ContentslistsavailableatScienceDirect
Journal
of
Pharmaceutical
and
Biomedical
Analysis
jou rn al h om e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / j p b a
Simultaneous
determination
of
insulin
and
its
analogues
in
pharmaceutical
formulations
by
micellar
electrokinetic
chromatography
Caroline
Lamalle
a,
Anne-Catherine
Servais
a,
Régis
P.
Radermecker
b,
Jacques
Crommen
a,
Marianne
Fillet
a,∗aLaboratoryofAnalyticalPharmaceuticalChemistry,DepartmentofPharmacy,CIRM,UniversityofLiege,CHU,B36,B-4000Liege,Belgium bDepartmentofDiabetes,NutritionandMetabolicdisorders,CHUSartTilman,UniversityofLiege,Belgium
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16October2014 Receivedinrevisedform 17December2014 Accepted19December2014 Availableonlinexxx Keywords: Counterfeitmedicines Insulin
Micellarelectrokineticchromatography Qualitycontrol
Stability
a
b
s
t
r
a
c
t
AsimpleandefficientMEKCmethodwasdevelopedtosimultaneouslydeterminehumaninsulin,itsfive analogues,themaindegradationproductsandtheexcipientsusuallypresentininjectionformulations. Averyfastmethodwithatotalanalysistimeof3minwasthensuccessfullyvalidatedfortheanalysisof humaninsulinandthequalitycontrolofcommercialformulationswascarriedout.
©2015ElsevierB.V.Allrightsreserved.
1. Introduction
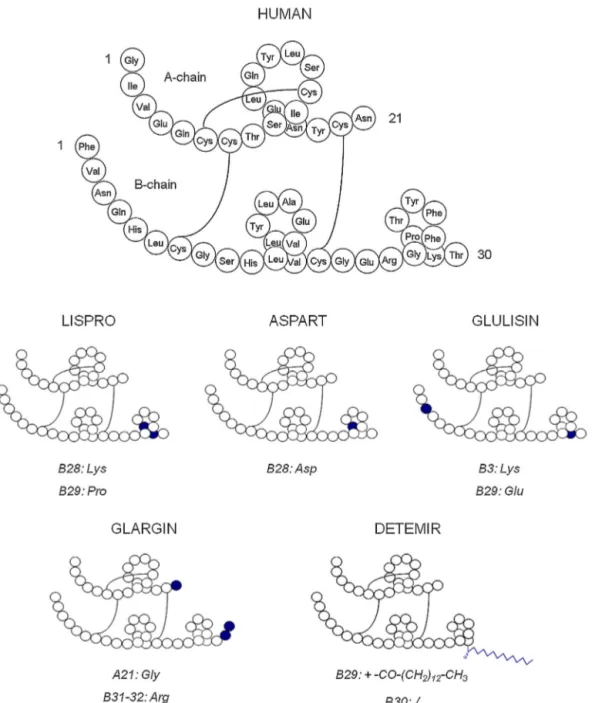
Insulinisanimportanthormonesecretedbypancreatic-cells regulatingprincipallyglucosemetabolism.Currentlysynthesised by recombinant DNA technology, this hormone is commonly administered by subcutaneous injection for the treatment of insulin-dependent diabetes mellitus.Human insulin consistsof two peptide chains – A and B – containing 21 and 30 amino acid residues, respectively, and connected via two disulphide bridges(Fig.1).Ascanbeseeninthisfigure,thedifferentinsulin variantsarequitesimilarsincetheydifferonlybyonetothree aminoacids.Lispro,aspartandglulisinarerapid-actinganalogues that mimic postprandial insulin secretion.Glargin and detemir are long-acting analogues that mimic basal insulin secretion.
Abbreviations: ACN, acetonitrile; BGE,background electrolyte; CM--CyD, carboxymethyl--CyD; DNA, deoxyribonucleic acid; EOF, electroosmoticflow; HDAS--CyD,heptakis(2,3-di-O-acetyl-6-O-sulfo)--CyD;HDMS--CyD,heptakis (2,3-di-O-methyl-6-O-sulfo)--CyD;IS,internalstandard;N,numberoftheoretical plates,separationefficiency;OS-␥-CyD,octakis(2,3-dihydroxy-6-O-sulfo)-␥-CyD; pI,isoelectricpoint;SBE--CyD,sulfobutylether--CyD;TM--CyD,heptakis (2,3,6-tri-O-methyl)--CyD.
∗ Correspondingauthor.Tel.:+3243664345;fax:+3243664347. E-mailaddress:Marianne.fillet@ulg.ac.be(M.Fillet).
Protamineissometimesassociatedwithhumaninsulin,lisproor asparttoprovideanintermediateactionprofile[1].Theexcipients ofpharmaceuticalinjectionsaremainlymeta-cresolandphenol.
Diabetesisoneofthemostcommonmetabolicdiseasesinthe worldandtheprevalenceisincreasingeveryyear,especiallyfor type2diabetes.Alotofinsulinformulationsarethenproduced; theyareexpensiveandrequireaprescription.Thereforetheyare animportanttargetforcounterfeiting.Eveniftheproportionof counterfeitdrugsissuperiorindevelopingcountries,itisworth notingthatitisaffectingthewholeworldandmoreparticularlythe e-commerceinmoreeconomicallydevelopedcountries. Counter-feitedinsulinscontributetotherapeuticfailuresandinsomecases canalsoleadtodeath.Therefore,itisessentialforpublichealthto strengthenthecontrolofpharmaceuticalproductssuchasinsulin formulationsbydevelopingefficientandeasilyapplicablequality controlmethods.
Thedeterminationofinsulinhasbeenalreadydescribedinthe literaturebutmostpapersreportimmunochemicalmethodsfor themonitoringofbiologicalsamples[2–11].Someinstrumental analytical methods based onLCor CEwere alsodeveloped for theanalysisofpharmaceuticalformulationsbutonlyforhuman insulinquantificationinformulationswithoutprotamine[12–15]. Recently,CEandMEKCwereappliedtotheseparationofvarious insulinanalogues [16–18].Indeed,CEisanattractivetechnique http://dx.doi.org/10.1016/j.jpba.2014.12.038
Fig.1.Structureofhumaninsulinanditsanalogues.
withitswell-knownadvantagessuchassimplicity,highseparation efficiency,shortanalysistimeand lowsampleand solvent con-sumption.ItisconsideredasapowerfulalternativetoHPLCandis frequentlyusedfortheseparationoflargebiomolecules[19,20].
Theaimofthis workwastodevelop aneasyandfastMEKC methodforthesimultaneousdeterminationofhumaninsulin,its fiveanaloguesandtheexcipientsusuallypresentincommercial formulations.Astabilitystudywasalsoperformedanddegradation productscouldbeseparatedwiththesamemethod.Themethod wasshortened,adjustedandwasthenfullyvalidatedforhuman insulinanalysis.Itwasalsoappliedtothequalitycontrolof phar-maceuticalformulations,includingthosecontainingprotamine. 2. Materialsandmethods
2.1. Chemicalsandreagents
Acetic acid, ammonia, HCl (37%), ACN, SDS and ZnCl2 were
purchasedfromMerck(Darmstadt,Germany).Protamine,sodium
benzoate,glycerol,phenoland meta-cresolwere obtainedfrom Sigma-Aldrich(St.Louis,MO,USA).␥-CyDwasfromSigma-Aldrich. OS-␥-CyDwaskindlyprovidedbyProfessorG.Vigh(TexasA&M University, TX, USA). CM--CyD, TM--CyD and HDMS--CyD werepurchased fromCyclolab (Budapest, Hungary).SBE--CyD wasfromCyDexPharmaceuticals(Lenexa,KS,USA)andHDAS- -CyDwasobtainedfromAntekInstruments(Houston,TX,USA).
Human insulin standard was obtained from Sigma-Aldrich. Different pharmaceutical formulations were also used: Humu-lineNPH®(humaninsulin)andHumalog®(insulinlispro)were obtained from Eli-Lilly (Indianapolis, IN, USA). Novomix 30® (insulinaspart)and Levemir®(insulindetemir)werepurchased from Novo Nordisk (Bagsvaerd, Denmark). Insuman Rapid® (humaninsulin),Apidra®(insulinglulisin)andLantus®(insulin glargin)wereobtainedfromSanofiAventis(Bridgewater,NJ,USA). Actrapid®andMixtard®(humaninsulins)werecollectedonthe internet.
Ultra-pure water was supplied by a Milli-Q equipment (Millipore, Bedford, MA, USA) and Chromafil® syringe filters
(0.20m) were purchased from Macherey-Nagel (Düren, Germany).
2.2. BufferandBGE
Thebufferwaspreparedbyaddingasolutionof50mMacetic acidtoasolutionof50mMammoniainordertoreachpH9.0.In thefinalconditions,theBGEwasmadebydissolving20mMSDSin thebuffer.Then,itwasmixedwithACN(13%v/v)andthesolution wasfilteredthroughasyringefilter.
ToprepareBGEwithotherreagentsmentionedinthestudy,the stepsweresimilarasmentionedabove.
2.3. Samplepreparation
2.3.1. BGEoptimizationandstabilitystudy
Pharmaceuticalformulations containing 100IU/ml of insulin were used for method development. This corresponds to 3.50mg/ml for human, lispro, aspart and glulisin insulins, 3.64mg/mlforglargininsulinand14.20mg/mlfordetemirinsulin. Human, lispro, aspart, glulisin and glargin formulations were diluted 80 fold in 0.01M HCl to obtain a concentration of approximately50g/ml(43.75or45.50g/ml).Unlessotherwise mentioned,detemirformulationwasdiluted160foldinthesame solvent(88.75g/ml).Themixtureofthe6insulinvariantswas alsopreparedasdescribedabove.Sodiumbenzoatewasselected asISandwaspreparedin0.01MHCl.Allanalysiswereperformed induplicate.
2.3.2. Validation
Calibrationandvalidationstandardsofhumaninsulinwere pre-paredin0.01MHCl,with100g/mlISand10%ACN.Calibration standardswerepreparedwithoutexcipientswhiletheexcipients (phenol,meta-cresol,glycerol,ZnCl2andprotamine)wereadded
inthevalidationstandards.Threequantificationlevelswere inves-tigated(80,100and120%),thetargetlevelof100%corresponding to4IU/ml(m=3).Fourreplicateswerepreparedateachleveland threeindependentserieswerecarriedout(k=3;n=4).
Calibrationstandardswereusedtosetupthecalibrationmodel forthemethodwhiletrueness,precision,accuracyandlinearity wereestimatedusingthevalidationstandards.
2.3.3. Qualitycontrol
Standardsolutionswerepreparedexactlylikecalibration stan-dards.Foursamplesolutionswerepreparedbydissolutionofthe pharmaceuticalformulationin0.01MHCltoobtainaconcentration of4IU/ml,takingintoaccounttheadditionofISandACN(n=4). For-mulationscontaininghumaninsulin(Actrapid®,InsumanRapid®) andhumaninsulinwithprotamine(Mixtard®,HumulineNPH®) contain100IU/ml.
2.4. Instrumentation
All the experiments were carried out on a HP3DCE system
(AgilentTechnologies, Waldbronn,Germany) equipped withan autosampler,anon-columnDADandatemperaturecontrol sys-tem(15–60◦C±0.1◦C).Chemstation(Hewlett-Packard,PaloAlto, CA,USA)wasusedforinstrumentcontrol,dataacquisitionanddata analysis.Fusedsilicacapillarieswereprovidedby ThermoSepara-tionProducts(SanJose,CA,USA).
2.4.1. BGEoptimizationandstabilitystudy
Electrophoreticseparationswerecarriedoutinuncoatedfused silicacapillarieswith50mi.d.and48.5cmtotallength(40cm effectivelength).Atthebeginningofeachworkingday,thecapillary waswashedwith1MNaOH,0.1MNaOHandwaterfor10mineach. Beforeeachinjection,thecapillarywassuccessivelywashedwith
1MNaOHfor2min,waterfor1minandwasthenequilibratedwith theBGEfor4min.Aftereachrun,thecapillarywaswashedwith 0.01MHClfor5minandwaterfor1min.Capillarywashcycleswere performedatapressureofapproximately1bar.Injectionswere madebyapplyingapressureof50mbarforaperiodof5s.During therun,theappliedvoltagewas20kVandthecapillarywas ther-mostatedat20◦C.Electropherogramswererecordedat200nm. 2.4.2. Validationandqualitycontrol
Toreduceanalysistime,theinjectionwasmadeattheshort cap-illaryend(8.5cmeffectivelength).Moreover,theoutletelectrode wasimmersedin0.01MHClaftereachinjection.
Validationdatawereprocessedusingthee.noval®3.0software (Arlenda,Liege,Belgium).
3. Resultsanddiscussion 3.1. Preliminarystudies
Abasicbuffercomposedof50mMammoniumacetate(pH9.0) wasselectedbecauseitinducedanimportantandstableEOFand itprovidedbetterpeakshapethanacidicbuffers.
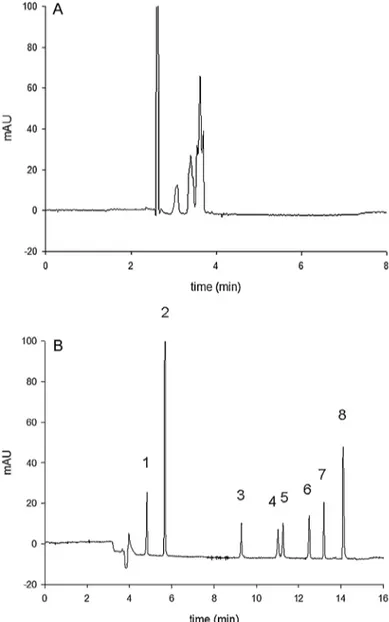
Fig.2. Electropherogramofthesixinsulinformulations.(A)BGE:50mM ammo-niumacetatepH9.0.(B)BGE:50mMammoniumacetatepH9.0+50mMSDS+15% ACN.OtherconditionsaredescribedinSection2.3.1.Peaks:1=phenol,2=m-cresol, 3=aspart,4=human,5=lispro,6=glulisin,7=glargin,8=detemir.
Assomeformulations containedprotamine, thesample dis-solution medium was also optimised. Indeed, protamine is a basic and aliphatic protein which forms withinsulin and zinc a complex insoluble at neutral pH. An acidic pH was then requiredtosolubiliseprotamine,butalsoglargin.Asolutionmade of 0.01M HCl was finally chosen as dissolution and dilution solvent.
3.2. BGEoptimizationfortheseparationofhumaninsulinandits analogues
Fig.2Ashowstheelectropherogramofthemixturecontaining sixpharmaceuticalformulations(humaninsulinanditsfive ana-logues)undertheconditionsmentionedinthepreliminarystudies. Ascanbeseeninthisfigure,alltheanalogueswerenotseparated.As theirmolecularweights(∼5800Da)andpIs(∼5.5)arequitesimilar, theyhavealmostidenticalelectrophoreticmobilitiesandtheir sep-arationbyconventionalCEisnoteasy.Therefore,someadditives wereusedtoimprovetheseparation.
First,differentCyDwerechosentoinvestigatetheirselectivity towardsinsulinsaccordingtotheirsizeandtheirdifferent sub-stituents:␥-CyD,OS-␥-CyD,CM--CyD,TM--CyD,HDMS--CyD, SBE--CyDandHDAS--CyD.TheCyDconcentrationstested(15 and30mM)areinagreementwiththosecommonlyusedinour laboratory.Themigrationprofilewasmodifiedbysomeofthem butthiswasnotsufficienttoobtainacompleteresolution(data notshown).
MEKCwastheninvestigated.SDSisthemostcommonlyused surfactant and was tested at different concentrations (35 and 50mM)inthepresence–ornot–ofanorganicmodifier(0–20% ACN).
Theadditionof50mMSDSledtotheseparationofthetwomajor excipients,phenolandmeta-cresol,butnotofallinsulins.Thesame observationwasmadewith10%ACNv/vaddedtotheSDSsystem. Goodresultswereobtainedwith15%ACNsincethesixinsulins werecompletelyseparatedwithin15min(seeFig.2B).Itisworth notingthatamultivariateapproachcouldnotbeappliedinthis studybecauseoftooextremeresponses(withon/offeffects)when afactorwasmodified.
Theseresultsare inaccordance withpreviousreports which indicatedthatsmallpeptidescouldbesuccessfullyseparatedby MEKC[21–24]andthattheadditionoforganicsolventwasrequired toseparatecloselyrelatedpeptideswithmorethan20aminoacid residues[25,26].Indeed, proteinsstronglyinteractwith surfac-tantsandtendtoformcomplexes.Theorganicsolventaddedto themicellarsolutioncanpreventthisinteraction.
Theoptimizedconditionledtothefollowing insulins migra-tionorder: aspart,human, lispro, glulisin, glarginand detemir. Indeed,withitsC14fattyacidchain,detemiristhemost hydropho-bicanalogueandthusinteractsmorestronglywiththesurfactant. Fortheothercompounds,aMEKCsystemwithanorganic modi-fiermakestheinterpretationverycomplexasalotofparameters areinvolvedinthemigrationbehaviour[27].Indeedthe interac-tionsdependonthelogPandthechargeoftheanalyte(possible ion-pair formation).However, thepresence of ACN in the BGE reduces the interactions with the micelles and the migration behaviourcanbesignificantlymodifiedfromoneanalytetothe others [27]. Attention can be paid to the resolution obtained betweenhumanandlisproinsulinswhichdifferonlybythe inver-sionoftwoaminoacids(Fig.1).Thissuggeststhatthesecondary structureof theproteincouldalsoplayan essentialrole inthe interactionwiththesurfactant,probablybecauseofdifferent expo-sitionsofhydrophobicsites.Theseparationofhumanandlispro insulins was also achieved thanks to the high peak efficiency (N≈150000).
3.3. Stabilitystudy
Astability studywascarriedout onthepharmaceutical for-mulationsstoredinarefrigerator(4–8◦C),atroomtemperature (20–25◦C)andat37±1◦Cduring3,7,14,21and28days.The per-centageofdegradationwasestimatedbycalculatingthepeakarea ratiobetweenthepossibledegradationproductandthesumofthe insulinandthepossibledegradationproduct.Nodegradationwas detectedafter28daysirrespectiveofthetemperature(datanot shown).
The insulin stability was also investigated in formulations diluted in 0.01MHCl and stored 24h, 48h and 6 days at the threepreviouslymentionedtemperatures(seeFig.3).No degra-dationwasobservedforthesampleskeptinrefrigerator.Asmall degradationwasdetectedforglulisinanddetemirstoredatroom temperaturebutonlyafter6days.Forthesamplesstoredat37◦C, degradationwasalreadyobservedafter24hforallinsulinsexcept aspartandglargin.ThissuggeststhatanacidicpHandaratherhigh temperature promote degradation. Degradation increased after 48hforthesameinsulinsandincreasedagainafter6daysforall insulinsexceptglargin.Thisexceptioncanbeexplainedbythefact thatthemaindegradationofinsulinoriginatesfromdeamidation atresidueA-21[28].Atthisposition,glarginhastheaminoacid GlyinsteadofAsn(Fig.1).Therefore,A-21deamidationcouldnot occurforglargininsulin.Fig.4demonstratestheselectivityofthe methodforimpuritydetection.
Thestabilityofhumaninsulinstandardwasalsostudiedafter dilutionin0.01MHClandstoragefor24h,48hand6daysatthe threepreviouslymentionedtemperatures.Despitetheabsenceof preservatives,thedegradationprofileofhumaninsulinstandard lookedquitesimilarcomparedtothatoftheformulation(seeFig.3). Insummary,samplesseemtoremainstableafter24or48hif storedatamaximaltemperatureof25◦C.
3.4. Methodvalidationforroutinequalitycontrolofformulations containinghumaninsulin
Todemonstratetheapplicabilityofourapproach forroutine analysis,amethodvalidationwascarriedoutforthequantification ofhumaninsulin.
Beforethevalidationand toreduceanalysistimeforroutine qualitycontrol,thepreviouslydevelopedmethodwasshortened. Theinjectionwasmadeattheshortcapillaryendleadingtoa 5-foldreductionoftheanalysistime.SmalladjustmentsoftheBGE composition(20mMSDSand13%ACN) wereprovided inorder toobtainsatisfactoryselectivitywiththedegradationproduct(cf. Fig.5A).10%ACNwasalsoaddedtothesamplestoguaranteethe stabilityofinsulinandpreventitsadsorption.
Itisworthnotingthatinthoseconditions,theseparationofthe 6insulinswithin3minwasalsoverygoodexceptthatbetween humaninsulinandlispro(Fig.5B).Itwasconsideredsufficientto performidentification,however,theinitialmethodcouldbeeasily appliedtoconfirmtheidentificationifindoubt.Selectivityofthe analyticalmethodwasassessedbyanalyzingreconstitutedblank solution(containingtheexcipientsincludedprotaminebutwithout insulin).Noendogenoussourceofinterferencewasobservedatthe migrationtimeofhumaninsulin.
Inordertoquantifyoneoftheseinsulins,sodiumbenzoatewas chosenasIS.Thenormalizedpeakarearatios(i.e.areasdividedby migrationtimes)betweentheactivecompoundandtheISwere calculated.
3.4.1. Selectionofthecalibrationmodel
Theacceptancelimitsweresetat±10%(cf.requirementofthe EuropeanPharmacopeaforHumanInsulinpreparations)andthe maximumrisk (1-) toobtainresultsoutside theseacceptance
Fig.3. Stabilitystudiesshowingthepercentageofdegradationasafunctionoftime.Pharmaceuticalformulationsdilutedin0.01MHCl.
limitswassetat5%.Differentregressionmodelswerefittedtothe calibrationstandardsandlinearregressionwaschosenasthemost appropriateresponsefunction(Fig.6).
3.4.2. Othervalidationcriteria
Table1presentsthevalidationresultsofhumaninsulin. True-nessofthemethodwasexcellentastheupperrelativebiasofthe methodwas1.272%.TheRSDvaluesforrepeatabilityand inter-mediateprecisionweresatisfactory(below3%),demonstratinga goodprecision.Themethodwasaccuratesincethelowerandupper
Fig.4. Effectof6daysstorageinHCl0.01Mandat37◦Conthesixinsulin for-mulations.BGE:50mMammoniumacetatepH9.0+50mMSDS+15%ACN.Other conditionsaredescribedinSection2.3.1.Peaks:*=degradationproduct.
tolerancelimitsdidnotexceedtheacceptancelimitsof10%over the consideredconcentration range. For all series, a regression linewasfittedtotheback-calculatedconcentrationsofthe vali-dationstandardsasafunctionoftheintroducedconcentrations. Theresultsattestingthemethodlinearity,namelytheregression
Fig.5. (A)Electropherogramofhumaninsulinwithitsdegradationproduct(2days storageinHCl0.01Mandat37◦C).Peaks:IS=sodiumbenzoate(IS),*=degradation
product,hum=humaninsulin.(B)Electropherogramofthesixinsulinformulations obtainedafteranalysisattheshortcapillaryend.BGE:50mMammoniumacetate pH9.0+20mMSDS+13%ACN.Peaks:1=phenol,2=m-cresol,3=aspart,4=human, 5=lispro,6=glulisin,7=glargin,8=detemir.Otherconditionsaredescribedin Sec-tion2.3.1.
Fig.6.Accuracyprofileofhumaninsulinobtainedbyconsideringlinearregression(range:3.2–4.8IU/ml).Plainline:relativebias,dashedlines:-expectationtolerance limits,dottedcurves:acceptancelimitsanddots:relativeback-calculatedconcentrations.
equationcorrespondingtothatrelationshipwiththecoefficientof determination,arepresentedinTable1.TheLODwasestimated at0.43IU/mlusingtheinterceptofthecalibrationmodelandthe residualvarianceoftheregression.Inthiscase,theLOQwasthe lowestconcentrationlevel,3.2IU/ml.
3.5. Qualitycontrolofhumaninsulinformulations
Aqualitativeandquantitativeanalysisoffourdifferent phar-maceuticalformulations(Actrapid®,Mixtard®,HumulineNPH® andInsumanRapid®)wascarriedoutaccordingtothevalidated method.Fourstandardsolutionsateachlevel(80,100and120%) andfoursamplesofeach formulationat4IU/mlwere indepen-dently prepared. To make the conversion from IU to mg and Table1
Validationresultsofhumaninsulin(k=3;m=3;n=4).
Day1 Day2 Day3
Responsefunction
Slope 0.1643 0.1594 0.1559
Intercept −0.04498 −0.01421 −0.005656
r2 0.9795 0.9739 0.9883
Level1 Level2 Level3
Trueness Relativebias(%) 1.272 0.4236 −0.06906 Precision Repeatability(RSD,%) 1.838 2.140 1.665 Intermediateprecision(RSD,%) 2.534 2.426 2.275 Accuracy
-expectationtolerancelimits(%) −5.877/8.421 −5.542/6.389 −6.445/6.307 Linearity Range(IU/ml) 3.2–4.8 Slope 0.9725 Intercept 0.1282 r2 0.9814 LOD(IU/ml) 0.43 LOQ(IU/ml) 3.2
k:numberofdaysofexperiments(series),m:numberofconcentrationlevels,n: numberofreplicatesperconcentrationlevelandperseries.
Table2
Analytical results for the quantification of human insulin in pharmaceutical formulations. Pharmaceutical formulations Concentration found(IU/ml) Percentageofthe claimedcontent(%) Repeatability (RSD,%) Actrapid® 3.90 97.3 1.34 Mixtard® 4.16 103.9 1.05 Humuline NPH® 4.29 107.2 0.91 Insuman Rapid® 4.02 100.5 3.84
thereforetoknowwhichweightofthestandardisneeded,theassay
inIU/mgandthelossondryingofthisstandardmustbetakeninto
account.
Theregressionequationwasusedtocalculatetheconcentration
ofthesamplesandthenthepercentageoftheclaimedcontent.The
meanofthepercentagesandtheRSDsaregiveninTable2.Allthe
testedformulationswereinsidethelimitsof90–110%. 4. Concludingremarks
Inthepresentstudy,MEKCwasusedtosimultaneouslyanalyse humaninsulinanditsfiveanalogues(lispro,aspart,glulisin,glargin anddetemir).AmethodwasdevelopedusingthefollowingBGE: 50mMammoniumacetatepH9.0,50mMSDSand15%ACN.Thesix insulinsandthetwomajorexcipientsofpharmaceutical formula-tionscouldbeseparatedwithin15min.Thismethodalsoexhibited selectivityregardingtheirprincipaldegradationproducts,sothat thesamplesstabilitycouldbestudiedasafunctionoftimeand temperatureofthestorage.
A fastmethodwith ananalysis time of 3minwas obtained byinjectingattheshortcapillaryendandbyusingthefollowing BGE: 50mM ammonium acetate pH 9.0, 20mM SDS and 13% ACN.Thismethodwasfullyvalidatedforhumaninsulinoverthe concentrationrangeof3.2and4.8IU/ml,withacceptancelimits of10%andariskof5%.Then,thequalitycontrolofformulations containinghumaninsulin,includingthose withprotamine, was successfully achieved. These investigations demonstrate the
interestingabilityofcapillaryelectrophoresisforquantificationof intactbiomolecules.
Acknowledgements
Manythanksare duetotheFondsNationaldela Recherche Scientifique(FNRS,Belgium)andtheFondsLéonFredericq(Liege, Belgium)fortheirfinancialsupport.
References
[1]S.Madsbad,Insulinandnewinsulinanalogueswithfocusontype2diabetes, in:C.E.Mogensen(Ed.),PharmacotherapyofDiabetes:NewDevelopments, Springer,US,2007,pp.53–65.
[2]W.Tong,E.S.Yeung,Determinationofinsulininsinglepancreaticcellsby cap-illaryelectrophoresisandlaser-inducednativefluorescence,J.Chromatogr.B: Biomed.Appl.685(1996)35–40.
[3]I.German,R.T.Kennedy,Rapidsimultaneousdeterminationofglucagonand insulinbycapillaryelectrophoresisimmunoassays,J.Chromatogr.B:Biomed. Sci.Appl.742(2000)353–362.
[4]V.Solinova,V.Kasicka,D.Koval,T.Barth,A.Ciencialova,L.Zakova,Analysis ofsyntheticderivativesofpeptidehormonesbycapillaryzone electrophore-sisandmicellarelectrokineticchromatographywithultraviolet-absorption andlaser-inducedfluorescencedetection,J.Chromatogr.B:Analyt.Technol. Biomed.LifeSci.808(2004)75–82.
[5]I.LePotier,G.Franck,C.Smadja,S.Varlet,M.Taverna,In-capillaryderivatization approachappliedtotheanalysisofinsulinbycapillaryelectrophoresiswith laser-inducedfluorescencedetection,J.Chromatogr.A1046(2004)271–276. [6]S.Descroix,I.LePotier,C.Niquet,N.Minc,J.L.Viovy,M.Taverna,In-capillary
non-covalentlabelingofinsulinandonegastrointestinalpeptidefortheir anal-ysesbycapillaryelectrophoresiswithlaser-inducedfluorescencedetection,J. Chromatogr.A1087(2005)203–209.
[7]M.Thevis,A.Thomas,P.Delahaut,A.Bosseloir,W.Schanzer,Qualitative deter-minationofsyntheticanaloguesofinsulininhumanplasmabyimmunoaffinity purificationandliquidchromatography–tandemmassspectrometryfordoping controlpurposes,Anal.Chem.77(2005)3579–3585.
[8]C.Guillo,M.G.Roper,Two-colorelectrophoreticimmunoassayfor simulta-neousmeasurementofinsulinandglucagoncontentinisletsofLangerhans, Electrophoresis29(2008)410–416.
[9]J.F.Dishinger,K.R.Reid,R.T.Kennedy,Quantitativemonitoringofinsulin secre-tionfromsingleisletsofLangerhansinparallelonamicrofluidicchip,Anal. Chem.81(2009)3119–3127.
[10]C. Guillo,T.M.Truong,M.G.Roper,Simultaneouscapillary electrophoresis competitiveimmunoassayforinsulin,glucagon,andisletamyloid polypep-tidesecretionfrommouseisletsofLangerhans,J.Chromatogr.A1218(2011) 4059–4064.
[11]C. Hess,A.Thomas, M.Thevis,B.Stratmann,W.Quester,D. Tschoepe,B. Madea,F.Musshoff,Simultaneousdeterminationandvalidated quantifica-tionofhumaninsulinanditssyntheticanaloguesinhumanbloodserumby
immunoaffinitypurificationandliquidchromatography–massspectrometry, Anal.Bioanal.Chem.404(2012)1813–1822.
[12]A.Kunkel,S.Günter,C.Dette,H.Wätzig,Quantitationofinsulinbycapillary electrophoresisandhigh-performanceliquidchromatography.Method com-parisonandvalidation,J.Chromatogr.A781(1997)445–455.
[13]P.Moslemi,A.R.Najafabadi,H.Tajerzadeh,Arapidandsensitivemethodfor simultaneousdeterminationofinsulinandA21-desamidoinsulinby high-performanceliquidchromatography,J.Pharm.Biomed.Anal.33(2003)45–51. [14]B.Deng,Z.Liu,G.Luo,H.Ma,M.Duan,Rapidquantitativedeterminationand assessmentofinsulininoilformulationbymicellarelectrokineticcapillary chromatography,J.Pharm.Biomed.Anal.27(2002)73–80.
[15]A.Staub,S.Rudaz,J.L.Veuthey,J.Schappler,Multipleinjectiontechniquefor thedeterminationandquantitationofinsulinformulationsbycapillary elec-trophoresisandtime-of-flightmassspectrometry,J.Chromatogr.A1217(2010) 8041–8047.
[16]K.Ortner,W.Buchberger,M.Himmelsbach,Capillaryelectrokinetic chro-matographyofinsulinandrelatedsyntheticanalogues,J.Chromatogr.A1216 (2009)2953–2957.
[17]M.Haunschmidt,K.Ortner,K.Hainz,E.Bradt,L.Sternbauer,W.Buchberger, C.W.Klampfl,Investigationsonthemigrationbehaviorofinsulinandrelated syntheticanaloguesinCZE,MEKCandMEEKCemployingdifferentsurfactants, Electrophoresis31(2010)1560–1564.
[18]H.H.Yeh,H.L.Wu,C.Y.Lu,S.H.Chen,Simultaneousdeterminationofregular insulinandinsulinaspartbycapillaryzoneelectrophoresisandapplicationin drugformulations,J.Pharm.Biomed.Anal.53(2010)145–150.
[19]N.F.Visser,M.vanHarmelen,H.Lingeman,H.Irth,On-lineSPE-CEforthe deter-minationofinsulinderivativesinbiologicalfluids,J.Pharm.Biomed.Anal.33 (2003)451–462.
[20]A.Staub,D.Guillarme,J.Schappler,J.L.Veuthey,S.Rudaz,Intactproteinanalysis inthebiopharmaceuticalfield,J.Pharm.Biomed.Anal.55(2011)810–822. [21]H.J. Liu, R.E. Strong, I.S. Krull, S.A. Cohen, Homogeneouspreparation of
fluorescent-derivatizedinsulinanditsapplicationtocompetitive chromato-graphicimmunoassays,Anal.Biochem.298(2001)103–111.
[22]H.Nishi,S.Terabe,Micellarelectrokineticchromatographyperspectivesindrug analysis,J.Chromatogr.A735(1996)3–27.
[23]B.Stanley,K.A.Mehr,T.Kellock,J.D.VanHamme,K.K.Donkor,Separationand determinationofcloselyrelatedlantibioticsbymicellarelectrokinetic chro-matography,J.Sep.Sci.32(2009)2993–3000.
[24]Y.Wu,J.Xie,F.Wang,Z.Chen,Separationofsmallmolecularpeptideswithsame aminoacidcompositionbutdifferentsequencesbycapillaryelectrophoresis, J.Sep.Sci.32(2009)437–440.
[25]T.Yashima,A.Tsuchiya,O.Morita,S.Terabe,Separationofcloselyrelatedlarge peptidesbymicellarelectrokineticchromatographywithorganicmodifiers, Anal.Chem.64(1992)2981–2984.
[26]C.Arcelloni,L.Falqui,S.Martinenghi,A.E.Pontiroli,R.Paroni,Capillary elec-trophoresisforsimultaneousquantificationofhumanproinsulin,insulinand intermediateforms,Electrophoresis19(1998)1475–1477.
[27]C.Lamalle,A.C.Servais,I.Fradi,J. Crommen,M.Fillet,Micellar electroki-neticchromatographysystemsfortheseparationofmixturesofchargedand unchargedcompounds,J.Sep.Sci.35(2012)1933–1939.
[28]J.Brange,L.Langkjaer,Chemicalstabilityofinsulin.3.Influenceofexcipients, formulation,andpH,ActaPharm.Nord.4(1992)149–158.