Comparison of Feeding Inorganic Sulfate Trace Minerals to Partial
Substitution of Organic Trace Minerals on Gilt Development,
Production and Longevity and Progeny Growth Performance
Mémoire
JEAN-PHILIPPE MARTINEAU
Maîtrise en sciences animales Maître ès sciences (M. Sc.)
Québec, Canada
Comparison of Feeding Inorganic Sulfate Trace Minerals to Partial
Substitution of Organic Trace Minerals on Gilt Development,
Production and Longevity and Progeny Growth Performance
Mémoire
JEAN-PHILIPPE MARTINEAU
Sous la direction de :
iii
RÉSUMÉ
A trial was conducted to compare the effect of partial substitution of inorganic (INO) trace minerals (Cu, Zn and Mn) by an organic source (ORG) on performance and physiological status of sows over 2 parities, and their progeny up to the end of nursery phase. Substitution was at the level of 60%, 33% and 40% for Cu, Zn et Mn respectively. Sow reproductive performance, antioxidant enzyme concentration, immunity transfer, milk composition and piglet growth were some of the measurements taken to assess differences between trace mineral sources. Outcomes were very similar between trace mineral sources although ORG trace mineral sows had increased pre-weaning mortality and lower piglet birth weight, but decreased back-fat loss during lactation and increased feed intake of the 2nd parity
iv
ABSTRACT
A trial was conducted to compare the effect of partial substitution of INO trace minerals (Cu, Zn and Mn) by an ORG source on performance and physiological status of sows over 2 parities, and their progeny up to the end of nursery phase. The trace mineral substitution provided 10, 50 and 20 ppm of Cu-AA, Zn-AA and Mn-AA respectively. These levels represented a substitution of 60%, 33% and 40% of the respective INO minerals in barley-DDGS based diets. A total of 1976 gilts of similar age and weight were allotted to one of two dietary treatments and followed during development phase (35 to 100 kg) from which a representative sample of 360 gilts were selected at the end of gilt development phase to be followed through 2 parities during gestation and lactation as well as their progeny.
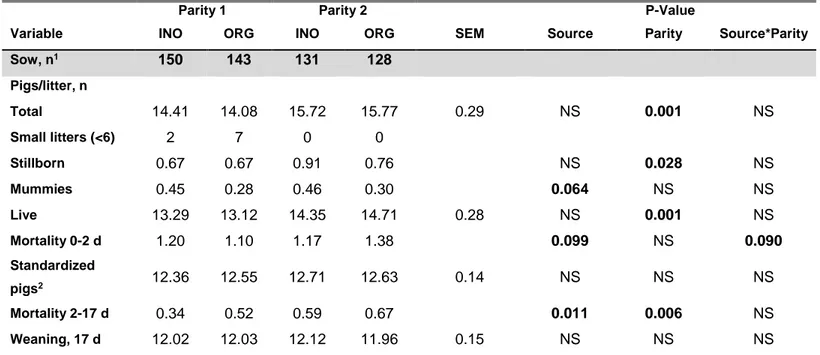
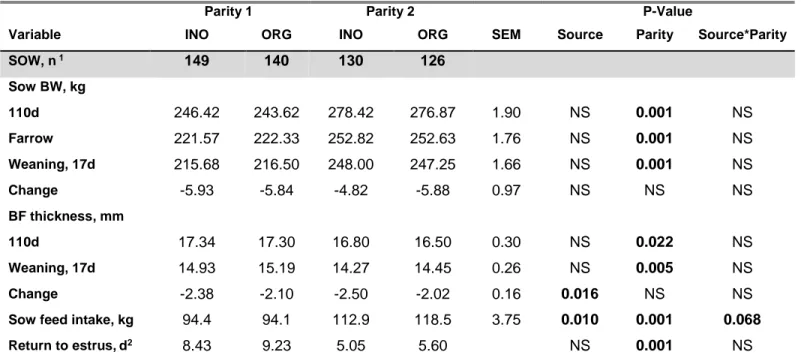
Average daily gain was improved for ORG trace mineral fed gilts during development phase (P<0.05). For the 2 first parities, sows from both treatments farrowed similar total and live piglets (P>0.10). During lactation, mummies tended to be reduced (P<0.10) in ORG trace minerals treated sows, but mortality tended to be increased during 0-2d (P<0.10) and also during 2-17d (P<0.05) associated with lower piglet birth weights (P<0.10). The number of weaned pigs was similar between treatments. Back-fat thickness loss during lactation was reduced for both parities (P<0.05) and feed intake tended to increase in parity 2 sows (P<0.10) using ORG trace mineral. Milk composition, plasma antioxidant enzymes, claw lesion or lameness in sows were similar between treatments. Although there was a difference in piglets’ birth weight, by the end of the nursery phase there was no carry over effect shown on weights (P>0.10).
Implications: These results suggest that partial substitution of trace minerals by an ORG source reduces back-fat thickness loss of sows during lactation, but during the 2 first parities the difference in performance of the sows and their progeny did not justify the use of ORG trace minerals.
v
TABLE OF CONTENT
Résumé ... iii
Abstract ... iv
Table of content ... v
List of tables ... vii
List of figures ... viii
Chapter 1 Introduction ... 1
2. Trace minerals in sow nutrition ... 6
2.1. Roles of trace minerals ... 6
2.1.1. Role of Zinc... 6
2.1.2. Role of Copper ... 9
2.1.3. Role of Manganese ... 10
2.2. Lameness/claw lesions in sows ... 11
2.3. Antioxidant Enzymes, copper, manganese and zinc ... 12
2.4. Inorganic/Organic Trace Mineral Terminologies ... 13
2.5. Mineral stability/Chelation Strength/Bioavailability ... 14
2.6. Effects of organic trace minerals on sow performance ... 18
2.7. Effect of organic trace mineral on lameness and claw lesion on sow performance ... 19
2.8. Effects of organic trace minerals on milk composition, yield and immune transfer ... 20
2.9. Hypothesis and objectives ... 23
2.10.REFERENCES ... 24
CHAPTER 3 ... 35
3. INTRODUCTION ... 37
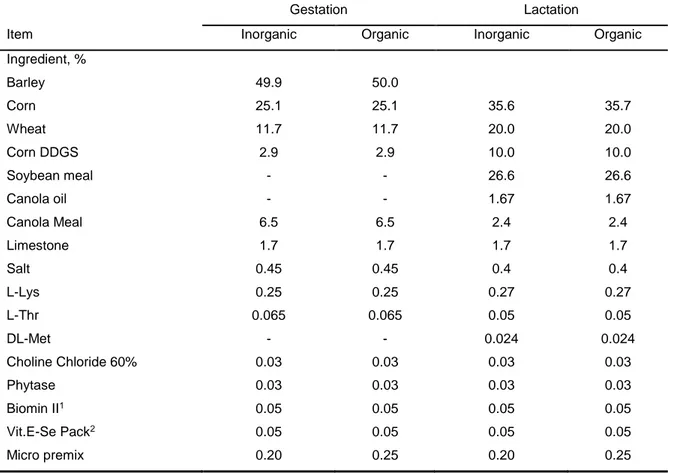
3.1. MATERIAL AND METHODS ... 38
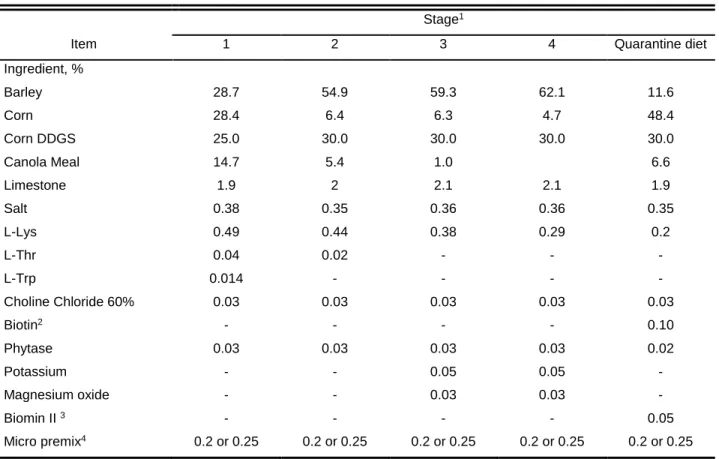
3.1.1. Experimental Diets ... 40
3.1.2. Animal management and experimental measurements ... 42
3.1.2.1.Gilt Development ... 42
3.1.3. Quarantine barn ... 44
3.1.4. Sow barn ... 45
3.1.4.1.Litters and sow management: ... 47
3.1.4.2.Blood handling and biochemical analysis ... 48
vi
3.1.6. Culling requirements ... 51
3.1.7. Statistical Analysis ... 52
3.2. Results ... 54
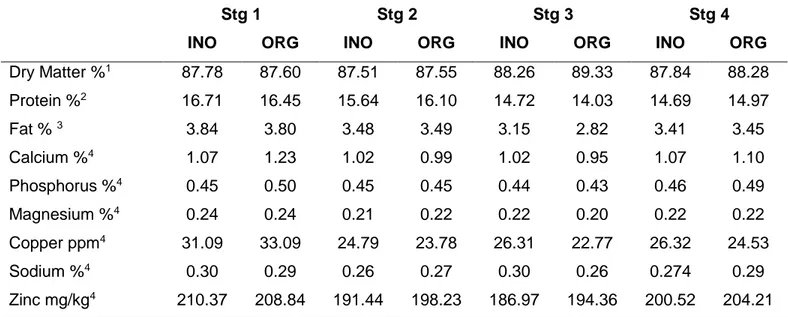
3.2.1. Gilt Development phase: ... 54
3.2.2. Reproductive performance ... 55
3.2.3. Piglet Performance ... 57
3.2.4. Sow Weight, Feed, and Backfat Measurements ... 59
3.2.5. Sow antioxidant enzymes at weaning ... 61
3.2.6. Sow milk composition ... 62
3.2.7. Sow feet lesions in (P1-2) ... 64
3.2.8. Sow feet lesions (P2 only) ... 66
3.2.9. Sow lameness incidence ... 67
3.2.10. Sow cull/removal rate ... 69
3.3. Discussion ... 71
3.3.1. Gilt Development Phase ... 71
3.3.2. Sow performance... 71
3.3.3. Progeny performance ... 74
3.4. Conclusion ... 76
vii
LIST OF TABLES
Table 1 Summary of the differences in trace minerals absorption for Inorganic vs Organic source ... 16 Table 2 Concentration (mg/kg) of the studied trace mineral content in complete feed. ... 39 Table 3 Ingredient composition of the gilt developer diets given from 39-100kg ... 41 Table 4 Ingredient composition of the gilt/sow diets ... 46 Table 5 Diet chemical composition during gilt ... 54 Table 6 Treatment response to trace mineral source on sow’s reproductive
performance ... 56 Table 7 Treatment response on piglet weight ... 58 Table 8 Effect of trace mineral source on gilt and sow weight feed and backfat
measurements ... 60 Table 9 Treatment effect on Antioxidant enzyme levels in sow’s serum after 17
days of lactation ... 61 Table 10 Treatment effect on sow milk composition and mineral content ... 63 Table 11 Effect of trace mineral source on claw lesion scores ... 65 Table 12 Effect of trace mineral source on claw lesion scores in second parity sows ... 66 Table 13 Treatment effect on lameness incidence ... 68 Table 14 Treatment effect on cull/removal ... 70
viii
LIST OF FIGURES
Figure 1 Relationship between IgG and Ig immunocrit ratios (Vallet et al., 2013) 21 Figure 2 Effect of immunocrit on pre-weaning mortality for piglets of different
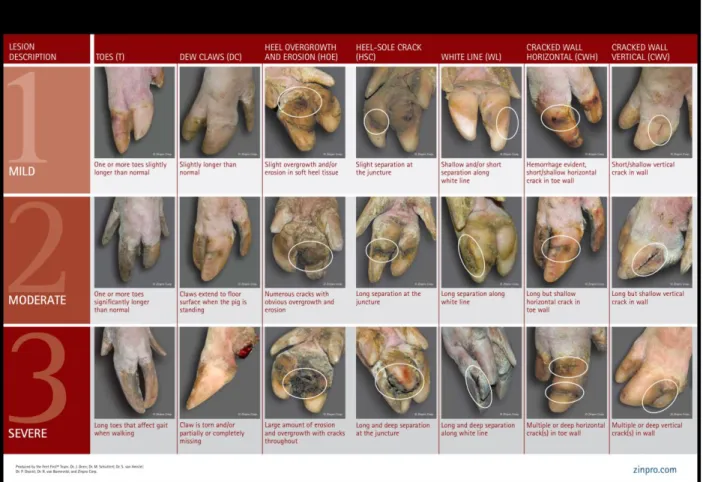
weight (Vallet et al., 2013) ... 22 Figure 3 Feetfirst claw lesion scale (Zinpro) ... 51
ix
To my wife and son for their love and support. To my parents for giving me the tools to accomplish my goals. To Dan Bussières for being a great mentor To my Prof, Frederic Guay that has never been further than a phone call
To the cooperators involved in this trial: Mark E. Wilson, Chantal Farmer and Zach Rambo.
1
CHAPTER 1 INTRODUCTION
The hog industry has significantly changed in the past 90 years. In 1921, pigs were found on fifty percent of farms, now only four percent of Canada’s farms report having pigs (Statistics Canada, 2011). Perhaps, this has to do with the production of pigs moving from the hobby farms to more specialized production units. While the number of farms reporting pigs has decreased from 452 935 to 7 371 during the period of 1921 to 2011, the hog production increased from 3 to 12.6 million pigs during that same period. The hog industry now plays a central role in Canada’s economy with cash receipts of 3.9 Billion annually. This is the 4th most important
sector in Canada’s agricultural market (Statistics Canada, 2015).
A significant increase in the price of the grains and proteins since the late 2000’s, lead hog production in Canada to drop by 15.7% during that period (Statistics Canada, 2011). To balance the extra feed costs and reduced profitability, the hog producers had to ensure efficacy of the systems with a focus on intensive genetic improvements and technological advances as well as research & development programs. These advancements have led to reproductive success with Swine Smart Systems (SMS, Fermont, NE, USA) reporting an increased productivity of 24.48 pigs weaned per sow per year (P/W/S/Y) in 2011 to 25.4 P/W/S/Y in 2016 for their database of 1.5 million sows throughout North America. That translates as an extra pig per sow year in a period of 5 years.
An increase in productivity is related to increase energy and protein demands in feed and, consequently, swine nutrition programs must adapt to the increase in requirements due to improved performance. Requirements for energy and amino acids are generally well defined and reported for sows, however, trace minerals are poorly defined even though it is known that an insufficient supply will affect the immune system, bone mineralization and reproduction system. Understanding micro-minerals is important especially with the current production increases and knowing that with advancing parities, the sows are having a higher depletion in trace minerals (Mahan and Newton, 1995).
2
Dietary trace minerals are most commonly provided to sows by simple INO salts (oxides and sulfates) which are the least expensive source of these minerals. However, INO trace minerals have lower bioavailability when compared to their ORG forms (Burkett et al., 2009; Jolliff and Mahan, 2012). This difference is shown to be especially true in diets that have high levels of phytic acids (Liu et al, 2014). Miles and Henry (2006) suggested that ORG minerals are chemically synthesized with amino-acid bonds creating a ring structure that increases their stability at low stomach pH and reduces unwanted chemical reactions in the gastrointestinal tract. Although bioavailability differences have been reported between both sources of minerals (Spears, 1996; Henry et al., 1992; Wedekind et al., 1992; Formigoni et al., 1993; Predieri et al., 2005), as Miles and Henry (2006) mention in their literature review on trace mineral bioavailability, no one can predict accurately what percentage of either source is actually absorbed. This variation is dependent on mineral intake, chemical forms, overall diet digestibility, particle size, interaction with other minerals and nutrients, chelators, inhibitors, physiological state of the animal, water quality, processing conditions to which the individual ingredients or complete feed has been exposed and the age of the animal.
This study investigated the effects of long term partial substitution of INO zinc (Zn), copper (Cu) and manganese (Mn) by the respective ORG sources. More specifically, these ORG substitute minerals were metal amino acid complexes provided by Availa-Sow™ manufactured by Zinpro (Eden Prairie, Minnesota, USA). The choice of going to partial substitution was based on potential profitability of Org vs INO trace minerals. The metal amino acid complexes are the result of soluble salts combined with an amino acid in a 1:1 ratio. This supplementation started during the gilt development phase and was followed throughout two parities. Effects on gilts’ growth, sows’ reproduction, oxidative stress enzymes, offspring’s growth, feet quality and milk characteristics of sows were the main parameters evaluated during this study. Only one other study has investigated the long-term effect of feeding ORG mineral to grower gilts and followed performance on several parties. In that study, it
3
was reported that sows receiving organic trace minerals (Se, Fe, Cu, Zn and Mn) farrowed 0.7 more live piglets (P<0.05) (Peters & Mahan, 2008). The current study was not only necessary to show repeatability of the previous findings, but also to factor in extra parameters such as claw lesion assessment between trace mineral sources.
In the following chapter of this master’s thesis, a literature review addresses the metabolic roles of the trace minerals, such as Zn, Cu and Mn. There is also an overview of previously reported differences in digestibility of trace mineral sources and their potential anti-nutritional factors that could influence their bioavailability. Finally, the last section of the literature review assesses the differences of trace mineral sources and their impact on growth, claw lesions, reproduction and milk production. The last chapter includes the experiment performed to evaluate the effects of Cu, Zn and Mn on different metabolic and physiological parameters in gilts and their progeny
4
References
Burkett, J. L., Stalder, K. J., Powers, W. J., Bregendahl, K., Pierce, J. L., Baas, T., J., Bailey, T., and Shafer, B. L. 2009. Effect of inorganic and organic trace mineral supplementation on the performance, carcass characteristics, and fecal mineral excretion of phase-fed, grow-finish swine. Asian-Australas. J. Anim. Sci. 22: 1279–1287.
Formigoni, A., Parisini, P. and Corradi, F., 1993. The use of amino acids chelates in high producing dairy cows. In: Ashmead, H.D. (Ed.), The Roles of Amino Acid Chelates in Animal Nutrition. Noyes Publication, Park Ridge, NJ, USA, pp. 170– 186.
Henry, P.R., Ammerman, C.B. and Littell, R.C., 1992. Relative bioavailability of manganese from a manganese–methionine complex and inorganic sources for ruminants. J. Dairy Sci. 75: 3473–3478.
Jolliff, J. S., and Mahan, D. C. 2012. Effect of dietary inulin and phytase on mineral digestibility and tissue retention in weanling and growing swine. J. Anim. Sci. 90: 3012–3022.
Liu, Y., Ma, Y. L., Zhao, J. M., Vazquez-Añón, M. and Stein, H. H. 2014. Digestibility and retention of zinc, copper, manganese, iron, calcium, and phosphorus in pigs fed diets containing inorganic or organic minerals. J. Anim. Sci. 92: 3407-3415.
Mahan, D. and Newton, C. A. 1995. Effect of initial breeding weight on macro- and micro-mineral composition over a three-parity period using a high-producing sow genotype. J. Anim. Sci. 73: 151-158.
Miles D. R. and Henry R. P. 2006. Relative trace mineral bioavailability, Cienc. Anim. Bras.1: 73-93.
Peters, J. C., and Mahan, D. C. 2008. Effects of dietary organic and inorganic trace mineral levels on sow reproductive performances and daily mineral intakes over six parities. Journal of animal science, 86: 2247-2260.
Predieri, G., Elviri, L., Tegoni, M., Zagnoni, I., Cinti, E., Biagi, G., Ferruzzi, S. and Leonardi, G., 2005. Metal chelates of 2-hydroxy-4-methylthiobutanoic acid in animal feeding. Part 2: further characterizations, in vitro and in vivo
investigations. J. Inorg. Biochem. 99: 627- 636.
Spears, J. W. 1996. Organic trace minerals in ruminant nutrition. Anim. Feed Sci. Technol. 58:151–163.
Statistics Canada, Census 2011, Catalogue no. 96-325-X: The Changing Face of the Canadian Hog Industry, ISSN 0-662-35659-9.
5
Statistics Canada, CANSIM table 002-0001 – Farm cash receipts, annual (dollars), last modified on: 2015-11-24
Swine Smart System (SMS) Fremont, NE, USA
Wedekind, K.J., Hortin, A.E. and Baker, D.H., 1992. Methodology for assessing zinc bioavailability: efficacy estimates for zinc-methionine, zinc sulfate and zinc oxide. J. Anim. Sci. 70: 178–187.
6
CHAPTER 2 LITTERATURE REVIEW
2. Trace minerals in sow nutrition
Essential trace minerals are involved in many functions and systems in the pig body. For instance, they are involved in enzyme systems, bone deposition, muscle development and reproduction (NRC 2012). It is known that as parities advances, there is a lack in mineral reserves in sows (Mahan and Newton, 1995). Their importance becomes even more relevant in herds where sows produce over 30 weaning pigs/year, where mineral outputs are greater. Supplementing with trace minerals over the NRC requirements (NRC, 2012) for the sow, has long been an approach to reduce suboptimal mineral status, but probably not that helpful knowing that mineral supplied in high amounts can interfere with the absorption of others, causing greater problems (Pu et al., 2016). Also, environmental issues with farmland loading with trace minerals have brought some countries, for instance countries part of the Europe Union, to legislate the amounts of trace minerals allowed in pig diets (legislation EC No 1334/2003). As a result of these multiple roles and interactions that trace minerals play in the pig metabolisms, it is important to understand the present knowledge on trace mineral nutrition to help reach swine optimal productivity and animal welfare. The present review will concentrate only on Zn, Cu and Mn with a focus on the sow model.
2.1. Roles of trace minerals
2.1.1. Role of Zinc
Zinc is an essential trace mineral involved in several enzyme activities such as metalloenzymes and is also essential for DNA synthetases, RNA synthetases, immune development and function, and hormone synthesis (Aggett and al., 1995). It is also involved in insulin production and has antioxidant effects (Pechova et al.,
7
2006). Because of all these vital roles, Zn is considered as an essential trace mineral. Its absorption is mainly through the duodenum and jejunum then marginally through the ileum by the zinc transporter families of SLC30 and SLC39 (Lee et al., 1989; Martin et al., 2013). Zinc’s absorption is influenced by other substances in the diet, for example phytate, which inhibits its absorption by forming insoluble complexes (Hambidge et al., 2010). Most of zinc’s content in body is found in muscles and bones (Jackson, 1989). Zinc is essential for healthy claws for its role in keratinization via the metalloenzymes (Tomlinson et al., 2004). Metallothionein (MT), a metalloprotein, binds zinc, as well as other divalent metals such as copper. Metalloprotein can serve as a short-term zinc pool (Tomlinson et al., 2004).
Zinc is a trace mineral that has been demonstrated to be essential to optimal growth (Tuerk and Fazel, 2009). Its role in growth is due to the fact that 10 percent of proteins require zinc for proper structure and function (Andreini and al., 2006). Bone deposition is also an important role of zinc. Indeed, zinc intake is positively correlated with bone mass as well as being involved in maintaining plasma vitamin A concentrations which is also related to bone development (Bouglé et al., 2004; Ovesen et al., 2009; Tuerk and Fazel, 2009).
Zinc also plays a role in immune functions (Tuerk and Fazel, 2009). It is an essential mineral for the maintenance of the integrity and barrier functions of the skin which may explain its positive effect on health status of mammary glands associated to a decrease in somatic cell counts (SCC) (Boland et al., 1996). SCC in milk is a measurement that gives an indication of immune system activation by bacterial infection, causing mastitis. In the case of mastitis infection, immune system is activated and releases leukocytes (white cells). Five different cells are classified as leukocytes: neutrophils, macrophages, lymphocytes, eosinophils and epithelial. In human, Zn deficiency is also associated to reduce immune functions (Fraker et al., 2000). Zinc oxides are commonly added to diets at pharmacological levels (2000-3000 mg/kg) in the early weanlings, to reduce the incidence of diarrhea and improve growth (Hedemann et al., 2006).
8
Deficiency effects of Zn in female rats are well documented and are associated to an abnormal ovarian development, disruption of the estrus cycle, frequent abortion, a prolonged gestation period, still-birth, difficulty in parturition and low birth weights (Bedwal et al. 1994).
Studies on the effect of zinc inclusion level on the effect of breeding and lactating performance in sows is not well established, other than it is accepted that needs should be increased in these animals compared to growing pigs to account extra trace mineral needs for fetal growth and milk production (NRC, 2012). At 33 ppm of zinc during gestation the sow has similar performance during lactation than 83 ppm (Hedges et al., 1976). Although 33 ppm appear sufficient for sow’s performance, feeding 83 ppm of Zn during gestation appears more adequate because new born piglets had more zinc content in organs and bones at birth and increased piglet performance (Hedges et al, 1976). More specifically, the authors of Hedges et al. (1976) have looked at the effect of two different Zn levels (33 and 83 ppm) on a long term trial (5 parities) and found that both 33 or 83 ppm of Zn allowed similar sow performance, but progeny had decreased ADG and FE up to 45 kg. This difference in performance appeared to be caused by a reduced Zn storage in liver and bone of new born piglets. A more recent study looked at the effect of increasing Zn to 200 ppm in a sow diet during pregnancy and compared performance to sows fed control of 100 ppm of Zn (Payne et al., 2006). Increasing Zn from 100 to 200 ppm increased litter size and weight which led to an increase in the number of pigs/weaned per litter (Payne et al., 2006).
At elevated inclusion rate in gilt developer and sow diets (5 000 ppm), Zn interferes with the absorption of Cu and Fe, shown by a decrease of serum Cu and hepatic Fe as Zn concentration increased (Hill et al., 1983). This results in a reduce growth during development phase, but does not affect litter size and weights (Hill et al., 1983). Although litter size is not affected, there appears to be an increase in the number of piglets born with abnormalities and reduces wean weight, when 5 000
9
ppm of zinc is fed during development and throughout 2 parities (Hill et al., 1983). The incidence of osteochondritis is higher in sows fed very high levels of zinc (5000 ppm) compared to 500 ppm (Hill et al., 1983).
Zn in milk in found bounded to citrate, serum albumin and to caseins (Lonnerdal et al., 1980; Lonnerdal et al., 1982; Harzer et al., 1982). Transport of Zn through milk is not well established, but Zn concentration appears to be highly regulated in milk (McCormick et al., 2014).
2.1.2. Role of Copper
Copper is mainly involved in the synthesis of hemoglobulin, formation of bone, development and degeneration of the nervous system, energy production and wound healing (Miller et al., 1979; Follis et al., 1955; Carnes et al., 1961; Hill et al., 1983). Copper is an essential trace mineral in pigs. Its absorption is by the Ctr1 protein, a high-affinity Cu+ transporter, which is located on the apical membrane of
the small intestine epithelial cells (Nose et al., 2010). Copper is mostly stored in the liver and to a smaller extent in the kidneys, therefore when differences in concentration needs to be studied, one way is to analyzed based on circulating Cu and cuproenzymes in plasma (Cromwell et al.,1993; Linder and Hazegh-Azam, 1996). Copper plays an important role of formation of structural tissues such collagen and elastin cross-linking. It serves as an essential catalytic cofactor for many cuproenzymes such as ceruloplasmin (Cp) and superoxide dismutase (SOD). Its role in energy is by the interim of the cytochrome c oxidase (CCO), which contains other than Zn, 3 copper ions, several proteins and Mg. Cytochrome c oxidase reduces O2
to H2O and generates a proton gradient that is necessary for ATP synthesis (Erdman
et al., 2012). Some roles involving copper in intracellular signaling for leukocyte trafficking have been reported (Salmi and Jalkanen, 2001). Copper is also essential for healthy claws in ungulates (O’Dell, 1990). Most of the copper in pig’s blood is found in ceruloplasmin (O’Dell, 1990). More specifically, the copper-dependant amine oxidase, lysyl oxidase (LO), plays a central role in initiating the formation of cross-links that stabilize elastin and collagen (Kagan and Li, 2003). When the activity
10
of LO decreases, it creates connective tissue disorders (e.g. osteoporosis or bone defects) occur in Cu deficient animals (Danks, 1988).
Copper is accumulated in infant fetus mainly at the end of gestation period, and stored in the liver of the fetus (Widdowson, 1974). This stored copper aids to the prevention of copper deficiency during the early age of life (Widdowson, 1974).
Feeding Cu to gestating and lactating sows at a high level (266 mg/kg of feed) has benefits compared to a lower inclusion rate (16 mg/kg of feed) according to a study by Cromwell et al. (1993). In that study, birth weights were increased by 9% and had a carryover effect of piglet’s wean weight of 6% (Cromwell et al., 1993). An increase birth weight response to increased concentration of copper in feed was also reported by Lillie and Frobish (1978) in sows fed 15, 30 and 60 ppm. In contrast to the two last studies cited, Thacker (1991) has seen no effect of increasing Cu in sow diets when those increases were done 1 week prior to farrowing and during lactation. This leads to the understanding that Cu needs to be given during gestation (long term; mineral loading) in order to have effect on piglet birth weights. Stillborns can be increased by low inclusion of copper in the diet during pregnancy, in fact pregnant sows fed 2 ppm of Cu farrowed more stillborns than sows fed 9.5 ppm (Kirchgessner et al., 1980).
2.1.3. Role of Manganese
Manganese is mainly involved in protein and fat metabolism, bone growth and immune function. Low Mn has been associated with osteoporosis and impaired wound healing (Klimis-Tavantzis, 1994). It is an essential element for its role as enzyme cofactors and is a constituent of several metalloenzymes (Leach and Harris, 1997). Same as Zn and Cu, Mn is essential for healthy claws in ungulates (Tomlinson et al., 2004). It is a trace mineral necessary for the function of a unique type of SOD; Mn-SOD. Manganese is essential to bone ORG matrix development (synthesis of glycosaminoglycans), which is essential for adequate bone mineralization and bone
11
growth (Andrieu, 2008). Requirements for sows according to NRC (2012) are 25 ppm for both gestating and lactating sows.
Long term feeding of a diet containing only 0.5 ppm of Mn affects negatively bone deposition, reproduction, piglet viability and milk production (Plumlee et al., 1956). According to Kirchgessner et al. (1981), pregnant sow’s requirement is 25 ppm of Mn. Litter birth weight is increased when Mn is increased from 10 ppm to 84 ppm (Rheaume and Chavaz, 1989). Piglet birth weight is affected by Mn content because it was shown that 10 or 20 ppm resulted in heavier piglet at birth than 5 ppm of Mn (Christianson et al., 1989; Christianson et al., 1990). Return to estrus time is reduced in sows fed 20 ppm versus 5 or 10 ppm (Christianson et al., 1989; Christianson et al., 1990).
2.2. Lameness/claw lesions in sows
These trace minerals, Zn, Cu and Mn, have closely related roles in bone deposition and growth and are important for healthy claws. A disturbance in one of these processes could possibly cause lameness, which is estimated to have a prevalence in sows of 10% to 15% (Boyle et al., 2010; Engblom et al., 2007). Economic consequences of lameness are important mainly because lame sows often have to be culled and they have higher piglet mortality (27.7% vs 12.4%) (Anil et al., 2009). This increase in piglet mortality is explained by an uncontrolled lying-down behavior that increases the amount of crushed pigs (Bonde et al., 2004). The most common causes for lameness are osteoporosis, arthritis and claw lesions (Bradley, 2010; Wendt, 2011).
Claw lesions include heel erosions, separations and cracks along the heel/sole junction, separations and cracks along the white line, horizontal and vertical wall cracks, skin lesions near the claw, and excessive (dew) claw length (Anil et al., 2007). With an impairment of mineral supply, and processes they are involved in, the dew claw is more susceptible to chemical, physical or microbial damage from the environment (Muelling, 2009; Tomlinson et al., 2004). Interrupted diffuse supply may
12
happen during mechanical overload and tissue compression (e.g. parturition) (Muelling, 2009). A recent review by van Riet (2013) on the impact of nutrition on lameness and claw health in sows has revealed that yet more research is needed to understand the specific roles of micro minerals in relation to lameness and claw integrity. The complexity of the regulatory mechanisms and the fact that nutritional components often interact with each other makes it hard to establish the optimal dietary intake per the same authors (van Riet et al., 2013).
2.3. Antioxidant Enzymes, copper, manganese and
zinc
Zn, Cu and Mn all have structural and functional roles in antioxidant enzymes and reactions. In the presence of reactive oxygen species (ROS) such as superoxide, hydroxyl and peroxyl radicals, antioxidant enzymes will play an essential role to protect cell membranes of peroxidation of lipids, denaturation of proteins and damage of nucleic acids (Blokhina et al., 2003). Endogenous antioxidants can reduce the impact of these substrates on oxidation reaction of cell membranes. Among those are found ascorbic acid, glutathione and tocopherols, but there are also ROS-interacting enzymes that can be metabolized by the body which are super-oxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx). The SOD family includes the cytosolic Cu/Zn SOD, mitochondrial Mn SOD and extracellular SOD (Zelco et al., 2002). The SODs transform free radicals to a molecular oxygen (O2-) and hydrogen peroxide (H2O2), while the GPx system removes the hydrogen peroxide by oxidizing reduced glutathione to oxidized glutathione (Cecarini et al., 2007; Jenkinson et al., 1982). Disturbance in the Cu/Zn SOD (caused by Cu or Zn deficiency) may result in higher fragility of the cell membranes due to the unsaturated lipids in the cell periphery, which are particularly vulnerable to oxidative damage (Tomlinson et al., 2004).
The metabolic pressure is increased in sows during late gestation and during lactation causing systemic oxidative stress during those periods (Berchieri-Ronchi
13
et al., 2011). The oxidative stress increase at those periods is caused by elevated placental production of ROS during pregnancy which results from high demands in energy and oxygen requirements (Casanueva and Viteri, 2003; Myatt and Cui, 2004; Chen and Scholl, 2005). In a study by Berchieri-Ronchi et al (2011), it was well demonstrated that oxidative stress is increased in sows in the second quarter of the gestational period, although the true effects on sow’s production is not yet well understood. In that last study, it was also shown that the increased oxidative stress is not fully recovered until the weaning period (Berchieri-Ronchi et al., 2011). One aspect that did get some attention related to antioxidant enzymes and sow productivity is that a deficiency in Cu/Zn SOD activity affects claw integrity, which we have seen earlier in this review to be related to productivity (Tomlinson et al., 2004).
2.4. Inorganic/Organic Trace Mineral Terminologies
Most commonly, trace minerals are added in the animal feed in their INO form, which is its natural form. The INO terminology refers to its salt form which can be made with an oxide or a sulfate as examples. The INO trace minerals have a relatively low bioavailability due to antagonisms and interactions with other components in the digesta (Leeson and Summers, 2001; O’Dell, 1989; Underwood and Suttle, 1999). Phytic acids, is one of those molecule that can act antagonistically against the trace minerals. Phytic acids are negatively charged molecules that bind to positively charged molecules such as INO trace minerals, which once bound together, precipitate at the neutral pH of the intestine and are not available to epithelial cells (Cheryan, 1980; Leeson and Summers, 2001).
While these trace minerals have been added to diets in their form of oxides and sulfates to prevent deficiencies, they present certain disadvantages such as high excretion, low bioavailability, high oxidative levels and alteration of nutrients in the diet (Batal et al., 1990; Huang et al., 2007; Yu et al., 2008). The use of ORG trace minerals have been looked as an alternative. This is where comes the interest of ORG trace minerals that have improved stability and decreased antagonistic effect
14
because their positive charges are reduced and sometimes even brought to neutral. This is the result of the ORG trace minerals being complexed with amino acids and other ORG compounds. This again increases their stability constants and facilitates its digestibility (Krebs, 2000; Lonnerdal, 2000). Once combined to a carbon chain (ex: AA, ORG acid), these complexes are known under the terminology of ORG mineral. Several types of ORG trace minerals exist. The Association of American Feed Control Officials (AAFCO, 1997), have classified the different types available on the market: 1) A Metal Amino Acid Chelate (57.142) is the product resulting from the reaction of a soluble metal salt with amino acids with a mole ratio of one mole of metal to one to three (preferably two) moles of amino acids to form coordinate covalent bonds. The average weight of the hydrolyzed amino acids must be approximately 150 daltons and the resulting molecular weight of the chelate must not exceed 800 daltons. 2) A Metal Amino Acid Complex (57.150) is the product resulting from complexing of a soluble metal salt with an amino acid(s). 3) A Metal
(Specific amino acid) Complex (57.151) is the product resulting from the chelation of
a soluble salt with a specific amino acid. 4) A Metal Proteinate (57.23) is the product resulting from the chelation of a soluble salt with amino acids and/or partially hydrolyzed protein. 5) A Metal Polysaccharide Complex (57.29) is the product resulting from complexing of a soluble salt with a polysaccharide solution declared as an ingredient as the specific metal complex.
2.5. Mineral stability/Chelation Strength/Bioavailability
Digestibility trials on grower and finisher pigs show consistency in improved mineral absorption of ORG trace minerals over INO trace minerals (Table 2.1). In most parts, ORG trace minerals are more bioavailable than INO trace minerals due to a reduce interaction antagonisms to phytic acids, fiber and excesses of Ca and or P, which results in more mineral getting to intestine in an absorbable form (Cao et al., 2000; Fly et al., 1989; Guo et al., 2001; Predieri et al., 2005; Wang et al., 2007; Yan and Waldroup, 2006). As an example, evidence indicates that zinc-histidine complexes are absorbed 30-40% more efficiently than zinc-sulfate sources in healthy men (Scholmerich and al., 1987). Another example is that Mn-HMTBa2 was
15
shown to be 150% as available as the Mn from manganous oxide in poultry (Dibner et al, 2004; Yan and Waldroup, 2006). Org Zn and Mn seem to consistently be shown as more bioavailable compared to their INO forms, Cu-SO4 on the other side, is often
shown as having a comparable bioavailability as to ORG complexes of Cu (Stansbury et al., 1990; Coffey et al., 1994; Apgar et al., 1995; Apgar and Kornegay, 1996).
16
Table 2.1 Summary of the differences in trace minerals absorption for Inorganic vs Organic source Treatment Group Difference absorption (%) Reference Cu
100 ppm from Cu-proteinate versus
250 ppm from CuSO4 Weanlings 6 to 17kg 16.0↑ Veum et al.,2004
50 ppm from Cu(HMTBa)2 versus
50 ppm from CuSO4 Feeders 31.1 to 47.5 kg 14.5↑ Liu et al., 2014
15 ppm from Cu-AA versus 15 ppm
from CuSO4 Feeders 44.4 to 60kg 4.5↑
Jolliff and Mahan, 2013
Zn
40 ppm from Zn(HMTBA)2 versus
40 pmm from ZnSO4
Weanlings 31.1 to 47.5
kg 10.7↑ Liu et al., 2014
140 ppm from Zn-AA versus 140
ppm from ZnSO4 feeders 44.4 to 60kg 2.3↑
Jolliff and Mahan, 2013 Mn 20 ppm Mn(HMTBA)2 versus 20 ppm from MnSO4 Weanlings 31.1 to 47.5 kg 7.21↑ Liu et al., 2014
10 ppm Mn-AA versus 10 ppm from
MnO feeders 44.4 to 60kg 6.6↑
Jolliff and Mahan, 2013
17
Although ORG trace minerals are recognized as more absorbed, there are several different ORG products on the market, which makes it difficult to establish which one brings the most benefits. To help, several varieties of assays have been developed to demonstrate differences in bioavailability. Bioavailability is defined as “the degree to which an ingested nutrient in a particular source is absorbed in a form that can be utilized by the metabolism in animals” (Ammerman et al., 1995). From the methods developed are the solubility test as described in Brown and Zeringue (1994), as well as analyzing the structural characteristics as described in Li et al., 2004. The concept behind the solubility test makes sense; minerals need to be soluble in order to be absorbed. Although in theory the concept of a mineral that is soluble will be available is true, some highly soluble minerals such as Zn, Cu and Mn sulfates have been shown to be very poorly correlated to bioavailability (Guo et al., 2001; Ledoux et al., 1995; Miles et al., 1998).
Another technique is to classify ORG trace minerals based on their degree of chelation, because it is hypothesized that the bioavailability differs based on the complex and chelation strength (Qf) (Ashmead, 1993). This chelation strength is a quantitative measure obtained by the calculation of half-wave potential (E1/2) and the shift in half-wave potential (∆E1/2) as described by Holwerda et al., (1995). As an example, the formula to calculate the chelation strength of Mn, as the formation quotient (Qf), is calculated from log (Qf) = (E1/2)/0.05916, where n = to the number
of electrons accepted by Mn2+ (Li et al., 2004). Using this method, Li et al., (2004)
looked at thirteen different sources of Mn, which includes proteinates, methionine complexes and amino acids (other than methionine). Qf ranged from 1.9 to 115.4.
The chelation strength is, according to that study, a better predictor of bioavailability compared to other technique such as solubility or structural integrity based on a Mn-SOD mRNA model in chicks (Li et al., 2004). Also, a distinction of the different ORG mineral strength and antagonistic substances to trace minerals found in the diets such as calcium and phytate needs to be understood. For instance, ORG Mn sources that have a moderate or strong chelation strength (Qf from 45.3 to 115.4) increased resistance to interference with high dietary calcium, but no difference at
18
low calcium levels (Li et al., 2005). Also, when using a diet with a lower concentration of phytic acid there are very little differences in the absorption of trace minerals between ORG versus INO trace minerals in comparison to a higher in phytic acid diet (Liu et al., 2014). This shows indications that not only mineral source affects bioavailability, but also antagonistic substances and mineral complex strength should be considered. Finally, biomarkers that evaluate the activity of a mineral dependent enzyme and proteins are commonly used to assess bioavailability of a trace mineral. SODs are some of those enzymes that, because their expression depends on the amount of the trace mineral absorbed, can give an indication of bioavailability (Cortinhas et al., 2010; Martin et al., 2011). Measuring the activity of enzymes is one of the method recognized to assess differences in trace mineral bioavailability.
2.6. Effects of organic trace minerals on sow
performance
As seen so far, Zn, Cu and Mn play essential roles in sow nutrition by their fundamental roles in the pig metabolism. Due to their higher availability, ORG trace minerals might reduce the incidence of deficiency in time of the cycle of production where sow’s needs are higher such at late gestation and during lactation, although requirements are not well established for those periods in sows and therefore bringing an advantage on performance. Peter and Mahan (2008) compared feeding INO to ORG trace minerals (Cu, Fe, Mn, Se and Zn) to developing gilts and followed them throughout six parities to evaluate effect on performance. Gilt growth and feed efficiency were not affected between mineral sources from 30 to 110 kg period. In that study, litter size was significantly improved with the use of ORG trace mineral going from 11.3 to 12.2 total born to the advantage of ORG source (P<0.05). Even though litters were greater on the ORG treatment, the piglets tended to have an improved ADG (P<0.10). These improvement numbers are promising, but the number of total born (12 pigs/litter) and weaned pigs (~10 pigs/litter) do not reflect
19
the full reproductive genetic potential of sows for a trial, which makes it hard to transpose results to a system weaning over 12 pigs/sow.
A combination of proteinates forms of Cu, Zn, Fe and Mn was shown to reduce the wean-to-estrus interval in sows (Spears and Flowers, 1995). Similarly, the percentage of sows bred by 7 days post weaning was increased when supplemental ORG Cu fed as Cu-proteinates complex was fed during lactation at 14 mg/day in a study from Yen et al. (2005). Supplemental Zn-AA (amino acid-chelated Zn) fed to gestating and lactating sow was shown to increase the number piglets born alive and weaned per litter (Payne et al., 2006; Caine et al., 2009).
2.7. Effect of organic trace mineral on lameness and
claw lesion on sow performance
Trace minerals can reduce the incidence of lameness by increasing claw integrity (Audrieu, 2008; Kessler et al., 2003; Tomlinson et al., 2004). A supply in sufficient bioavailable trace minerals is even more important in high demanding periods such as late gestation and early lactation (McDowell, 2003; NRC, 2012). Although mechanisms are not yet well established, lameness and claw lesions do not affect the sow the same way. While lameness affects the farms’ productivity because of a reduction in the sow longevity, claw lesions in contrast show direct effect on reproduction parameters (Pluym et al., 2013). For instance, Fitzgerald et al. (2012) reported a negative correlation between toes overgrowths on feed intake during lactation, which is well known to reduce subsequent litter performance. Also, white lines significantly increase the odds of stillborns (P=0.036), the heel lesions are associate which an increase of crushed piglets (P=0.017) and mummified piglets are more present when the sow claw has wall cracks (P=0.044) (Pluym et al., 2013).
Feeding ORG trace minerals (Zn, Cu and Mn) to sows has been shown to decrease the severity of lesions compared to an INO trace mineral group (Nair, 2011). This same study observed a reduction of stillborns/litter and increased piglet
20
wean weight (P<0.05). A reduction of claw disorders in sows has also been reported when INO Zn, Cu and Mn were partially replaced by an ORG source (Varagka et al., 2016).
2.8. Effects of organic trace minerals on milk
composition, yield and immune transfer
In addition to potential benefits to sow’s performance, an increase uptake of trace minerals, with their role in oxidative enzymes, have been shown to enhance the defense mechanisms of the mammary gland in preventing mastitis (Weiss and Wyatt, 2002; Ashmead and Samford, 2004). This is a result of greater circulations of Zn and Cu has been associated with higher antioxidant capacity of SOD and GPx activity. When these oxidative enzymes are increased, it has been shown to reduce somatic cell counts (SCC) in cows (Spain et al., 1993; Weiss and Wyatt, 2002). Although the SCC have at some occasions been reduced with the use of ORG trace minerals in cows, to our knowledge, the effect of mineral source on SCC in sows has not yet been studied.
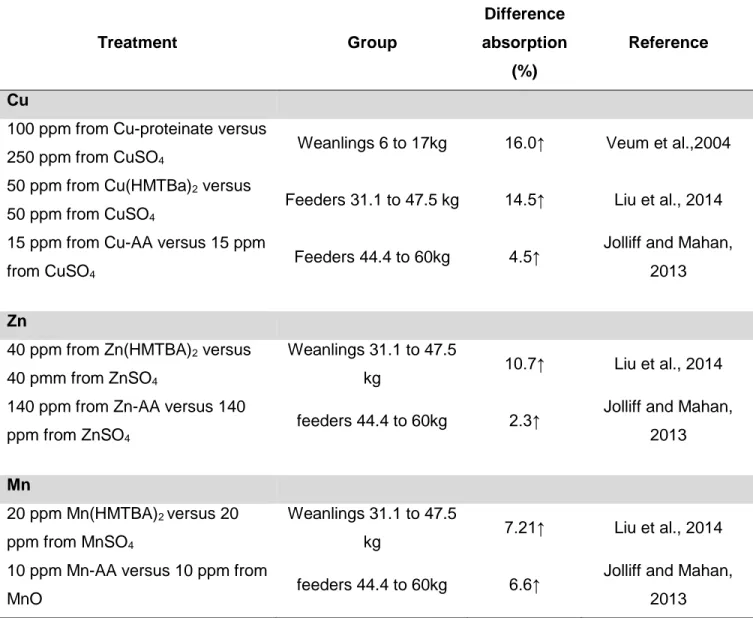
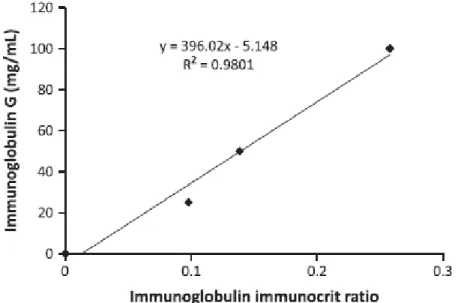
Organic Zn, Cu and Mn has been shown in cows to increase immunoglobulins (Igs) in colostrum by 19% (Formigoni et al., 2011). A reduction of colostrum Igs decreases the effectiveness of passive immunity transfer of immunoglobulins to piglets (Salmon et al., 2009). Vallet et al. (2013) have developed a method to estimate Igs in piglet. This method provides a ratio (Ig immunocrit ratio) that can be transformed to an IgG value (Fig 2.1) Igs in piglet serum during first hours of life is highly correlated to piglet survivability (Fig 2.2).
21
22
Figure 1.2 Effect of immunocrit on pre-weaning mortality for piglets of different weight (Vallet et al., 2013)
23
2.9. Hypothesis and objectives
While effect of ORG trace minerals has well been documented in weanlings and grower pigs, the effect of long term feeding of ORG trace Zn, Cu and Mn on the sow’s performance and progeny has not well been established. Also, sow’s performance has increased considerably over the years and the importance of minerals source might be more important from having sows that are nutritionally challenged because of increased mineral requirements as reproductive performance increases. In addition to sow’s performance parameters, evaluating trace mineral source effect on oxidative stress status, milk composition, claw lesion and immunity transfer would bring strength to relate effects to factors in such trial.
The objective of this trial was to study the effect of partial substitution of INO Zn, Cu and Mn by an ORG trace mineral in a highly performing commercial sow line.
Hypothesis:
1. Long term feeding of a diet containing partial substitution of ORG trace minerals will increase piglet’s birth weight, increase litter size and will have positive effects on progeny’s growth performance.
2. A reduction in the incidence of sow lameness and claw lesion will be observed in sows fed ORG minerals after their 2nd parturition.
3. Sow’s immune system will be improved and will be shown by a reduction of linear point (SCC) in milk and an increase in antioxidant enzymes in blood stream of sow after 17 days of lactation.
24
2.10.
REFERENCES
Aggett, P. J. and Comerford, J. G. 1995. Zinc and human health. Nutrition reviews 53: 16-22.
Ammerman, C. B., Baker D. H. and Lewis, A. J. Introduction to bioavailability of nutrients for animals. In: Ammerman, C. B., Baker, D. H., Lewis, A.J., editors. Bioavailability of Nutrients for Animals: Amino Acids, Minerals, and Vitamins. 1st ed. San Diego, CA, USA: Academic Press; 1995.
Andreini, C., Banci, L. and Bertini, I. 2006. Counting the zinc proteins encoded in the human genome . J. Proteome Res. 5: 196–201.
Andrieu, S., 2008. Is there a role for organic trace element supplements in transition cow health? Vet. J. 176: 77–83.
Anil S.S., Anil L., Deen, J., Baidoo, S.K. and Walker, R.D., 2007. Factors
associated with claw lesions in gestating sows. Swine Health Prod. 15: 78– 83.
Anil, S.S., Anil, L. and Deen, J., 2009. Effect of lameness on sow longevity. J. Am. Vet. Med. Assoc. 235: 1–5.
Anil, S.S., Deen, J., Anil, L., Baidoo, S. K., Wilson, M. E. and Ward, T. L., 2010. Evaluation of the supplementation of complexed trace minerals on the number of claw lesions in breeding sows. In: Manipulating Pig Production XII, Australian Pig Science Association, Cairns, Australia.
Apgar, G. A., E. T. Kornegay, M. D. Lindemann and D. R. Notter. 1995. Evaluation of copper sulfate and a copper lysine complex as growth promotants for weanling swine. J. Anim. Sci. 73: 2640-2646.
Apgar, G. A., and E. T. Kornegay. 1996. Mineral balance of finishing pigs fed copper sulfate or a copper-lysine complex as growth promoting levels. J. Anim. Sci. 74: 1594-1600.
Ashmead, D., 1993. The Role of Metal Amino Acid Chelate. Academic Press, San Diego, CA.
Ashmead, H.D., Samford, R.A., 2004. Effects of metal amino acid chelates or inorganic minerals on three successive lactations in dairy cows. Intern. J. Appl. Res. Vet. Med. 2: 181–188.
Varagka, N., Lisgara, M., Skampardonis, V., Psychas, V., Leontides, L., 2016. Partial substitution, with their chelated complexes, of the inorganic zinc, copper and manganese in the sow diets reduced the laminitic lesions in the
25
claws and improved the morphometric characteristics of the hoof horn of sows from three Greek herds. Porcine Health Manag. 2: 26-39
Batal, A. B., Parr, T. M. and Baker, D. H. 1990. Zinc bioavailability in tetrabasic zinc chloride and the dietary zinc requirement of young chicks fed a soy concentrate diet. Poult. Sci. 80: 87–90.
Bedwal, R. S. and Bahuguna A., 1994. Zinc, copper and selenium in reproduction. Experientia, 50: 626-640.
Berchieri-Ronchi, C. B., S. W. Kim, Y. Zhao, C. R. Correa, K. J. Yeum, and A. L. A. Ferreira. 2011. Oxidative stress status of highly prolific sows during
gestation and lactation. animal 5: 1774-1779.
Blokhina, O., Virolainen, E., and Fagerstedt, K. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany 91: 179-194.
Boland M. P., O’Donnell G. O. and O’Callaghan D. 1996: The contribution of mineral proteinates to production and reproduction in dairy cattle. In: Biotechnology in the Feed Industry, Proceedings of the 12th Annual Symposium (T.P. Lyons and K. A. Jacques, eds.). Nottigham University Press, Loughborough, Leics, UK, pp. 95-102.
Bonde M., Rousing T., Badsberg JH and Sorensen J.T. 2004. Associations
between lying-down behaviour problems and body condition, limb disorders and skin lesions of lactating sows housed in farrowing crates in commercial sow farms. Livestock Prod. Sci. 87: 179-187.
Bouglé, D. L., Sabatier, J. P., Guaydier-Souquieres, G., Guillon-Metz, F., Laroche, D., Jauzec, P. and Bueau, F., 2004. Zinc status and bone mineralisation in adolescent girls. J. Trace Elem. Med. Biol. 18: 17-21.
Bradley, C. L., 2010. Evaluating the Impact of Dietary Inorganic or Organic Trace Mineral Supplementation on Gilt Development and Sow Reproduction, Lameness, and Longevity. University of Arkansas, Fayetteville1–398. Burkett, J. L., Stalder, K. J., Powers, W. J., Bregendahl, K. Pierce, J. L., Baas, T.
Bailey, J. T., and Shafer. B. L. 2009. Effect of inorganic and organic trace mineral supplementation on the performance, carcass characteristics, and fecal mineral excretion of phase-fed, grow-finish swine. Asian-Australas. J. Anim. Sci. 22: 1279–1287.
Boyle, L. A., Bullo, E. and Cota Nolan, T., 2010. Lameness and limb lesions in replacement gilts on a commercial farm. Adv. Anim. Biosci. 1: 198.
Caine, W. R., Metzler-Zebeli, B. U., McFall, M., Miller, B., Ward, T. L., Kirkwood, R. N., Mosenthin, R., 2009. Supplementation of diets for gestating sows with
26
zinc amino acid complex and gastric intubation of suckling pigs with zinc-methionine on mineral status, intestinal morphology and bacterial
translocation in lipopolysaccharide-challenged early-weaned pigs. Res. Vet. Sci. 86: 453–462.
Cao, J., Henry, P. R., Guo, R. Holwerda, R. A., Toth, J. P., Littell, R. C., Miles R. D. and Ammerman, C. B. 2000. Chemical characteristics and relative bioavialability of supplemental organic zinc sources for poultry and ruminants. J. Anim. Sci. 78: 2039-2054.
Carnes, W. H., Shields, C. S., Cartwright, C. E. and Winthrop, M. M. 1961. Vacular lesions in copper-deficient swine. Federation Proceedings 20:118 (abstr.) Casanueva, E. and Viteri, F. E. 2003. Iron and oxidative stress in pregnancy. J.
Nutr. 133: 1700S–1708S.
Cecarini, V., Gee, J., Fioretti, E., Amici, M., Angeletti, M. and Eleuteri, A. M. 2007. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim Biophys Acta. 1773: 93–104.
Chen, X. and Scholl, T.O. 2005. Oxidative stress: changes in pregnancy and with gestational diabetes mellitus. Current Diabetes Reports 5: 282–288.
Cheryan, M. 1980. Phytic acid interactions in food systems. Crit. Rev. Food Sci. Nutr. 13: 297-335.
Christianson, S. L., Peo, E. R. and Lewis, A. J. Jr., 1989. Effects of dietary
manganese levels on reproductive performance of sows. J. Anim. Sci. 67: 251 (abstr.)
Christianson, S. L., Peo, E. R., Lewis, A. J. Jr. and Giesemann M. A. 1990. Influence of dietary manganese levels on reproduction, serum cholesterol and milk manganese concentration of sows. J. Anim. Sci. 68: 368 (abstr.). Coffey, R. D., G. L. Cromwell, and H. J. Monegue. 1994. Efficacy of a
copper-lysine complex as a growth promotant for weanling pigs. J. Anim. Sci. 72: 2880-2886.
Cortinhas, C. S., B. G. Botaro, M. C. A. Sucupira, F. P. Renno, and M. V. Santos. 2010. Antioxidant enzymes and somatic cell count in dairy cows fed with organic source of zinc, copper and selenium. Liv. Sci. 127: 84-87.
Cromwell, G. L., H. J. Monegue and T. S. Stahly. 1993. Long-term effects of feeding a high copper diet to sows during gestation and lactation. J. Anim. Sci. 71: 2996-3002.
27
Danks , D.M. 1988. Copper defi ciency in humans. Annu. Rev. Nutr. 8: 235 – 257 . Dibner, J. J., Trehy, M. L., Schasteen C. S. and Hume, J. A. 2004. Use of
2-hydroxy-4 (methylthio) butanoic acid (HMTBa) as a ligand for organic trace minerals. Poult. Sci. 83: 436.
Engblom, L., Lundeheim, N., Dalin, A., Andersson, K., 2007. Sow removal in Swedish commercial herds. Livest. Sci. 106: 76–86.
Final report of the EU-Concerted Action. AROMIS, KTBL Publication No 432, Darmstadt, Germany
Fly, A. D., Izquierdo, O. A., Lowry, K. R. and Baker, D. H. 1989. Manganese bioavailability in a Mn methionine chelate. Nutr. Res. 9: 901-910.
Fitzgerald, R. F., Stalder, K. J, Karriker, L. A, Sadler, L. J, Hill, H. T, Kaisand, J. and Johnson A. K. 2012. The effect of hoof abnormalities on sow behaviour and performance. Livestock Sci. 145: 230-238.
Formigoni, A., Fustini, M., Archetti, L., Emanuele, S., Sniffen, C. and Biagi, G. 2011. Effects of an organic source of copper, manganese and zinc on dairy cattle productive performance, health status and fertility. Anim. Feed Sci. And Tech., 164: 191-198.
Follis, R. H., Bush, Jr, J. A., Cartwright, G. E. and Wintrobe, M. M. 1955. Studies of copper deficiency in swine. Bulletin of Johns Hopkins Hospital 97:405. Fraker, P. J., King, L. E., Laakko, T., Vollmer, T. L., 2000. The dynamic link
between the integrity of theimmunesystem and zinc status. J. Nutr. 130: 1399S–1406S.
Guo, R., Henry, P. R., Holwerda, R. A., Cao, J., Littell, R. C., Miles R. D. and Ammerman, C. B. 2001. Chemical characteristics and relative bioavailability of supplemental organic copper sources for poultry. J. Anim. Sci. 79: 1132-1141.
Hambidge , K. M., Miller, L. V. and Westcott, J. E. 2010. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 91: 1478S – 1483S .
Harzer, G. and Kauer, H.1982. Binding of zinc to casein. Am. J. Clin. Nutr. 35: 981–987.
Hedemann, M. S., Jensen, B. B. and Poulsen H. D. 2006. Influence of dietary zinc and copper on digestive enzyme activity and intestinal morphology in weaned pigs. J. anim. Sci. 84: 3310-3320.
28
Hedges, J. D., Kornegay, E. T., and Thomas, H. R. 1976. Comparison of dietary zinc levels for reproducing sows and the effect of dietary zinc and calcium on the subsequent performance of their progeny. J. anim. Sci. 43: 453-463. Hill, G. M., Ku, P. K., Miller, E. R., Ullrey, D. E., Lostly, T. A. and O’Dell, B. L.
1983. A copper deficiency in neonatal pigs induced by a high zinc maternal diet. J. Nutr. 113: 867-872.
Hill, G. M., Miller, E. R. and Stowe, H. D. 1983. Effect of dietary zinc levels on health and productivity of gilts and sows through two parities. J. anim. Sci. 57: 114-122.
Holwerda, R. A., Albin, R. C. and Madsen, F. C. 1995. Chelation effectiveness of zinc proteinates demonstrated. Feedstuffs 67: 12−13, 23.
Huang, Y. L. 2007. An optimal dietary zinc level and relative bioavailabilities of organic zinc sources for broiler chicks fed a corn-soybean meal diet. PhD Dissertation, Chinese Academy of Agricultural Science, Beijing, P. R. China Jackson , M. 1989. Physiology of zinc: general aspects . In C.F. Mills (ed.), Zinc in
Human Biology . Springer - Verlag, New York , pp: 1 – 14 .
Jenkinson, S. G., Lawrence, R. A., Burk, R. F. and Williams, D. M. 1982. Effects of copper deficiency on the activity of the selenoenzyme glutathione
peroxidase and on excretion and tissue retention of 75SeO3(2-). J. Nutr. 112: 197-204.
Jolliff, J. S. and Mahan, D. C.. 2013. Effect of dietary calcium and phosphorus levels on the total tract digestibility of innate and supplemental organic and inorganic microminerals in a corn-soybean meal based diet of grower pigs. J. Anim. Sci. 9: 2775-2783.
Kagan , H. M. and Li , W. 2003. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell . J. Cell Biochem. 88: 660 – 672 .
Kessler, J., Morel, I., Dufey, F. A., Gutzwiller, A., Stern, A., Geyes, H., 2003. Effect of organic zinc sources on performance, zinc status, and carcass, meat, and claw quality in fattening bulls. Livest. Prod. Sci. 81: 161–171.
Kirchgessner, M., Mader, H. and Grassman, E. 1980. Zur Fruchtbarkeitslwistung von Saven bei unterschiedlicher Cu-Versorgung. Zuchtungskunde 52: 46-53.
Kirchgessner, M., Roth-Maier, Doraa and Spörl, R. 1981. Untersuchungen zum Trächtigkeitsanabolismus der Spurenelemente Kupfer, Zink, Nickel und Mangan bei Zuchtsauen. Archiv für Tierernaehrung 31: 21–34
29
Klimis - Tavantzis , D. J. 1994. Manganese in Health and Disease. CRC Press, Boca Raton, FL.
Krebs, N. F. 2000. Overview of zinc absorption and excretion in the human gastrointestinal tract . J. Nutr. 130: 1374S – 1377S.
Leach , R.M. , Jr , and Harris , E.D. 1997. Manganese . In B.L. O ’ Dell and R.A. Sunde (eds), Handbook of Nutritionally Essential Minerals . Marcel Dekker , New York, pp. 335 – 355 .
Ledoux, D. R., Pott, E. B. Henry, P. R. Ammerman, C. B. Merritt A. M. and Madison. J. B. 1995. Estimation of the relative bioavailability of inorganic copper sources for sheep. Nutr. Res. 12: 1803-1813.
Lee, H. H., Prasad, A. S. and Brewer, G. J. 1989. Zinc absorption in human small intestine. Am J Physiol 256, G87 – 91.
Leeson, S., and Summers, J.D. 2001. Scott’s Nutrition of the Chicken, 4th ed. University Books, Guelph, Ont.
Li, S., Luo, X., Liu, B., Crenshaw, T. Kuang, X., Shao, G. and Yu, S. 2004. Use of chemical characteristics to predict the relative bioavailability of supplemental organic manganese sources for broilers. J. Anim. Sci. 82: 2352-2363.
Li, S.F., Luo, X.G., Lu, L., Crenshaw, T.D., Bu, Y.Q., Liu, B., Kuang, X., Shao, G.Z. and Yu, S.X. 2005. Bioavailability of organic manganese sources in broilers fed high dietary calcium. Anim. Feed Sci. Technol. 123: 703–715.
Lillie, R. J., and Frobish, L. T. 1978. Effect of copper and iron supplements on performance and hematology of confined sows and their progeny through four reproductive cycles. J. Anim. Sci. 46:678.
Linder , M.C. and Hazegh - Azam , M. 1996. Copper biochemistry and molecular biology . Am. J. Clin. Nutr. 63: 797S – 811S.
Liu, Y., Ma, Y. L., Zhao, J. M., Vazquez-Añón, M. and Stein, H. H. 2014. Digestibility and retention of zinc, copper, manganese, iron, calcium, and phosphorus in pigs fed diets containing inorganic or organic minerals. J. Anim. Sci. 92: 3407-3415.
Lönnerdal, B., Stanislowski, A. G., Hurley, L. S. 1980. Isolation of a low molecular weight zinc binding ligand from human milk. J. Inorg. Biochem. 12: 71–78. Lönnerdal, B., Hoffman, B., Hurley, L.S. 1982. Zinc and copper binding proteins in
30
Lonnerdal , B. 2000. Dietary factors infl uencing zinc absorption. J. Nutr. 130: 1378S – 1383S .
Mahan, D. and Newton, C. A. 1995. Effect of initial breeding weight on macro- and micro-mineral composition over a three-parity period using a high-producing sow genotype. J. Anim. Sci, 73: 151-158.
Martin, R. E., Mahan, D. C., Hill, G. M., Link, J. E. and Jolliff, J. S. 2011. Effect of dietary organic microminerals on starter pig performance, tissue mineral concentrations, and liver and plasma enzyme activities. J. Anim. Sci 89: 1042-1055.
Martin, L., Lodemann, U., Bondzio, A., Gefeller, E.-M., Vahjen, W., Aschenbach, J. R., Zentek, J. and Pieper, R. 2013. A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets. J. Nutr. 143: 1205-1210.
McCormick, N.H., Hennigar, S.R., Kiselyov, K. and Kelleher, S.L., 2014. The biology of zinc transport in mammary epithelial cells: implications for
mammary gland development, lactation, and involution. J. Mammary Gland Biol. Neoplasia 19: 59–71.
McDowell, L.R.. 2003. Minerals in Animal and Human Nutrition. Elservier Science B.V., The Netherlands.
Miles, R. D., O’Keefe, S. F. Henry, P. R., Ammerman, C. B. and Luo. X. G. 1998. The effect of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability and dietary prooxidant activity. Poult. Sci. 77: 416-425.
Miles D. R. and Henry R. P. 2006. Relative trace mineral bioavailability, Ciencia Animal Brasileira, 1: 73-93.
Miller, E. R., Stowe, H. D., Ku, P. K. and Hill, G. M. 1979. Copper and zinc in swine nutrition. P. 109 in National Feed Ingredients Association Litterature Review on Copper and Zinc in Animal Nutrition. West Des Moines, IA: National Feed Ingredients Association.
Muelling, C. K. W. 2009. Nutritional influences on horn quality and hoof health. WCDS Adv. Dairy Technol. 21: 283–291.
Myatt, L. and Cui, X. 2004. Oxidative stress in the placenta. Histochemistry and Cell. Biology 122: 369–382.
31
NAHMS USDA. 2007. Swine 2006, Part 1: Reference of Swine health and management Practices in the United States. USDA:APHIS:VS, Center for Epidemiology and Animal Health, Fort Collins, CO.
NAIR, SAS. 2011. Epidemiology of lameness in breeding female pigs. Dissertation submitted to the falculty of the graduate school of University of Minnesota. Nose, Y., Wood, L. K., Kim, B.-E., Prohaska, J. R., Fry, R. S., Spears, J. W. and
Thiele D. J. 2010. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J. Bio. Chem. 285: 32385-32392.
NRC, 2012. Nutrient Requirements of Swine, Eleventh Revised Edition National Academic Press, USA.
O’Dell, B. L. 1989. Mineral interactions relevant to nutrient requirements. J. Nutr. 119: 1832-1838.
O’Dell, B. L. 1990. Copper. In: Brown, M.L. (Ed.), Present Knowledge in Nutrition. , 6th ed. ILSI Press, Washington, DC, USA, pp. 261–267.
Ovesen, J., Møller-Madsen, B., Nielsen, P. T., Christensen, P. H., Simonsen, O., Hoeck, H. C., Laursen, M. B., Thomsen, J. S. 2009. Differences in zinc status between patients with osteoarthritis and osteoporosis. J. Trace Elem. Med. Biol. 23: 1–8.
Payne, R. L., Bidner, T. D., Fakler, T. M., Southern, L. L. 2006. Growth and intestinal morphology of pigs from sows fed two zinc sources during gestation and lactation. J. Anim. Sci. 84: 2141–2149.
Pechova, A., Pavlata, L. and Lokajova, E. 2006. Zinc supplementation and somatic cell count in milk of dairy cows. Acta Veterinaria Brno. 75: 355-361.
Peters, J. C., and Mahan, D. C.. 2008. Effects of dietary organic and inorganic trace mineral levels on sow reproductive performances and daily mineral intakes over six parities. J. Anim. Sci. 86: 2247-2260.
Plumlee, M. P., Thrasher, D. M. Beeson, W. M., Andrews, F. N. and Parker, H.E. 1956. The effects of a manganese deficiency upon the growth, development and reproduction swine. J. Anim. Sci. 15: 352-368.
Pluym, L., Van Nuffel, A., Van Weyenberg, S. and Maes, D. 2013. Prevalence of lameness and claw lesions during different stages in the reproductive cycle of sows and the impact on reproduction results. Animal 7: 1174-1181. Predieri, G., L. Elviri, M. Tegoni, I. Zagnoni, E. Cinti, G. Biagi, S. Ferruzza and G.
32
animal feeding, Part 2: Further characterizations, in vitro and in vivo investigations. J. Inorg. Biochem. 99: 627-636.
Present Knowledge in Nutrition, Tenth Edition. Edited by John W. Erdman Jr, Ian A. Macdonald and Steven H. Zeisel. © 2012 International Life Sciences Institute. Published 2012 by John Wiley & Sons, Inc.
Pu, J., Tian, G., Li, B. Chen, D., He, J., Zheng, P., Mao, X., Yu, J., Huang, Z. and Yu, B. 2016. Trace Mineral Overload Induced Hepatic Oxidative Damage and Apoptosis in Pigs with Long-Term High-Level Dietary Mineral Exposure. J. Agri. and food Chem. 64: 1841-1849.
Rheaume, J. A. and Chavaz, E. R. 1989. Trace mineral metabolism in non-gravid, gestating and lactating gilts fed two dietary levels of manganese. J. Trace
Elements and Electrolytes in Health and Disease 3: 231-242.
Salmi , M. and Jalkanen, S. 2001. VAP - 1: an adhesin and an enzyme . Trends Immunol 22 : 211–216.
Salmon, H., Berri, M., Gerdts, V. and Meurens, F. 2009. Humoral and cellular factors of maternal immunity in swine. Developments in Comparative Immunology 33: 384–393.
Scholmerich, J., Freudemann, A. and Kottgen, E. 1987. Bioavailability of zinc from zinc – histidine complexes. I. Comparison with zinc sulfate in healthy men . Am. J. Clin. Nutr. 45: 1480–1486.
Spain, J. N., Stevens, B. J., Hardin D. K. and Thorne J. G. 1993. Effects of Bioplex Zinc or zinc oxide on mastitis incidence in lactating dairy cows. Pages 53– 60 in Biotechnol. Feed Ind., Proc. Alltech’s 9th Annu. Symp., Nicholasville, KY.
Spears, J. W., and Flowers, W. L. 1995. Effect of metal proteinates on baby pig growth and survival and sow reproductive performance. Chelated Minerals Corp. Tech. Data. Salt Lake City, UT.
Stansbury, W. F., Tribble, L. F. and Orr, D. E., Jr. 1990. Effects of chelated copper sources on performance of nursery and growing pigs. J. Anim. Sci. 68: 1318-1322.
Taylor-pickard, J. A., Nollet, L. and Geers, R. 2013. Performance, carcass characteristics and economic benefits of total replacement of inorganic minerals by organic forms in growing pig diets. Vol. :2.
Thacker, P. A. 1991. Effect of high levels of copper or dichlorvos during late gestation and lactation on sow productivity. Can. J. him. Sci. 71:227-233.
33
Tomlinson, D. J., Mulling, C. H. and Fakler, T. M. 2004. Invited review: formation of keratins in the bovine claw: roles of hormones, minerals and vitamins in functional claw integrity. J. Dairy Sci. 87: 797–809.
Tomlinson, D. J., Mulling, C. H. and Fakler, T. M. 2004. Invited review: formation of keratins in the bovine claw: roles of hormones, minerals and vitamins in functional claw integrity. J. Dairy Sci. 87: 797–809.
Tuerk , M. J. and Fazel, N. 2009. Zinc deficiency. Curr. Opin. Gastroenterol. 25: 136–143.
Underwood, E. J. and Suttle, N. F. 1999. The mineral nutrition of livestock, 3rd ed. CABI Publishing, New York, N.Y.
van Riet, M. M. J., Millet, S., Aluwé, M. and Janssens G. P. J. 2013. Impact of nutrition on lameness and claw health in sows. Livestock Sci. 156: 24-35. Vallet, J., Miles, Jr., L. Rempel, and L. Kuehn. 2010. The "immunocrit," a simple
measure of passive transfer, is a useful predictor of nursing ability and preweaning mortality of piglets. J. Dairy Sci. 93: 501-501.
Vallet, J. L., Miles, J. R., and Rempel, L. A.. 2013. A simple novel measure of passive transfer of maternal immunoglobulin is predictive of preweaning mortality in piglets. Vet. J. 195: 91-97.
Veum, T. L., Carlson, M. S., Wu, C. W., Bollinger, D. W. and Ellersieck, M. R. 2004. Copper proteinate in weanling pig diets for enhancing growth performance and reducing fecal copper excretion compared with copper sulfate. J. Anim. Sci. 82:1062-1070.
Wang, Z., Cerrate, S., Coto, C., Yan, F. and Waldroup, P. W. 2007. Evaluation of Mintrex Copper as a source of copper in broiler diets. Int. J. Poult. Sci. 5: 308-313.
Weiss, W.P., Wyatt, D.J., 2002. Effects of feeding diets based on silage from corn hybrids that differed in concentration and in vitro digestibility of neutral detergent fiber to dairy cows. J. Dairy Sci. 85: 3462–3469.
Wendt, M., 2011. Risk factors and prevention of lameness. In: Proceedings of the European Symposium of Porcine Health Management, Espoo, Finland, pp. 24–34.
Widdowson, EM. 1974. Trace elements in foetal and early postnatal development. Proc. Nutr. Soc. 33: 275–84.
34
Yan, F. and Waldroup, P. 2006. An evaluation of MINTREX organic trace mineral as a source of manganese in broiler diets. International Poultry Scientific Forum, Atlanta, Ga.
Yen, J. T., Ford, J. J. and Klindt, J. 2005. Effect of supplemental copper proteinate on reproductive performance of first- and second-parity sows. Can. J. Anim. Sci. 85: 205-210
Yu, Y. Study on characteristics and mechanisms of absorptions of zinc from different zinc sources in the small intestine of broilers. 2008. PhD
Dissertation, Chinese Academy of Agricultural Science, Beijing, P. R. China Zelko, IN, Mariani T. J., Folz, R. J. 2002. Superoxide dismutase multigenefamily: a
comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 33: 337–349