STN, with the output from the STN to the GPi/SNr being excitatory. Therefore activation of the direct
12
pathway inhibits basal ganglia output, therefore disinhibiting thalamocortical interactions while activation of the indirect pathway has an opposite effect. The two pathways are under differential control exerted by the dopaminergic nigrostriatal pathway, whereby D1 dopamine receptor activation stimulate the direct pathway, while D2 receptor activation inhibits the indirect pathway (Figure 5a)(Smith et al., 2011). In PD, the degeneration of dopaminergic neurons of the SNpc leads to functional changes in the network. The most relevant alterations affect the output nuclei of the circuit, the GPi/SNr, which become hyperactive. Such hyperactivity is sustained by the enhanced glutamatergic inputs that output nuclei receive from the STN (Figure 5b).
Deep brain stimulation (DBS) in basal ganglia target nuclei and particularly the subthalamic nucleus is considered as an effective therapy that helps to reduce severe motor complications in advanced PD stages. Therefore, this surgical procedure is applicable on patients exhibiting motor fluctuations and drug-induced dyskinesias that are significantly disabling and is usually conducted approximately between 11 and 13 years after diagnosis. An increased interest in this procedure, as a treatment of PD, began in 1987 (Benabid et al., 1987) after it was observed that a high frequency stimulation of the ventral intermediate thalamic nucleus results in a significant relief of tremor of PD patients. Since then, the improvements in the imaging and neurophysiological recording techniques contributed to its further amelioration and refinement. The underlying therapeutic effect is still unclear, however observations have suggested that a high frequency electrical stimulation of target regions can mimic, in a reversible and adjustable way, a lesion-like effect suggesting a mechanism of functional inhibition (Chen et al., 2013). DBS also reduces the therapeutic treatment dosage required which
Figure 5. Direct and indirect basal ganglia circuitry in normal state (a) and in PD (b). Excitatory glutamatergic connections are indicated by blue arrows; inhibitory GABAergic connections are indicated by red arrows. Change in thickness of the arrows indicates an increase (thick) or decrease (thin) in firing rates. The dashed arrows indicate the partial lesion of the dopaminergic connections from the SNc to the putamen in Parkinson’s disease. CM, centromedian nucleus; CMA, cingulate motor area; GPe, globus pallidus, external segment; GPi, globus pallidus, internal segment; M1, primary
motor cortex; PD: Parkinson’s disease; PMC, pre-motor cortex; PPN, pedunculopontine nucleus; SMA, supplementary motor a rea; SNc, substantia nigra pa rs compacta; SNr, substantia nigra pa rs reticulata; STN, subthalamic nucleus; VA/VL, ventral anterior/ventral lateral nucleus (Modified from(Smith et al. 2011)
13
results in a decrease in the associated side effects (Deuschl et al., 2006). Several studies demonstrated significant motor enhancements after the surgical intervention resulting in marked improvements in the quality of life of patients with advanced PD. However, recent long-term studies have reported a gradual decline in the effectiveness of STN-DBS on gait disturbances (Fasano et al., 2010; Merola et al., 2011). Therefore this strategy could be helpful in relieving severe motor conditions, yet it is not enough to ensure the total elimination of all motor deficits at a long term.
3. Restorative cell therapy: Historical view and current status
The poor understanding of PD aetiology constitutes a major obstacle in the development of curative treatments, and thus current therapeutic options are only symptomatic. Subsequently, the absence of restorative medications and the well-defined anatomical property of the nigrostriatal pathway are two main reasons that make PD a perfect candidate for cell therapy as an approach to restore the lost dopaminergic neurotransmission and to ensure long-lasting motor enhancement.
Initial experimental and clinical transplantation studies were first conducted in the 1970s, since then, major advances in the field of transplantation were accomplished (Figure 6). Different types of tissues were used in the transplantation, for example early trials involved transplantation of adrenal medullary tissue as a source of direct dopamine release. Then, in the aim to restore the physiological synaptic dopamine release, foetal dopaminergic neurons derived from the ventral mesencephalon (VM) region were transplanted, first as tissue blocks and later as cell suspensions. However, inconsistencies obtained in clinical outcomes suggested the requirement of further ameliorations in the transplantation procedures. The use of foetal VM cells, together with the ectopic
In tr o d u ct io n
Figure 6. The Progress of cell replacement therapy in the treatment of PD. 1Olson and Malmfors, 1970; 2Olson and Seiger, 1972; 3Perlow et al., 1979; 4Bjorklund and Stenevi, 1979; 5Björklund et al., 1980; 6Dunnett et al., 1981a; 7Dunnett et al., 1981b; 8Dunnett et al., 1981c; 9Madrazo et al., 1988; 10Lindvall et al., 1988; 11Lindvall et al., 1989; 12Zhou and Chiang, 1995; 13Olanow et al., 2003; 14Freed et al., 2001; 15Kawasaki et al., 2000; 16Perrier et al., 2004 ;17Gaillard et al., 2009; 18Thompson et al., 2009; 19Díaz-Martínez et al., 2013. PD: Parkinson’s disease.
14
placement of the transplanted cells are two major challenges that could contribute to this variability. Consequently, alternative sources of dopaminergic cells that are ethically acceptable and widely available, such as embryonic stem cells (ESC) and induced pluripotent stem cells (iPS cells) are currently studied (Barker et al., 2015). Moreover, homotopic intranigral transplantation that would ensure a proper anatomical reconstruction of the damaged circuitry is considered (Sayles et al., 2004).
3.1.Early studies: transplantation of Adrenal Chromaffin Cell
Adrenal medulla chromaffin cells were the first autologous dopamine cell-source substituent considered in cell replacement studies. In addition to escaping the ethical problems, another advantage of an autologous graft is avoiding an immune reaction that would be faced otherwise with allogenic foetal transplants. Chromaffin cells of the adrenal medulla are characterized by high level of phenotypic plasticity. They produce dopamine as an intermediate product in the synthesis of adrenalin. When dissected from the surrounding adrenal cortex and cultured in vitro, chromaffin cells display a sympathetic neuronal-like phenotype forming neurites and shifting their catecholamine synthesis toward a more dopaminergic phenotype (Unsicker et al., 1985; Hansen et al., 1989). The major drawback of using these cells is that chromaffin cells provide a direct diffuse release of dopamine rather than synaptic transmission. Olson was the first to perform a successful graft of chromaffin cells in the anterior eye chamber of rats (Olson and Malmfors, 1970; Olson and Seiger, 1972) proving that these transplanted cells can survive and grow neurites (Olson et al., 1980). Later on Freed et al. reported important motor ameliorations two months after intraventricular transplantation of adrenal medullary tissue in rats with unilateral 6-OHDA lesions. As the transplanted tissue did
15
not innervate the striatum it was suggested that the observed effects resulted from a diffusion of released catecholamines (Freed et al., 1981). Soon after the work of Freed, and based on primitive preclinical studies, clinical trials have shown less impressive effects of autologous unilateral adrenal medulla transplants into the caudate nucleus or putamen of patients with advanced PD (Backlund et al., 1985; Lindvall et al., 1987). Yet, another publication appeared in 1987 by Madrazo et al, reporting an improvement in the motor symptoms of PD in two patients after autografting adrenal medulla pieces in a pre-made cavity in the head of the caudate nucleus (Madrazo et al., 1987). However, subsequent studies by other groups did not report important long-term improvements after transplantation and the approach was subsequently abandoned as it induced only poor and transient recovery and was associated with high levels of mortality and morbidity (Goetz et al., 1991). Although this transplantation procedure did not provide sufficient clinical outcomes to be justified as a routine therapeutic procedure, yet it paved the way for other transplantation procedures recruiting other sources of dopamine. Particularly the use of embryonic ventral mesencephalic tissue was being studied in both experimental and clinical trials.
3.2.Ectopic Transplantation of Foetal Tissue: achievements and challenges
The first experimental studies involving transplantation of foetal VM cells were conducted in 1979 (Perlow et al., 1979; Bjorklund and Stenevi, 1979). Grafts of embryonic VM as solid tissue blocks were placed ectopically in the lateral ventricle, or in a created dorsal cortical cavity. Both studies reported favourable functional and histological outcomes. Later on, dopaminergic neurons derived from the developing VM were used as cell suspension preparations and were transplanted directly in the
16
denervated striatum (Figure 7) (Björklund et al., 1980). Extensive experimental studies on rodent models using intrastriatal grafts provided evidence of the capacity of foetal VM cell transplants to survive and integrate in the striatal region, providing important dopamine release and contributing to behavioural ameliorations (Dunnett et al., 1981a; Dunnett et al., 1981b; Dunnett et al., 1981c; Schmidt et al., 1983; Dunnett et al., 1983); Björklund, 1992). In 1985, the approval of using human fœtal tissue in transplantation procedures was issued in Sweden. Consequently, preclinical trials were conducted using foetal VM cells, obtained from aborted foetuses of 6.5 to 9 weeks age, transplanted in the striatum of immunosuppressed 6-OHDA rat models. Favourable effects were observed, the host striatum was innervated, resulting in dopamine release, which reduced motor deficits in the grafted animals. These promising results have lead the way for the initiation of the early clinical trials (Brundin et al., 1986).
The first clinical trial was conducted in 1987 in Sweden. Early clinical studies involved small groups of patients and were designed as uncontrolled open label trials i.e. both patients and researchers know which clinical intervention is being administered. The outcomes were evaluated based on 18F-fluorodopa uptake by positron emission tomography (PET) scans that provided objective indications of the survival and growth of the grafted neurons. In addition, core assessment program for intracerebral transplantations (CAPIT protocol) (Langston et al., 1992), that is a quantitative functional assessment protocol was developed based on the work of Lindvall et al., (1989). Significant long lasting benefits in some patients were recorded: the grafted tissue survived, and striatal dopamine levels increased, resulting in sustained amelioration of the motor function. This was supported by post-mortem data that revealed graft survival
Figure 7. Cell replacement therapy in a rodent model of PD. After unilateral 6-OHDA injection in the substantia nigra pars compacta, foetal VM cells derived from E12.5 rodents are transplanted in the denervated striatum. Coronal sections at the level of the striatum show the transplanted cells, and at the level of the substantia nigra demonstrating the unilateral lesion. SNPc: substantia nigra pars compacta; VM: ventral mesencephalon (Modified from Guerra-Crespo et al., 2011).
E12.5 rodent brain
Rostral
Dorsal
17
and good innervation of the grafted striatum. However, in some patients no major clinical effects were noted after transplantation (Madrazo et al., 1988; Lindvall et al., 1988; Lindvall et al., 1989; Lindvall et al., 1994; Lindvall et al., 1990; Hagell et al., 1999; Lindvall et al., 1992; Reviewed in Barker et al., 2013). Since then cell therapy of PD has moved to a new level with the initiation of two placebo-controlled double-blind studies, the Colorado/Columbia trial and the Tampa/Mount Sinai/Rush trial, that were reported in 2001 and 2003 (Olanow et al., 2003; Freed et al., 2001). The outcomes of these trials were disappointing in terms of poor efficacy and the appearance of graft induced dyskinesia in some patients, and development of Lewy bodies in transplanted cells (Li et al., 2008).
The negative results obtained in the controlled trials and the variability in clinical outcomes of the open label trials constituted major drawback in the restorative therapy procedures of PD. Here it is important to note that many factors could contribute to the efficacy of transplantation, such as transplantation protocols, composition and preparation of the foetal tissue, immunosuppressive treatment, and patient selection (reviewed by Gaillard and Jaber, 2011). Therefore, several aspects of cell therapy procedures should be further normalized and studied in experimental models. In this regard, two major limitations of the intrastriatal foetal VM cells grafting technique have led to the shift of cell therapy procedure to a different direction. First, the intrastriatal grafts aims to create a ‘short circuit’ instead of rewiring nigrostriatal pathway (Collier, 2014). Therefore, the transplanted dopaminergic neuroblasts are deprived from both trophic factors expressed in their homotopic location that would be essential for their proper differentiation and subsequent optimal function, and from the afferent connections
18
that they should normally receive in the substantia nigra (Gaillard and Jaber, 2011). In addition, the use of foetal tissue is associated with major technical and ethical issues. Each patient requires optimally between eight and twelve foetuses for bilateral transplantation, therefore the limited tissue availability is a major obstacle in the adoption of transplantation as a widespread routine procedure. Thus, alternative sources of dopaminergic cells for therapeutic applications are currently under investigation.
3.3. Alternative sources of dopaminergic cells
Current efforts are devoted to generate large numbers of dopaminergic neurons for cell replacement therapies through standardized preparations that aim to enhance reproducibility and safety of the procedure. Different sources that are either allogeneic or syngeneic have been considered so far to obtain dopaminergic neuroblasts including embryonic stem cells (ESC) (Kirkeby et al., 2012), expanded neural precursor cells derived from foetal VM (Delcroix et al., 2011), bone marrow derived mesenchymal stem cells (Offen et al., 2007), and induced pluripotent stem (iPS) cells (Hallett et al., 2015), as well as induced neurons obtained by direct reprogramming of somatic cells (Caiazzo et al., 2011) (Figure 8).
Transplantation studies in animal models have demonstrated an important capacity of these cells to survive and integrate in the striatal tissue, and good results were particularly obtained using ESC. ESC are pluripotent cells derived from the inner cell mass of the blastocyst (Martin, 1981; Evans and Kaufman, 1981). These cells have the potential to produce cells of the three germ layers ectoderm, mesoderm, and endoderm. Lee et al. (2000) was the first to produce dopaminergic neurons from mouse ESC, these neurons expressed TH (Lee et al., 2000). Extensive transplantation studies using mouse ESC-
Figure 8. Schematic representation of different dopaminergic cell sources for transplantation in Parkinson’s disease. On the left part of the figure: hESCs and fetal tissue sources, which both result in allografts. On the right part of figure: the recently discovered iPS and induced neuronal cells that could be sources of syngeneic donor tissue originating from the patient. iN: induced neuronal cells; iPS: induced pluripotent stem cells; hESC: human embryonic stem cells; Allogeneic: genetically dissimilar; Syngeneic: genetically identical (Modified from Brundin et al., 2010).
Expanded neural precursor cells
19
derived dopaminergic neurons (Kim et al., 2002; Kawasaki et al., 2000; Björklund et al., 2002) led to the development of human ESC-derived dopaminergic neurons (Perrier et al., 2004; Zeng et al., 2004; Grealish et al., 2014). Further progress towards the optimization of restorative therapy has been achieved through generation of patient-specific autologous neuroblasts from iPS cells bypassing many obstacles that have been faced through the use of ESC (Soldner et al., 2009). In particular, the immunosuppressive therapy necessary for reducing the immune reaction induced by the use of allografts is not required with the use of iPS cells; this is in addition to eliminating the ethical complications. iPS cells are obtained by reprogramming mouse and human somatic cells to a pluripotent state through viral transduction of four transcription factors, OCT4, KLF4, SOX2, and c-MYC (Takahashi et al., 2007; Soldner et al., 2009). Experimental studies on animal models have been carried out using iPS cells and promising results have been obtained (Han et al., 2015). However as these cells are derived from PD patient thus they still hold the same genetic profile, therefore the same susceptibility to develop PD pathogenesis. In addition, the presence of residual reprogramming viral factors in these cell preparations is associated with alterations in the differentiation potential of the iPS cells. Therefore iPS cells free of reprogramming factors should be obtained for transplantation (Hargus et al., 2010; Soldner et al., 2009). Moreover, different protocols used for generating dopaminergic neurons, produced different percentages of cells expressing TH, however only small proportions of these cells expressed the midbrain dopaminergic markers as the transcription factor LMX1A, which could explain modest functional effects after transplantation (Barker et al., 2015).
20
Finally, the use of both ESC and iPS cells is associated with the risk of tumour development. Therefore, before moving to the clinical phase, extensive experimental studies are required to develop efficient differentiation protocols that allow the production of sufficient numbers of dopaminergic neurons of the midbrain phenotype devoid of undifferentiated tumour-forming cells.
3.4.Rewiring the pathway through Intranigral transplantation
So far, the vast majority of both experimental and clinical transplantation strategies were focused on the placement of dopaminergic cells directly in the target striatal region, in the aim of restoring direct synaptic neurotransmission. However, this ectopic placement could be a major contributor to the suboptimal outcomes of intrastriatal grafting, through the deprivation of these transplanted cells from the trophic factors of their physiological environment and the axonal afferents they should normally receive. Therefore in the aim to augment the functional effects of transplantation, it is important to re-construct the pathway anatomically. However homotopic intranigral trials transplanting VM dopaminergic neuroblasts into the substantia nigra have had only modest success. These grafts did not show extensive de novo axonal growth towards the striatum along the pathway (Nikkhah et al., 1994; Collier et al., 2002; Mukhida et al., 2001; Starr et al., 1999; Bentlage et al., 1999; Mendez et al., 1996). This failure was attributed to the non-permissive capacity of the adult brain to support long distance axonal growth and extension of the transplanted cells (Bentlage et al., 1999). However, another possible explanation to this poor innervation of the striatum could be the modest visualization techniques that were used to check the extent of axonal growth. Indeed, studies using xenografts from human or porcine origin, transplanted in the substantia nigra in rat
21
models provided evidence of substantial axonal growth towards the striatum, using species specific markers of the host tissue (Isacson and Deacon, 1996; Isacson et al., 1995; Wictorin et al., 1992). Moreover, the tracking strategies further improved through the use of transgenic donor animals that overexpress green fluorescent protein (GFP) under the promoters of beta-actin or the tyrosine hydroxylase (TH) a marker of dopaminergic cells (Sawamoto et al., 2001). Subsequent to this amelioration, experimental studies on rodents revealed that homotopic intranigral transplants of dopaminergic VM cells derived from transgenic GFP-donors succeeded to produce important striatal innervations. In addition to striatum, important projections connecting to other target regions like the frontal cortex, the NAcc and the subventricular zone have been detected, therefore providing evidence of a permissive capacity of the adult brain. This proper anatomical repair was translated by enhanced functional outcomes (Gaillard et al., 2009; Thompson et al., 2009). Yet, still striatal re-innervation remained below the level of the intact brain, which requires additional efforts to improve the level of connectivity from these grafts before moving to clinical trials. This could be accomplished by incorporating growth promoting factors in the transplantation procedures to enhance axonal growth and innervation efficiency.
3.5.Bridge transplantation procedures
Intranigral transplantation has been associated with bridging procedures in order to enhance the axonal growth and extension and subsequently the target innervation outcomes (Zhou and Chiang, 1995). These techniques were used to create growth supporting medium along the pathway from substantia nigra to the striatum by incorporating growth promoting factors or attractive cues towards the striatum. Many
22
studies have incorporated some trophic factors such as the glial cell-derived neurotrophic factor (GDNF), a trophin known to promote the survival and axonal growth of dopaminergic neurons and to promote axonal regeneration after lesion of the nigrostriatal pathway (Brizard et al., 2006). Bridges were created by direct injection of GDNF in the striatum or along the pathway (Wang et al., 1996; Redmond et al., 2009; Thompson et al., 2009), where it was shown to act as chemoattractant increasing the outgrowth of fibers from foetal VM cells transplanted in the SN. In additional transplantation of tissues that provide a source of factors was also considered. For example, intrastriatal transplantation of GDNF-secreting Schwann cells with E14 ventral mesencephalic grafts in rat model of PD improved graft survival and axonal outgrowth (Wilby et al., 1999). In addition, foetal kidney tissue expressing high levels of GDNF was also transplanted along the pathway forming a bridge coupled to intranigral transplant of foetal VM tissue. Fibers expressing TH were detected in the striatum and behavioural ameliorations were observed proving the efficacy of kidney tissue as a source of trophic factors (Chiang et al., 2001). Bridge grafts of Fibroblast growth factor 4-secreting cells were also combined with intranigral graft of foetal VM cells which enhanced the graft survival, anatomical repair and functional outcome (Brecknell et al., 1996). In addition, co-transplantation was also performed using embryonic straiatal tissue transplanted along the pathway, as a source of growth promoting and attractive cues (Dunnett et al., 1989). In this study TH expressing fibres have been observed to extend along the path towards the striatum. Moreover, nigrostriatal innervation was observed after double, intranigral and intrastriatal, transplants of foetal VM cells (Mendez et al., 1996; Mendez and Hong, 1997),. Homotopically implanted grafts also succeed to connect to the striatal targets after an
23
ibotenic and kainic acid injection along the pathway. Therefore proving that an excitotoxic lesion can promote axonal growth (Zhou and Chiang, 1995). Despite important enhancements attributed to these different types of bridges, yet the amount of striatal reinnervation remained modest. Bridging procedures so far have only incorporated growth promoting and attractive mechanisms to assist the axonal extension after transplantation. However, going back to the developmental stages, it is well established that inhibitory and repulsive mechanisms are as essential as the attractive and growth-promoting effects for the axonal guidance of neurons (Collier, 2014). Among the molecules able to direct the axons of grafted cells, are axon guidance cues. These cues are expressed along the nigrostriatal pathway during development and direct the construction of the nigrostriatal pathway by both attractive as well as repulsive effects and act through temporal and spatial gradients (reviewed by Prestoz et al., 2012). Therefore these cues constitutes obvious candidates for characterizing mechanisms involved in the anatomical repair in animal model of PD, and thus development of ideal transplantation strategies would require a proper understanding of these complex cellular and molecular mechanisms of dopaminergic axon guidance.
24
II. Lessons from the developing nigrostriatal dopaminergic
pathway
1. Different families of axon guidance molecules drive axonal navigation During embryonic development of the nervous system, axons are guided to their proper synaptic targets along precise pathways forming defined neuronal networks. Varieties of molecules, called axon guidance molecules (GMs), are present in the extracellular environment and exert either attractive and permissive effects or repulsive and inhibitory effects on the growing axons. These guidance cues can either be membrane-bound or diffusible. Membrane bound cues operate in a proximal contact dependent manner while diffusible cues are secreted in specific regions generating gradients that act over longer distances (Goodman, 1996; Wen and Zheng, 2006). Therefore these cues acting through different mechanisms constitute road directional signs that define specific routes for axonal navigations (Figure 9a). For example adhesive cues that are expressed on neighbouring cell surface, such as the cell adhesion molecules or in the extracellular matrix such as laminin and fibronectin, constitute a pathway support or a “roadway” along which the axons grow, while repulsive molecules expressed at the surface constitute the growth limiting boundaries or the “roadway guard rails”. As for diffusible molecules that are released along the pathway, they provide “road signs” that induce steering of the growing axon through attractive or repulsive effects (Lowery and Vactor, 2009).
GMs receptors are expressed on the growth cone which is a palm-like structure at the distal end of the axon (Figure 9b). In this structure guidance signals detected along the pathway are integrated and translated into directional movement, therefore the growth
25
b a
Figure 9. Mechanisms of axon guidance during development. a: Long-range cues and short-range cues are expressed in the extracellular environment. They exert either attractive or repulsive effects on the axonal growth cones, defining specific navigation routes for the growing axons. b: The motility of the growth cones is mediated through the cytoskeleton which consists of long bundled actin filaments (F-actin bundles) forming the filopodia, and branched F-actin networks forming lamellipodia, and a core structure composed of bundled microtubules extending from the axon shaft. BMP: bone morphogenetic protein; BDNF: brain-derived neurotrophic factor; CAM: cell adhesion molecules; ECM: extracellular matrix; LRR: Leucine rich repeats; SHH: sonic hedgehog (Modified from (Lowery and Vactor, 2009).
25
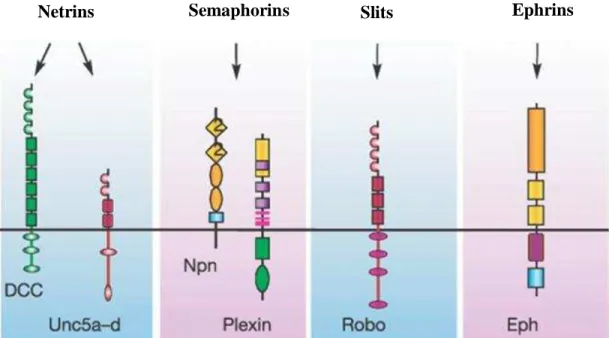
cone acts both as a motor vehicle and as a guiding navigator for the axonal growth (Lowery and Vactor, 2009). Most of the signalling cascades triggered by guidance cues eventually converge onto the cytoskeleton leading to the motility of the growth cone. The cytoskeleton of the growth cone consists of long bundled actin filaments (F-actin bundles) forming the filopodia, in addition to branched F-actin networks forming lamellipodia, and a core structure composed mainly of bundled microtubules. Several GMs and their receptors have been identified and organized into four main classical families that are conserved across different species, reflecting an evolutionary conservation of axon guidance mechanism (Chilton, 2006). These four families include Slits and Robo receptors, netrins and deleted in colorectal cancer (DCC)/UNC5a receptors, semaphorins and neuropilin/plexin receptors, and finally ephrins and Eph receptors (Figure 10).
1.1.Family of Netrins
The first conserved guidance cue identified was netrin (Hedgecock et al., 1990). The name netrin is derived from the Sanskrit “Netr” meaning 'guide'. Five members have been identified in mammals, three secreted netrins (netrins-1, 3, and 4), and two glycosylphosphatidylinositol (GPI)-anchored membrane proteins netrins-G1 and G2. Netrin-Gs bind to the transmembrane netrin G ligands NGL1 and 2 and function in regulating synaptic interactions between neurons. Receptors for the secreted netrins (netrin1, 3 and 4) include the DCC family and the UNC-5 homolog family in mammals: Unc5A, B, C and D (Rajasekharan and Kennedy, 2009). Secreted netrins are bifunctional, acting as attractants for some cell types and repellents for others, the function being determined by different combinations of receptors (Round and Stein, 2007). Netrin-1
Figure 10. Four conserved families of axon guidance molecules. Ephrins bind to their Eph receptors, netrins bind to DCC and UNC5 receptors, semaphorins bind to plexin and neuropilin receptors and Slits bind to Robo receptors (Modified from Carmeliet and Tessier-Lavigne, 2005).