B.Mikic N.Todreas PFC/RR-79-15
FUSION TECHNOLOGY PROGRAM
Massachusetts Institute of Technology Cambridge, MA 02139
ABSTRACT
In previous work(',2), a design window approach has been applied to a liquid metal cooled, stagnant lithium breeding blanket, where the cooling tubes are spaced such that they all have the same heat flux per unit length (constant q'). This report is partly supplemental in that it is a detailed clarification of the equations and assumptions used, inclu-ding several refinements. However, it also includes documentation for a revised version of the WINDOW code used to generate the design windows, and (as an example of the usefulness of the design window approach) a comparison of lithium cooling to sodium cooling of this blanket. The results confirm the desirability of lithium as a coolant.
Table of Contents
Page
Abstract ii
Table of Contents iii
Nomenclature iv
1. Introduction
2. Design Window Analysis 3
3. Program WINDOW 11
4. Comparison of Lithium and Sodium Cooling 13
5. Conclusions 21
6. References 23
Appendix A: Source Listing of Program WINDOW 24
a - radial half-width of region cooled by i th cooling tube (m)
b- azimuthal half-width of region cooled by ith cooling tube (m)
B - magnetic field strength (T) C - defined by Eqn. (10)
C - specific heat of coolant (J/kg-0C) C1 - pumping power ration
DhDt - header and cooling tube diameter (m)
f - defined by Eqns. (23) and (24)
Fc - allowance factor in pressure loss calculations h - heat transfer coefficient (W/m2-oC)
kc,kLi ks thermal conductivity of coolant, structure and lithium pool (W/m-OC) L - major on-axis circumference of reactor (m)
M - blanket energy multiplication factor n - number of coolant tubes per header
N - number of blanket modules (or headers) azimuthally Nt - total number of coolant tubes
Nu - Nusselt number
APh'AEt - header and total pressure loss (Pa)
q' - heat load per uniit length (W/m)
01
2.
- first wall neutron energy loading (W/m
)
q"'(r) - volumetric heat generation rate in blanket (W/m3
Q
- total power removed per blanket module (W)r - radial distance from first wall
(m)-Ri - radial position of ith coolant tube (m)
Rw - first wall radius (m)
Rwo - reference first wall radius for neutronics calculations (m)
S - coefficient in q"'(r) (1/p)
Si - fitted reference coefficient from neutronics calculations (m)
th~tt
- header tube and coolant tube thickness (m)Tin - inlet temperature ( C)
Tm - maximum lithium pool temperature (0C)
ATc - coolant temperature rise across blanket (0C)
ATf - film temperature drop (0C)
ATm - temperature drop between coolant tube and maximum in pool (0C)
ATw - wall temperature drop
(
0C)Uh - coolant velocity at inlet (m/s)
v - fitted coefficient in q'''(r) (1/m)
x - axial length of coolant tubes (m)
z - radial thickness of blanket (M)
'ac , - fraction of blanket volume occupied by coolant and structural
c, s material (exclusive of header region)
- coolant viscosity (kg/m-s)
p - coolant density (kg/m3
ac'Ys - electrical conductivity of coolant and structure (1/a-m)
Clh - hoop stress (Pa)
1. Introduction
An interesting fusion reactor coolant/breeding material combination is liquid metal cooling with a stagnant lithium pool breeding region. A design methodology is developed to identify the constraints on a constant q' blanket model (Figure 1). Here, coolant tubes (running parallel to the main toroidal field to minimize MHD pressure loss) are distributed to
match the volumetric heating rate. Each tube receives the same heat input. This design emphasizes fewer tubes and lower thermal stresses.
The design window approach is to take basic thermal-hydraulic, structural and neutronic constraints, and use them to define limit lines in design parameter space. Here the length of the coolant tubes (x) and the number of tubes per module header (n) are used as the unspecified para-meters that must be chosen consistent with the constraints and design ob-jecti ves.
The limiting constraints in this blanket model are:
- maximum lithium pool temperature (vapor pressure becomes too large);
- maximum coolant temperature (limited by corrosion of-tube material);
- minimum coolant temperature (coolant must be liquid, and hot enough for
useful energy generation efficiency);
- maximum stress (primary membrane stresses must be less than the
struc-tural material yield strength);
- maximum neutron fluence (limited by materials damage);
N 2 B n Xh
I
coolant ,stagnant Li - tube - headerI1
coolant Constant q' Blanket Modeln(
- maximum number of cooling tubes (reliability decreases with complexity);
- maximum pumping power to heat removal ratio (economic limit);
- maximum header diameter (limited by space).
There is sufficient information available to choose values for
most of these constraints, irrespective of reactor parameters. For example, the maximum lithium pool temperature is about 9000C. This allows a margin of safety from the boiling point (13000C), and keeps the vapor pressure
low (less than 13 kPa) such that the blanket module need not be pressurized. The maximum pool temperature and other such constraints are implicitly in-cluded in the design window calculations.
The remaining constraints, notably the maximum neutron fluence, the maximum number of coolant tubes and the maximum header diameter, are more susceptible to other design considerations not included here.
Accor-dingly, these constraints are drawn as contours or limit lines, n = n(x), in the n-x design parameter space, and bound the acceptable design window.
2. Design Window Analysis
In this analysis of the constant q' blanket, materials properties are assumed known and temperature independent, some typical or reference
reactor parameters are used, and values of the constraints are taken as given. In addition, a fitted function is used to describe the volumetric heating rate through the blanket. The intent is to derive expressions for n = n(x) based explicitly on the total number of coolant tubes (Nt), the header diameter (Dh) and the first wall neutron loading (q").
w
The first limit line n = n(x) follows easily from the expression for the total number of coolant tubes,
Nt = NnL/x (
where N is the number of blanket modules (azimuthally), and L is the major circumference on axis.
The remaining equations require considerably more work. From neutronics calculations, the volumetric heating rate q"'(r) is expressed approximately as
q"'(r) = Sq"e-vr (2)
where S and v are fitted coefficients, and r is the distance from the first wall. Since the calculations are nominally for some reference first wall radius Rwo, Eqn. (2) must be generalized to handle other Rw. Now for
the same wall loading and total blanket volume
q"' = q".27rR L/volume (3)
w w
while for the same wall loading and blanket thickness
q"' = q" 2TrRL/{r(R +z)w w 2-TR }L (4)
In either case, q"' increases as R increases, so
q"'(r) !W- S q"e-vr (5)
which strictly is only correct for constant blanket volume. Note also that while q"'(r) may be correct for any fraction of lithium coolant in the blanket, the fitted coefficients will not be correct over a wide range of coolant volume fraction for other liquid metals.
I Given this heat generation rate, determine the size and location of the coolant tubes. Consider an arbitrary value of n. The tubes must
be radially spaced such that the constant q' condition is met. Since q"' decreases with r, the tubes are spaced further apart at the outer blanket edge than near the plasma. In fact, the radial distance between tubes is given by
2a. = 2a Rw+Ri e v(R i-R1) (6)
1 1 Rw+R.
where 2a is the radial width of the blanket volume "covered" by the cooling tube at radius R. from the first wall. It is in the region around the
outer tubes that the maximum blanket temperature occurs, and here
a Dt R e-vDt/2 evRn (7)
n 2 Rn+Rw
which assumes Dt (tube diameter) << 2Rw and, more significantly, that a1 = R = Dt/2. This latter implies that the tubes are very closely
spaced (radially) near the first wall.
Now an = z - R where z is the blanket thickness. So by deter-mining Dt from other considerations, Eqn. (7) can be solved for Rn' Ideally, Dt comes from the following system of equations:
n z = E 2a. (8a) i=l 2a D R e-vDt/2 evRi (8b) Rw+R R = £ 2a.-a. (8c)
However, this is a complex problem. But Dt is only needed in the calcula-tion of the maximum pool temperature, and there it is not a dominant factor. Accordingly, use an approximation (accurate for large n) to get reasonable
results. Rearrange z = E 2a. = Z 2a(R.) to obtain,
z
z (
(dR 2a(R) 0 2a(R)
Therefore, using Eqn. (6),
n evDt/2 _vz 1-(l+vz)e- vz vDt/2 c (10)
Dt R v Dt v
where c is the expression in brackets.
Now Dt is expected to be small, so Eqn. (10) can be reduced to give D - c/nv. But for a little more accuracy, expand the exponential,
Dt(I - vDt/2) - c/nv. Solving the quadratic in Dt and taking the correct root
Dt (1-/1--2c/n)/v (1
Using this value of Dt, Eqn. (7) can be solved for Rn, which is needed in calculating the maximum lithium pool temperature, Tmax*
In particular, the maximum temperature difference between the lithium pool and the coolant is given by(l,2)
im=2 f (Rn) 2 ( (b D2a(b_ ) + r b n n 2an(12)
Dt Rwe
-vDt/2
vRn where anRw+Rnbn = r(Rw+Rn )/N
(this assumes bn > an - otherwise simply interchange their definitions).
This can be written as
ATm = q". f (13)
w
where f is a function of known quantities.
The maximum blanket temperature is T max, where
Tm =T + ATc + ATf + ATW + ATm (14)
For given Ti and ATc (coolant temperature rise), and ATm from Eqn. (13), only the film (ATf) and wall (ATw) temperature drop need be found. The wall temperature drop is easily obtained as
Nu k 2t
AT -2 k ln(l+ ) AT (15)
where tt and ATf are still undetermined. (Under the blanket conditions, Nu is approximately constant over a wide range of flow velocities.)
The total power absorbed (and extracted) from each blanket module can be expressed in terms of the coolant temperature rise,
D2
Q = U pc AT (16)
or in terms of the heat transfer rate to the coolant,
or in .terms of the pumping power to heat removal ratio C, D2
Q = APt Puh 4 c (18)
or in terms of the absorbed energy flux
Q
q"(r) x 2f_ (Rw+r) dr0 N
Here Uh is the coolant velocity in the headers, and APt is the total pres-sure drop. Using Eqn. (5), Eqn. (19) becomes
NQ = q" 2TRwxM (20)
S
=wo vz {1+Rwv-(w+vz+R v)e-v (21)
where M is the effective blanket energy multiplication factor since q" 2rrR x is the incident neutron power. (It is assumed that the first wall thermal load is removed by a separate cooling system.)
Returning to the film temperature drop, combine Eqns. (17) and (20) to obtain
AT = q" 2RWM / Nn Nu kc (22)
Since Tmax is a known constant, Eqns. (13), (14), (15) and (22) can be rearranged to yield
q _ = Tmax - Tin - ATc
w 2Rw M -i+ Nu kc ln(+ tt) +f(23) nN Nu knc kNs 12 D t
The ratio tt Dt comes from the hoop stress limit, which is (because liquid metal coolants operate at low pressure)
Dt Ap
Y(24)
t h-y
and from combining Eqns. (16) and (18) to give
EPt Gpc ATC (25)
So Eqn. (23) gives the second limit line in terms of the wall loading. It is independent of x because the temperature constraints can be met for arbitrary x by suitable flow rates.
This brings up the final limit line which, by using a maximum hoop stress and a header diameter limit, restricts the maximum possible flow rate. In particular, it relates the maximum flow velocity to the maximum pumping power ratio through the pressure drop.
A general expression for the total pressure drop would include
(1) MHD pressure loss in the header where flow is perpendicular to the
B field, (2) friction pressure loss, (3) MHD pressure loss at corners,
and (4) MHD losses through regions of changing B field. Simple
satis-factory solutions are available for the first two terms, but not for the rest. Fortunately, usually only the-first term is significant. For the purposes of this analysis, total pressure drop is taken as the MHD pres-sure loss in the header region multiplied by an allowance factor, FC
AP = Fc A (26)
This factor is intended to encompass all other pressure loss terms and can be improved as better models become available. From calculations
An approximate expression for the header pressure drop, valid for large Hartmann number flow (H=B(Dt/2) /ac/u ) is
H2 4 2tac
-AP h ~ 1 2T thOc DO (Dh/h 2)2 ztotal (27)
Dh s
where
Oh
is the average header velocity and ztotal is the total header length (blanket plus shield). The blanket inlet header velocity Uh is of particular interest since it determines the total coolant mass flow, and here it is assumed that Uh - Oh, which is correct if the header is tapered towards the first wall. It is also assumed that the blanket and shield are of comparable thickness so that ztotal 2z.For the same maximum hoop stress in headers and coolant tubes, and allowing the pressure--and hoop stress-- to be equal everywhere if a tube becomes blocked, then
Dh/th = Dt/tt (28)
Equations (24) to (28) can be rearranged to yield the maximum header velocity consistent with Gh 5 Oy
u - (7r/8) Clpc pAT c +y_9
uhmax F B2 c
[
a. a -c s (29)where allowance has been made for pressure drop in the two headers. At this maximum velocity, a restriction on the header diameter would give a maximum flow rate pUhmax .- Dh. If the header is straight, then the maximum Dh is
Dh < 2rrRW/N (30) but if it tapers in towards the first wall,
Dh < 27(Rw+z)/N (31)
where the diameter of the headers is restricted at the entrance to the blanket module.
The maximum amount of energy that can be removed is then given by Eqn. (16). Combining this with Eqns. (20) and (23) then the third
limit line is 2RWMQ 1+ . ln(l+ -2tt n 1 2 ks D t n = Nu kc '{2nRwMx (T max-T in- ) - fNQ (32) where Q = L'hsmax -Dh2 . pc ATc
So Eqns. (1), (23) and (32) are of the form n = n(x) and describe the limit lines for the design window. These equations are incorporated into a program WINDOW (described in the next section) and are applied to lithium and sodium coolants in the final section.
3. Program WINDOW
The design window analysis has been implemented in the computer program WINDOW. In particular, WINDOW solves the limit line n = n(x) from Eqn. (32), and in the p-rocess yields values for the other two limit
lines given by Eqns. (1) and (23). It also calculates and prints other interestinq design variables such as AT f.
The program is a short FORTRAN program, with the input specifi-cally put on cards in the deck itself. The data needed includes materials data, reactor parameters, limiting parameters and fitted coefficients for q"'(r). These are listed (along with typical values) in the next section. A source listing of the program is enclosed, which indicates exactly how the data if formatted.
The output consists of a list of reactor parameters evaluated at the limit line from Eqn. (32) -- the Dh limit line -- at a range of values of n. Plotting n versus x gives this limit line. Since q" = q"(n) only, and since q"(n) values are output, then the second set of limit lines
can also be drawn. The final set is obtained easily from Eqn. (1) -- the N t limit line. A typical output is enclosed.
The input variables are explained in the program. The output variables are:
N - number of coolant tubes per blanket module (or header);
UH - header inlet velocity;
DELTAP - total pressure drop through blanket; DT - coolant tube diameter;
TT - coolant tube thickness;
RN - distance of last coolant tube from first wall;
TM - temperature difference between last coolant tube and maximum temperature in lithium pool;
TF - film temperature drop at last coolant tube's exit; TW - wall temperature drop at last coolant tube's exit; QW - first wall neutron loading;
QTOT - total reactor thermal power;
X - length of blanket module (without headers);
ALPHAS - percentage of structure in blanket volume, assuming straight headers;
ALPHAC - percentage of coolant in blanket volume, assuming straight header
4. Comparison of Lithium and Sodium Cooling
Liquid metals such as lithium and sodium are good, low pressure, high temperature, radiation damage resistant blanket coolants. Lithium is of special interest because it also moderates neutrons and breeds
tritium, has low induced activity, and is quite compatible with the refrac-tory metals. There is still interest, however, in liquid sodium as a near-term reactor coolant, especially in fusion-fission hybrids (5,6). This is because there is more experience with sodium (i.e., the LMFGR program), it is less corrosive to Fe and Ai based alloys (and stainless steel is the most likely near-term structural material), it is cheaper than lithium, it
(3) is inert to tritium, and it is somewhat less reactive
This section compares the two liquid metals as heat transfer agents. The purpose is to further quantify the relative merits of these
coolants and to illustrate the design window methodology just developed. Table 1 lists the parameters examined in this report, and compares them with representative values from some roughly comparable detailed reactor studies. Table 2 lists the materials properties used. While changing these parameters (e.g. TZM rather than stainless steel structure, or N = 20 rather than 100) will quantitatively change the conclusions, it is not believed that this will substantially affect the qualitative con-clusions regarding lithium versus sodium coolant.
The volumetric heat generation rate is taken as : q"'I(r) = 4.67 ( ) e-4.256r (r, Rw in m)(
T i s r t 4.6 y3 li .
)
qt pool/ ri(rium n, (33) This is strictly only true for lithium pool/lithium cool ant, but is assumed00 0 0 00 0) O LO C 00C C M 0 C CNOma LAO C- 00 * M r- 0 -- 0 0 00 Al 0 C c V? Vi to CD 00 Ln r 0 Or 00 C C" C) I CO * . r O 0 * LO O 0) I M C) 1*- 94- D It*- r-M: ON L .O O LAO C 0 r Nr- I - I e M LO . " 0 * 0) .J I J -- Lo. M O W kD m 0 r-. CN 00) LO LA 0 O r-r- CO 0 0 CO Or- C t os * o CO C V C) 0 LO Rdto M C J r-Mr- CJ LO 0 CO 9- 4-et to a E .C 0 Ou er-2 4.) F- N <3 :- 3: t F-L. - E .) :5 r- = a E2- C a) a 0) 0.) 0.) .C- E - -o -- c -n .- 2 C.- 0 C1 . w C E - -r- Q) :3 ( 4) -ld C S- () W) 3 - 4 *S- C - 2 4.J 4-C e o - D M 0 0 .4 a QJ ,.... # . ad CJ C * E 4.) a ni ) E C E- 40 -~C 4- U) r- =n CL. W. (-a 0 S. -r-. 0) 3 0e 4. 5. .x 0u 1- 0) 0 - 4 C 4 0- 4 r- 0) .. ro4- . cu r a a ed 1- 0 C 4 ) 2 -- U S. C. C ) r- C ) W -r- 3 *r- a) 2 3 .0 U 20O 4- - C C. A r~ 0 -0 C 1. '-- 4# 2 4. r- C. 4- 4- C . 0 -r- %a 0) &E 0 --- 3 ) C 4.) 3 = Q = m C L 06. C =.L 0) 1- .)# .2 4. 0u 4.) 0) 0 0) 2 C 0 in C 0) r~ in 0. .0 .0 0O--0 r- t - 0 r-C C -r- 2 2 2 0J rX 0 0 L. C3 c C *- CL 2 a): 0 0 r-2- 0 ~ - ) -J .-J in /) -Y -.#-A W LmC in C u 0 o a) -4 .-4. 0)4J) SS-CL "9- Ln 00 r- 4J ro Ca r-,-C) 0> 0-~ 4J 4) C 1- 1 41 0 c E C) S- r 00> C4 ---CL C) --r- C ui C rj ci - *.-4-) S.. ..C a) '-d 4 . to o 4- 0 -r- to4-C 3: cO 3 a 0 N C4-e Q ac 0 = 4 c~4 .) In 0) Lii C'.. Co - 0 O. 4J LA G) r-..0 0) .--I a) 9- 0.4.- 1-4. 0 4J 5. CC 4) U 4-a) C 0) S.. 9--. 0) 9- I- r- --0) .r- 4.) 4.) rv r-E 0 0 C s- c. = C 4J 1- SJ) 4.) 1-a, 4-3 a) E C. 0. 4. C 9-0 0 C..
C) CIC) r---. C, C)NS 0:t Xo x fs- LA CLO en 0.0 co C -S e O Co C.. S CD C CO o-- 1. C.O C C) . co x LO x Co X C)* %0- LA OD CD t. LA1 mo r- RIt LA* x m. -- 0 E I -3-4U1 -r-0 0 U 0r 4.) u u .C >- > .-- *- 4.)--*.- -. ro o 1 4.) 0 -C ej 0-ro C. F--* M 4-a 4J Co ,4-.o S 0 4 0 C 4.) C--0- .r-o M n x LC LO E 0 CD. - Un C) j O r-o In I -- O L- ) o ~C -0 LA ro a 4-- o 9. c C 4-) Ca) o Q 4. 4 - 4- C* Vo4) In 4. In 0 4) >i 41 4- > '-.I- -r- 4J C > > d I .rI- ."- 4 0 vn .u 4. 0 o 0 r-4) 4W a)- In . 4 r- -) -LL V) r -C -C I--o o A-4C) 4-) tv M Cn 0. C .-- F-- -0-Co C) EO 3 LO .- S 00 m' EO .) 0 - LO .r-0C --o C), 0) 4-) a) 2 C, -- S-U, 0) . di 0g
5.-reasonable for small volume fractions of sodium coolant (in the examples shown here, there is typically about 6% coolant, 0.5% structure). Further-more, since the pure lithium case had a breeding ratio of about 1.4,0)
the small amount of sodium coolant should not reduce the breeding unaccep-tably low.
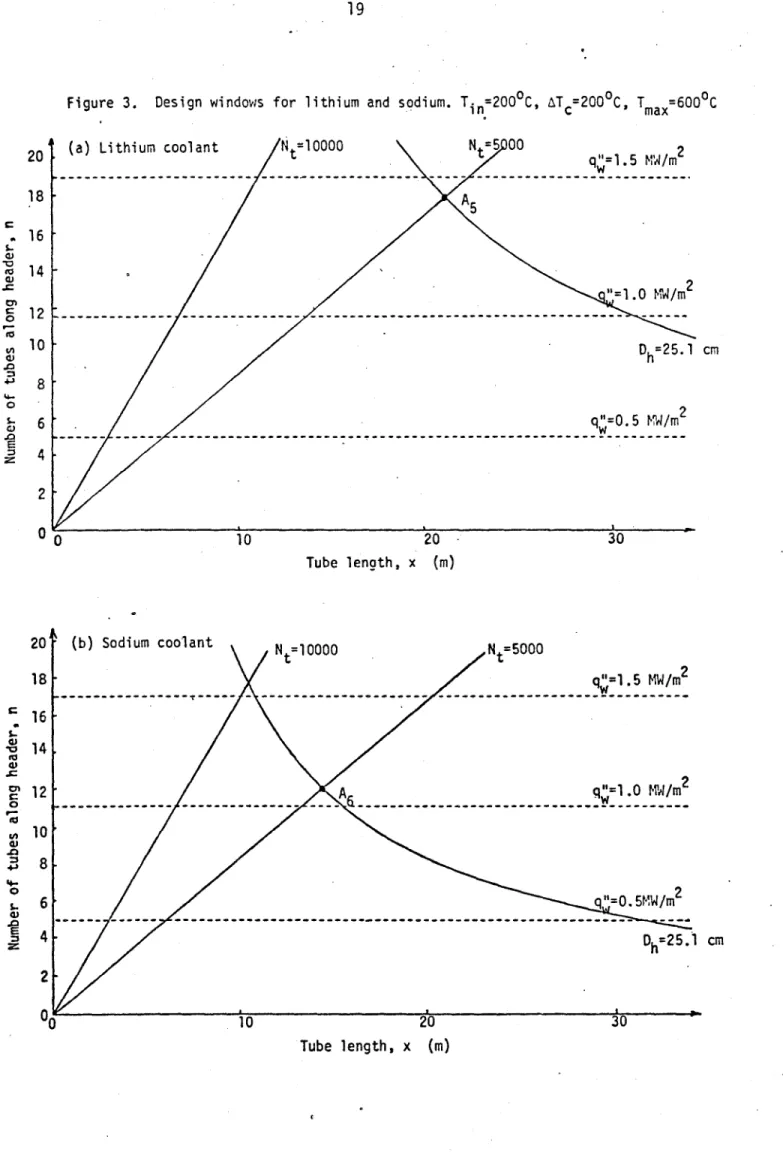
Figures 1, 2 and 3 show the design windows for lithium and sodium coolant. Figure 1 uses identical reactor parameters (notably Ti = ATc =
2000C, Tmax = 9000C). The shaded area is the acceptable design window
for Nt < 10000, Dh < 0.251 m and q" > 1.0 1W/m 2. Figure 2b takes
ad-vantage of the possible higher operating temperature of sodium /steel as compared with lithium/steel). Only the Dh limit line is appreciably changed, and two cases of T. = 2300C, AT c = 3000C and T. = 3000C, ATC = 2000C are shown. Figure 2a is identical with Figure la and is
repeated to simplify comparison of the lithium and sodium curves. Figure 3 again uses identical reactor parameters, but Trmax is dropped to 6000C and Nt reduced to 5000, to represent near-term reactor objectives. Table 3 compares the blanket parameters at point A--the maximum first wall loading design consistent with the constraints (note, though, that n is an integer, so some leeway was taken with the Nt line).
From all results, it is quite clear that lithium is a better coolant than sodium. Not only does it lead to larger option spaces, but it allows higher wall loading operation, higher total thermal power, and even fewer tubes for operating at a given wall loading. The higher possible tempera-ture of the sodium/steel system allows the optimum sodium design to approach the optimum cooler lithium/steel system (Case 3 compared with Case 1) but is
T =9000C N < 10000 q">1.0 MW/m 2 0 h<25.1 cm. max t w 2 Nt=1000 0 Nt=5000 q"=4.0 MW/m 20 --- --- --- ---18.
16 A (a) Lithium coolant
S14, I--c 10 q"=2.0 Kd/m2 4-) 6 4J q"=1.0 r-:d1m '4-D 2 5.1 cm E 2 h 0 0 20 30 Tube length, x (m) N t=10000 N =5000 -q2=4.0 MW/r 2 18 S16- (b) Sodium coolant 14 o,12-10 2 - q"=2.0 MA/m q"1. W A/ 40 8 --- -- -- --- --- --- ---42 '4- 06-0 EW Dh =25.1 cm 00 0 20 3o . 10 Tube length, x (m)
Nt=10000 Nt=5o06 4.0 l 2
20 N~ ~=4.o Nt q RA/m2
20 --- --- --- '---
---
---18
6 A (a) Lithium coolant
14 0 ~l2 .10, q"=2.0 Kd/m2 0 8 6. Sq"1=1.0 t-7/m2 4 --- --- --- ----2 =2 5.1 cm T. =2000C AT =2000C 10 ' 20 30 Tube length, x (m) 20 Nt=10000 Nt=5000 18 q"=3.0 MW/rm2 16-A 14 3 (b) Sodium coolant 0 2 S12A q"=2.0 MW/m S10 4J '4-T=20 T30 o8.i .0 4-10 20 30 Tube length, x (m)
Figure 3. Design windows for lithium and sodium. Tin=200in C, AT c=200C C, T max=600 C
(a) Lithium coolant Nt=10000 Nt=5 00 2
A5 2 "=1.0 MW/m w i--- ----.---- -- -- ---D h=25.1 cm q"=0.5 MWd/m2 10 20 30 Tube lenath, x (m) (b) Sodium coolant Nt=1000 0 Nt=50 00 q"=1.5 MW/m 2 2 A q"=1 .0 HW/m q"=0. 5MW/m2 Dh=2 5.1 cm 10 20 30 Tube length, x (m) 20
1
18 -0 0 -16 14 12 10 8 6 4 2' 0* 20 18 16 14 12 10 1-0 --0 81 4 2 U 0 0Table 3: Comparison of Lithium and Sodium Coolants for Maximum q" Design
Parameter Case 1 Case 2 Case 3 Case 4 Case 5 Case 6
Figure la lb 2b 2b 3a 3b Coolant Li Na Na Na Li Na Ti (in0C) 200 200 200 300 200 200 AT C(C) 200 200 300 200 200 200 T (0C) 900 900 900 900 600 600 max Nt 10300 10500 10300 10500 5100 5000 q" (MW/m2) 3.3 2.6 2.6 2.2 1.4 1.1 Qtotal (MW) 678 353 503 334 678 353 n 16 11 15 12 18 12 x(m) 9.4 6.3 8.8 7.0 21. 15. uh(m/s) 0.32 0.31 0.31 0.31 0.32 0.31 APt (MPa) 4.3 2.3 3.2 2.2 4.3 2.3 Dt(cm) 1.6 2.4 1.7 2.2 1.6 2.2 tt(cm) 0.058 0.045 0.046 0.040 0.058 0.042 ATm (C) 427 452 356 360 171 181 ATf(0C) 45. 31. 26. 28. 18. 12. AT w(C) 27. 16. 17. 12. 11. 6.6 a S() 0.86 0.66 0.69 0.58 0.48 0.38 ac( 6.0 8.7 6.4 8.0 3.3 4.9
still -not superior. So while sodium is still a viable coolant--all con-straints considered here can be met--these results show that lithium coolant is definitely the thermal-hydraulic choice.
These results confirm the conclusion that could be drawn directly from the -materials properties (Table 2) themselves. For liquid metal coolants in magnetic fields, two rough figures of merit are PC and a. The first is a measure of the coolant's ability to absorb heat, and the second is a measure of the resistance to flow in a magnetic field. Ideally, the first should be large and the second small. At 3000C, (PCP)Li = 2.2x106 J/rm- 0C ,
(PCp)Na = l.1x106 J/m3- 0C, and aLi =3.3x10' (Q-m)1 , a = 5.7xl06
(2-m)-Clearly lithium is superior in both respects. The results of this design window analysis illustrate exactly how these material advantages affect the blanket design.
5. Conclusions
The analysis in this report clarifies and refines the analysis of a liquid metal cooled, stagnant lithium breeding blanket with constant q' coolant tube spacing using a general design window methodology. In parti-cular, expressions are obtained relating basic reactor parameters to con-straint curves in n-x space, for the particular constraints of maximum number of coolant tubes (reliability limit), first wall neutron load
(design objective) and header diameter (physical geometry limit). A com-puter program, WINDOW, to calculate the design window curves is documented.
This analysis is then applied to a general comparison of lithium and sodium coolants. The results confirm that lithium is a better heat
transfer agent (3.3 MW/m2 maximum first wall loading as compared with
2.6 MW/m2, for the best cases considered here), although sodium is still shown to meet the basic constraints considered here. This application also serves to illustrate the usefulness of the design window approach.
6. References
1. J. Chao, B. Mikic and N. Todreas, "Thermal-Hydraulic and Neutronic Considerations for Designing a Lithium-cooled Tokamak Blanket,"
PFC/RR-79-ll, MIT, 1979.
2. J. Chao, B. Mikic and N. Todreas, "A Parametric Study of a Lithium Cooled Tokamak Blanket," Nud.. Tech., 42, January 1979, p. 22. 3. B. Badger et al., "UVMAK-I, A Wisconsin Toroidal Fusion Reactor Design,"
UWFDM-68, University of Wisconsin, Vol. I, 1974, p. IV-A-3.
4. M. Hoffman and G. Carlson, "Calculational Techniques for Estimating the Pressure Losses for Conducting Fluid Flows in Magnetic Fields," UCRL-51010, Lawrence Radiation Laboratory.
5. "Proceedings of the Second Fusion-Fission Energy Systems Review Meeting, Vol. I, November 1977," CONF-771155, DOE, July 1978, p. 149.
6. G. Moses, R. Conn and S. Abdel-Khalik, "Laser Fusion Hybrids - Technical, Economic and Proliferation Considerations," UUFDM-273, University
of Wisconsin, November 1978.
7. B. Gore and E, Murphy, "Current Fusion Power Plant Design Concepts," BNWL-2013, Battelle Pacific Northwest Laboratories, September 1976. 8. B. Badger et al., "UNMAK-III, A Non-circular Tokamak Power Reactor
Design," EPRI ER-368, Vol. I, July 1976.
9. R, Werner, "ORNL Fusion Power Demonstration Study: The Concept of the Cassette Blanket," ORNL/TM-5964, Oak Ridge National Laboratory, October 1977.
10, D. Rigney, S. Kapelner and R. Cleary, "The Electrical Resistivity of Lithium and Columbium-1 Zirconium Alloy to 14300C," Pratt and Whitney Aircraft (CANEL), TIM-854 (1965).
11, J. Ballif et al., "Lithium Literature Review: Lithium's Properties and Interactions," HEDL TC-1000 (1978).
12. G. Holden and J. Tokor, "Thermophysical Properties of Sodium," ANL-7323, Argonne National Laboratory, August 1967.
13. "Nuclear System Materials Handbook, Vol. 1, Design Data," TID-26666, Book 1, Hanford Engineering Development Laboratory, revised to June 15, 1979.
Appendix A
Le L Z n -j I .U B X - 1-Z _j 0. .- <-_: 4,* U-. e I-w S -- ~ ~ ~ .j- L w * j- Zm' C- I * )-LJ . L) <- I Ed 44UI W U LD I Li- -' - I * ~ % Z - IU..Z 0 o I4 ) U W ) - 0 o o.- . it UI L).W .-of u - ~ ~ ~ ~ ~ ~ .~ .. ~ U w U LU w . Za . I U. -. t. U -. . U I. -M LI ) W N I. C- .; w C, Ucc al ! in <. Lt*U t* 2.. - x w W . . - _j . - U. 0-- *. 5- u -<.L - ... < :LL. 0 . I--. -W I I Z~ V " C, 43 M U 0--= -o 0 UZ.U- I Uj C- 3 WwU I " *.a - .C<-.. = '-z - I U 0 0 OrZ r.,'-- - .< . .4 X c- *0>- U I c & --uL Li. 7U 2 (U X < r.<" I = a -c<
- UOL'UE. * 4J x <.U" n <J I.-*
U*- = - C .4 *U -r- V: -=-Q Us VI =S- - C woe.JL0..; %. uj' .4 u2 - 1n 0. 4 a-LL6 *.0 CJ U -U .. cc . Z0 z N < !e U <1. OUJjo *l-)(CI* I - U W -j tZ W LL<t V. '. 7- 0* z .0 1 M:E L. fLU0Z -- ~~ * UI e l I -4~ <~- L"tI - ~ ~ ~ ~ , *. zp- WU, U 0 ~ .W U . I
.-U C.I - -o -in> C U - = = *nU n
*U .. ~ = I-. V) .0 C 7 '-J Ij m -. u 4 e
Z' Z.~U~ . Z LU-- j% 0 - I ~ I LJ
~" - .. - _' *,)3 .', I.,- <.- > -jI ,IA _
Z l C.) 'A x a. x~ -- op Ll-U U < -J Z) -. < ie
-j w CC =- mf -- a..i- * - U 1<n n I;_ o Z > AU.i..DL
.j I . *0 *..i.- X ; -e L JJ~
-j<U
4.
U U *) U zu
-%U%-i~a C, .Z.U r< *Pj <-'j d:L
*< 4 -- L U1~ Z tLi0~ l <~u U ) l U. J
IAI LI)L. <W < - X'. ... U U * I ,;..~4 L 'U ZU. y
4IU_0_<-0 = M.U r uz. c: J. .J.J 21 1 7 11 1 71 01 1 1 0 U, U 1-J Q Ll U ci (J'3 U 'U- U 10 -) -U 0 . iU'-) U 0-1 0C C .~. .*% I C C.) c~ O r~
-'o L) > ItL3 I. I I0, - IW L). . 4 CL LAI1 N ) 0 =1 0l .1 L. u- i LLI -zi 4. 0 > X I -C UZ)i I/ M :) - * 0 LL i-n CC +~ 0. U . + b.-i' -I Z* ti,-J Ii . v 0 . - * If W = = 4 :L > x 4 .7Z I ) tf t I W. U., Li * .- - - *i z ; z- + 0 4n -- w)I' 6n v L) -*.-4 J-L 0 C :e -3 = mC NcI - OL 4, Nj4 :- NU 0Z- 7= -17 MLiZ- f ' _j 01 11. N.~ X. Li ne2 J% t 7 11 11 f I - -4 1 U 11 1 1 1 Z 11 1 _ j Z I t. , -1 0z -1 11 t ItN1 i W4t .. j 1 t 4 % - LiJ = I.j J 04 ... **j* Z I- ~~~~ Z Z Li.ZLi~ -->I w x .. o 4 ji t* Q + < . Li 0 - _, 0 . 0 0~ "' 0-r0 i~ ^ -Z 0 PCU ' .- ' - 3 0 - N 3' ~ ~ ~ ~ ~.C ~ i ~ ~ - Z- ~ n ) i00 (. en 0 -: 3 - e -q 0 .. ~ 0 Li ) 0 0 0 ~
000 30oCC~o Oe~0O 0~0Li -U000c~000000
-4 I a. z U- x I& I r - - 4 CP N N 0 o * -+ UJ 0 -4. .J r * 0 - 4. 0 A . U. 2- CP *f C4-) 9
Appendix B
Sample output of program WINDOW This output is for Case 5 of Table 3.
co LU 4na .0 U" N 4 2: -4 I'-.0 4 2: -4 0 y2 400 Z--4 .4 )4 2' %A- . 2:_j~~J0 .cJ nO~ 0 -4 0J U. N N' L- -1c < LU If zb VI *2 0 CC U' 0nZ LA. Li. -j _i -. X N N r .'v 4 w 'r en N 4- r. t - -N tQ ~ ~ ~ ~ ~ ~ 0C ' a. .'a.F r-- - 1N0.p N C 0 ON C-C, 0- Ln' M C. - N
w4-en- I. " en~. f en en a'n rn fn
<
- - - - -0 - -0 - 0-