HAL Id: tel-01941719
https://tel.archives-ouvertes.fr/tel-01941719
Submitted on 2 Dec 2018
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
moléculaires et évaluation de leur implication dans la
réponse thérapeutique
Dunja Sobot
To cite this version:
Dunja Sobot. Nanoparticules squalenisées et lipoprotéines plasmatiques : caractérisation des inter-actions moléculaires et évaluation de leur implication dans la réponse thérapeutique. Pharmacie galénique. Université Paris Saclay (COmUE), 2016. Français. �NNT : 2016SACLS419�. �tel-01941719�
NNT : 2016SACLS419
T
HESE DE DOCTORAT
DE
L’U
NIVERSITE
P
ARIS
-S
ACLAY
PREPAREE A
L’U
NIVERSITE
P
ARIS
-S
UD
E
COLED
OCTORALE N° 569
Innovation Thérapeutique - du fondamental a l’appliqué
Specialité : Pharmacotechnie et Biopharmacie
Par
Mlle Dunja Sobot
Nanoparticules squalenisées et lipoprotéines plasmatiques : caractérisation des
interactions moléculaires et évaluation de leur implication dans la réponse
thérapeutique
Thèse présentée et soutenue à Châtenay-Malabry, le 21 Novembre 2016 Composition du Jury :
Pr. Elias Fattal Professeur des universités, Université Paris-Sud Président Pr. Stefaan De Smedt Professeur des universités, Universiteit Gent Rapporteur Pr. Joseph Ciccolini Maître de conférences, Université Aix-Marseille Rapporteur Dr. Sylvain Huille Chercheur, Sanofi R&D Examinateur Dr. Andrey Klimchenko Directeur de recherche, Université de Strasbourg Examinateur Pr. Patrick Couvreur Professeur des universités, Université Paris-Sud Directeur de thèse Dr. Simona Mura Maître de conférences, Université Paris-Sud Co-directeur de thèse
Table of Contents
Abbreviations ... 3 Acknowledgements ... 6 General Introduction ... 8 Introduction Générale ... 10 Introduction ... 12Nanoparticles: Blood Components Interactions ... 12
Abstract ... 13
Introduction ... 13
Interaction with blood proteins and the complement system ... 15
Interaction with platelets and coagulation system ... 20
Interaction with lipoproteins ... 22
Conclusion ... 24
References ... 25
Chapter 1 ... 28
Squalenoylation of gemcitabine, a unique approach which exploits endogenous lipoproteins for drug delivery ... 30
Abstract ... 31 Introduction ... 32 Results ... 34 Discussion ... 39 Methods... 43 References ... 53 Supplementary Information ... 59 Chapter 2 ... 70
Circulating lipoprotein: a Trojan horse guiding squalenoylated drugs to
LDL-accumulating cancer cells ... 72
Introduction ... 73
Materials and methods ... 75
Results and Discussion ... 81
Conclusion ... 94
References ... 95
Supplementary Material ... 102
General discussion ... 114
Squalenoyl-Gemcitabine Nanoparticles Design ... 116
SQGem NPs formulation ... 116
SQGem NPs labeling tools ... 116
SQGem Nanoparticles and Circulating Lipoproteins ... 119
Investigating the interaction between nanoparticles and lipoproteins ... 119
SQGem NPs distribution among lipoproteins... 120
Mechanism of SQGem NPs interaction with LPs ... 122
Biological Implication of the Interaction Between SQGem NPs and LDL ... 124
SQGem targeting towards LDL-accumulating cancer cells – in vitro ... 124
SQGem targeting towards LDL-accumulating tumor xenografts – in vivo ... 124
The overall impact of SQGem NPs interaction with LDL ... 128
Towards clinical trials ... 128
Towards industrial production ... 129
Supplementary Material ... 131
References ... 133
General Conclusion and Future Perspectives ... 140
Abbreviations
ABC accelerated blood clearance
ALAT alanine transaminase
AP alternative pathway
ASAT aspartate transaminase
ATP adenosine triphosphate
Apo-E apolipoprotein-E
aPTT activated partial thromboplastin time
BBB blood-brain barrier
CM chylomicrons
CNRS Centre National de la Recherche Scientifique
CP classical pathway
CRM confocal Raman microscopy
Ctrl control
EDTA ethylenediaminetetraacetic acid
ERC European Research Council
ERK extracellular signal–regulated kinases
ESP electrostatic potential
FBS fetal bovine serum
FDA Food and Drug Administration
FRET Förster Resonance Energy Transfer
GMP Good Manufacturing Practice
HC high cholesterol
HDL high density lipoproteins
hENT1 human equilibrative nucleoside transporter 1
IC50 inhibitory concentration 50
IgE immunoglobulin E
IgM immunoglobulin M
LDL low density lipoproteins
LDLR low density lipoproteins receptor
LP lectin pathway
LPDF lipoprotein-deficient fraction
LPDS lipoprotein-deficient serum
LPs lipoproteins
MAPK mitogen-activated protein kinases
MD molecular dynamics
MFI mean fluorescence intensity
MPS mononuclear phagocyte system
MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Mw molecular weight
NPs nanoparticules
PAMAM polyamidoamine
PBCA polybutylcyanoacrylate
PBS phosphate buffered saline
PC phosphocholine
PDI polydispersity index
PEG poly(ethyleneglycol)
PI3K Phosphoinositide 3-kinase
PK pharmacokinetic
PLA polylactic acid
PLGA poly(lactic-co-glycolic acid)
PMFs profiles of mean force
POPC 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
PT prothrombine time
SC standard cholesterol
SDS sodium dodecylsulfate
SQGem squalenoyl-gemcitabine
SQ squalene
Acknowledgements
I would first like to thank to Pr. Stefaan De Smedt and Prof. Joseph Ciccolini for taking the time and effort to review this manuscript. I also thank to Prof. Elias Fattal, Dr. Sylvaine Huille and Dr. Andrey Klimchenko who did me the honor of accepting the invitation to be the part of my PhD jury.
At the end of these three years, I would like to express my gratitude to all the people in Galien Institute and especially to the director Prof. Elias Fattal who allowed me to spend my PhD in a dynamic and multicultural environment.
A special gratitude is expressed to my PhD director Prof. Patrick Couvreur who accepted me in his group and gave me the great opportunity to work on a variety of exciting scientific projects in a multidisciplinary environment. Thank you for guiding me through this first experience as an independent researcher, for teaching me how to cope with both success and failure, for boosting me with optimism even when everything seemed to be negative, for bringing out the best in me, for endless discussions whenever I needed them (this list could continue on several pages)… This PhD work would not be possible without my co-director Dr. Simona Mura. I am grateful for your scientific advice, for helping me to overcome every obstacle and supporting me in every experiment (even if it meant staying until 23h in Châtenay-Malabry), for being always available and comprehensive.
Many other people enriched the scientific value of this project with their contribution for which I am very thankful. Many thanks to Didier, Eric, Sinda and Sandrine who always responded to our crazy requests for chemical synthesis, Maike and Branko for Raman images, Semen and Christophe for computational studies, Bernard and Grégory for radioactive products, Prof. Delphine Borgel for human blood samples, Laura for her great work during Master 2 internship that built the foundation of my PhD project. I would also like to thank to Fanny, the best Master 2 student for her hard work, critical thinking and great autonomy in conducting the experiments. Special thanks to Prof. Jean-louis Paul who was always there to help us overcome our lack of expertise in lipoproteins with his valuable advice and to provide us with lipoprotein samples whenever we needed them.
her dynamic character and always energetic help with cell culture experiments, Celine for proteomics experiments and to people from animal facility for taking care of our mice and rats. Many thanks to all the permanent staff from Institut Galien, especially Juliette, Hervé, Julien and particularly Nicolas for his precious advice how to deal with PhD life on daily bases.
I am particularly grateful to all the PhDs and post-docs that I met during these three years because they create the core of Galien Institute and its worm and friendly atmosphere and they made my entire PhD experience particularly unforgettable! Special thanks to “les filles” Anaelle, Nadia, Nadege, Naila and Teresa for our never boring discussions. Finally, I am particularly grateful to Anaelle and Gianpiero for putting up with me during the last days of my PhD (when I was probably unbearable).
Great contribution to the success of my PhD (maybe not scientific, but as equally important) belongs to my family. First of all, I am infinitely grateful to my parents Slavko and Slavica and my brother Sinisa who were always there for me and even though far from home, I never felt the lack of their love and support (nor home-made food). Special thanks to my adorable ants Milena, Ljubica and Slavica who are like my “second mothers”. I am also grateful to my Belgium (Diana and David) and French (Milica, Thibault, Largo, Zorica and Bogdan) families who made me feel like home during these three years. I would like to dedicate this work to my uncle Patrick Moriau who had a special role in me becoming who I am today and even though he is no longer with us I know he would have been particularly proud.
Finally, I would especially like to thank to my other half, Milos. Thank you for all the love, affection, constant encouragement, endless discussions (sometimes scientific, sometimes motivational) as well as the artistic contribution to my PhD work. Thank you for being patient during my “nothing is working” crisis and for understanding me better than anyone else. I think that after our journey of two PhDs, we are strong and ready for the next exciting step…
General Introduction
Cancer is one of the leading causes of death in modern society with the number of new cases expected to rise by about 70% over the next two decades. Despite the increasing understanding of molecular mechanisms behind cancer development, curative treatment often remains uncertain. The failure of systemic chemotherapy is frequently related to (i) very poor delivery of anticancer agent to the tumor tissue and (ii) the insurgence of toxicity as a consequence of the non-specific action on healthy tissues. However, by taking advantage of some unique features of cancer cells, selective delivery (i.e., targeting) of anticancer drugs can be achieved.
In this context, some creative solutions have emerged along with the progress in the nanotechnology field and its application to medicine. A broad range of drug delivery systems (i.e., nanomedicines) has been developed in order to overcome the limits associated to the traditional chemotherapy.
This PhD thesis is part of the TERNANOMED European Research Council (ERC) Advanced Grant project in which, terpenoids have been used as biomaterials for the design of nanomedicines to improve the treatment of severe diseases including cancer.
In the TERNANOMED project a variety of active molecules has been conjugated to terpenoids and the resulting bioconjugates self-assembled in form of nanoparticles in water. In this PhD research, we focused on the bioconjugate obtained by the chemical linkage of the squalene (a triterpene, precursor of the cholesterol’s biosynthesis) to the anticancer drug gemcitabine. Our main objective was to investigate whether, due to the similarity between squalene and cholesterol, such nanoparticles could spontaneously interact with the circulating lipoproteins. Once the interaction was established, we have then evaluated if it could be exploited to indirectly target the cancer cells by taking advantage of the increased need of fast proliferating cells for endogenous lipoproteins.
The first introducing chapter of this thesis is a bibliographic review which will address the series of events that may occur in a complex biological environment after intravenous administration of nanoparticles. The main types of interactions between nanoparticles and blood components will be discussed focusing on their potential implications in the toxicity and in vivo fate of nanoparticles.
The experimental work is divided in two chapters. Chapter 1 will provide a detailed investigation of the interaction between squalene-gemcitabine (SQGem) nanoparticles (NPs) and circulating lipoproteins through in vitro, in vivo and in silico experiments. Once the interaction with LPs has been verified, it was worth exploring its potential implication on the pharmacological activity of SQGem NPs. Thus, the Chapter 2 of this thesis was devoted to elucidating whether the spontaneous interaction between SQGem NPs and lipoproteins could mediate a so-called “indirect” targeting towards cancer cells, displaying high accumulation of endogenous lipoproteins.
All chapters are in form of articles that have been published, are already submitted or are currently in preparation. In annex of this thesis, three published articles can be found attached: (i) a bibliographic review on the use of nanomedicines for overcoming cellular-based anticancer drug resistance and two research articles describing (ii) the synthesis of a deuterated probe for the Confocal Raman Microscopy imaging of SQGem nanoparticles and (iii) the investigation of the transport mechanism of squalenoyl-adenosine nanoparticles across the blood-brain barrier. The research leading to these results has received funding from the ERC under the European Community's Seventh Framework Programme FP7/2007-2013 (Grant Agreement No. 249835).
Introduction Générale
Le cancer est l'une des principales causes de mortalité dans la société moderne avec une incidence qui ne cesse d’augmenter. Malgré une meilleure compréhension des mécanismes à l’origine de son développement, les résultats thérapeutiques sont souvent limités. L’échec des chimiothérapies anticancéreuses conventionnelles est dû (i) à la faible quantité de médicament administré qui atteint le tissu tumoral et (ii) à la forte toxicité systémique engendrée par une distribution non spécifique touchant les tissus sains. Ainsi, l’adressage de molécules thérapeutiques vers les cellules cancéreuses constitue aujourd’hui un défi majeur pour le traitement du cancer.
Dans ce contexte, les nanotechnologies et leurs applications dans le domaine de la médecine ont pris un essor considérable au cours des dernières années. Un grand nombre de nanomédicaments vectorisant des molécules anticancéreuses ont été développés afin de surmonter les limites associées à la chimiothérapie conventionnelle.
Ce travail de thèse s’inscrit dans le projet European Research Council (ERC) Advanced Grant TERNANOMED, dont le but est de développer l’utilisation des terpènes comme biomatériaux dans la conception de nanomédicaments afin d’améliorer le traitement des maladies graves, y compris le cancer.
Dans le projet TERNANOMED, des molécules actives ont été couplées de façon covalente aux terpénoïdes afin de former des bioconjugués ayant la capacité de s'auto-organiser sous forme de nanoparticules en milieu aqueux. Dans ce projet de thèse, nous nous sommes plus particulièrement intéressés au bioconjugué obtenu suite à la liaison covalente du squalène (triterpène, précurseur de la biosynthèse du cholestérol) à la gemcitabine, une molécule anticancéreuse. Notre objectif principal était de déterminer si la similarité structurale entre le squalène et le cholestérol pouvait favoriser la prise en charge et le transport des nanoparticules de gemcitabine-squalène (NPs de SQGem) par les lipoprotéines plasmatiques (LPs). Après avoir confirmé l’interaction entre le bioconjugué et ces protéines, nous avons évalué s’il était possible de l’exploiter pour cibler de façon indirecte les cellules cancéreuses. En effet, de nombreux cancers surexpriment les récepteurs
aux lipoprotéines plasmatiques qui pourraient servir de porte d’entrée à la SQGem intéragissant avec ces macromolecules.
La partie introductive de cette thèse correspond à un travail bibliographique qui porte sur la série d'événements qui peuvent se produire dans un environnement biologique complexe après administration intraveineuse de nanoparticules. Les principales interactions entre les nanoparticules et les composants sanguins seront détaillées en mettant l'accent sur les implications potentielles dans la toxicité et le devenir des nanoparticules in vivo.
La partie expérimentale est divisée en deux chapitres. Le Chapitre 1 décrira l’évaluation détaillée de l'interaction entre les NPs de SQGem et les LPs à travers des expériences in vitro, in vivo et in
silico. Après vérification de l'interaction avec les LPs, il était important de comprendre son
implication dans l'activité biologique des NPs de SQGem. Ainsi, le Chapitre 2 de cette thèse sera consacré à élucider si l'interaction spontanée entre les NPs de SQGem et les LPs pourrait permettre un ciblage indirect des cellules cancéreuses présentant une forte accumulation de LPs endogènes. L’ensemble des chapitres sont sous forme d'articles qui ont été publiés, déjà soumis ou qui sont actuellement en préparation. En annexe de cette thèse, trois articles publiés sont joints: (i) une étude bibliographique sur l'utilisation des nanomédicaments pour contourner la résistance aux médicaments anticancéreux et deux articles de recherche décrivant (ii) la synthèse d'une sonde deutérée pour la microscopie confocale Raman des NPs de SQGem et (iii) l'étude sur le mécanisme de transport de nanoparticules d’adénosine-squalène à travers la barrière hémato-encéphalique. Ce projet a bénéficié d'un financement de l'ERC au titre du septième programme-cadre FP7 de la Communauté européenne / 2007-2013 (accord de subvention n° 249835).
Introduction
Nanoparticles: Blood Components Interactions
Encyclopedia of Polymeric Nanomaterials, Springer Berlin Heidelberg, (2014), pp. 1-10.
Dunja Sobot, Simona Mura, Patrick Couvreur *
Université Paris-Sud, Faculté de Pharmacie, 5 rue Jean-Baptiste Clément, 92296 Châtenay-Malabry cedex, France
CNRS UMR 8612, Institut Galien Paris-Sud, 5 rue Jean-Baptiste Clément, 92296 Châtenay-Malabry cedex, France
*To whom correspondence should be addressed. E-Mail address: patrick.couvreur@u-psud.fr
Tel: +33 1 46 83 53 96 Fax: +33 1 46 83 55 11
Abstract
Blood compatibility is a mandatory requisite for the safe intravenous administration of drug-loaded nanoparticles. In this context, the assessment of nanoparticles interactions with blood components (i.e., blood cells, proteins, lipoproteins, components of the coagulation system) is needed. Indeed, the interaction of nanoparticles with blood components might trigger pathophysiological events and modify nanoparticle physico-chemical properties thus determining their in vivo fate and influencing their biodistribution, pharmacokinetic, therapeutic efficacy and potential toxicity.
Introduction
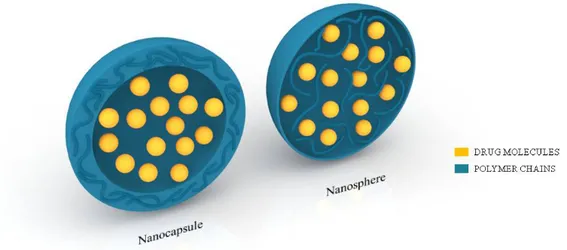
Over the past 40 years, owing to the impressive progress in the nanotechnology field and its application in medicine, a broad range of drug delivery systems (i.e., nanomedicines) has been developed in order to overcome the limits associated to the traditional drug delivery modalities. Nanomedicines can enhance drug properties by (i) offering protection from degradation, (ii) enabling controlled spatio-temporal release and distribution and (iii) increasing bioavailability. After loading, the fate of the drug is no more dependent on its physicochemical properties but relies on those of the carrier: specific distribution at tissue, cellular and even subcellular level can be achieved by opportune nanocarrier design thus also reducing side effects associated to nonspecific drug distribution. Among the various materials (e.g., lipids, polymers, inorganic materials) employed for drug delivery purposes, polymers have attracted a great deal of interest due to the high versatility of the synthesis methods, the extreme variety of their properties and composition as well as their ease of functionalization [1]. Polymer-based nanocarriers include: (i) nanoparticles, (ii) polymeric micelles and (iii) polymersomes. In this chapter, in the interest of brevity, we will mainly focus on polymer nanoparticles. Nanoparticles are defined as colloidal-sized particles with diameter range from 1 to 1000 nm. In nanoparticles, the drug can be either physically dispersed, or dissolved, or even chemically bound to the polymer chains. According to the preparation method, nanospheres or nanocapsules can be obtained (Fig. 1). Nanospheres are matrix-like systems in which the drug is dispersed within the polymer throughout the particle. On the contrary, nanocapsules are formed by a drug-containing liquid core (aqueous or lipophilic) enwrapped within a single polymeric membrane thus creating a vesicular nanodevice, acting as a “reservoir” system [2].
Safe application in the biomedical field of such drug-loaded nanoparticles requires assessment of their biocompatibility, expression that refers to the “benignity of the relationship between a material and its environment” [3]. Specifically, for intravenously administered nanomedicines, a major safety issue is related to the blood compatibility (that is, hemocompatibility), since it is the first tissue encountered after administration.
Once in the bloodstream, nanoparticles, indeed encounter a complex physiological environment which includes blood cells, proteins, lipoproteins as well as components of the coagulation system. Interaction with these components can trigger pathophysiological events such as complement activation and blood clotting. In addition, such interactions might modify nanoparticle physico-chemical properties, thus determining their in vivo fate and influencing their biodistribution, pharmacokinetic, therapeutic efficacy and potential toxicity.
The main types of interaction of nanoparticles with the blood components will be discussed in this chapter.
Fig. 1. Morphology of nanospheres and nanocapsules. Nanocapsules: drug-containing liquid
Interaction with blood proteins and the complement system Opsonization and complement activation
After intravenous administration, nanoparticles, as any other foreign element, come into contact with the plasma proteins by random Brownian motion and undergo a phenomenon called “opsonization”, which consists in nanoparticles labeling by proteins called opsonins [4, 5]. Major opsonins include blood serum proteins (e.g., laminin, fibronectin, C-reactive protein, type-I collagen), immunoglubulins (i.e., IgG and IgM) as well as opsonic fragments derived from C3 complement protein cleavage. The nature of the opsonins forming a corona at the nanoparticles (NPs) surface influences their fate in the bloodstream. Opsonized nanoparticles are mainly taken up by the specialized phagocytic cells of the mononuclear phagocyte system (MPS), mostly represented by the Kupffer cells of the liver, the macrophages of the bone marrow and spleen, hence, these organs are the main sites of accumulation of the nanoparticles [2, 5].
Opsonization is strongly dependent on nanoparticles physico-chemical surface properties, shape and size [6]. For instance, it has been observed that (i) nanoparticles with hydrophobic surfaces, or positively/negatively charged show higher tendency to opsonization and (ii) polystyrene worm-like nanoparticles are phagocyted to a lesser extent compared to spherical ones of equal volume [7, 8].
Despite the fact that the exact mechanisms involved in the opsonization process are rather complicated and still have not been completely elucidated, it has been demonstrated that the complement system plays a crucial role [5, 9, 10].
The complement system includes more than thirty distinct plasma proteins that can be activated
via three different pathways: (i) classical (CP), (ii) alternative (AP) and (iii) lectin (LP) one [4, 6].
Although these pathways use different recognition molecules to detect a foreign particle, they all converge to the same step: formation of the C3 convertase enzymes and cleavage of the complement protein C3 into the active fragments, C3a and C3b. This represents the main event of the complement activation [6].
While the CP, manly involved in the defense of the host from pathogen invasion, is activated by immune complexes antigen/specific antibody, the LP is triggered by the recognition of carbohydrate molecules on pathogens surfaces by mannose-binding lectin and L- and H-ficolins. On the other hand, a wide range of foreign materials, endowed with different physico-chemical properties, can activate the AP. Although this pathway is constantly kept at a low level of activity by the spontaneous hydrolysis of a small amount of the C3 protein in the plasma, it is also directly activated by C3b-opsonized materials and is therefore able to amplify the C3 conversion, initially triggered by the classic and lectin pathways. It results in an increased amount of C3b-mediated tagging of the foreign elements making them visible to the MPS, which mediates their clearance through specific receptor-ligand interactions [4]. Noteworthy is that all the three pathways can be responsible for phagocytosis of the foreign materials and hence of nanoscale drug delivery systems [11]. Indeed, in spite of the fact that the AP can trigger direct opsonization of the nanoparticles, the other pathways can be activated as function of the protein components of the nanoparticle corona and the nanoparticle surface properties such as presence of different functional groups and hydrophilic/hydrophobic domains [4, 12].
NPs opsonization and rapid clearance by macrophages highlight the crucial role of the complement system to protect the organism from foreign materials. However, complement activation could also induce important damages to self-tissues by triggering strong inflammatory and immune responses. In clinical practice, symptoms such as hypertension or hypotension, dyspnea, flushing, rash, etc., have been observed in a high percentage of patients (up to 45%) after NPs administration, whereas a fatal cardio-pulmonary syndrome may happen in rare cases. It has to be noted that these symptoms, which occur after the first treatment without prior sensitization and IgE production, have the tendency to resolve after repeated administrations, thus differing from a type I allergy and being defined as “pseudoallergic reaction”. It is believed that this reaction is initiated by the formation of potent anaphylatoxines (i.e., C3a, C4a and C5a), which can trigger the release of secondary mediators from immune cells (Fig. 2). Careful control of the rate of NPs perfusion as well as premedication with antihistamines and steroids are generally used in clinical practice to lessen these symptoms which remain very difficult to predict [6].
Fig. 2. Nanoparticles and the complement system. Nanoparticles can trigger any of the three
activation pathways and they all lead to the lytic complex formation. Important consequences of this activation are nanoparticle opsonization and rapid clearance by macrophages as well as generation of anaphylatoxins C3a and C5a which are responsible for the appearance of the symptoms of pseudoallergic reaction. Adapted with permission from ref. [12].
Escaping phagocytosis
Over the past three decades several strategies have been developed to interfere with NPs opsonization thus avoiding the rapid uptake by MPS, increasing the blood circulation time and enabling NPs to reach their biological target [10]. Among them, surface modification with hydrophilic polymers such as poly(ethyleneglycol) (PEG) and PEG-containing polymers has been shown to successfully achieve these effects through a process called dysopsonisation [5, 13]. PEGylation can be achieved (i) by physical adsorption or covalent grafting of PEG chains at the surface of preformed nanoparticle or (ii) by using a block copolymer in which the PEG moiety is linked to the hydrophobic block (e.g., poly(lactic acid), poly(lactic-co-glycolic) acid, or poly(alkylcyanoacrylates)) prior self-assembly in form of nanoparticles. The former approach presents, however, the inconvenience of a possible desorption of the PEG shell after injection and subsequent exposure of the nanoparticle surface thus allowing opsonin attack [2, 5].
The widely accepted theory to explain the stealth protective effect conferred by PEG coating is based on the formation of a dense, hydrophilic shell and the steric stabilization of the NPs.
Hydrophilic and flexible PEG chains assume an extended conformation in aqueous medium and start to be compressed in presence of serum proteins. This compression generates repulsive forces which are able to counteract the attractive forces between the opsonins and the particle surface [14].
The ability of the PEG chains to hinder NP opsonization is correlated to (i) PEG molecular weight (Mw), (ii) surface density and (iii) chain conformation. Several authors reported that a more effective protection could be achieved with high Mw PEG chains (2,000 to 5,000 Da) than with shorter ones due to their higher flexibility and the creation of a thicker shell [5, 13]. At low density, the PEG chains have greater freedom of motion and assume a “mushroom” conformation in which they are close to the NPs surface. However, in this configuration, some spaces may remain between the chains so that the surface may become accessible for small protein binding. On the other hand, increasing the chain density, the PEG chains assume a “brush” conformation and assure the complete coverage of the particles surface, thus blocking the access to proteins. Nevertheless, PEG chains in this conformation display reduced flexibility which compromises the steric repulsive effect of the PEG layer. Therefore, prevention of nanoparticle-protein interaction can be achieved with a conformation intermediate between the “mushroom” and the “brush” one, in which the PEG density assures adequate surface coverage maintaining a certain freedom of motion [10, 15].
Potential PEG immunogenicity
Despite the widely diffused usage of PEG to design stealth drug delivery systems, a debate about its potential immunogenicity has arisen. Several researchers reported that, upon repeated administration, the PEG coating might trigger the clearance of PEGylated drug delivery systems by the MPS cells. This so called “accelerated blood clearance” (ABC) phenomenon, leads to rapid liver uptake and blood clearance of the second and following doses of PEGylated nanocarriers. Initially described after administration of PEGylated liposomes, it has been also observed for other injected PEGylated nanocarriers such as polymer nanoparticles later on. The ABC phenomenon, which is believed to be very similar to hypersensitivity reactions, is composed of two phases: the induction and the effectuation. The former implies the interaction between the PEGylated NPs administered as first dose and the immune system, triggering the production of a large amount of
anti-PEG immunoglobulin M antibodies (IgM) by splenic B-cells. Consequently, anti-PEG IgM present in the bloodstream would recognize and bind at the surface of PEGylated NPs administered consecutively within a short time interval, thus causing complement activation, NPs opsonization and, finally, enhanced uptake by Kupffer cells [16]. The parameters which play a crucial role in NPs phagocytic uptake seem to be involved also in the insurgence of ABC phenomenon. For instance, it was observed that small polymeric micelles (mean diameter <31.5 nm) were not able to induce ABC phenomenon unlike larger ones (mean diameter >50.2 nm). In addition, surface hydrophobicity, which promotes the interaction with the blood components, the PEG molecular weight and the surface chain density appear highly important as well [16]. ABC has been reported to be triggered by both PEG-modified PLA and PLGA NPs, reaching the highest extent one week after the first administration [17-18]. For the former, the effect was clearly related to particle size (smaller NPs induced a weaker response) and PLA molecular weight but independent on the PEG content.
The accelerated blood clearance represents a major clinical concern since it could be responsible for a reduced therapeutic efficacy of the loaded drugs. However, several studies demonstrated that such process does not occur after the administration of particles carrying a cytotoxic therapeutic agents (e.g., Doxil®, doxorubicin-loaded PEGylated liposomes), probably as consequence of the possible toxic effect of the loaded drugs on the hepatosplenic macrophages [16].
In addition to the ABC process, it has been recently observed that PEG chains could also induce complement activation via the lectin pathway, due to the structural analogy between PEG segments and a region of the D-mannose moiety. Once again, the nanocarrier size plays a pivotal role and it has been demonstrated that, due to the limited surface available for LP-convertase deposition, NPs with a diameter lower than 30 nm promoted weaker complement activation compared to the larger ones [6].
It is evident that the insurgence of the previously described events imposes severe limitations to the introduction of PEG-modified drug delivery systems in clinical practice and there is an urgent need of new strategies to design long circulating non immunogenic nanocarriers. In this view, a promising unique squalene-based and PEG-free nanomedicine with systemic long circulating
properties was recently proposed. It is believed that the ability to evade clearance mechanisms is due to elongated morphology of these nanoassemblies and subsequent opposition to interaction with phagocytes. However, the exact mechanism of such long half-life in plasma is not fully understood and needs to be further investigated [19].
Interaction with platelets and coagulation system
Interaction of materials applied in the biomedical field with blood components such as platelets and coagulation factors might be responsible for severe side effects due to the activation of the coagulation cascade and thrombi formation. Mainly focused on cardiovascular devices such as stents, catheters and cardiopulmonary bypass [20], little attention has been dedicated to drug delivery systems, even though similar polymeric materials are being used. Noteworthy is that in the case of nanoscale carriers, the extent of interaction and the potential deleterious effects can be highly amplified due to high surface to volume ratio which results in a large surface area available to react with the surrounding environment.
The coagulation system includes cellular (i.e., platelets, endothelial cells, leukocytes) and protein components (i.e., blood coagulation factors). Activated platelets release in the blood different mediators such as growth factors, ATP and serotonin and trigger the activation of leucocytes and endothelial cells [21]. Since blood coagulation factors mainly circulate in the form of zymogens (i.e., inactive enzyme precursors), they require proteolytic activation in order to exert their function [20, 21].
Initiation of clotting occurs by two distinct pathways, the intrinsic and extrinsic one, which then converge into the common pathway, leading to thrombin formation and subsequent conversion of soluble fibrinogen in fibrin, the building block of a hemostatic plug. The fibrin clot is stabilized by the thrombin-activated factor XIII [20, 22]. The extrinsic pathway is activated upon tissue damage and blood vessel exposure to the tissue factor (TF). On the other hand, the intrinsic pathway is initiated upon activation of high molecular weight kininogen (HMWK), prekallikrein and Factor XII, following exposure to negatively charged surfaces (e.g., collagen) [21, 22].
Activated partial thromboplastin time (aPTT) and prothrombine time (PT) are used as markers of intrinsic and extrinsic pathway performance, respectively.
Mainly, biomaterials trigger activation of the coagulation cascade by the intrinsic pathway, due to the adsorption of blood proteins which endow them with a negative charge. But, it has been reported that biomaterials could induce TF expression by monocytes resulting in activation of the extrinsic pathway as well [20]. Still, precise identification of the activated pathway seems puzzling [20, 22].
Some nanoparticles have been specifically engineered to interact with the coagulation cascade in order to treat coagulation disorders. Two main categories can be identified: (i) systems that trigger thrombus formation to prevent bleeding and (ii) systems that are intended to inhibit coagulation (i.e., antithrombotic) [21]. In this chapter, attention will be focused on non-intentional nanoparticles/coagulation cascade interactions which might be responsible of severe toxicity. However, elaboration of a general conclusion remains rather difficult due to the few and often contradictory data available.
In the context of a toxicological evaluation of urban particulate matter, first studies on the thrombogenic potential of polymer nanoparticles have been performed, mainly to assess the effects of the passage of inhaled nanoparticles in the systemic circulation [23, 24]. For instance, the role of surface chemistry was investigated using neutral polystyrene NPs and tuning the surface charge
via amino and carboxylate modifications in order to have NPs positively and negatively charged,
respectively. In this study, only amine-modified NPs enhanced thrombus formation and ADP-mediated platelets activation after systemic administration [24]. Similar results were observed with cationic polyamidoamine (PAMAM) dendrimers. The absence of influence on both aPTT and PT suggested that the effect of the positively charged nanocarriers was related to interaction with negatively charged platelets, disruption of lipid membrane integrity and internal cytoskeletal structures, thus facilitating platelet to platelet interactions [25, 26].
Opposite results were obtained when carboxylate-modified polystyrene NPs were tested by other groups, who reported a significant increase in platelets aggregation [27, 28]. However, not any
effect on coagulation was triggered by sulphonate-modified NPs, thus suggesting that a negative charge by itself was not sufficient for the intrinsic pathway activation to occur. Oslakovic and coworkers also reported an inhibitory effect of amine-functionalized NPs on blood coagulation, which was inversely correlated to NPs size. Proposed mechanism relies on plasma depletion of coagulations factor FVII and FIX, adsorbed on NPs, thus hindering thrombin generation via the intrinsic pathway. Hence, smaller nanoparticles were more efficient due to the higher surface-to-volume ratio and their high reactivity [27]. In addition, examples of stronger platelet aggregation with larger particles are reported [25].
Interestingly, it was shown that cationic PAMAM dendrimers caused significant decrease in thrombin generation as well, by binding to platelets surface and, hence, disabling procoagulant protein binding to phospholipids exposed on platelets surface. Nevertheless, they induced platelet aggregation by different mechanisms previously mentioned [26].
To be noted that surface modification of nanoparticles with neutral, hydrophilic polymer brushes (e.g., PEG chains) has been used as a strategy to overcome the pro-coagulant effect by hindering surface adsorption of coagulation-related proteins.
To our knowledge, and as discussed above, only conflicting results are currently available, which points out the complexity to evaluate the influence of drug nanocarriers on the coagulation cascade and their interaction with platelets. In addition, it is worth to remember that surface properties of nanoparticles are modified in the bloodstream, thus the proposed mechanisms might be a simplified version of the sequence of events occurring in vivo.
Interaction with lipoproteins
Another class of blood components that can highly influence drug pharmacokinetic and toxicity are lipoproteins. In 2002, the US Food and Drug Administration (FDA) encouraged the introduction of lipoprotein-drug distribution studies for every novel drug formulation containing hydrophobic moieties [29]. For nanoscale systems, the impact of the nanocarrier itself on the interaction of the loaded drug with lipoproteins needs to be investigated.
Lipoproteins are lipid/protein complexes which transport water insoluble lipids in the biological fluids. They consist of a lipid core, mainly made of triglycerides and cholesterol esters and an amphiphilic surface composed of phospholipids and free cholesterol. The hydrophilic part of the lipoproteins is represented by proteins (i.e., apopoproteins) embedded in the phospholipid layer, which confer structural stability and capacity of interacting with specific receptors. According to their density and electrophoretic mobility, lipoproteins are assigned into four classes: chylomicrons (CM), very low density lipoproteins (VLDL), low density lipoproteins (LDL) and high density lipoproteins (HDL). The CM are the largest and the least dense particles, whereas the HDL show the highest density and the smallest diameter. Lipoprotein classes differ in the lipid composition, the type of apopoprotein they contain and their lipid to protein ratio [29].
Lipoprotein-based systems, endowed with targeting capacity due to the interaction with their specific receptors, have been proposed as drug delivery vehicles [30]. However, the complex composition and fragile nature of lipoproteins impose many limitations such as expensive industrial production, complicated isolation from human blood and difficult storage. Altogether, these limitations have hindered the further development of these drug delivery systems. On the other hand, limited attention has been devoted to the interaction of nanocarriers with the lipoproteins physiologically present in the circulatory system. In addition, studies were mainly focused on liposomes, due to their lipid composition and structural analogy with the lipoproteins, rather than on polymer nanoparticles. Nevertheless, the ability of NPs to interact with lipoproteins and adsorb apoproteins at their surface as result of an exchange process has been demonstrated [31]. For instance, preferential adsorption of Apo-E was observed for Tween®80-modified polybutylcyanoacrylate (PBCA) nanoparticles [2]. Furthermore, not only apopoproteins, but entire HDL particles could be found in some copolymer nanoparticles corona [32].
Lipoprotein-nanoparticles interaction could be very advantageous from several reasons. Firstly, the presence of the lipoprotein receptors on the blood-brain barrier (BBB) as well as on the various types of tumor cells makes this interaction a potential tool for improving drug delivery to cancer tissues and brain parenchyma [33]. Secondly, due to an inhibitory effect of apopoproteins A-I and A-II on the polymerization of the complement protein C9, nanoparticle/lipoprotein interactions could potentially play a regulatory and modulatory role on the complement system, thus mitigating the undesired side effects already discussed before [6]. Finally, the binding of physiological
lipoproteins onto nanoparticles could explain why they are not recognized as foreign material by the host organism and do not elicit toxic immune response [34].
Conclusion
As briefly described in this chapter, the interactions of nanocarriers with blood components might have important consequences on the pharmacokinetic and toxicological profile of the loaded drugs. Moreover, it is expected that the fast progresses in material science will introduce, in the near future, a large variety of new materials in the biomedical field. Therefore, the complexity of interactions will likely increase, hence, unpredicted outcomes may impose serious health and financial issues.
It is worth highlighting that interaction with NPs can trigger multiple reciprocal reactions among the various physiological blood components as mentioned above. For instance, activated platelets are capable of potentiating the inflammatory response which strongly depends on complement activation as well [28]. Furthermore, thrombin itself could be involved in complement activation [6]. Although in the interest of clarity, the coagulation and complement activation cascades have been here discussed separately, it is evident that they appear to be involved in a complex cross-talk activity, which results in inflammation and thrombotic responses [22]. In other words, an intricate sequence of interactions generally occurs concomitantly, so that they should be considered as a whole in order to claim the hemocompatibility of any drug delivery nanodevice. Hemocompatibility of polymer nanoparticles seems to be related to specific properties such as size, surface hydrophobicity and charge. Still, the limited number of existing studies hinders the elaboration of universal conclusions. Undeniably, there is an urgent need for standardized and rigorous studies which would enable to assure the safety of any novel drug delivery system, thus probably making shorter the step toward clinical translation of laboratory based nanomedicines.
References
[1] Nicolas J, Mura S, Brambilla D et al (2013) Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev 42(3):1147-235.
[2] Couvreur P, Hillaireau H (2006) Polymeric Nanoparticles as Drug Carriers. In: Uschegbu SA (ed) Polymers in Drug Delivery. CRC Press Taylor and Francis group.
[3] Kohane DS and Langer R (2010) Biocompatibility and drug delivery systems. Chemical Science 1(4):441-446.
[4] Johnson RJ (2004) Immunology and the Commplement System. In: Ratner B, Hoffman A, Schoen F, Lemons J (ed) Biomaterials Science; An Introduction to Materials in Medicine. Elsevier Academic Press
[5] Owens DE and Peppas NA (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 307(1):93-102.
[6] Moghimi SM, Andersen AJ, Ahmadvand D et al (2011) Material properties in complement activation. Adv Drug Deliv Rev 63(12):1000-7.
[7] Lynch I, Cedervall T, Lundqvist M et al (2007) The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv Colloid Interface Sci 134–135(0):167-174.
[8] Ernsting MJ, Murakami M, Roy A et al (2013) Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release 172(3):782-94
[9] Peracchia MT, Vauthier C, Passirani C et al (1997) Complement consumption by poly(ethylene glycol) in different conformations chemically coupled to poly(isobutyl 2-cyanoacrylate) nanoparticles. Life Sci 61(7):749-761.
[10] Storm G, Belliot SO, Daemen T et al (1995) Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Delivery Rev 17(1):31-48.
[11] Nilsson B, Ekdahl KN, Mollnes TE et al (2007) The role of complement in biomaterial-induced inflammation. Mol Immunol 44(1-3):82-94.
[12] Lettiero B, Andersen AJ, Hunter AC et al (2012) Complement system and the brain: Selected pathologies and avenues toward engineering of neurological nanomedicines. J Control Release 161(2):283-289.
[13] Gref R, Minamitake Y, Peracchia MT et al (1994) Biodegradable long-circulating polymeric nanospheres. Science 263(5153):1600-3.
[14] Jeon SI, Lee JH, Andrade JD et al (1991) Protein—surface interactions in the presence of polyethylene oxide: I. Simplified theory. J Colloid Interface Sci, 142(1):149-158.
[15] Vonarbourg A, Passirani C, Saulnier P et al (2006) Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 27(24):4356-4373.
[16] Abu Lila AS, Kiwada H and Ishida T (2013) The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J Control Release 172(1):38-47.
[17] Ishihara T, Takeda M, Sakamoto H et al (2009) Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res 26(10):2270-9. [18] Saadati R, Dadashzadeh S, Abbasin Z et al (2013) Accelerated blood clearance of
PEGylated PLGA nanoparticles following repeated injections: effects of polymer dose, PEG coating, and encapsulated anticancer drug. Pharm Res 30(4):985-95.
[19] Maksimenko A, Dossio F, Mougin J et al (2014) A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. Proc Natl Acad Sci U S A 2:2.
[20] Gorbet MB and Sefton MV (2006) Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. In: Williams DF (ed) Biomaterials: Silver Jubilee Compendium, D.F. Elsevier Science: Oxford.
[21] Ilinskaya AN and Dobrovolskaia MA (2013) Nanoparticles and the blood coagulation system. Part I: benefits of nanotechnology. Nanomedicine 8(5):773-84.
[22] Amara U, Rittirsch D, Flierl M et al (2008) Interaction between the coagulation and complement system. Adv Exp Med Biol 632:71-9.
[23] Ilinskaya AN and Dobrovolskaia MA (2013) Nanoparticles and the blood coagulation system. Part II: safety concerns. Nanomedicine 8(6):969-981.
[24] Nemmar A, Hoylaerts MF, Hoet PH et al (2002) Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Respir Crit Care Med 166(7):998-1004.
[25] Dobrovolskaia MA, Patri AK, Simak J et al (2012) Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol Pharm 9(3):382-93.
[26] Jones CF, Campbell RA, Brooks AE et al (2012) Cationic PAMAM Dendrimers Aggressively Initiate Blood Clot Formation. Acs Nano 6(11):9900-9910.
[27] Oslakovic C, Cedervall T, Linse S et al (2012) Polystyrene nanoparticles affecting blood coagulation. Nanomedicine 8(6):981-986.
[28] McGuinnes C, Duffin R, Brown S et al (2011) Surface derivatization state of polystyrene latex nanoparticles determines both their potency and their mechanism of causing human platelet aggregation in vitro. Toxicol Sci 119(2):359-68.
[29] Wasan KM, Brocks DR, Lee SD et al (2008) Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: implications for drug discovery. Nat Rev Drug Discov 7(1):84-99.
[30] Bricarello DA, Smilowitz JT, Zivkovic AM et al (2011) Reconstituted lipoprotein: a versatile class of biologically-inspired nanostructures. ACS Nano 5(1):42-57.
[31] Rahman M (2013) Nanoparticle and Protein Corona. In: Martinac B (ed) Protein-Nanoparticle Interactions: The Bio-Nano Interface. Springer
[32] Hellstrand E, Lynch I, Andersson A et al (2009) Complete high-density lipoproteins in nanoparticle corona. FEBS 276(12):3372-81.
[33] Kim DH, Lijima H, Goto K et al (1996) Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem 271(14):8373-80.
[34] Linkov I, Steevens J (2009) Interactions with lipids. In: Linkov I, Steevens J (ed) Nanomaterials : risks and benefits. Springer: Dodrecht.
Chapter 1
In the first introducing part we have given a brief insight into the complexity of the possible interactions which can occur between nanoparticles and the blood components, highlighting their potential impact on the in vivo fate of nanoparticles. It emphasized the importance of identifying and characterizing these interactions with every novel nanoscale drug delivery system before its introduction into clinical trials. Thus, in Chapter 1 we have investigated whether the squalene-gemcitabine (SQGem) nanoparticles (NPs), previously developed by our research group, interacted (or not) with circulating lipoproteins (LPs). The rationale behind the particular interest in lipoproteins relies on the lipid nature of SQ, a natural triterpene and precursor of the cholesterol’s biosynthesis which, similarly to cholesterol, might be transported by plasma LPs in the blood stream. We have demonstrated that, after intravenous administration, nanoparticles made of the squalene derivative of gemcitabine (SQGem) strongly interacted with LPs, which indirectly enabled targeting of cancer cells with high LP receptors expression. In vitro and in vivo experiments revealed a preeminent affinity of SQGem towards LP particles with the highest cholesterol content and in silico simulations further demonstrated their incorporation into the hydrophobic core of LPs. To the best of our knowledge, the use of squalene to induce drug insertion into LPs for “indirect” cancer cell targeting is a novel concept in drug delivery and it has been further explored in Chapter 2.
Chapitre 1
Dans la partie introductive, nous avons donné un aperçu de la complexité des interactions entre les nanoparticules et les composants sanguins et nous nous sommes intéressés à leur possible impact sur le devenir in vivo des nanoparticules. Ces éléments ont permis de montrer l'importance de l’identification et de la caractérisation de ces interactions pour tout nouveau nanomédicament, notamment avant son passage en phase clinique. Ainsi, dans le Chapitre 1, nous avons cherché à savoir si les nanoparticules (NPs) de gemcitabine-squalène (SQGem), préalablement développées au sein de notre équipe, interagissaient (ou non) avec les lipoprotéines plasmatiques (LPs). L'intérêt particulier pour les LPs repose sur la nature lipidique du squalène (SQ), triterpène naturel et précurseur de la biosynthèse du cholestérol, qui pourrait, comme le cholestérol, être transporté par les LPs dans la circulation sanguine. Nous avons pu mettre en évidence, après injection intraveineuse, l’interaction spontanée entre la SQGem et les LPs, ce qui pourrait permettre de cibler indirectement les cellules cancéreuses exprimant fortement les récepteurs aux LDL. Les résultats obtenus in vitro et in vivo ont montré que l’association préférentielle de la SQGem aux LPs était directement corrélée avec la quantité de cholestérol présente dans ces dernières. De plus, les simulations in silico ont montré que l’incorporation de la SQGem avait lieu dans le noyau hydrophobe des LPs. A notre connaissance, le couplage du squalène dans le but d’induire l'insertion de molécules thérapeutiques dans des LPs, pour un ciblage "indirect" des cellules cancéreuses, représente une stratégie innovante et potentiellement révolutionnaire dans le traitement expérimental du cancer. Cette hypothèse sera explorée dans le Chapitre 2.
Squalenoylation of gemcitabine, a unique approach which exploits
endogenous lipoproteins for drug delivery
Article under revision in Nature Communications
Dunja Sobot1, Simona Mura1, Semen O. Yesylevskyy2, Laura Dalbin1, Didier Desmaele1, Sinda Lepetre-Mouelhi1, Fanny Cayre1, Bernard Rousseau3, Jean-Louis Paul4, 5, Christophe Ramseyer6, Patrick Couvreur1
1Institut Galien Paris-Sud, UMR 8612, CNRS, Univ Paris-Sud, Université Paris-Saclay, Faculté de Pharmacie, 5 rue Jean-Baptiste Clément, F-92296 Châtenay-Malabry cedex, France. 2Department of Physics of Biological Systems, Institute of Physics of the National Academy of Sciences of Ukraine, Prospect Nauky 46, 03028, Kyiv, Ukraine. 3CEA Saclay, iBiTecS-S/SCBM, Labex LERMIT, 91191 Gif-sur-Yvette, France. 4AP-HP, Hôpital Européen Georges Pompidou, Service de Biochimie, 75015 Paris, France. 5Lip(Sys) 2, Athérosclérose: homéostasie et trafic du cholestérol des macrophages, Univ Paris-Sud, Université Paris-Saclay, 92296 Châtenay-Malabry, France. 6Laboratoire Chrono Environnement UMR CNRS 6249, Université de Bourgogne Franche-Comté, 16 route de Gray, 25030-Besançon, Cedex, France.
Correspondence and request for materials should be addressed to P.C. (email: patrick.couvreur@u-psud.fr)
Abstract
Once introduced in the organism, nanoparticles immediately interact with a plethora of biomolecules thereby acquiring certain biological identity, which can strongly impact their in vivo fate. Here we demonstrated that, after intravenous administration, nanoparticles made of the squalene derivative of the gemcitabine (SQGem) strongly interacted with lipopoproteins (LPs), which indirectly enabled targeting of cancer cells with high LP receptors expression. In vitro and
in vivo experiments revealed a preeminent affinity of squalenoyl-gemcitabine towards LP particles
with the highest cholesterol content and in silico simulations further displayed their incorporation into the hydrophobic core of LPs. Such indirect targeting of cancer cells was then confirmed both in cell culture and in an experimental tumor model in mice. To the best of our knowledge, the use of squalene to induce drug insertion into LPs for indirect cancer cell targeting is a novel concept in drug delivery. It represents a flexible, highly versatile platform that would enable efficient drug delivery by simply exploiting endogenous lipoproteins without the need of complex nanoparticles surface functionalization or artificial lipoproteins production.
Introduction
Gemcitabine (Gem) is a nucleoside analogue widely used in clinical practice for the treatment of various solid tumors [1, 2]. However, the anticancer activity of gemcitabine is hampered by serious limitations such as: (i) short biological half-life due to rapid blood metabolization, (ii) intracellular diffusion, which is restricted to the expression of the nucleoside transporter hENT1 and (iii) emergence of various mechanisms of resistance [3, 4, 5]. Consequently, developing improved gemcitabine formulations is an important challenge in cancer drug discovery. In this context, we have developed an original approach relying on the introduction of squalene (SQ), a natural triterpene and precursor of the cholesterol’s biosynthesis, as a biocompatible material for drug delivery purposes. The concept of “squalenoylation” consists in the use of squalene as a building block for the synthesis of SQ-drug bioconjugates, which demonstrated the ability to self-assemble in aqueous medium in the form of nanoparticles (NPs), without the need of any other transporter material [6, 7]. The bioconjugates obtained by the covalent linkage of SQ to gemcitabine (SQGem) resulted in the spontaneous formation of nanoparticles in water, with a diameter of about 100 - 200 nm and high drug loading (~40%) [8]. Preclinical evaluation of these nanoparticles revealed reduced blood clearance and metabolisation after intravenous administration and greater in vitro and in vivo anticancer activity, compared to free Gem, against both solid subcutaneously grafted tumors and aggressive metastatic leukemia [9, 10, 11]. It has been also reported that SQGem NPs can interact with cellular membranes [12, 13]. But, in the absence of any specific ligand, how these NPs could accumulate into cancer cells remained totally unexplored. Therefore, despite promising results, the introduction of this therapeutic concept into clinical trials has been hindered by a lack of knowledge concerning the exact mechanism behind tumor recognition and anticancer activity of SQGem NPs. Accordingly, current efforts need to be focused on the identification and elucidation of such mechanism of action.
Once introduced in the organism, nanoparticles encounter a complex biological environment composed of a plethora of endogenous molecules. In function of the composition of the surrounding biological environment (depending on the administration route), as well as the nanoparticles physico-chemical properties (e.g., material, size, surface charge and functionalization), nanoparticles will immediately interact with a specific set of biomolecules,
thereby acquiring certain biological identity [14- 17]. This identity will govern the in vivo fate of nanoparticles in terms of biodistribution, pharmacokinetics, therapeutic efficacy and potential toxicity [18, 19, 20]. The interacting biomolecules might (i) hinder the nanoparticles recognition by the targeted cells or, on the contrary, (ii) increase the specific interaction of the nanoparticles with the corresponding biological target [18, 21, 22].
Among proteins capable of interacting with nanoparticles, apolipoproteins have received special attention [19, 23-26]. Apolipoproteins are amphipathic molecules, which associate with different plasma lipids to form complex structures called lipoproteins (LPs), acting as macromolecular vehicles of water-insoluble lipids in the circulation (e.g., cholesterol, triglycerides, etc) [27]. Lipoproteins display various structures and functions and according to their ultracentrifugation flotation density and electrophoretic mobility, they can be classified into chylomicrons (CM), very low density lipoproteins (VLDL), low density lipoproteins (LDL) and high density lipoproteins (HDL). Apolipoproteins play a major role in determining LPs structural stability, metabolism, as well as interaction with cells since they act as ligands for LP receptors [28, 29].
Despite observing the presence of these apolipoproteins at the NPs surface after intravenous administration, only few studies investigated the interactions of nanoparticles with blood lipid components or with the lipoprotein particles as a whole [30, 31] and examined the relevance of such interactions for interpreting the in vivo fate of NPs. The association to LPs in the blood stream has been largely described for many hydrophobic drugs and it is well known that it can have a strong impact on the drug disposition and biological activity. Accordingly, since 2002, the US Food and Drug Administration (FDA) has recommended the introduction of lipoprotein-drug distribution studies for every novel drug with hydrophobic character [32]. In addition, LPs have been described as excellent carriers for targeted delivery of various drugs and imaging agents, due to their endogenous, non-toxic, long-circulating nature and to their ability to be recognized and taken up via LP receptors [33-35]. However, the development of LPs for drug delivery has been hampered by the difficulty to prepare reproducible drug loaded endogenous LPs [36].
The capacity of lipoproteins to transport hydrophobic molecules, the lipid nature of squalene as well as its structural similarity with cholesterol (Supplementary Fig. 1) altogether led us to believe
that the interaction between the SQGem nanoparticles and lipoproteins deserves to be deeply explored. In the present study, we demonstrated that SQGem bioconjugates in NPs could be captured and hence transported by plasma lipoproteins, in particular via cholesterol-rich ones, both
in vitro in human blood and in vivo in rodents. We have also analyzed the ability of SQGem to
spontaneously interact with LDL on a molecular level. It was discovered that endogenous LDL particles may function as carriers for SQGem, thus allowing the indirect targeting of cancer cells displaying high expression and activity of LDL receptors, without the need to functionalize NPs surface with hydrophilic PEG (polyethylene glycol) chains and/or with specific ligands.
Results
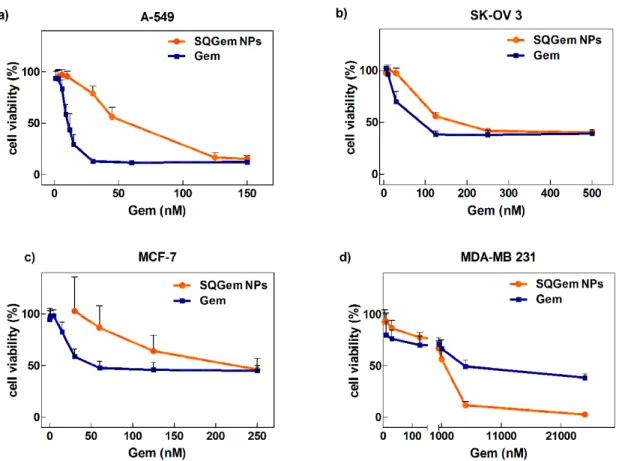
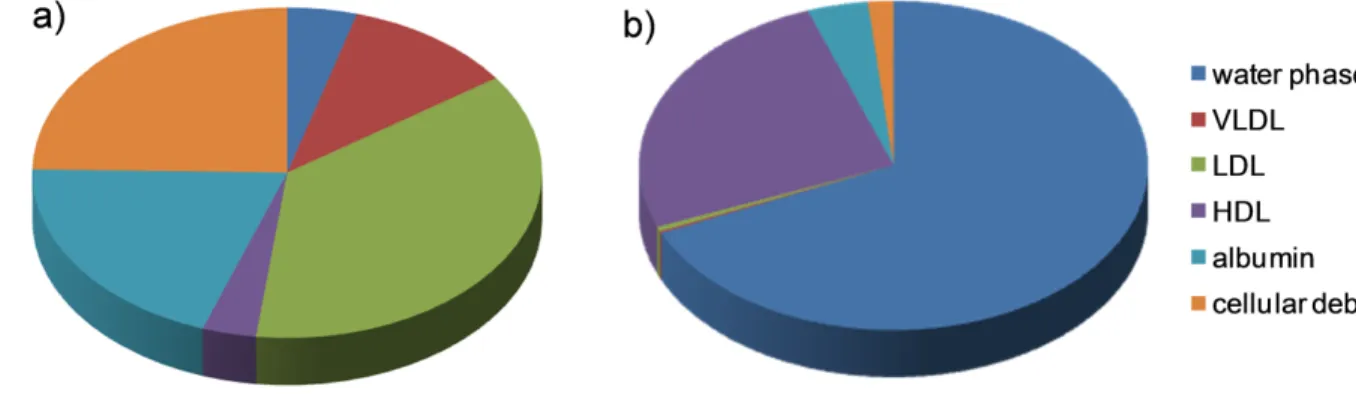
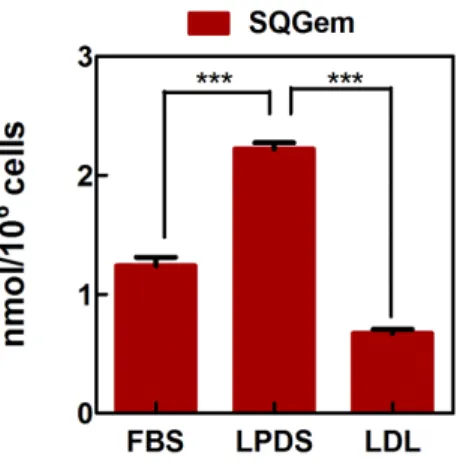
In vitro distribution of SQGem and Gem in human plasma fractions. The interaction of SQGem NPs with the different plasma fractions was first investigated in vitro after incubation with human blood samples at 37°C for 5 min. In order to track the distribution of SQGem in the different fractions, radiolabeled 3H-SQGem NPs were prepared and the radioactive signal was monitored. After blood centrifugation and plasma separation from the blood cells, it was found that most of 3H-SQGem remained in the plasma (~80%) (Supplementary Fig. 2), which was then analyzed for 3H signal into: (i) the lipoprotein (LP) fractions (i.e. very density lipoprotein (VLDL), low-density lipoprotein (LDL) and high-low-density lipoprotein (HDL)), (ii) the albumin fraction and (iii) the water phase. The latter corresponded to unbound 3H-SQGem. To be noted that after ultracentrifugation step, a small pellet was observed at the bottom of the ultracentrifugation tube, which was attributed to cellular debris still present in the plasma. The results expressed as a percentage of the overall plasma signal revealed that, once in contact with human blood, 3 H-SQGem was mainly distributed between LP fractions (51 %) and albumin (20 %) with some amount associated to the cellular debris (25 %) and very small quantity present in the water phase (4 %) (Fig. 1a). Remarkably, among the LP fractions, the LDL was the predominant 3 H-SQGem-interacting lipoprotein (37 %) followed by VLDL (11 %) and HDL (3 %). On the other hand, free 3H-Gem was almost entirely recovered in the water phase (70 %), even if some radioactivity was also measured in HDL (24 %). Interaction of 3H-Gem with albumin and LDL was almost negligible (3 % and below 1%, respectively) (Fig. 1b). The 3H-SQGem NPs and 3H-Gem distribution among the different plasma fractions was further tested after longer blood incubation times (t = 15, 30,
60, 90 and 120 min) but the distribution profile remained unchanged overtime for both formulations (Supplementary Fig. 3).
Figure 1. 3H-SQGem and 3H-Gem distribution in plasma fractions – in vitro. (a, b) The
distribution of 3H among the different plasma fractions was analyzed after incubation (5 min) of 3H-SQGem NPs (a) or 3H-Gem (b) with human blood. Results are expressed as a percentage of total plasma radioactivity (mean values, n=3).
The molar concentration of 3H-SQGem found in each fraction was then related to the molar concentration of albumin, LDL and HDL in each patient’s blood sample (Fig. 2). Results revealed a dramatic affinity of 3H-SQGem for LDL (i.e., 43 moles of Gem per mole of LDL), while more than a 50-fold lower affinity was observed towards HDL and albumin. The very low molar ratios of Gem confirmed the negligible interaction of the free drug with plasma components.
Figure 2. Molar concentration of SQGem (green bars) and Gem (blue bars) per mole of
LDL (a), HDL (b) and albumin (c) after incubation with total human blood (5min). Bars represent mean ± standard error of the mean (s.e.m.) (n=3).
In silico modeling of the SQGem and Gem interaction with lipoproteins. Atomic details of the interaction of LDL with single SQGem and Gem molecules were obtained by means of molecular dynamics simulations. A simplified atomistic model, which reproduces the hydrophobic properties of the lipid cores of LDL particles, was used. We computed the potential energy profiles of mean force (PMFs) of transferring Gem and SQGem molecules from bulk water to the lipid core of LDL particle (Fig. 3). It was clearly observed that interaction of Gem with LDL was energetically unfavorable while SQGem had strong affinity towards LDL and accumulated in their hydrophobic core.
Figure 3. Potentials of mean force of transferring individual Gem (red line) and SQGem (blue line) molecules from bulk water to the lipid core of model LDL particle. The plots are
superimposed onto a snapshot of the equilibrated LDL system. 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) lipids are shown in blue, 1- palmitoyl -2-hydroxy-sn-glycero-3-phosphocholine (lyso PC) in red, cholesterol in orange, cholesterol oleate in gray and glyceryl trioleate in green.
In vivo distribution of SQGem and Gem in rat plasma fractions. The distribution of SQGem between the different plasma fractions was then investigated after intravenous injection of 3 H-SQGem NPs to rats with plasma collection 5 min post administration. Twenty fractions (200 µl each), from the top to the bottom of the tube, were separated on the basis of their hydrated density by NaBr gradient ultracentrifugation method. According to the measured density and the


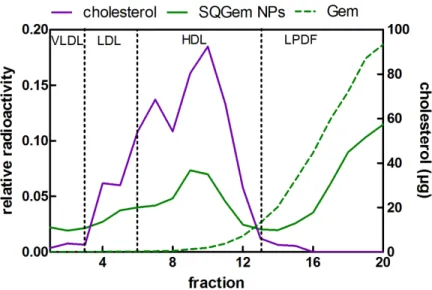
![Table 1. Composition of the system used for molecular dynamics simulations of LDL. The number of lipid molecules corresponds to 1/10 of the whole LDL particle which has been simulated in coarse-grained simulation [57]](https://thumb-eu.123doks.com/thumbv2/123doknet/15051547.695347/49.918.271.646.203.591/composition-molecular-dynamics-simulations-molecules-corresponds-simulated-simulation.webp)