1 23
International Journal of
Biometeorology
ISSN 0020-7128
Int J Biometeorol
DOI 10.1007/s00484-018-1507-5
The effects of early age thermal
conditioning and vinegar supplementation
of drinking water on physiological
responses of female and male broiler
chickens reared under summer
Mediterranean temperatures
Zahra Berrama, Soraya Temim, Baya
Djellout, Samir Souames, Nassim Moula
& Hassina Ain Baziz
1 23
Your article is protected by copyright and
all rights are held exclusively by ISB. This
e-offprint is for personal use only and shall not
be self-archived in electronic repositories. If
you wish to self-archive your article, please
use the accepted manuscript version for
posting on your own website. You may
further deposit the accepted manuscript
version in any repository, provided it is only
made publicly available 12 months after
official publication or later and provided
acknowledgement is given to the original
source of publication and a link is inserted
to the published article on Springer's
website. The link must be accompanied by
the following text: "The final publication is
available at link.springer.com”.
ORIGINAL PAPER
The effects of early age thermal conditioning and vinegar
supplementation of drinking water on physiological responses of female
and male broiler chickens reared under summer Mediterranean
temperatures
Zahra Berrama1&Soraya Temim1&Baya Djellout1&Samir Souames1&Nassim Moula2&Hassina Ain Baziz1
Received: 7 November 2016 / Revised: 30 October 2017 / Accepted: 23 January 2018 # ISB 2018
Abstract
The effects of early age thermal conditioning (ETC), vinegar supplementation (VS) of drinking water, broilers’ gender, and their interactions on respiratory rate, body temperature, and blood parameters (biochemical, hematological, and thyroid hormones) of broiler chickens reared under high ambient temperatures were determined. A total of 1100 1-day-old chicks were divided into four treatments: theBcontrol^ which were non-conditioned and non-supplemented; Bheat-conditioned^ which were exposed to 38 ± 1 °C for 24 h at 5 days of age;Bvinegar supplemented^ which were given drinking water supplemented with 0.2% of commercial vinegar from 28 to 49 days of age; andBcombined^ which were both heat conditioned and vinegar supplemented. All groups were exposed to the natural fluctuations of summer ambient temperature (average diurnal ambient temperature of about 30 ± 1 °C and average relative humidity of 58 ± 5%). ETC and broiler gender did not affect the respiratory rate or body temperature of chronic heat-exposed chickens. VS changed the body temperature across time (d35, d42, d49) (linear and quadratic effects,P < 0.05) without changing respiratory rate. Heat-conditioned chickens exhibited lower levels of glycemia (P < 0.0001) and higher hematocrit and red blood cell counts (P < 0.05). Furthermore, the greatest effects of VS, alone or associated with ETC, were the lowering of cholesterol and triglyceride blood concentrations. A significant (P < 0.05) effect of ETC, gender, and ETC×gender on T3:T4 ratio was observed. Finally, some beneficial physiological responses induced by ETC and VS, separately or in association, on chronically heat-stressed chickens were observed. However, the expected cumulative positive responses when the two treatments were combined were not evident.
Keywords Heat stress . Heat acclimation . Broiler . Vinegar supplementation . Blood parameters
Introduction
In recent years, ambient temperature increases have been greater than those seen during the last century, due to climate change (Meehl and Tebaldi2004). Some microclimate re-searchers have predicted more intense, larger, and frequent extreme heat episodes in the future (Meehl and Tebaldi
2004; Bernabucci et al.2010). In Algeria, this possibility is heightened due to its geographic position, where a large part of the country is in the Mediterranean basin—a Bhot spot^ of climate change (Sahnoune et al.2013). The hot season, which lasts almost 6 months of the year (May to October), and the sunshine time over the quasi-totality of the national territory, which exceeds 2000 h annually (Stambouli2011), have led the effective environmental temperature to exceed the recom-mended standards of microclimate conditions for modern commercial chickens which stipulates their thermoneutral zone to be 18–25 °C (Dei and Bumbie2011).The predicted increase in intensity and duration of heat waves could cause chronic heat stress that would compromise productivity and lead to economic losses (St-Pierre et al.2003) which may be aggravated by the abandonment of poultry production by pro-ducers as is common during these periods of the year. The adverse effect of heat stress on productivity has been
* Zahra Berrama
zahra_berrama@yahoo.fr
1 Laboratoire de recherches Santé et Productions Animales, Ecole
Nationale Supérieure Vétérinaire, rue Issad Abbes, El Alia, Oued Smar, Alger, Algerie
2
Department of Animal Production, Faculty of Veterinary Medicine, University of Liege, 4000 Liege, Belgium
International Journal of Biometeorology
https://doi.org/10.1007/s00484-018-1507-5
explained by a reduction in feed intake and growth rate (Lara and Rostagno2013) and by an impaired immune system that has led to an increase in the mortality rate (Quinteiro-Filho et al.2010). When the ambient temperature is above the upper critical threshold value (30 °C) (Donkoh1989), the bird may experience disturbances in homeostatic mechanisms. It is well known that thyroid hormones play a fundamental role in growth rate and are indispensable to maintaining metabolic homeostasis (McNabb1995; Reyns et al.2002). At high am-bient temperatures, reducing thyroid activity (Bowen and Washburn1985) is one way to reduce metabolic heat produc-tion. Generally, chickens have responded to heat stress by decreasing the thyroid hormone secretion rate (Sokolowicz and Herbut1999; Star et al. 2008). A negative correlation between plasma triiodothyronine (T3) concentration and am-bient temperature has been reported (May et al.1986; Yahav et al.1995). However, there is a discrepancy between results concerning plasma concentration of thyronine (T4). Although Geraert et al. (1996) found no effect of the environmental temperature on T4 concentration, Cogburn and Freeman (1987) reported a T4 concentration increase in birds exposed to the thermal challenge. In contrast, Sohail et al. (2010) re-ported that chronic heat stress reduced T4 levels. Likewise, the levels of several hematological and biochemical parame-ters were susceptible to environmental temperature changes (Vecerek et al.2002). Researchers have noticed a reduction in the blood concentration of hemoglobin and the hematocrit (Deaton et al.1969; Borges et al.2004) due to heat stress. Also, it was reported that total serum protein decreased (Khan et al.2002; Baker2009) and the concentration of plas-ma energy nutrients, such as glucose, cholesterol, and triglyc-erides, increased in the blood with arise in ambient tempera-ture (Garriga et al. 2006; Kataria et al. 2008; Rashidi et al.2010). In the same way, Yeh1992observed an increased level of serum Ca during hot temperatures.
Various nutritional strategies have been developed and tested as management tools to minimize the nega-tive effect of heat stress on zootechnical and physiolog-ical parameters of broiler chickens. However, there is an increasing trend in the poultry industry of using organic acids, such as acetic acid, as additives to diet and drink-ing water (Abde-Fattah et al. 2008; Král et al. 2011; Ghazalah et al. 2011). According to Daskiran et al. (2004), acidified water is more effective than an acidi-fied diet because of an increase in water intake and a decrease in feed consumption during heat stress. Few results are available concerning the effects of drinking water acidifiers on growth performances and physiology in heat-stressed broilers (Hassan et al. 2009; Heidari et al. 2013). Acetic acid is the principal biologically active component of vinegar (Ren et al. 1997). Recently, during warm summer periods, acetic acid in the form of vinegar was being widely used by
Algerian poultry producers to reduce productivity losses without being based on scientific investigation. Previous scientific reports have shown beneficial effects of supplementing diet and drinking water with acetic acid on egg productivity of laying hens (Farran et al. 2005), growth performances, and some physiological parame-ters of broilers reared under thermoneutral conditions (Abdel Fattah et al. 2008). Kadim et al. (2008) reported that acetic acid supplementation at a level of 400– 600 ppm in drinking water improved egg quality char-acteristics during the hot season.
Another way to alleviate the detrimental effects of heat stress on chickens is early age thermal conditioning (ETC) of chicks. This practice consists of exposing birds to an elevated ambient temperature during their first week of life to modify their thermoregulatory response and devel-op the capacity to cdevel-ope with heat stress during the grow-ing period (Dunngrow-ington and Siegel 1984; Yahav and Hurwitz 1996). It has been shown that early age thermal conditioning of chicks may change behavioral, physiolog-ical, and metabolic responses to high ambient temperature (Arjona et al.1990; Lin et al. 2004). Positive impacts of this technique on the survivability rate, short and long-term thermotolerance, and growth performances of broiler chickens challenged by acute heat stress under simulated and natural tropical climatic conditions have been report-ed (De Basilio et al. 2001; Yahav and Plavnick 1999; Günal 2013), and also under our Mediterranean summer climate (Temim et al. 2009; Boudouma and Tefiel 2012). The results of all the above studies, and the needs of Algerian poultry producers for simple, safe, and inexpen-sive practices, have incited our interest in vinegar supple-mentation as a nutritional practice, and in early heat con-ditioning as management technique, mainly as an alterna-tive to reduce the detrimental effects of chronic heat stress during the long summer months. To our knowledge, the effects of the ETC technique combined with vinegar sup-plementation on physiological responses of heat-exposed chickens remain to be elucidated. The present study will contribute to previous research that has assessed the ef-fects of early heat acclimation on broiler thermotolerance to acute heat stress over the short term by assessing the benefit and the usefulness of this management technique in reducing and ameliorating the physiological responses of commercial broilers during the prolonged and natural fluctuations of summer ambient temperatures. Therefore, this study was conducted to examine the effects of early age heat conditioning, vinegar supplementation, and the possible interaction effects of the combined approaches on the physiological and metabolic responses (respiratory frequency, body temperatures, blood contents, and thyroid hormone concentrations) of female and male broilers chal-lenged by chronic heat stress.
Int J Biometeorol
Materials and methods
Birds and experimental design
A total of 1100 1-day-old mixed-gender HUBBARD broiler chicks obtained from a local hatchery were used for a trial period of 49 days. The chicks were equally and randomly divided into 20 pens of 55 birds each with sim-ilar average body weights (39 ± 0.68 g). From 1 to 5 days of age, all chicks of the 20 pens were reared in the same house under similar rearing conditions. At the beginning of the fifth day of age, chicks of ten pens were removed from the rearing house, transferred in plastic crates to another juxtaposed rearing house, and subjected to ther-mal conditioning (38 ± 1 °C for 24 h; ambient temperature was progressively elevated over 5 h). At the beginning of the sixth day of age, thermal-conditioned birds were returned to their respective pens in the initial rearing house. From 28 to 49 days of age, birds from ten pens (five pens of thermal-conditioned birds and five pens of non-thermal-conditioned birds) were supplemented by 2 mL of vinegar per liter of drinking water. Indeed, four treatments were assigned as follows: (1) Control group (NETC-NVS), none of the chicks were heat conditioned or vinegar supplemented during all periods of the study;(2) Conditioned group (ETC), chicks were exposed to 38 ± 1 °C for 24 h at 5 days of age; (3) Supplemented group (VS), chicks were supplemented by 2 mL of vine-gar per liter of drinking water from 28 to 49 days of age; (4) Conditioned and supplemented group, chicks were heat conditioned (38 ± 1 °C for 24 h) at 5 days of age and supplemented by 2 mL of vinegar per liter of drinking water from 28 to 49 days of age. During the whole ex-perimental period, all the chicks were fed a standard diet adapted to their age: a starter diet (2800 kcal ME/kg, 20% crude protein) from 1 to 10 days, a grower diet (2900 kcal ME/kg, 19% crude protein) from 11 to 42 days, and a finisher diet (2930 kcal ME/kg, 17% crude protein) from 43 to 49 days. All chicks were given ad libitum access to diet and water, were kept under similar rearing conditions, and were exposed to the natural fluctuations of the sum-mer ambient temperature (average diurnal ambient tem-perature of about 30 ± 1°C and average relative humidity of 58 ± 5%).
Measurements and laboratory analysis
Body temperature and respiratory rate
Rectal temperature and respiratory rate were measured from the same birds (20 of each treatment: ten females and ten males) at 35, 42, and 49 days of age. Cloacal temperature
was obtained by introducing a probe thermometer about 3 cm into the rectum. Respiratory rate was measured by counting the time required for ten successive inspirations (panting breaths) (t10) during a sequence of panting, which was defined as an opening of the mouth (Perez et al.2006).
Laboratory analysis
At the end of the experimental period (49 days), 16 birds (eight females and eight males) were sampled from each ex-perimental group and used for determination of blood param-eters; the birds were fasted for approximately 12 h before blood collection. Blood was collected post-slaughter into EDTA tubes and heparinized tubes.
Blood collected into EDTA tubes was used to measure the hematocrit, hemoglobin concentration, total red blood cells (RBC), mean corpuscular volume (MCV), mean cor-puscular hemoglobin (MCH), mean corcor-puscular hemoglo-bin concentration (MCHC), and platelet count (PLT) on the same day using a ERMA PCE-210 automated hema-tology analyzers (Post et al.2003).
Blood collected into heparinized tubes was centrifuged at 3000 rpm for 10 min. The blood plasma samples obtained were collected and stored at − 20 °C until analysis. Plasma glucose, total protein, total cholesterol, triglycerides, and cal-cium were determined by using a spectrophotometer (LKB Novastec) and available commercial kits (SPINREACT, SA, Espagne). Glucose concentration was determined by using the GOD-POD method; total protein concentration was analyzed by the Biuret Method (colorimetric test); plasma cholesterol concentration was determined using the CHOD-PAP method; plasma triglyceride concentration was determined using the GPO-PAP enzymatic method; and plasma calcium concentra-tion was determined using the o-Cresophtalein v/v complex method. Total plasma triiodothyronine (T3) and thyroxin (T4) concentrations were measured by radioimmunoassay (RIA).
Statistical analysis
All analyses were performed using statistical software from the SAS Institute Inc. The body temperature and respiratory rate data were analyzed as repeated measures using the general linear model (GLM) procedure of SAS. The significance of trend over time was determined using orthogonal polynomial analysis, whereas all laboratory data analysis was performed using the GLM procedure of SAS 9.3 as a 2 × 2 × 2 factorial arrangement design. The effects of early thermal conditioning (ETC), vinegar supplementation (VS), broiler gender, and their interactions were considered. The experimental unit was a pen of 55 chicks with five replicates. The statistical significance was set atP < 0.05. Least squares analyses were used to detect the differences.
Int J Biometeorol
Results
The effects of ETC, VS, and gender on the body temperature and respiratory rate at 35, 42, and 49 days of age in chronically heat-stressed broiler chickens are shown in Table1. ETC and broiler gender did not affect (P > 0.05) body temperature and respiratory rate of broiler chickens submitted to hot summer conditions. However, VS did affect body temperature without changing the respiratory rate. Indeed, body temperature of supplemented broilers changed linearly (P = 0.002) and qua-dratically (P = 0.012) over time, corresponding to broiler age. The effects of ETC, VS, gender, and their combination on blood biochemistry of chronically heat-stressed broiler chickens are presented in Table2.
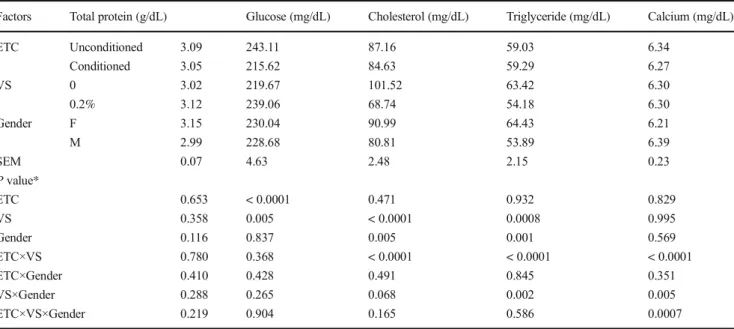
Statistical analysis showed that ETC and VS did not affect plasma total protein concentration (P > 0.05) but did influence glucose concentration. Glycemia was signifi-cantly decreased (− 11%, P < 0.0001) when birds were early heat conditioned, and increased (+ 9%, P < 0.01) when they were supplemented with 0.2% vinegar in drinking water. A significant interaction (P < 0.05) on to-tal cholesterol and triglyceride concentrations was ob-served by combining the ETC and VS. The same trend of significant decrease in cholesterol (− 32%, P < 0.0001) and triglyceride (− 15%, P = 0.014) concentrations was recorded for VS birds. Also, significant (P < 0.0001) in-teraction between ETC and VS in terms of calcium con-centration was recorded. The results showed no signifi-cant effects (P > 0.05) of gender in chronically stressed broilers on the total protein, glucose, and calcium concen-trations. However, higher cholesterol and triglyceride values were recorded for females than for males. In addi-tion, the interaction between VS and gender resulted in a
significant effect on triglyceride and calcium profiles, whereas only plasma calcium level was affected by the interaction of the three factors.
The effects of ETC, VS, and gender of chronically heat-stressed broilers on hematological parameters are presented in Table3. The number of circulating red blood cells (RBC) and hematocrit was significantly increased by ETC (+ 6%,P < 0.05 and + 6.5%, P = 0.012, respectively); this factor decreased MCHC level without simultaneous reduction in hemoglobin values (P = 0.215). Additionally, a significant effect of gender was observed on the number of red blood cells (P = 0.03), MCV (P < 0.001), and MCHC (P = 0.0017). No significant (P > 0.05) differences in all measured hematological parameters were ob-tained by the VS treatment or by the several interactions be-tween ETC, VS, and gender of broilers reared under hot climate. The effects of ETC, VS, broiler gender, and their combina-tion on thyroid hormones (T3; T4) are presented in Table4. Separately, ETC of chicks and VS resulted in insignificant var-iations in T3 and T4 levels. However, the T3:T4 ratio showed a significant (P = 0.04) reduction by ETC. Also, no difference in T3 hormones was observed related to the gender. However, plasma T4 concentrations and T3:T4 ratios were influenced by gender. Females presented higher blood levels of T4 (P = 0.04) and lower T3:T4 ratios (P = 0.016) than males. The thyroid hormones and T3:T4 ratio were not affected by all double or triple interaction combinations except for the interaction of ETC and gender where the T3:T4 ratio showed an effect (P = 0.045).
Discussion
Body temperature and respiratory rate can be used as physio-logical parameters that reflect the effectiveness of preventive
Table 1 The effect of early thermal conditioning (ETC), vinegar supplementation (VS), and gender (F and M) on body temperature and respiratory rate at 35, 42, and 49 days of age in broiler chickens reared under chronic high summer temperatures
Body temperature (°C) Respiratory rate (breaths/min) P* value orthogonal polynomials Body temperature Respiratory rate 35 days 42 days 49 days 35 days 42 days 49 days L Q L Q ETC Unconditioned 42.39 42.72 42.56 141.00 167.28 208.62 0.372 0.732 0.133 0.440 Conditioned 42.32 42.66 42.62 139.02 166.49 216.37 VS 0 42.44 42.58 42.52 141.78 163.75 213.39 0.002 0.012 0.785 0.063 0.2% 42.28 42.80 42.69 138.24 170.02 211.60 Gender F 42.29 42.61 42.55 141.32 166.32 214.30 0.842 0.732 0.878 0.372 M 42.43 42.77 42.67 138.70 167.45 210.69 L linear, Q quadratic *Significant differences (P < 0.05) Int J Biometeorol
measures against heat stress. In this study, body temperatures did not differ significantly by ETC or by broiler gender at different periods of age (35, 42, and 49 days). Previous studies have reported that the main physiological response due to early age heat acclimation was the attenuation of the increase in rectal temperature (May et al.1987; Teeter et al.1992; De
Basilio et al.2003). Most of these studies were conducted in order to elucidate the effect of this technique on either short- or long-term thermotolerance acquisition by measuring rectal temperature of broilers challenged by acute thermal stress a few hours or days after heat acclimation or later at marketing age (6 weeks). In our study, the lack of differences of body
Table 2 The effects of early thermal conditioning (ETC), vinegar supplementation (VS), and gender (F and M) on biochemical parameters at 49 days of age in broiler chickens reared under chronic high summer temperatures
Factors Total protein (g/dL) Glucose (mg/dL) Cholesterol (mg/dL) Triglyceride (mg/dL) Calcium (mg/dL)
ETC Unconditioned 3.09 243.11 87.16 59.03 6.34 Conditioned 3.05 215.62 84.63 59.29 6.27 VS 0 3.02 219.67 101.52 63.42 6.30 0.2% 3.12 239.06 68.74 54.18 6.30 Gender F 3.15 230.04 90.99 64.43 6.21 M 2.99 228.68 80.81 53.89 6.39 SEM 0.07 4.63 2.48 2.15 0.23 P value* ETC 0.653 < 0.0001 0.471 0.932 0.829 VS 0.358 0.005 < 0.0001 0.0008 0.995 Gender 0.116 0.837 0.005 0.001 0.569 ETC×VS 0.780 0.368 < 0.0001 < 0.0001 < 0.0001 ETC×Gender 0.410 0.428 0.491 0.845 0.351 VS×Gender 0.288 0.265 0.068 0.002 0.005 ETC×VS×Gender 0.219 0.904 0.165 0.586 0.0007
Values are least square treatment means SEM standard error of the mean *Significant differencesP < 0.05
Table 3 The effect of early thermal conditioning (ETC), vinegar supplementation (VS), and gender (F and M) on hematological parameters at 49 days of age in broiler chickens reared under chronic high summer temperatures
Factors Hct % RBC × 106(μL) Hmg (g/dL) MCV (fL) MCH (pg) MCHC (g/dL) PlTs × 106(μL) ETC Unconditioned 26.52 2.56 8.74 104.21 34.13 32.96 15.11 Conditioned 28.25 2.70 9.06 104.54 33.54 32.07 15.32 VS 0 27.46 2.64 8.84 104.51 33.49 32.24 16.07 0.2% 27.31 2.62 8.97 104.25 34.19 32.78 14.35 Gender F 27.19 2.71 9.10 101.93 33.63 33.08 16.14 M 27.57 2.55 8.70 106.83 34.05 31.93 14.29 SEM 0.49 0.05 0.18 0.60 0.33 0.24 1.13 P value* ETC 0.015 0.042 0.215 0.701 0.215 0.013 0.894 VS 0.833 0.819 0.617 0.764 0.143 0.126 0.289 Gender 0.587 0.033 0.119 < 0.001 0.376 0.0017 0.251 ETC×VS 0.260 0.399 0.676 0.652 0.567 0.099 0.201 ETC×Gender 0.842 0.423 0.409 0.235 0.785 0.256 0.125 VS×Gender 0.969 0.541 0.980 0.05 0.295 0.830 0.425 ETC×VS×Gender 0.481 0.367 0.509 0.791 0.778 0.721 0.088
SEM standard error of the mean *Significant differences (P < 0.05) Int J Biometeorol
temperature between heat-conditioned and unconditioned broilers may have been the response of natural acclimation (acclimatization), which could have occurred because the birds were reared under chronic heat stress (summer condi-tions). The same trend in our results was shown by Hassan and Reddy 2012who reported that the responses of broilers subjected to early age heat stress were similar to those adapted to chronic heat stress. Also, Collin et al. (2007) failed to signify a long-lasting improvement in thermotolerance as determined by rectal temperature measured at 28, 35, and 41 days of age due to the thermal manipulation technique during early and late embryogenesis. To our knowledge, there are no published data on the effects of vinegar water supple-mentation on respiratory frequency and rectal temperature of broilers exposed to high environmental temperature. In the present study, VS has changed broilers’ body temperatures across the time of the measures both linearly and quadratical-ly. There was an increase observed after 2 weeks of supple-mentation during the growing period, followed by a decrease after 3 weeks of supplementation. This suggests a possible contribution of VS in improving thermotolerance acquisition of chronically heat-stressed broilers over time. These results agree with those hypotheses that have suggested that supplementing drinking water or diet with acids can restore blood pH to normal values in an attempt to maintain and correct thermal balance (Hilmann et al. 1985). However, adding vinegar to drinking water failed to show any positive effects on respiratory rate in this study.
Biochemical parameters can also be used to assess the heat tolerance of broiler chickens. Blood plasma proteins play an important role in homeostasis. Previous studies have demon-strated that the plasma protein profile in birds varied with changes in their thermal environment (Zhou et al.1999; Lin et al.2006). Also, it has been suggested that heat stress re-sponse can be modulated by early age thermal acclimation of chicks, leading to changes in blood contents that are a part of thermoregulatory responses (Yahav et al.1997). In the present study, total plasma protein concentration did not change in the blood by ETC in birds reared under hot summer temperatures. This result is consistent with those reported by Toplu et al. (2014), but did not agree with those of other studies (Zhou et al. 1999; Al-Zghoul et al.2015) that reported that either pre-or post-natal heat acclimation increased total plasma proteins during exposure to heat stress, thus suggesting an improve-ment in humoral immunity and also protection from muscle injury induced by heat stress (Al-Zghoul et al.2015). In addi-tion, the present results showed that the plasma protein profile was not affected either by VS, broiler gender, or any of the studied interactions. Similarly, no significant variation in the blood protein profile was observed to be associated with die-tary acidification in broiler chickens reared under thermoneutral conditions (Abdel-Fattah et al. 2008; Nourmohamadi et al.2010).
Separately, ETC of birds attenuated the negative effects of hot ambient temperatures on plasma glucose levels. These findings confirmed those previously reported by Toplu et al. (2014). However, VS increased glucose levels. Some investi-gations in humans and rabbits have revealed that vinegar pos-sesses an antiglycemic activity (Ostman et al.2005; Setorki et al.2010; Mahmoodi et al.2013), but some cases of discrep-ant effects have also been reported. Johnston et al. (2010) observed that the post-prandial hypoglycemic proprieties of vinegar were influenced by the collection periods of blood, and this antiglycemic characteristic did not persist 5 h after vinegar consumption.
In this study, blood lipid profiles in terms of total choles-terol and triglyceride concentrations were decreased by VS and by VS×ETC. However, there are several reports that, in thermoneutral conditions, adding acidifiers to the diet of chickens caused a decrease in blood cholesterol, total lipids, and triglycerides (Abdo 2004; Abdel-Fattah et al. 2008). Furthermore, the hypolipidemic and hypocholesterolemic properties of vinegar have been reported previously (Shishehbor et al.2007; Setorki et al.2010; Capcarova et al.
2014). Several mechanisms have been suggested to explain the effects of vinegar on the lipid profile. Fushimi et al. (2006) studied the efficacy of vinegar for prevention of hyperlipid-emia in rats, and suggested that acetic acid, the active ingre-dient of vinegar, may control these blood parameters through different mechanisms: inhibition of metabolic pathways of cholesterogenesis and lipogenesis in the liver, enhancement
Table 4 The effect of early thermal conditioning (ETC), vinegar supplementation (VS), and gender (F and M) on thyroid hormones parameters at 49 days of age in broiler chickens reared under chronic high summer temperature
Factors T3 (pm) T4 (pm) T3:T4 ETC Unconditioned 2.41 7.52 0.59 Conditioned 2.57 9.43 0.35 VS 0 2.60 7.78 0.54 0.2% 2.39 9.17 0.40 Gender F 2.39 9.69 0.33 M 2.59 7.26 0.61 SEM 0.24 0.82 0.08 P value ETC 0.632 0.107 0.04 VS 0.545 0.238 0.212 Gender 0.567 0.043 0.016 ETC×VS 0.213 0.639 0.906 ETC×Gender 0.483 0.774 0.045 VS×Gender 0.584 0.575 0.071 ETC×VS×Gender 0.515 0.897 0.242 SEM standard error of the mean
*Significant differences (P ≤ 0.05)
Int J Biometeorol
of fatty acid oxidation, and also stimulation of fecal bile acid excretion. Also, the inhibitory effect of AMPK (AMP-activat-ed protein kinase), produc(AMP-activat-ed by acetic acid metabolism in the liver, on fatty acid and sterol synthesis has been previously reported (Winder and Hardie1999). Chronically heat-stressed male broilers have shown higher lipid profiles than females in terms of cholesterol and triglyceride content in plasma. There is a lack of information concerning gender’s effect on com-mercial broiler chickens’ metabolite changes in hot climates.
In this study, neither ETC nor VS had any effect on calcium concentrations in the blood. This result agrees with the report by Hassan and Reddy (2012) for thermal conditioned (24 h at 5 days of age) birds and for chronically stressed birds. Also, along the same lines, Al-Zghoul et al. (2015) stated that ther-mal manipulation during broiler embryogenesis did not affect the concentration of calcium in blood. Studies conducted by Kishi et al. (1999) revealed that a diet containing acetic acid or vinegar did not influence serum calcium of ovariectomized r a t s . B o l i n g - F r a n k e n b a c h e t a l . , 2 0 0 1a n d A b d o ,
2004reported an increase of blood Ca levels in broiler chickens reared in thermoneutral conditions when organic acids were added to the diet. This response was generally thought to be explained by lowering of gastrointestinal tract pH by the acid enhancing the absorption and improving the digestibility (Abdel-Fattah et al. 2008) of this mineral. Furthermore, in this study, plasma calcium concentration showed different responses due to the significant interactions between ETC and VS (P < 0.001), between VS and gender (P = 0.005), and between ETC, VS, and gender (P = 0.0007). Several studies have stated that decreases in hemoglo-bin concentration with a rise in ambient temperature were generally accompanied by decreased hematocrit values and red blood cell counts (Sturkie and Griminger 1986; Zhou et al.1999). This decrease has been variously attrib-uted to difficulties in oxygen uptake and transport (Phillips et al.1985), depression in erythropoiesis (Donkoh1989), and/or as the result of hemodilution following increases in water consumption induced by heat stress (Darre and Harisson1987). Furthermore, it has been noted that early age thermal conditioning may affect the changes in blood parameters of heat-stressed birds and cause rises in hemat-ocrit, number of circulating erythrocytes, and hemoglobin concentration (Yahav and Plavnick1999). In this respect, the blood results obtained in the present study revealed an increase of hematocrit value and red blood cell numbers, with an insignificant (P = 0.215) slight rise of hemoglobin concentration by ETC. The increases in these parameters could be an adaptive response to the ETC, which let us suggest an eventual reduction of the depression in eryth-ropoiesis caused by heat stress. However, an opposite ef-fect was noted in mean corpuscular hemoglobin concen-trations (MCHC), which were decreased by ETC. The lev-el of blood platlev-elets (PLT), MCV, and MCH did not show
any fluctuation due to ETC. Also, no significant differences of hematological parameters were noted with VS, either alone or in interaction with ETC. There have been few studies examining the effects of dietary or water acidifier supplementation on the hematology parameters of broilers reared under thermoneutral or high temperature conditions. However, Tollba (2010) indicated that there was no significant effect on RBC count when citric acid was added to the diets of broilers under heat stress. The effect of gender on RBC count, MCV, and MCHC values was significant (P < 0.05). Females submitted to chronic heat stress expressed a higher RBC count (P = 0.03), an insignificant increase in hemoglobin level (P = 0.12), low-er MCV (P < 0.001), and highlow-er MCHC (P = 0.002) than males reared in the same ambient conditions. These fe-males’ hematological status indicated that females were less stressed than males and confirmed that male broilers were more vulnerable to environmental changes than fe-males (Fernandes et al.2016). No interaction effects of the three factors were observed on the measured hematologi-cal parameters.
In the present study, there were no effects of ETC and VS on plasma T3 and T4 concentrations. However, a significant de-crease in the T3:T4 ratio was observed with ETC. In this re-spect, May et al. (1986) have suggested that early short-term acclimation does not change circulating hormones, but pro-vokes other endocrine and physiological responses. Bowen and Washburn (1985) opined that the increased resistance to heat stress previously observed by Bowen and Washburn (1984) in handled birds was not due to changes in thyroid function, but may have been associated with a decrease of corticosterone levels. Additionally, Yalcin et al. (2009) reported that birds submitted to prenatal acclimation were less stressed and showed better heat resistance when compared to non-acclimated individuals, while showing an insignificant increase in serum T3 concentrations. These authors estimated that the numerical rise of blood T3 levels was due to the lesser demand of T3 necessary for oxidative metabolism; thus, less T3 was absorbed by the liver, resulting in more T3 remaining in the plasma. In contrast, several studies have reported that early age thermal acclimation of birds improved thermotolerance to heat stress by reducing plasma triiodothyronine (T3) concentration (Tanizaw et al. 2014; Dalkilic et al. 2015). Alternatively, Abdel-Fattah et al. (2008) and Nourmohammadi et al. (2010) stated that dietary addition of acidifiers, such as acetic acid and citric acid, to broiler chickens reared under thermoneutral con-ditions increased serum T3 without changing T4 levels. There was a significant effect of gender on T4 (P = 0.043) concentra-tion and the T3:T4 ratio (P = 0.016). The highest level of T4 and the lowest ratio of T3:T4 with a simultaneous insignificant (P > 0.05) decrease in T3 concentration was observed in female broilers, demonstrating that females were more adaptable than males to prolonged high temperatures.
Int J Biometeorol
In conclusion, the two tested strategies to cope with the chronic high summer temperature, conducted on female and male broilers, resulted in some beneficial physiological re-sponses. Indeed, the exposure of chicks at 5 days of age to 24 h at 38 °C led to improved glycemia, some hematological parameters (hematocrit and red blood cell count) of broilers at 49 days of age, and T3:T4 ratios. Moreover, the greatest im-provement of adding 2% vinegar to the drinking water, alone or in interaction with early heat acclimation, was observed on the lipid profile of the chickens.
Compliance with ethical standards
Ethical approval All applicable international, national, and/or institu-tional guidelines for the care and use of animals were followed. Conflict of interest The authors declare that they have no conflict of interest.
References
Abdel-Fattah SA, El-Sanhoury MH, El-Mednay NM, Abdel-Azeem F (2008) Thyroid activity, some blood constituents, organs morphol-ogy and performance of broiler chicks fed supplemental organic acids. Int J Poult Sci 7(3):215–222.https://doi.org/10.3923/ijps. 2008.215.222
Abdo MAZ (2004) Efficacy of acetic acid in improving the utilization of low protein-low energy broiler diets. Egypt Poult Sci 24:123–141 Al-Zghoul M-B, El-Bahr SM, Al-Rukibat RK (2015) Biochemical and
molecular investigation of thermal manipulation protocols during broiler embryogenesis and subsequent thermal challenge. BMC Vet Res 11(1):292.https://doi.org/10.1186/s12917-015-0609-0 Arjona AA, Denbow DM, Weaver WD (1990) Neonatally-induced
ther-motolerance: physiological responses. Comp Biochem Physiol 95(3):393–399.https://doi.org/10.1016/0300-9629(90)90238-N Baker DH (2009) Advances in protein–amino acid nutrition of poultry.
Amino Acids 37(1):29–41. https://doi.org/10.1007/s00726-008-0198-3
Bernabucci U, Lacetera N, Baumgard LH, Rhoads RP, Ronchi B, Nardone A (2010) Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 4(07):1167–1183. https://doi.org/10.1017/S175173111000090X
Boling-Frankenbach SD, Snow JL, Parsons CM, Baker DH (2001) The effect of citric acid on the calcium and phosphorus requirements of chicks fed corn-soybean meal diets. Poult Sci 80(6):783–788. https://doi.org/10.1093/ps/80.6.783
Borges SA, Fischer da Silva AV, Majorka A, Hooge DM, Cummings KR (2004) Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult Sci 83(9):1551–1558.https:// doi.org/10.1093/ps/83.9.1551
Bowen SJ, Washburn KW (1984) Preconditioning to heat stress by a nontemperature stressor. Poult Sci 63(5):917–919.https://doi.org/ 10.3382/ps.0630917
Boudouma D, Tefiel H (2012) Performances du poulet de chair acclimaté et élevé en conditions chaudes dans le Nord de l'Algérie. Livest Res Rural Dev 24
Bowen SJ, Washburn KW (1985) Thyroid and adrenal response to heat stress in chickens and quail differing in heat tolerance. Poult Sci 64(1):149–154.https://doi.org/10.3382/ps.0640149
Capcarova M, Kalafova A, Hrncar C, Kopecky J, Weis J (2014) Comparative analysis of acetic and citric acid on internal milieu of broiler chickens. Potravinarstvo 8(1):190–195.https://doi.org/10. 5219/379
Cogburn LA, Freeman RM (1987) Response surface of daily thyroid hormone rhythms in young chickens exposed to constant ambient temperature. Gen Comp Endocrinol 68(1):113–123.https://doi.org/ 10.1016/0016-6480(87)90066-9
Collin A, Berri C, Tesseraud S, Rodon FER, Skiba-Cassy S, Crochet S, Duclos MJ, Rideau N, Tona K, Buyse J, Bruggeman V, Decuypere E, Picard M, Yahav S (2007) Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult Sci 86(5):795–800.https:// doi.org/10.1093/ps/86.5.795
Dalkilic B, Simsek UG, Ciftci M, Baykalir Y (2015) Effect of dietary orange peel essential oil on physiological, biochemical and metabol-ic responses of Japanese quails as affected by early age thermal conditioning and fasting. Rev Med Vet 166:154–162
Darre MJ, Harrison PC (1987) Heart rate, blood pressure, cardiac output, and total peripheral resistance of single comb white leghorn hens during an acute exposure to 35 c ambient temperature. Poult Sci 66(3):541–547.https://doi.org/10.3382/ps.0660541
Daskiran M, Teeter RG, Vanhooser SL, Gibson ML, Roura E (2004) Effect of dietary acidification on mortality rates, general perfor-mance, carcass characteristics, and serum chemistry of broilers ex-posed to cycling high ambient temperature stress1. J Appl Poultry Res 13(4):605–613.https://doi.org/10.1093/japr/13.4.605 Deaton JW, Reece FN, Tarver WJ (1969) Hematocrit, hemoglobin and
plasma protein levels of broilers reared under constant temperatures. Poult Sci 48(6):1993–1996.https://doi.org/10.3382/ps.0481993 De Basilio V, Vilarino M, Yahav S, Picard M (2001) Early age thermal
conditioning and a dual feeding program for male broilers chal-lenged by heat stress. Poult Sci 80(1):29–36
De Basilio V, Requena F, Leon A, Vilarino M, Picard M (2003) Early age thermal conditioning immediately reduces body temperature of broiler chicks in a tropical environment. Poult Sci 82(8):1235– 1241.https://doi.org/10.1093/ps/82.8.1235
Dei HK, Bumbie GZ (2011) Effect of wet feeding on growth performance of broiler chickens in a hot climate. Br Poult Sci 52(1):82–85. https://doi.org/10.1080/00071668.2010.540230
Donkoh A (1989) Ambient temperature: a factor affecting performance and physiological response of broiler chickens. Int J Biometeorol 33(4):259–265.https://doi.org/10.1007/BF01051087
Dunnington EA, Siegel PB (1984) Thermoregulation in newly hatched chicks. Poult Sci 63(7):1303–1313.https://doi.org/10.3382/ps. 0631303
Farran MT, Halaby WS, Barbour GW, Uwayjan MG, Sleiman FT, Ashkarian VM (2005) Effects of feeding ervil (Vicia ervilia) seeds soaked in water or acetic acid on performance and internal organ size of broilers and production and egg quality of laying hens. Poult Sci 84(11):1723–1728.https://doi.org/10.1093/ps/84.11.1723 Fernandes J, Santos TC, Kaneko IN et al (2016) Effect of thermal
em-bryonic manipulation on the quality of male and female broiler meat submitted to thermal stress pre-slaughter. Rev Bras Cienc Avic 18(2):343–350.https://doi.org/10.1590/1806-9061-2015-0073 Fushimi T, Suruga K, Oshima Y, Fukiharu M, Tsukamoto Y, Goda T
(2006) Dietary acetic acid reduces serum cholesterol and triacylglyc-erols in rats fed a cholesterol-rich diet. Br J Nutr 95(5):916–924. https://doi.org/10.1079/BJN20061740
Garriga C, Hunter RR, Amat C, Planas JM, et al (2006) Heat stress increases apical glucose transport in the chicken jejunum. AJP: Regulatory Integr Comp Physiol 290:R195–R201.https://doi.org/ 10.1152/ajpregu.00393.2005
Geraert PA, Padilha JCF, Guillaumin S (1996) Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: Int J Biometeorol
growth performance, body composition and energy retention. Br J Nutr 75(02):195–204.https://doi.org/10.1017/BJN19960124 Ghazalah AA, Atta AM, Elkloub K, Moustafa MEL, Shata RFH (2011)
Effect of dietary supplementation of organic acids on performance, nutrients digestibility and health of broiler chicks. Int J Poult Sci 10(3):176–184.https://doi.org/10.3923/ijps.2011.176.184 Günal M (2013) The effects of early-age thermal manipulation and daily
short-term fasting on performance and body temperatures in broiler exposed to heat stress: heat stress, thermal manipulation, fasting, broiler. J Physiol Animal Nutr doi: https://doi.org/10.1111/j.1439-0396.2012.01330.x
Hassan AM, May AbdelA H, Reddy PG (2009) Effect of some water supplements on the performance and immune system of chronically heat-stressed broiler chicks. Int J Poult Sci 8(5):432–436
Hassan AM, Reddy PG (2012) Early age thermal conditioning improves broiler chick’s response to acute heat stress at marketing age. Am J Anim Vet Sci 7(1):1–6.https://doi.org/10.3844/ajavsp.2012.1.6 Heidari M, Moeini MM, Nanekarani SH (2013) Effect of vitamin C,
acetylsalicylic, NaHCO3 and KCL supplementation on the perfor-mance of broiler chickens under heat stress condition. J Agr Technol 9:323–331
Hilmann PE, Scott NR, Van Tienhoven A (1985) Physiological responses and adaptations to hot and cold environments. In: Yousef MK (ed) Stress physiology in livestock. CRC Press, Boca Raton, FL, pp 1–71 Johnston CS, Steplewska I, Long CA, Harris LN, Ryals RH (2010) Examination of the antiglycemic properties of vinegar in healthy adults. Annals of. Nutr Metab 56(1):74–79.https://doi.org/10. 1159/000272133
Kadim IT, Al-Marzooqi W, Mahgoub O et al (2008) Effect of acetic acid supplementation on egg quality characteristics of commercial laying hens during hot season. Int J Poult Sci 7:1015–1021
Kataria N, Kataria AN, Gahlot AK (2008) Ambient temperature associ-ated variation in serum hormones and interrelassoci-ated analysis of broiler chickens in arid tract. Solv Vet Res 45:127–134
Khan WA, Khan A, Anjum AD, Urrehman Z (2002) Effects of induced heat stress on some biochemical values in broiler chicks. Int J Agric Biol 4:74–75
Kishi M, Fukaya M, Tsukamoto Y et al (1999) Enhancing effect of dietary vinegar on the intestinal absorption of calcium in ovariectomized rats. Biosci Biotechnol Biochem 63(5):905–910.https://doi.org/10. 1271/bbb.63.905
Král M, Angelovičová M, Mrázová Ľ et al (2011) Probiotic and acetic acid of broiler chickens performance. Sci Papers Animal Sci Biotechnol 44:149–152
Lara L, Rostagno M (2013) Impact of heat stress on poultry production. Animals 3(2):356–369.https://doi.org/10.3390/ani3020356 Lin H, Jiao HC, Buyse J, Decuypere E (2006) Strategies for preventing
heat stress in poultry. World’s Poultry Sci J 62(01):71–86.https:// doi.org/10.1079/WPS200585
Lin H, Malheiros RD, Moraes VMB et al (2004) Acclimation of broiler chickens to chronic high environmental temperature. Arch Geflügelkd 68:39–46
Mahmoodi M, Hosseini-zijoud SM, Hassanshahi G et al (2013) The effect of white vinegar on some blood biochemical factors in type 2 diabetic patients. J Diabetes Endocrinol 4:1–5.https://doi.org/10. 5897/JDE12.015
May JD, Deaton JW, Branton SL (1987) Body temperature of acclimated broilers during exposure to high temperature. Poult Sci 66(2):378–
380.https://doi.org/10.3382/ps.0660378
May JD, Deaton JW, Reece FN, Branton SL (1986) Effect of acclimation and heat stress on thyroid hormone concentration. Poult Sci 65(6): 1211–1213.https://doi.org/10.3382/ps.0651211
McNabb FM (1995) Thyroid hormones, their activation, degradation and effects on metabolism. J Nutr 125:1773–1776
Meehl GA, Tebaldi C (2004) More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305(5686):994–997. https://doi.org/10.1126/science.1098704
Nourmohammadi R, Mohammad HS, Farhangfar H (2010) Effect of dietary acidification on some blood parameters and weekly perfor-mance of broiler chickens. J Anim Vet Adv 9(24):3092–3097. https://doi.org/10.3923/javaa.2010.3092.3097
Östman E, Granfeldt Y, Persson L, Björck I (2005) Vinegar supplemen-tation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr 59(9):983–
988.https://doi.org/10.1038/sj.ejcn.1602197
Pérez M, De Basilio V, Colina Y, Oliveros Y, Yahav S, Picard M, Bastianelli D (2006) Evaluation du niveau de stress thermique par mesure de la température corporelle et du niveau d’hyperventilation chez le poulet de chair dans des conditions de production au Venezuela. Rev Elev Med Vet Pays Trop 59(1-4):81–90.https:// doi.org/10.19182/remvt.9959
Phillips JG, Butler PJ, Sharp PJ (1985) Physiological strategies in avian biology. Blackie, Glasgow
Post J, Rebel J, ter Huurne A (2003) Automated blood cell count: a sensitive and reliable method to study corticosterone-related stress in broilers. Poult Sci 82(4):591–595. https://doi.org/10. 1093/ps/82.4.591
Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sa LRM, Ferreira AJP, Palermo-Neto J (2010) Heat stress im-pairs performance parameters, induces intestinal injury, and de-creases macrophage activity in broiler chickens. Poult Sci 89(9): 1905–1914.https://doi.org/10.3382/ps.2010-00812
Rashidi AA, Ivari YG, Khatibjoo A, Vakili R (2010) Effects of dietary fat, vitamin E and zinc on immune response and blood parameters of broiler reared under heat stress. Res J Poultry Sci 3:32–38 Ren H, Endo H, Watanabe E, Hayashi T (1997) Chemical and sensory
characteristics of Chinese, Korean and Japanese vinegars. J Tokyo Univ Fish 84:1–11
Reyns GE, Janssens KA, Buyse J, Kühn ER, Darras VM (2002) Changes in thyroid hormone levels in chicken liver during fasting and refeeding. Comp Biochem Physiol B: Biochem Mol Biol 132(1): 239–245.https://doi.org/10.1016/S1096-4959(01)00528-0 Sahnoune F, Belhamel M, Zelmat M, Kerbachi R (2013) Climate change
in Algeria: vulnerability and strategy of mitigation and adaptation. Energy Procedia 36:1286–1294.https://doi.org/10.1016/j.egypro. 2013.07.145
Setorki M, Nazari B, Asgary S et al (2010) Acute effects of apple cider vinegar intake on some biochemical risk factors of atherosclerosis in rabbits fed with a high cholesterol diet. Qom Univ Med Sci 3:11–18 Shishehbor F, Mansouri A, Sarkaki A et al (2007) The effect of white vinegar on fasting blood glucose, glycosylated hemoglobin and lipid profile in normal and diabetic rats. Iran. J Endocrinol Metab 9:69–75 Sohail MU, Ijaz A, Yousaf MS, Ashraf K, Zaneb H, Aleem M, Rehman H (2010) Alleviation of cyclic heat stress in broilers by dietary supple-mentation of mannan-oligosaccharide and Lactobacillus-based pro-biotic: dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult Sci 89(9):1934– 1938.https://doi.org/10.3382/ps.2010-00751
Sokołowicz Z, Herbut E (1999) Effect of chronic high temperature stress on thyroid activity and metabolic rate of broiler pullets cockerels. Ann Anim Sci 26:377–383
Stambouli AB (2011) Promotion of renewable energies in Algeria: strat-egies and perspectives. Renew Sust Energ Rev 15(2):1169–1181. https://doi.org/10.1016/j.rser.2010.11.017
Star L, Decuypere E, Parmentier HK, Kemp B (2008) Effect of single or combined climatic and hygienic stress in four layer lines: 2. Endocrine and oxidative stress responses. Poult Sci 87(6):1031– 1038.https://doi.org/10.3382/ps.2007-00143
Int J Biometeorol
St-Pierre NR, Cobanov B, Schnitkey G (2003) Economic losses from heat stress by US livestock industries. J Dairy Sci 86:E52–E77. https://doi.org/10.3168/jds.S0022-0302(03)74040-5
Sturkie PD, Griminger P (1986) Body fluids: blood. In: Sturkie PD (ed) Avian physiology. Springer-Verlag, New York.Inc, pp 102–129. https://doi.org/10.1007/978-1-4612-4862-0
Tanizawa H, Shiraishi J, Kawakami S-I et al (2014) Effect of short-term thermal conditioning on physiological and behavioral responses to subsequent acute heat exposure in chicks. J Poult Sci 51(1):80–86. https://doi.org/10.2141/jpsa.0130040
Teeter RG, Smith MO, Wiernusz CJ (1992) Research note: broiler acclimation to heat distress and feed intake effects on body temperature in birds exposed to thermoneutral and high ambi-ent temperatures. Poult Sci 71(6):1101–1104. https://doi.org/ 10.3382/ps.0711101
Temim S, Bedrani L, Ain Baziz H et al (2009) Effet de l'acclimatation précoce sur les performances de croissance et la morphométrie intestinale des poulets de chair élevés en conditions estivales méditerranéennes. Eur J Sci Res 38:110–118
Tollba AAH (2010) Reduction of broilers intestinal pathogenic micro-flora under normal or stressed condition. Egypt. Poult Sci 30:249–270 Toplu HDO, Tunca R, Aypak SU et al (2014) Effects of heat conditioning
and dietary ascorbic acid supplementation on heat shock protein 70 expression, blood parameters and fear-related behavior in broilers subjected to heat stress. Acta Sci Vet 42:1179
Vecerek V, Strakova E, Suchy P, Voslarova E (2002) Influence of high environmental temperature on production and haematological and bio-chemical indexes in broiler chickens. Czek J Anim Sci 47:176–182
Winder WW, Hardie DG (1999) AMP-activated protein kinase, a meta-bolic master switch: possible roles in type 2 diabetes. Am J Phys 277:1–10
Yahav S, Plavnick I (1999) Effect of early-age thermal conditioning and food restriction on performance and thermotolerance of male broiler chickens. Br Poult Sci 40(1):120–126. https://doi.org/10.1080/ 00071669987944
Yahav S, Goldfeld S, Plavnik I, Hurwitz S (1995) Physiological responses of chickens and turkeys to relative humidity during exposure to high ambient temperature. J Therm Biol 20(3):245–253.https://doi.org/ 10.1016/0306-4565(94)00046-L
Yahav S, Hurwitz S (1996) Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age. Poult Sci 75(3):402–406.https://doi.org/10.3382/ps.0750402
Yahav S, Shamay A, Horev G, Bar-Ilan D, Genina O, Friedman-Einat M (1997) Effect of acquisition of improved thermotolerance on the induction of heat shock proteins in broiler chickens. Poult Sci 76(10):1428–1434.https://doi.org/10.1093/ps/76.10.1428 Yalcin S, Bruggeman V, Buyse J, Decuypere E, Cabuk M, Siegel PB
(2009) Acclimation to heat during incubation: 4. Blood hormones and metabolites in broilers exposed to daily high temperatures. Poult Sci 88(9):2006–2013.https://doi.org/10.3382/ps.2008-00505 Yeh CC (1992) Effect of acute heat stress on the blood characteristics of
Taiwan country chickens and broilers. J Chin Soc Anim Sci 21:57–66 Zhou WT, Fujita M, Yamamoto S (1999) Effects of ambient temperatures on blood viscosity and plasma protein concentration of broiler chickens (Gallus domesticus). J Therm Biol 24(2):105–112. https://doi.org/10.1016/S0306-4565(98)00045-X
Int J Biometeorol