JUAN DIEGO URRJAGO SUAREZ
Les réponses comportementales
de P oursin Tetrapygus niger face aux étoiles de mer
prédatrices Meyenaster gelatinosus
et Heliaster helianthus
Mémoire présenté
à la Faculté des études supérieures de l'Université Laval dans le cadre du programme de maîtrise en biologie pour l'obtention du grade de Maître es Sciences (M.Sc.)
DEPARTEMENT DE BIOLOGIE FACULTÉ DES SCIENCES ET DE GÉNIE
UNIVERSITÉ LAVAL QUÉBEC
2010
J'ai mené des expériences afin d'étudier les réponses de l'oursin Tetrapygus niger à la prédation des étoiles de mer. L'oursin était capable de différencier les étoiles de mer prédatrices des non-prédatrices mais également de distinguer différents niveaux de risques associés aux étoiles de mer, Heliaster helianthus et Meyenaster gelatinosus. Les oursins soumis à un haut niveau de risque hérissaient rapidement leurs épines, puis étendaient leurs pieds ambulacraires pour fuir le prédateur. L'oursin associait un risque plus grand à M. gelatinosus. J'ai également démontré l'existence de la chimiodétection à distance des prédateurs. La micro-distribution des oursins sur les surfaces élevées semble représenter une stratégie pour limiter la prédation par les étoiles de mer. Le nombre de celles-ci étant plus réduit sur ces surfaces élevées, leur capacité à capturer les oursins est moindre et les oursins peuvent se détacher pour éviter d'être mangé. Enfin, des expériences avec entravement indiquent que le taux de survie est plus élevé pour les animaux situés sur les surfaces élevées.
11
Abstract
I conducted field experiments to examine responses of the sea urchin Tetrapygus niger to sea star predators. The urchin distinguished between predatory and non-predatory sea stars and also recognized different levels of risk associated with the predatory sea stars, Heliaster helianthus and Meyenaster gelatinosus. Urchins under high prédation risk rapidly raised their spines, and then extended podia and fled. The urchin associates the strongest risk with M. gelatinosus. I further demonstrated distance chemodetection of predators. The urchin's micro-distribution on elevated surfaces appears to represent a strategy for limiting prédation by sea stars because on elevated surfaces there are fewer sea stars, the ability of sea stars to capture urchins is reduced, and the urchins can detach to avoid being eaten. Finally, tethering experiments indicate that survival rate is greater on elevated surfaces.
Avant-propos
Ce mémoire comporte quatre chapitres : une introduction générale, deux chapitres dans lesquels les résultats obtenus au cours de mon étude sont présentés et discutés (corps du mémoire) et une conclusion générale. Les chapitres qui constituent le corps du mémoire ont été rédigés en anglais sous la forme d'articles scientifiques. J'ai été l'instigateur et le réalisateur de chacune de ces études. Les Drs. John H. Himmelman (directeur du mémoire) et Carlos F. Gaymer (codirecteur) ont participé à la rédaction de ces articles.
Chapitre II. Urriago J.D. Himmelman J.H., Gaymer CF., 2010. Responses ofthe black sea urchin Tetrapygus niger to its sea star predators Heliaster helianthus and Meyenaster gelatinosus under field conditions. JEMBE (soumis)
Chapitre III. Urriago J.D. Himmelman J.H., Gaymer CF., 2010. Does the distribution of sea urchins Tetrapygus niger on elevated surfaces represent a strategy for avoiding prédation by sea stars? En préparation.
Les résultats obtenus au cours de cette thèse ont été présentés lors de congrès dont la liste apparaît ci-dessous:
Urriago J.D., Himmelman J.H., Gaymer CF., 2010. Responses of the black sea urchin Tetrapygus niger to its sea star predators Heliaster helianthus and Meyenaster gelatinosus. Canadian Society of Zoology. Vancouver, Canada.
Urriago J.D., Himmelman J.H., Gaymer CF., 2010. Responses of the black sea urchin Tetrapygus niger to its sea star predators Heliaster helianthus and Meyenaster gelatinosus. The 5th Annual meeting of the Canadian Society for Ecology and Evolution.
Quebec City, Canada.
Urriago J.D., Himmelman J.H., Gaymer CF., 2010. Too close for comfort: distance detection of predators by sea urchins. lere édition du Colloque du Département de
biologie de l'Université Laval. Quebec City, Canada.
Urriago J.D., Himmelman J.H., Gaymer CF., 2009. Too close for comfort: distance detection of predators by sea urchins. Forum québécois en sciences de la mer. Rimouski, Canada.
IV
First of all I would like to thank my director John Himmelman who has been like a father to me in this country so far from home. He has been an excellent academic advisor, who during these 3 years in Canada has taught me with, patience and wisdom, a clear way to do research. My thanks for your advice, dedication, friendship and support.
I sincerely thank my co-director Carlos Gaymer for his trust and support in my work. His patience and relevant inputs during the development of my master's research has been essential during the two field seasons (2008 - 2009) and during the previous year working on different Ecology and Marine Conservation projects (2007) in Chile. I especially want to thank him for his sincere friendship and the good energy extended to me since we met.
I want to especially thank Clément Dumont, my friend and future Ph.D. co-director in Hong Kong, for giving me my first job as a marine biologist. He introduced me to Carlos and then suggested John as a potential Master's supervisor. Clement and Carlos are two more members ofthe extended Himmelman family which I have had the privilege to join as John's last student.
This work would not have been possible without the important logistical support of my Elite diving team Etienne Renaud-Roy and Mayra Natalia Munoz Pinilla. Thanks for sharing with me continuous days of diving in the beautiful, cold, rough Chilean waters. I cannot leave out my grandfather Dario who also helped me logistically during several days of diving in Chile.
The content of this thesis has been improved by the pertinent comments of my evaluation committee Ladd Johnson and Helga Guderley. Thanks to Ladd for his support and for shared pleasant moments in Quebec City and its surroundings. To Helga, the beautiful woman with an eternal smile and nice energy, thanks for her maternal friendship. I appreciated sharing Helga and John's love for nature and healthy living.
Merci à Hélène Crépeau qui à été une personne clé dans la vérification des analyses statistiques. Je remercie Flavienne Bruyant pour les corrections de texte en Français. Je remercie le CRSNG (Canada) et le FONDECYT (Chile) pour leur soutien financier et logistique au projet. J'ai bénéficié pour ma part de bourses du Gouvernement du Québec, de Québec-Océan et du Département de Biologie de l'Université Laval.
Department) during my masters, especially my roommate Nicolas Martin ("mon petite c") who introduced me to his family in Shawinigan Sud and with whom in one way or another I shared thousands of experiences. I cannot put aside the many evenings of good coffee, accompanied by chess and great friends. Among them are: Hernân Pérez ("el especial"), the fancy English Samuel Collin ("my p. cow") a man of confidence, special musical preferences and with whom I shared really good times. Special thanks to David Pâez (liAustralianus maximus colombiensis") for his contributions and reviews on the articles in
this thesis, but especially since my arrival to share good chess, good coffee, hard workouts in the gym and sauna evenings to discuss life.
Finally, I specially want to thanks my mom Cecilia, my dad Dario and my grandmother Ruth for giving me unconditional support and trust. Thanks to them for encouraging me with tons of love which has kept me motivated. They will be forever in my heart.
VI
Table des matières
Page
CHAPITRE I. Introduction générale 1
CHAPITRE IL Responses of the black sea urchin Tetrapygus niger to its sea-star predators Heliaster helianthus and Meyenaster gelatinosus under field
conditions 5
Résumé 6 Abstract 7 Introduction 8 Methods 11
Responses to different sea stars 11 Responses relative to the level of risk of predatory sea stars 12
Density effects on the sea urchin's response 12
Statistical analyses 13
Results 14 Responses to different sea stars 14
Responses relative to the level of risk of predatory sea stars 16
Density effects on the sea urchin's response 18
Discussion 21
CHAPITRE III. Does the distribution of sea urchins Tetrapygus niger on elevated
surfaces represent a strategy for avoiding prédation by sea stars? 26
Résumé 27 Abstract 28 Introduction 29 Methods 31
Distribution of sea urchins and sea stars 31 Responses of sea urchins to sea stars on different types of bottom 32
Sustained (simulated) attacks on vertical walls 32
Sustained attacks on aggregations 33 Survival on high and low surfaces 33
Statistical analyses 34
Results 34 Distribution of sea urchins and sea stars 34
Responses of sea urchins to sea stars on different types of bottom 35
Sustained (simulated) attacks on vertical walls 35
Sustained attacks on aggregations 38 Survival on high and low surfaces 40
Discussion 43
CHAPITRE IV. Conclusion générale 48
1
CHAPITRE I Introduction générale
La prédation est une interaction entre espèces au cours de laquelle une des espèces, le prédateur, se nourrit de l'autre espèce, la proie. La proie n'est pas nécessairement tuée au cours de la prédation. Les prédateurs comprennent les herbivores, les carnivores et les parasites (Krebs, 2009). La prédation peut indirectement affecter le comportement de la proie, sa distribution, son abondance ainsi que la structure de sa communauté (Paine, 1980; Kerfoot et Sih, 1987; Estes et al., 1998; Broom et al., 2010). Les espèces proies présentent des réponses comportementales évoluées et diverses à l'égard des prédateurs. Ces réponses peuvent être groupées en deux catégories, l'évitement et la fuite (Lima et Dill, 1990). L'évitement minimise les rencontres avec les prédateurs, il s'agit par exemple des réponses comme le camouflage, l'aposématisme, la présence d'armures de protection ou bien des mécanismes de défense chimique (Nelson et Vance,
1979; Cronin et Hay, 1996; Saporito et al., 2006). La fuite tend à réduire la probabilité de blessures ou de mort après l'éventualité d'une rencontre avec un prédateur (Feder, 1963; Legault et Himmelman, 1993; Markowska et Kidawa, 2007; Gaymer et Himmelman, 2008).
Dans le milieu marin la chimiodétection (à distance ou bien au contact) est un facteur important dans la médiation des interactions proie-prédateur. Les prédateurs peuvent souvent détecter l'odeur des espèces proies (McClintock et al., 1984; Rochette et al., 1994; Dale, 1997; Thompson et al., 2005), de même, les proies peuvent souvent détecter certaines substances excrétées par les prédateurs (Alexander et Covich, 1991; Covich et al., 1994; Svensen et Kiorboe, 2000; Markowska et Kidawa, 2007). Par exemple, les substances chimiques excrétées par les étoiles de mer prédatrices (asterisaponins; Gameau et al., 1989) déclenchent fréquemment des réponses défensives de la part de leur proies (Kats et Dill, 1998; McClintock et al., 2008b). Les espèces proies peuvent alors larguer dans le milieu naturel des substances chimiques qui alertent leurs congénères d'un danger potentiel (Smith, 1992; Chivers et Smith, 1998). Certains oursins de mer fuient lorsque exposés à des substances chimiques provenant d'étoiles de mer ou de congénères blessés (Dayton, 1975; Scheibling et Hamm, 1991). La plupart des études examinant la réponse des oursins aux risques de prédation ont été conduite en laboratoire, dans des conditions d'eau calme (Jensen, 1966; Bernstein et al., 1981; Tegner et Levin, 1983; Mann et al.,
1984; Scheibling et Hamm, 1991; Legault et Himmelman, 1993; Hagen et Mann, 1994; Rodriguez et Ojeda, 1998; Matassa, 2010) et de courant unidirectionnel (Phillips, 1978; Moitoza
études réalisées sur le terrain l'ont généralement été dans des conditions de courant unidirectionnel ou en eaux calmes (Snyder et Snyder, 1970; Rosenthal et Chess, 1972; Dayton et al., 1977; Bernstein et al., 1983; Parker et Shulman, 1986; Vadas et al., 1986; Andrew et Macdiarmid, 1991; Vadas et Elner, 2003). Une seule étude rapporte les réponses des patelles au contact d'étoiles de mer prédatrices dans des conditions agitées (Espoz et Castilla, 2000). Aucune étude dans le milieu naturel n'examine la chimiodétection à distance dans des conditions agitées (présence de vagues), alors qu'en laboratoire, une étude par Gagnon et al. (2003) montre que l'étoile de mer Asterias vulgaris détecte sa proie, les moules, et se dirige vers elle dans un bac soumis à des vagues.
La présente étude évalue les réponses de l'oursin de mer noir Tetrapygus niger envers deux de ces principaux prédateurs, les étoiles de mer Heliaster helianthus et Meyenaster gelatinosus. La présence de vagues est une caractéristique constante de la plupart des habitats où l'on trouve T. niger dans le centre et le Nord du Chili. Dans cette région, T. niger est aussi l'oursin
•y
le plus abondant (-40 ind. m" ; Vasquez et Buschmann, 1997). Les activités de broutage intensif de T. niger ont transformé de nombreuses zones sublittorales en zone dénudées. T. niger limite aussi souvent la distribution de la laminaire Lessonia trabeculata aux zones de faibles profondeurs et a provoqué des extinctions locales de la laminaire sublittoral Macrocystis integrifolia (Vega et al., 2005). Des études faites dans d'autres parties du monde ont également démontré que le broutage intensif par T. niger a provoqué le dénuement de zones marines et réduit la diversité et la biomasse des macroalgues (Himmelman et al., 1983; McClanahan et Shafir, 1990; Alcoverro et Mariani, 2002; Shears et Babcock, 2002).
Les prédateurs peuvent affecter le comportement, la densité et la structure des populations des oursins de mer (Tegner et Levin, 1983; Sala et al., 1998; Tuya et al., 2004; Guidetti, 2006). La plupart des études sur les prédateurs des oursins se concentrent sur les étoiles de mer (Jensen, 1966; Rosenthal et Chess, 1972; Dayton et al., 1977; Moitoza et Phillips, 1979; Legault et Himmelman, 1993; Rodriguez et Ojeda, 1998; Hagen et al., 2002). Cependant, d'autre types de prédateurs, comme par exemple les loutres de mer (Estes et al., 1998), les poissons (Sala, 1997), les homards (Andrew et Macdiarmid, 1991) et les crabes (Scheibling et Hamm, 1991), ont été identifiés comme se nourrissant sur les populations d'oursins.
De nombreux prédateurs sont identifiés comme consommateurs de T. niger, incluant les poissons Semicossyphus maculatus (Fuentes, 1981), Graus nigra (Fuentes, 1982), Pinguipes chilensis (Rodriguez et Ojeda, 1998), Cheilodactylus variegatus et Oplegnathus insignis (Medina et al., 2004), et l'étoile de mer Luidia magellanica (Gaymer et Himmelman, 2008), cependant, les deux prédateurs les plus fréquemment observés se nourrissant de T. niger sont les étoiles de mer H. helianthus et M. gelatinosus (Barrios et al., 2008; Gaymer et Himmelman, 2008). Ces étoiles de mer ont été décrites comme des espèces prédateurs clés pour les communautés rocheuses peu profondes du Nord du Chili (Gaymer et Himmelman, 2008; Barahona et Navarrete, 2010). H. helianthus est un prédateur ubiquiste qui consomme ses proies en fonction de leur disponibilité (Gaymer et Himmelman, 2008; Barahona et Navarrete, 2010), alors que M. gelatinosus est un prédateur sélectif qui préfère consommer T. niger (Gaymer et Himmelman, 2008). Dans le Nord du Chili la densité moyenne de H. helianthus est de 3 ind. m" et celle de M. gelatinosus est <0.5
■y
ind. m" . Les deux espèces sont endémiques le long de la côte Ouest de l'Amérique du Sud (Dayton et al., 1977; Tokeshi et al., 1989; Gaymer et Himmelman, 2008; Navarrete et Manzur, 2008). H. helianthus possède un corps robuste et aplati avec de nombreux bras (jusqu'à 40), elle peut atteindre 32 cm de diamètre (Tokeshi et al., 1989). En revanche, M. gelatinosus a un corps mou avec 6 larges bras et peut atteindre jusqu'à 56 cm de diamètre (Dayton et al., 1977; Gaymer et Himmelman, 2008).
Deux études se concentrent sur les réponses comportementales de T. niger envers ses étoiles de mer prédatrices. Dayton et al. (1977) ont examiné les réponses de plusieurs invertébrés à la présence de M. gelatinosus dans des bassins créés par la marée; ils se sont particulièrement concentrés sur les réponses de la variété commerciale d'oursin Loxechinus albus. Ils mentionnent que T. niger est capable de détecter M. gelatinosus à une distance de 50 à 130 cm et de répondre presque immédiatement par une fuite vers des surfaces élevées. Rodriguez et Ojeda (1998) ont réalisé des études dans des bacs de laboratoire pour examiner la réponse des oursins à la présence de M. gelatinosus et à celle du poisson P. chilensis. Ils rapportent que T. niger augmente son taux de déplacement lorsque ces prédateurs sont proches et qu'une proportion plus grande d'individus répond lors des tests avec M. gelatinosus (92 %) que lors des tests avec P. chilensis (67 %).
L'objectif général de cette thèse est d'examiner les réponses comportementales de l'oursin de mer noir Tetrapygus niger face aux étoiles de mer Heliaster helianthus et Meyenaster gelatinosus, dans les conditions naturelles. J'ai conduit des expériences dans le milieu naturel
le Nord du Chili. Dans le chapitre II, j'ai examiné les réponses de T. niger à des degrés variés de proximité avec ses deux prédateurs. Plus spécifiquement j'ai (1) évalué la capacité de l'oursin à différencier entre deux étoiles de mer prédatrices et une non prédatrice, (2) enregistré les réponses comportementales de l'oursin soumis à des attaques simulées de H. helianthus et M. gelatinosus, (3) déterminé la distance de réaction de l'oursin face à ses deux étoiles de mer prédatrices et (4) étudié dans quelle mesure la densité de congénères modifie les réponses des oursins envers leurs prédateurs, ce qui suggérerait l'utilisation de signaux d'alarme. Dans le chapitre III, j'ai regardé si la micro-distribution de T. niger sur les surfaces surélevées réduisait les risques d'attaque par les étoiles de mer prédatrices H. helianthus et M. gelatinosus. Spécifiquement j'ai (1) examiné l'association des oursins et des étoiles de mer sur les surfaces en hauteur et plus basses, (2) examiné les réponses des oursins aux étoiles de mer dans différentes situations et (3) réalisé des essais avec des oursins entravés pour comparer les risques de prédations sur les surfaces surélevées versus les surfaces plus basses.
CHAPITRE II
Responses of the black sea urchin Tetrapygus niger to its
Nous avons utilisé des expériences dans le milieu naturel pour étudier les réponses de l'oursin de mer noir Tetrapygus niger à la prédation des étoiles de mer. Des essais impliquant des attaques simulées (un ou plusieurs bras d'étoile de mer placés sur une moitié de l'oursin) ont montrés que les oursins étaient capables de différencier les étoiles de mer prédatrices Heliaster helianthus and Meyenaster gelatinosus des étoiles de mer non-prédatrices Stichaster striatus, et qu'ils ne montraient presque aucune réponse dans le cas d'une fausse étoile de mer. Les réponses des oursins à différents niveaux de menace représentés par les deux étoiles de mer prédatrices ont également été comparées. Le plus haut niveau de menace est l'attaque simulée, puis un simple contact, et enfin des étoiles de mer placées à différentes distances de l'oursin. Tous les oursins ont réagis aux attaques simulées et à la mise en contact avec les deux espèces d'étoiles de mer. La proportion de réponse diminue avec la distance et ce plus rapidement pour H. helianthus (0 % à une distance de 30 cm) que pour M. gelatinosus (33 % à une distance de 50 cm). A chaque niveau de menace présentant une réponse pour chacune des espèces prédatrices, les oursins ont répondus plus rapidement dans le cas de M. gelatinosus que dans celui de H. helianthus. Dans une troisième expérience, une étoile de mer prédatrice était ajoutée dans une aire circulaire (1-m de diamètre) dans laquelle 4 à 8 ou 11 à 19 oursins (non dérangés) étaient présents. Les oursins fuyaient la zone plus rapidement dans le cas de M. gelatinosus, mais le taux de fuite ne variait pas avec la densité (comme ce serait le cas s'il y avait communication entre les oursins par des signaux d'alarme). Nos observations suggèrent que M. gelatinosus représente une menace de prédation plus importante que H. helianthus. Ceci est en accord avec des observations faites dans le milieu naturel montrant que les oursins sont consommés plus fréquemment par M. gelatinosus. Ces expériences en milieu naturel sont les premières démontrant la chimiodétection à distance chez un invertébré marin sous des conditions de courant bidirectionnel induit par les vagues.
ABSTRACT
We ran field experiments to examine the responses of the black sea urchin Tetrapygus niger to predatory sea stars. Trials involving simulated attacks (one or several arms of a sea star being placed on top of half the urchin) showed that the urchin differentiated between the predatory sea stars, Heliaster helianthus and Meyenaster gelatinosus, and a non-predatory sea star, Stichaster striatus, and showed almost no response to a sea star mimic. We further compared the responses ofthe urchin to different threat levels presented by the two predatory sea stars. The highest threat level was a simulated attack, then mere contact, and subsequently sea stars being placed at different distances from the urchin. All urchins responded to simulated attacks and contact with both sea stars. The proportion responding decreased with distance and more rapidly in trials with H. helianthus (0 % at a distance of 30 cm) than with M. gelatinosus (33 % at a distance of 50 cm). At each of the threat levels where there was a response to both sea stars, the urchins responded more rapidly to M. gelatinosus than to H. helianthus. In a third experiment where a predatory sea star was added to a circular area (1-m diameter) in which either 4-8 or
11-19 undisturbed urchins were present, the urchins fled the area more rapidly when the added sea star was M. gelatinosus, but the rate of fleeing did not vary with density, as might occur if there was communication among urchins using alarm signals. Our observations suggest that M. gelatinosus presents a stronger predatory threat than H. helianthus. This corresponds to field observations showing that the urchins are more frequently consumed by M. gelatinosus. These are the first field experiments demonstrating distance chemodetection by a marine invertebrate under back-and-forth water flow from wave activity.
INTRODUCTION
Prédation is an important ecological factor because of its effects on prey behaviour and survival, and ultimately community structure (Paine, 1980; Estes et al., 1998; Gaymer and Himmelman, 2008). Natural selection should lead predators to select prey that increase their fitness and lead prey to develop defenses that decrease the risk of being eaten (Kerfoot and Sih, 1987). The diverse behavioural responses to predators (Legault and Himmelman, 1993; Sih and Wooster, 1994; Rochette et al., 1998) can be grouped into two categories, avoidance and escape adaptations (Lima and Dill, 1990). Avoidance adaptations act to reduce encounters with predators, for example cryptic and aposematic coloration, protective armor and chemical defenses (Cronin and Hay, 1996; Terlau et al., 1996; Gaymer et al., 2001a; 2001b). Escape adaptations minimize the likelihood of death when a predator is encountered, for example flight responses upon contact with predators and morphologies that reduce the predator's handling efficiency (Jensen, 1966; Vadas, 1977; Bernstein et al., 1983; Miller and Byrne, 2000; Furrow et al., 2003; McClintock et al., 2008b).
Chemodetection is often involved in interactions among marine animals. For example, numerous studies show that sea stars can detect substances exuded by potential prey (McClintock et al., 1984; Rochette et al., 1994; Dale, 1997) and that prey can detect the odours from predators (Phillips, 1978; Vadas et al., 1986; Alexander and Covich, 1991; Covich et al., 1994; Svensen and Kiorboe, 2000; Markowska and Kidawa, 2007). Chemical signals from predatory sea stars often trigger particular defensive responses (Kats and Dill, 1998; Nishizaki and Ackerman, 2005) and in some systems chemicals released by prey being attacked alert conspecifics to the danger (Smith, 1992; Chivers and Smith, 1998). For example, numerous asteroids, ophiuroids and echinoids exhibit defensive behaviours when exposed to chemicals from predators or injured conspecifics (Snyder and Snyder, 1970; Dayton, 1975; Parker and Shulman, 1986; Vadas et al.,
1986; Scheibling and Hamm, 1991; Legault and Himmelman, 1993; Rodriguez and Ojeda, 1998; Rosenberg and Selander, 2000). A few studies also demonstrate alarm signaling among prey species in response to an attack by a common predator species (Brown and Godin, 1997). All species within a prey guild should benefit from such alarm signals (thus have a reduced risk of prédation), independent of the species that produces the signal (Crawl and Covich, 1990; Alexander and Covich, 1991; Covich et al., 1994; McClintock et al., 2008a).
A number of studies report that escape responses by sea urchins can reduce prédation risk (Snyder and Snyder, 1970; Bernstein et al., 1981; Bernstein et al., 1983; Parker and Shulman,
1986; Vadas et al., 1986; Scheibling and Hamm, 1991; Hagen and Mann, 1994). Several studies suggest that sea urchins form aggregations that decrease the vulnerability to predators (Garnick, 1978; Mann, 1982; Bernstein et al., 1983). However, this hypothesis is contested because the aggregative behaviour may be related to the patchy distribution of food (Vadas et al., 1986; Himmelman and Nedelec, 1990; Scheibling and Hamm, 1991; Hagen and Mann, 1994; Rodriguez and Ojeda, 1998; Vadas and Elner, 2003), bottom topography (Laur et al., 1986), or juveniles seeking protection under adults (Nishizaki and Ackerman, 2005). Most experiments examining contact or distance chemodetection in predator-prey interactions among marine organisms have been ran in the laboratory (Lima and Dill, 1990; Chivers and Smith, 1998; Drolet and Himmelman, 2004; Jackson and Kiorboe, 2004; Thompson et al., 2005; McClintock et al., 2008a; Himmelman et al., 2009). Also, the studies examining responses of sea urchins to predatory risk have been mainly conducted under still water conditions (Jensen, 1966; Bernstein et al., 1981; Tegner and Levin, 1983; Mann et al., 1984; Scheibling and Hamm, 1991; Legault and Himmelman, 1993; Hagen and Mann, 1994; Rodriguez and Ojeda, 1998; Matassa, 2010) or unidirectional flow (Phillips, 1978; Moitoza and Phillips, 1979; Campbell et al., 2001; Hagen et al., 2002; Nishizaki and Ackerman, 2005). Most of the studies made in the field were executed under unidirectional flow or calm conditions (Snyder and Snyder, 1970; Rosenthal and Chess, 1972; Dayton et al., 1977; Bernstein et al., 1983; Parker and Shulman, 1986; Vadas et al., 1986; Andrew and Macdiarmid, 1991; Vadas and Elner, 2003). An ability to identify the risk associated with different predatory sea stars has been shown for a variety of animals, including gastropods (Feder, 1963, 1972; Phillips, 1977; Fishlyn and Phillips, 1980; Rochette et al., 1998; Mahon et al., 2002; Markowska and Kidawa, 2007), cnidarians (Weightman and Arsenault, 2002) and sea stars that are prey (McClintock et al., 2008b). These species usually flee when there is contact with the predatory sea star or detection from a distance. One study executed in northern Chile reports escape responses of intertidal limpets resulting from contact with sea stars under wave conditions (Espoz and Castilla, 2000). No studies have examined distance chemodetection under wave conditions in the field, although one laboratory study (Gagnon et al., 2003) shows that the sea star Asterias vulgaris detects and moves towards food (mussels) in a wave tank.
Wave surge is a consistent feature of most habitats of the sea urchin Tetrapygus niger in central and northern Chile. This sea urchin is the most conspicuous benthic grazer in Chile (ranging from 2 to 85 ind. m"2; Rodriguez, 2003) and its intensive grazing causes the formation of
extensive urchin barrens (Vasquez and Buschmann, 1997). The two predators most frequently observed feeding on T. niger are the sea stars Meyenaster gelatinosus and Heliaster helianthus (Barrios et al., 2008; Gaymer and Himmelman, 2008). These sea stars have been described as keystone predators in shallow rocky communities in northern Chile (Gaymer and Himmelman, 2008; Barahona and Navarrete, 2010). The multi-armed sea star H. helianthus is a generalist predator that consumes prey according to availability (Gaymer and Himmelman, 2008; Barahona and Navarrete, 2010), whereas M. gelatinosus is a highly selective predator that prefers the urchin T niger and the sea star H. helianthus (Gaymer and Himmelman, 2008). Dayton et al. (1977) examined responses of a variety of invertebrates to M. gelatinosus in tide pools, but focused on the responses ofthe commercially-harvested urchin Loxechinus albus. They mention that T. niger could detect M. gelatinosus at a distance (50 to 130 cm) and responded within seconds with a fleeing response. Laboratory studies by Rodriguez and Ojeda (1998) showed that the addition of M. gelatinosus or the predatory fish Pinguipes chilensis to tanks caused T. niger to move faster, but not to aggregate. The proportion of urchins showing increased movement was higher in the trials with M. gelatinosus (92 %) than in those with Pinguipes chilensis (67 %) (Rodriguez and Ojeda, 1998).
The present study examines the responses of the black sea urchin T niger to varying degrees of proximity with the predatory sea stars M. gelatinosus and H helianthus, representing different levels of predatory risk. All trials were made under field conditions involving continuous back-and-forth wave movement. Specifically, we (1) examined the ability of the urchin to discriminate different sea stars, two predators and a non predator, (2) evaluated the behavioural responses of the urchin to simulated attacks by, and to contact with, the two predatory sea stars, (3) determined the distance over which the urchin reacts to the two predatory sea stars and (4) investigated whether the density of conspecifics modifies the responses of the urchin to its predators, which would suggest the use of alarm responses.
11
METHODS
Our study was conducted during June through August in 2008 and 2009 in the shallow subtidal zones at Cisnes (27°14'50"S, 70°57'34"W) and La Herradura (29°28'r'S, 71°21'18"W) Bays in northern Chile. All manipulations were made using SCUBA diving in wave-exposed environments between 2 and 10 m in depth. The two sites were moderately sloped bottoms (down to -10 m depth and at -30 m from shore) that supported rocky barrens communities dominated by the sea urchin Tetrapygus niger. At both sites the sea stars Heliaster helianthus and Meyenaster gelatinosus were present at densities of <3 m2. During our trials,
water temperature ranged from 12 to 14 °C In all experiments, divers collected sea stars randomly from among individuals that were stationary and not feeding. In each trial different sea stars, urchins and urchins aggregations were used.
Responses to different sea stars
Our first experiment was ran on a relatively smooth horizontal platform (bedrock with few surface irregularities) at La Herradura Bay in 2008 and examined the ability of the urchin to differentiate among different sea stars representing varying degrees of predatory risk. For this, we recorded the responses of isolated urchins (5-6 cm in diameter) to simulated attacks by H. helianthus (9-14 cm in radius) and M. gelatinosus (13-21 cm), as well as by the sea star Stichaster striatus (8-11 cm), which does not prey on urchins (Viviani, 1978), and by sea star mimics (5 balloons filled with sand and covered with synthetic leather, -10 cm in radius). For each simulated attack, the sea star (one arm in attacks with M. gelatinosus, S. striatus and the mimic, and several arms in attacks with H. helianthus) was placed on top of half the target urchin (the target urchin was separated from other urchins at least 20 cm) and held in this position for up to 120 s. The sea star was maintained in the initial position, even as the urchin moved away. Preliminary trials indicated that if there were a response it would occur within 120 s. For each attack, we recorded if and when the urchin (1) raised its spines, (2) started displacing, and (3) severed contact with the sea star. In this way, 20-26 simulated attacks were made for each of the three sea stars and the mimic. The proportion of urchins responding and reaction times for each ofthe three parameters were analyzed separately. The same body-size range o i H helianthus, M. gelatinosus and T. niger was used in subsequent experiments.
Responses relative to the level of risk of predatory sea stars
We evaluated the response time of undisturbed urchins (time until the urchin displaced itself) subjected to situations representing different levels of predatory risk by H. helianthus and M. gelatinosus. The highest level of risk was to simulated attacks (as described above) and the next level was to mere contact with the tip of one arm of the sea star (we touched spines on the sides of the urchin). The subsequent risk levels involved placing sea stars at different distances from the urchin. The first distance was 10 cm and then distances were increased by 10-cm intervals until the urchins no longer responded, or up to a maximum of 50 cm. In each trial the sea star was placed at the desired distance along the axis of the wave activity and held there until the urchin responded or for a maximum of 120 s. For each sea star we made 40 trials with simulated attacks, 14-15 trials with mere contact, and 14-15 times for each ofthe distances (new sea stars and urchins were used in every trial at each threat level, including at each distance). These experiments were conducted on a relatively smooth horizontal bedrock platform at Cisnes Bay in August 2009.
Density effects on the sea urchin's response
We conducted experiments in 2008 at Cisnes Bay aimed at determining if the density of conspecifics affects the urchin's behavioural responses to the two predatory sea stars. We recorded the time to respond to a sea star being placed nearby for urchins at low or high densities. In each trial, we first placed a 1-m diameter circular hoop (made with 5-mm thick wire) around urchins found on a relatively flat rocky platform (we chose areas where there were no urchins in the center of the hoop) and removed urchins when necessary so that there were either 4-8 (low density) or 11-19 (high density) urchins within the area defined by the hoop. Then we placed a sea star, collected haphazardly from the surrounding area, in the empty space at the center of the hoop (the sea star was always at least 5 cm from the urchins) and recorded the departure times for urchins leaving the area defined by the hoop during a 6-min period. The urchins readily climbed over the wire hoop. The sea star was maintained at the center of the hoop during each trial. This procedure was repeated 5 times with M. gelatinosus and H. helianthus, and 8 times without sea stars (control trials), at both low and high densities of T. niger (each trial was on a new experimental area). An increased response at a higher density would imply that the urchins were responding to some sort of alarm signal.
13
Statistical analyses
For the experiments evaluating the ability of the urchin to differentiate between different sea stars representing varying degrees of predatory risk, we quantified the proportion of urchins showing a response to the sea stars and, response times, for the three variables, (1) raising the spines, (2) active displacement and (3) severing contact. A £2 test was used to test if the
proportion of urchins responding to the different sea stars and the mimic varied. When the global test was significant we followed with pairwise comparisons using the same test. A one-way ANOVA was performed to compare the mean time of urchins responding to the different sea stars and the mimic and followed with pairwise comparisons using protected Fisher least square difference tests (LSD). These two analyses were performed separately for each variable (spines raising, displacement and severing contact). Data were log transformed when necessary to meet the assumptions of normality and homogeneity of variance. Normality was tested using the Shapiro-Wilk's test (SAS, 2008) and homogeneity of variances using the Levene test (Snedecor and Cochran, 1989).
A generalized linear model (using the GENMOD procedure with the binomial distribution and the logit link was used in SAS, 2008) was then used to compare the proportion of urchins responding to different levels of predatory risk represented by the two predatory sea stars. In this model there were two fixed factors, the sea star and the level of predatory risk. This analysis was only applied to the data for distances of 20 and 30 cm because the urchin responses to the two sea stars were the same (100%) in simulated attacks, with mere contact and at 10 cm and the trials for distances of 40 and 50 cm we performed only with M. gelatinosus. A two-way ANOVA, with the same fixed factors, was used to compare the urchin response time to situations representing different levels of predatory risk. The MIXED procedure (SAS, 2008) was used to model the heterogeneity observed in each combination of the factors. In this case, no transformation was necessary to meet the normality assumption. To test if the mean response time of urchins varied in the trials with different levels of predatory risk from M. gelatinosus, a one-way ANOVA was performed. The fixed factor was predatory risk. The MIXED procedure was also used because of the heterogeneity observed in each combination of predatory risk test (SAS, 2008). The log-transformation was used to meet the normality assumption.
To determine if the density of conspecifics modified the responses of the urchin to M. gelatinosus and H. helianthus, we first compared the proportion of urchins leaving the circular
area using a generalized linear model (GENMOD procedure as described above). The two fixed factors were the sea star species and urchin density. Then, a conditional analysis was applied to the time when the urchins left the area defined by the hoop. Trials in which no urchins left the hoop were not included in the analysis. A two-way ANOVA was used to compare the effect of urchin density and the sea star species. No transformation was necessary.
RESULTS Responses to different sea stars
The simulated attacks with three species of sea stars and the mimic sea star showed that the sea urchin Tetrapygus niger differentiates between predatory and non predatory sea stars (Fig. 2.1). The urchin's first response to Heliaster helianthus and Meyenaster gelatinosus was raising the spines, which occurred after about 3 s of exposure. Displacement followed after about 7 s and the displacement resulted in severing of contact after about 28 s. The urchins used their spines and podia to move away from the sea stars. There were no differences in the numbers of urchins responding and response times for the trials with the two sea stars (Fig. 2.1; /*>0.05). The time until contact was severed differed significantly (j°=0.038), although the mean time differed by only 21%. In contrast, few urchins responded to the sea star mimic and reaction times were much greater, as only six urchins (30 %) showed displacement within the 120-s observation period and only one of these (5 %) raised its spines and severed contact with the mimic. Responses to the non-predatory sea star S. striatus were intermediate. Although 88 % of the urchins raised their spines and displaced themselves, and 48 % severed contact with S. striatus, the reaction times were almost as slow as in the trials with the mimics (Fig. 2.1).
In 2009, we also made trials to record the time required for the urchin to extend its podia. The responses did not vary between the two predatory sea stars, either for the number of urchins responding (all responded) or the response time (P=0.84). Podia extension took place in about 8.7 s in response to attacks by H. helianthus and in 8.5 s in attacks by M. gelatinosus, thus at almost the same time as when displacement began in the trials with simulated attacks made in 2008.
15 100 "5 80 -60 c o a a c o c o a 8 20 -a. 40 0 3 5 3 B %& 100 80
i
ol
o a. </> o cr 60 40 20 n=26 n H. helianthus n = 26 m M. g e l a t i n o s u s " = 25 os.striatus n = 20 EiMimic'■
m
m
m
I
i
I
i
* m
Raising spines Displacement Severing contact
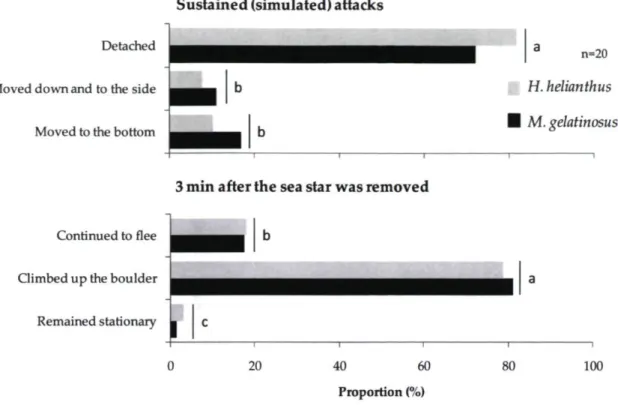
Figure 2.1. Proportion (%) of sea urchins (Tetrapygus niger) responding within 120 s and response time for three variables (spines raising, displacement and severing contact) used to evaluate the urchin's response to simulated attacks by the sea stars Heliaster helianthus, Meyenaster gelatinosus, Stichaster striatus and a mimic sea star. Values are means ± SE. Each variable was analyzed separately; bars not sharing the same letter are different (P<0.05).
Responses relative to the level of risk of predatory sea stars
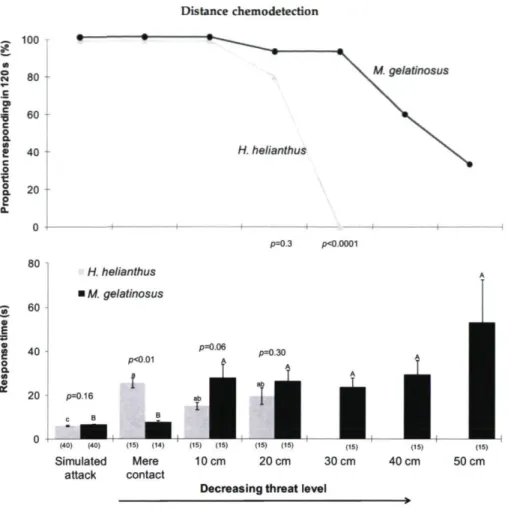
All urchins moved away in the trials representing to the first three levels of risk (simulated attacks, mere contact and a distance of 10 cm) ofthe two predatory sea stars (Fig. 2.2). Then, with further increases in distance (and decreased risk) the percent displacing declined. The decrease was more rapid in the trials with H. helianthus than with M. gelatinosus, for example, no urchins responded when H. helianthus was placed at 30 cm from the urchin, whereas 33.3 % ofthe urchins still responded in the trials in which M. gelatinosus was placed at 50 cm. To further define the range of detection of H. helianthus, we performed additional trials at 25 cm and found that 29 % ofthe urchins (6/21) detected the sea star (mean response time 43.3 s, SE±13.0).
In the simulated attacks, there was no difference in response time in the trials with the two predatory sea stars (P=0.16). In contrast, with mere contact the response time was notably more delayed in the trials with H. helianthus than with M. gelatinosus (P<0.01). At distances of 10 and 20 cm response times to the two sea stars were similar (P>0.05). The response time in trials with M. gelatinosus was notably higher at 50 cm than at closer distances, but the increase was not significant (P>0.05).
17 80 ^ 60 «u 8 o a «/> 40 20 Distance chemodetection — 100 • • > ^ ^ ^ ^ JS g 80 S. M. gelatinosus «^ e a | 60 c o a. S 40 H. helianthus c o t â 20 S a n i 1 — 1 1 — ■ 1 1 p=0.3 pO.0001 H. helianthus i M. gelatinosus p O . 0 1 î p=0.06 p=0 30 p=0.16 c B
II I
(40) (40) (15) (14) (15) (15) (15) (15) ( 1 5 ) ( 1 5 ) ( 1 5 ) Simulated Mere 10 cm 20 cm 30 cm 40 cm 50 cm attack contactDecreasing threat level
Figure 2.2. Proportion (%) of sea urchins (Tetrapygus niger) responding within 120 s and response time in trials in which the urchin was exposed to the sea stars Heliaster helianthus and Meyenaster gelatinosus at different threat levels: simulated attacks, mere contact (with the tip of one arm ofthe sea star) and distances (10-50 cm) from the urchin. Values are means ± SE. The P values give the probability that the urchin responded in the same way to the two sea stars. For each sea star, bars not sharing the same letters are different (PO.05). The number of urchins used in each treatment is indicated in parenthesis.
Density effects on the sea urchin's response
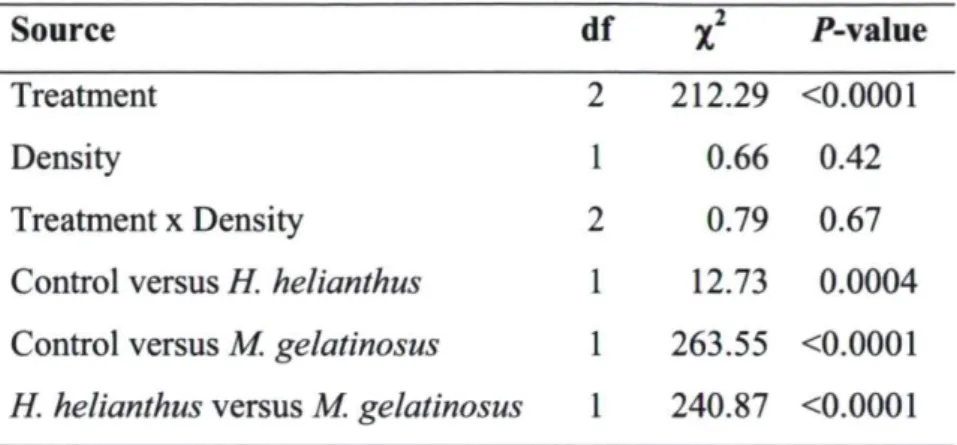
The final experiment in which we measured the fleeing of urchins from circular areas where we added a predatory sea star (H. helianthus or M. gelatinosus) or nothing (control), showed no effect of density but an effect ofthe treatments (Table 1). In fact, in the trials with each sea star, the percentage of urchins leaving the hoop and departure times were similar for the two densities of urchins (Table 1; Fig. 2.3). The rate of fleeing was much greater in the trials with M. gelatinosus than with H. helianthus. For example, >95 % of the urchins left the hoop within 4 min in the trials with M. gelatinosus, compared to <40 % at 6 min in the trials with H. helianthus. In the trials with H. helianthus, the urchin often only moved a short distance and not enough to leave the hoop; and very few urchins left the hoop near the end of the trials (between 4 and 6 min). In contrast, in the trials with M. gelatinosus almost all urchins (99%) moved outside the hoop within 6 min. In the control trials (where nothing was added) most urchins remained stationary or only moved slightly. Only -10 % ofthe urchins left the hoop in 6 min and some urchins from outside moved into the area defined by the hoop (Fig. 2.3).
19
100
Time (min)
Figure 2.3. Cumulative proportion of sea urchins (Tetrapygus niger) leaving a circular area at different time intervals after a sea star, Heliaster helianthus or Meyenaster gelatinosus, was added to the center area. The initial number of urchins in the area was 4-8 (low density) or 11-19 (high density). Values are means ± SE. At the end of the trials (at 6 min), the three treatments differed (PO.05).
Table 1. Logistic regressions for the proportion of sea urchins (Tetrapygus niger) leaving the circular areas defined by a 1 -m diameter hoop (GENMOD procedure; SAS, 2008). The variables were density (low and high) and the three treatments (the sea stars, Heliaster helianthus and Meyenaster gelatinosus and the control, without a sea star). Statistical differences in proportions were identified using Pearson x2 tests. Means were compared using LSD tests.
Source df
x
2 P-valueTreatment 2 212.29 O.0001
Density 1 0.66 0.42
Treatment x Density 2 0.79 0.67
Control versus H helianthus 1 12.73 0.0004 Control versus M. gelatinosus 1 263.55 O.0001 H helianthus versus M. gelatinosus 1 240.87 O.0001
21
DISCUSSION
Our study is the first to examine responses of the black sea urchin Tetrapygus niger to predators under field conditions. Given that the back-and-forth flow from waves is a prevalent feature of most habitats where T. niger is found along the coast of northern Chile, wave action should be considered in evaluating the urchin's responses to predators. Our field data demonstrate that T. niger can (1) differentiate between predatory and non-predatory sea stars, (2) distinguish between different threat levels presented by predatory sea stars, and (3) detect predatory sea stars at a distance.
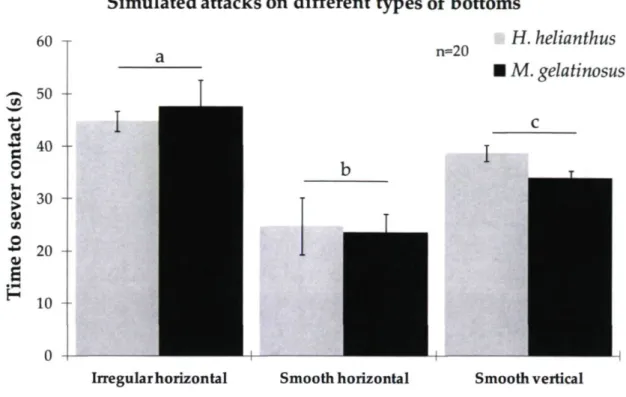
The behavioural responses of T. niger to simulated attacks by predatory sea stars consisted of raising the spines (3 s), followed by displacement and elongation of podia (both at about 8 s). The displacement led to severing contact with the predator (in about 30 s). We have also observed this sequence when sea stars naturally come into contact with sea urchins in the field, though we did not quantify reaction times. This sequence and similar reaction times have been reported for the sea urchin Strongylocentrotus droebachiensis in response to contact with its predators, the sea stars Leptasterias polaris and Crossaster papposus (Legault and Himmelman, 1993). The first response for that urchin was bending of spines away from the area of contact (occurring almost immediately as for T. niger), then elongation of podia and displacement (about 10 s later). They do not report the time when contact with the sea star was severed. In the field T. niger is likely to escape predatory sea stars unless its movement is blocked by other urchins or by bottom irregularities (Dayton et al., 1977). T. niger can more quickly sever contact with H. helianthus and M. gelatinosus on smooth horizontal platform than on irregular horizontal bottom (Chapter II). Any inability to flee generally allows the sea star to wrap its arms around the urchin and begin digesting it externally (JDU, observations).
Our trials using simulated attacks demonstrate that T. niger differentiates between predatory and non-predatory sea stars. The response was strongest and similar for the two predatory sea stars. We recorded a 100 % response for the three variables evaluated (spine raising, displacement and severing of contact) in the trials with both H. helianthus and M. gelatinosus. Further, the reaction time of the urchins was rapid and similar for the two sea star predators, but the urchins tended to sever contact more rapidly with H. helianthus than with M. gelatinosus. Only a few urchins responded to the mimic sea star, and the reaction time for those that did respond was very slow. Most of the urchins remained in contact with the mimic. In the
trials with the non-predatory sea star, S. striatus, although a large proportion of urchins (88 %) raised their spines and displacement, only half (48 %) severed contact. Also, the response time to respond for all three variables was long, and indeed similar to those with a mimic. The ability of animals to differentiate between predators and non-predatory species has been demonstrated in many species (Sih et al., 1985; Chivers and Smith, 1998), including several species of urchins (Snyder and Snyder, 1970; Parker and Shulman, 1986; Hagen et al., 2002). The high proportion of urchins raising their spines and displacing in response to attacks by S. striatus is possibly because S. striatus, being an asteroid echinoderm, shares morphological and some chemical characteristics with H helianthus and M. gelatinosus. However, the low proportion severing contact suggests that S. striatus represents a low risk of prédation. Other studies also report no response of urchins to mimic sea stars (Legault and Himmelman, 1993; Gaymer et al., 2002). In northern Chile, the responses of the limpet Lottia orbignyi to its predators parallel those of the urchins. It vigorously reacts to the sea star H helianthus, a known limpet consumer, but shows almost no reaction to S. striatus, which has never been reported to prey on limpets (Espoz and Castilla, 2000). M. gelatinosus is also a predator of H. helianthus, but field trials involving simulated attacks showed that H. helianthus takes 134 s to sever contact with M. gelatinosus (Gaymer and Himmelman, 2008). This was four times longer than the average time T. niger took to sever contact with M. gelatinosus (31 s). The slower response may be because H. helianthus can avoid total prédation by autotomizing arms, and thus can take higher risks in interactions with M. gelatinosus, or alternatively because H. helianthus is a slower moving animal.
Further understanding of the responses of T. niger to its predators was provided by our field trials involving situations with difference levels of prédation risk. All urchins tested responded to the higher risk levels (attack and contact). Although the reaction time to simulated attacks was similar for the two sea stars, the reaction time to contact was much shorter in the trials with M. gelatinosus than those with H. helianthus. The high reaction time to simulated attacks by these two sea stars suggests that the urchin perceives such a high risk, irrespective of the predator, that it responds with the maximal response. The maintenance of a short reaction time in the trials with mere contact to M. gelatinosus suggests this species is consistently perceived as a high risk. In contrast, the slower response to mere contact with H. helianthus, compared to the response time in the simulated attacks, suggests that just touching this predator is perceived as a lower risk. The capacity of T. niger to distinguish between H. helianthus and M.
23
gelatinosus could decrease the cost of disrupted foraging activity and metabolic alterations resulting from defense and escape responses.
The experiments where predators were placed at different distances provided further evidence of a stronger response to M. gelatinosus than to H. helianthus. In the trials with H. helianthus the percentage of urchins responding began to drop at a distance of 20 cm and was null at 30 cm (additional trials made at 25 cm, not shown in Fig. 2.2, show a 29 % response at this distance). In contrast, the major drop in the percentage of urchins responding to M. gelatinosus began at 40 cm, and 33 % of the urchins still responded at 50 cm, the furthest distance studied. Trials were not conducted at greater distances with M. gelatinosus because it was rare to find urchins separated from other urchins by distances of >50 cm. The different perception of the urchin to sea-star threats is also indicated by observations of the feeding of the two sea stars in the field. M. gelatinosus is a highly selective predator that strongly chooses echinoderm prey, such as T. niger and H. helianthus, whereas H. helianthus is a generalist feeder that consumes a wide variety of prey according to availability (Gaymer and Himmelman, 2008; Barahona and Navarrete, 2010). As a means of limiting energy expenditure, H. helianthus probably feeds on animals that are easier to attack than T. niger, as for example, the mussel Semimytilus algosus or the abundant gastropod Turritella cingulata (Gaymer and Himmelman, 2008). The weaker responses of T. niger to H. helianthus compared to M. gelatinosus could be one reason why urchins are often found in close proximity to H. helianthus but not to M. gelatinosus (JDU, observations). A closer association with H. helianthus than with M. gelatinosus was also indicated by the analysis of survey data involving sampling of 1-m2 quadrats
at five locations (Gaymer, unpubl. data). There was a significant positive correlation between the density ofthe urchin and that oiH. helianthus but not with M. gelatinosus.
Numerous previous studies have documented the ability of urchins, and other prey species, to detect predators at a distance, and some studies using extracts from predatory sea stars suggest urchins have the capacity to detect these chemicals (e.g., saponins; Phillips, 1978; Garneau et al., 1989). Our observations are useful because we evaluated the urchin's responses to intact sea stars in the field. In natural environments, wave action and associated turbulence would be expected to reduce the effectiveness of distance chemodetection (Jackson and Kiorboe, 2004). The ability of T. niger to detect and distinguish between predators, even at a distance and under wave surge, should permit it to decrease the probability of encounters. This may lead to changes
in its distribution and association with other species (e.g., T. niger appears to aggregate on elevated surfaces to escape from sea stars; Chapter III).
We showed that urchins in groups disperse when H. helianthus or M. gelatinosus is placed nearby. The movement away from the predator was considerably more rapid in the trials with M. gelatinosus than in those with H. helianthus. However, the rate of dispersion did not vary with urchin density. During these experiments, we noted that the urchins closest to the sea star reacted faster than urchins at greater distances, although this was not quantified. Probably odours from the sea star were strongest for the closest urchins.
Our field experiments support the laboratory observations by Rodriguez and Ojeda (1998) indicating that T. niger is able to recognize the presence of M. gelatinosus at a distance. They observed that urchins in 7-individual aggregations (large individuals as in our trials) reacted by moving away from the sea star; 19 % ofthe urchins tested remained in the vicinity 5 min after the introduction of M gelatinosus in the middle of a 90 x 100 cm tank (with a continuous inflow of sea water). In contrast, in our field study <3 % ofthe urchins in the 1-m diameter hoops remained in the hoop 5 min after the addition of M gelatinosus. Thus, although the experimental area, and the density and size of urchins were similar in our study and that by Rodriguez and Ojeda (1998), the urchins fled more rapidly in our study. Presumably the slower flight by the urchins in their study was due to the artificial conditions in the laboratory.
The grazing by the high densities of the sea urchin T. niger in Chile has strong impacts on benthic communities and can cause extinction of kelp beds in localized areas (Vega et al., 2005). Since kelp beds are important larval recruitment areas and nursery grounds for a number of commercial invertebrates and vertebrates, understanding the factors that regulate urchin populations is crucial. Several factors favor the high numbers of T. niger. 1) this urchin is not harvested commercially, 2) it may have several reproductive events per year (Rodriguez and Ojeda, 1993), 3) it has a broad diet (e.g., it feeds on kelp, algal turfs, crastose coralline algae and drifting algae; Contreras and Castilla, 1987; Rodriguez, 2003) and 4) its main predators (the predatory sea stars H. helianthus and M. gelatinosus), although abundant, only slightly reduce its densities (Gaymer and Himmelman, 2008). The escape responses of T. niger to sea stars, as observed during our study, probably further contribute to its high densities. Further studies are needed to extend our knowledge about the urchin's interaction with its predators, such as tethering experiments and studies using video filming.
25
Predator-prey interactions usually involve co-evolution between the prey and predator: the prey develops mechanisms to improve escaping from predators and the predator develops mechanisms to facilitate the capture of prey. Distance detection of predators by prey, as we show for T. niger, should allow prey to adjust escape behaviours to predatory risk so that wasteful expenditure of energy is limited. This should improve prey survival and fitness.
CHAPITRE III
Does the distribution of sea urchins Tetrapygus niger on elevated surfaces represent a strategy for avoiding prédation by sea stars?
27
RÉSUMÉ
Nous avons utilisé des expériences dans le milieu naturel pour vérifier si la micro-distribution des oursins de mer Tetrapygus niger sur les surfaces surélevées représente une stratégie permettant de limiter la prédation par les étoiles de mer Heliaster helianthus and Meyenaster gelatinosus. Plusieurs évidences soutiennent cette hypothèse. (1) Un examen de la distribution des oursins et des deux espèces d'étoiles de mer montre que les oursins sont majoritairement situés sur les surfaces surélevées, et les étoiles de mer sur les surfaces plus proches du fond. (2) Lors d'essais impliquant des attaques simulées, le temps nécessaire aux oursins pour briser le contact avec les étoiles de mer était deux fois plus court pour les oursins situés sur les surfaces surélevées que pour ceux situés sur le fond. (3) Lors d'essais impliquant des attaques simulées soutenues (le plus haut niveau de risque de prédation) les oursins pouvaient se détacher pour éviter d'être mangés. Enfin, des expériences au cours desquelles les oursins étaient entravés, indiquent que ceux-ci ont un plus haut taux de survie lorsque situés sur les surfaces surélevées que quand ils sont sur les surfaces plus basses. Nos observations indiquent que M. gelatinosus représente une menace plus forte pour T. niger que H. helianthus.
ABSTRACT
We ran field experiments to examine if the micro-distribution ofthe sea urchin Tetrapygus niger on elevated surfaces represent a strategy for limiting prédation by the sea stars Heliaster helianthus and Meyenaster gelatinosus. Several lines of evidence supported this hypothesis. (1) A survey of the distribution of the urchin and two sea stars showed that urchins occur manly on elevated surfaces, and sea stars on low surfaces. (2) In trials involving simulated attacks the time needed by the urchin to sever contact with the sea stars was 48 % less on elevated surfaces than on the bottom. (3) In trials involving sustained simulated attacks (high predatory risk) the urchins could detach themselves from the elevated surfaces to avoid being eaten. Finally, tethering experiments indicated that the urchin had a higher survival rate on elevated than low surfaces. Our observations confirm that M. gelatinosus represents a stronger predatory threat to T. niger than H. helianthus.
29
INTRODUCTION
Animals have a variety of anti-predatory strategies (Sih et al., 1985; Chivers and Smith, 1998; Ruxton et al., 2004; Caro, 2005) that can be divided in two main categories: (1) avoiding encounters with predators and (2) avoiding being eaten once there has been an encounter (Lima and Dill, 1990). For example, the aposematic coloration ofthe poison frog Dendrobates pumilio decreases the probability of encounters with predators (Saporito et al., 2006) and the autotomizing of arms by the sea star Heliaster helianthus when under attack by the sea star Meyenaster gelatinosus decreases the probability of death (Gaymer and Himmelman, 2008). Predators not only affect prey directly by eating them, but also indirectly by changing their behaviour. Bottom structures, such as crevices, can reduce the probability of encounters with predators and thus increase survival. For several species of sea urchins, the juveniles hide in crevices to reduce the probability of predatory attacks (Scheibling and Hamm, 1991; Rodriguez and Ojeda, 1993; Hereu et al., 2005). Shears and Babcock (2002) show that prédation on the sea urchin Evechinus chloroticus is most intense for juveniles that are beginning to leave crevices for open habitats.
Sea urchins are important in benthic communities because of their intensive grazing. Locations supporting high densities of urchins are often transformed into barrens with a reduced diversity and biomass of macroalgae (Himmelman et al., 1983; McClanahan and Shafir, 1990; Alcoverro and Mariani, 2002; Shears and Babcock, 2002). Predators can affect the density, behaviour and population structure of urchins (Tegner and Levin, 1983; Sala et al., 1998; Tuya et al., 2004; Guidetti, 2006). A variety of predators are known to feed on urchins, including sea otters (Estes et al., 1998), fishes (Sala, 1997), lobsters (Andrew and Macdiarmid, 1991), crabs (Scheibling and Hamm, 1991) and sea stars (Himmelman and Dutil, 1991). Relationships between sea stars and urchins are the best studied of these interactions (Jensen, 1966; Rosenthal and Chess, 1972; Dayton et al., 1977; Moitoza and Phillips, 1979; Legault and Himmelman,
1993; Rodriguez and Ojeda, 1998; Hagen et al., 2002).
The black sea urchin Tetrapygus niger is the most abundant urchin in central and northern Chile (Vasquez and Buschmann, 1997). Its grazing has converted many subtidal areas into barrens. It usually limits the depth distribution of the subtidal kelp Lessonia trabeculata to shallow water and has caused local extinctions of the subtidal kelp Macrocystis integrifolia (Vega et al., 2005). A number of predators are reported to consume T. niger, including the fishes
Semicossyphus maculatus (Fuentes, 1981), Graus nigra (Fuentes, 1982), Pinguipes chilensis (Rodriguez and Ojeda, 1998), Cheilodactylus variegatus, and Oplegnathus insignis (Medina et al., 2004) and the sea star Luidia magellanica (Gaymer and Himmelman, 2008). However, the predators that likely have the greatest impact on the abundance of T. niger are the sea stars H helianthus and M. gelatinosus (Barrios et al., 2008; Gaymer and Himmelman, 2008). These sea stars have been described as keystone predators in shallow rocky communities in northern Chile; (Gaymer and Himmelman, 2008; Barahona and Navarrete, 2010). H helianthus is a generalist feeder consuming prey according to their availability (Gaymer and Himmelman, 2008; Barahona and Navarrete, 2010), whereas M. gelatinosus is a selective feeder that prefers consuming the urchin T. niger (Gaymer and Himmelman, 2008). Both H. helianthus and M. gelatinosus are endemic along the west coast of South America and in northern Chile their average densities are 3 ind. m2 and <0.5 ind. m2, respectively (Dayton et al., 1977; Tokeshi et al., 1989; Gaymer and
Himmelman, 2008; Navarrete and Manzur, 2008). H. helianthus has a robust and flattened body with as many as 40 arms and can attain up to 32 cm in diameter (Tokeshi et al., 1989). In contrast, M. gelatinosus has a soft body with 6 thick arms and can attain 56 cm in diameter (Dayton et al., 1977; Gaymer and Himmelman, 2008).
Three studies have reported the behavioural responses of T. niger to its sea star predators. Dayton et al. (1977) mentioned that T. niger can detect M. gelatinosus at a distance and indicated that it responds rapidly by fleeing. Rodriguez and Ojeda (1998) examined the urchin's responses to M. gelatinosus and the fish Pinguipes chilensis in the laboratory and reported that the urchin increases its rate of displacement when these predators were nearby and that a higher proportion of individuals respond in trials with M. gelatinosus (92 %) than with P. chilensis (67 %). Finally, Urriago et al. (Chapter II) quantified the urchin responses to predatory sea stars, and showed that the urchin can differentiate between predatory (H. helianthus and M. gelatinosus) and non-predatory (Stichaster striatus) sea stars, can distinguish between different threat levels associated with the predatory sea stars and can detect predatory sea stars at a distance. They indicated that M. gelatinosus presents a stronger predatory threat to urchins than H. helianthus.
T niger frequently occur on elevated surfaces (e.g., boulder tops) within the barrens communities that predominate in shallow rocky subtidal areas along the coast of central and northern Chile. As food resources are less abundant on elevated surfaces than on the bottom, we reasoned that this micro-distribution of T. niger might limit attacks by the sea stars H. helianthus
31
and M. gelatinosus. The present study examines this hypothesis. We first documented the preference of the urchins for elevated surfaces, then examined the urchin's responses to sea stars in a variety of situations (predatory attacks), and finally conducted a tethering experiment to compare the survival on high and low surfaces (i.e. top and bottom of boulders).
METHODS
Our study was conducted during June, July and August in 2008 and 2009 in the subtidal zone at Obispito Bay (26°48'22"S, 70°47*05"W), Cisnes Bay (27°14'50"S, 70°57'34"W) and El Frances Bay (30°5'42"S, 71°22'47"W) in northern Chile. All manipulations were made using SCUBA diving at depths of 2 and 9 m in wave-exposed environments (i.e., with continuous back and forth water movement). At the three bays, the bottom was moderately sloped (down to -10 m depth at -30 m from shore) and supported a barrens community, with a scarcity of fleshy macroalgae. The sea urchin Tetrapygus niger was abundant and the sea stars Heliaster helianthus and Meyenaster gelatinosus were present in much lower numbers. During our trials, water temperatures ranged between 12 and 14 °C In the various experiments, the sea stars were taken at random from among individuals that were stationary and not feeding. Different urchins and sea stars were used in each trial.
Distribution of sea urchins and sea stars
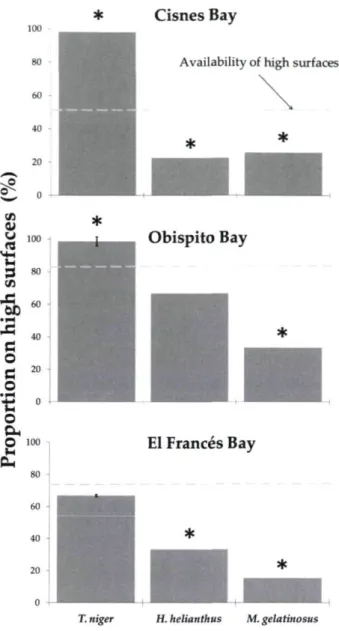
We made a field survey at Obispito, Cisnes and El Frances Bays to characterized the abundance and distribution of T. niger and the two predatory sea stars H helianthus and M. gelatinosus. For each urchin and each sea star encountered we recorded its position in two categories, high and low surfaces. We also quantified the percentage cover of high and low surfaces. High surfaces included from the tops of boulders and bedrock outcrops to half way down the vertical faces ofthe structures, and low surfaces were mainly flat areas of pebbles, shell debris and small cobbles but also included surfaces extending half way up the sides of boulders and outcrops. The boulders were 1.0-1.5 m in height and the outcrops 4-5 m. In 2008, we systematically surveyed an entire cove at Cisnes Bay (using five 50-m transects running from the shore seaward, and spaced at 6m intervals) whereas in 2009 we sampled 79 randomly placed 1 -m2 quadrats at Obispito Bay and 72 at El Frances Bay.
We made trials in which we placed predatory sea stars on boulder tops to determine whether they would remain there or move to lower positions. In each trial we placed a sea star on a boulder top not covered by sea urchins and held it there until it was attached (<1 min). Then, after 5 min we recorded its position. We ran 20 trials for both M. gelatinosus and H. helianthus at El Frances Bay in 2009.
Responses of sea urchins to sea stars on different types of bottom
We further performed a number of short-term field experiments at Cisnes Bay in 2009 to provide insights into the responses of the sea urchin to the two predatory sea stars, H. helianthus (9-14 cm in radius) and M. gelatinosus (13-21 cm), on three types of bottom. We first quantified the time it took isolated urchins (5-6 cm in diameter and at least 20 cm from other urchins) to sever contact from a simulated attack by a sea star. A simulated attack consisted of holding a sea star so that an arm, or several arms in the case of H. helianthus, covered half of the target urchin. The sea star was maintained in the initial position, even as the urchin moved away. In each trial on each type of bottom we first placed an urchin on the substratum and allowed it 4-5 min to attach (this was done because urchins were rarely found on irregular horizontal surfaces). The urchins always remained very close to where they were placed. Then we initiated the simulated attack. We executed 20 simulated attacks with both H. helianthus and M. gelatinosus (1) on irregular horizontal bottoms, (2) on relatively smooth horizontal platform (bedrock with few surface irregularities), and (3) on relatively smooth vertical walls (side of a bedrock outcrop or large boulder).
Sustained (simulated) attacks on vertical walls
We further ran experiments at Cisnes Bay in 2008 to examine the responses of undisturbed sea urchins to a sustained simulated attack (hereafter referred to as "sustained attack") by predatory sea stars on vertical walls. In each trial we first selected a target urchin at the lower edge of an aggregation at the top of a wall. The walls were 4-5 m in height and the distance between the target urchin and the aggregation at the top varied from 50 to 80 cm. We then initiated a sustained attack from below the urchin and advanced the sea star so that its arm (or several arms with H. helianthus) always covered half the urchin. Each trial was continued