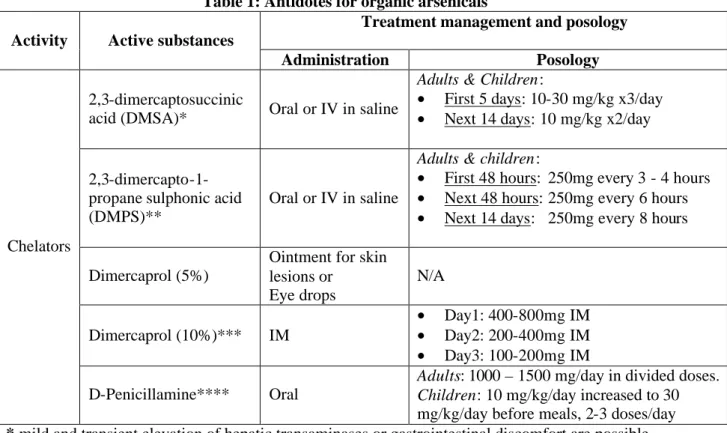
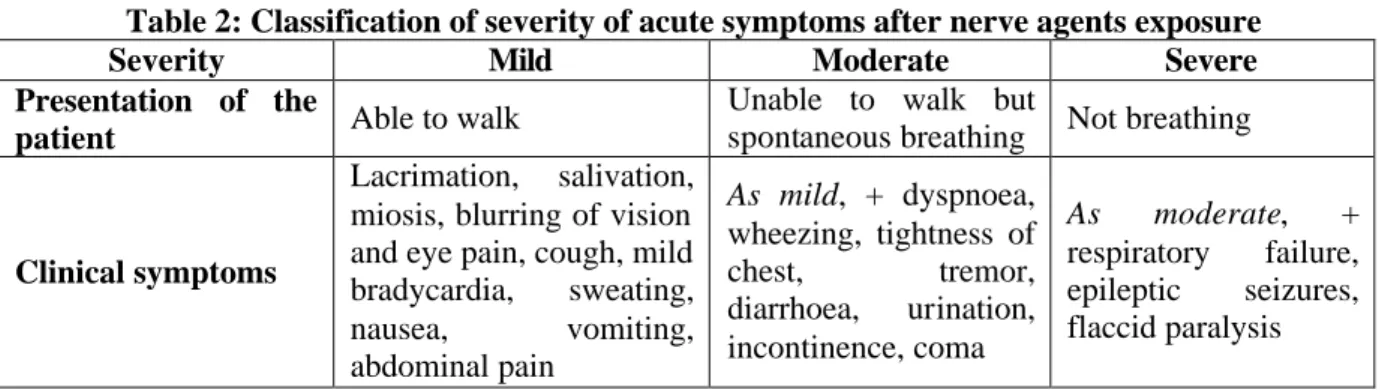
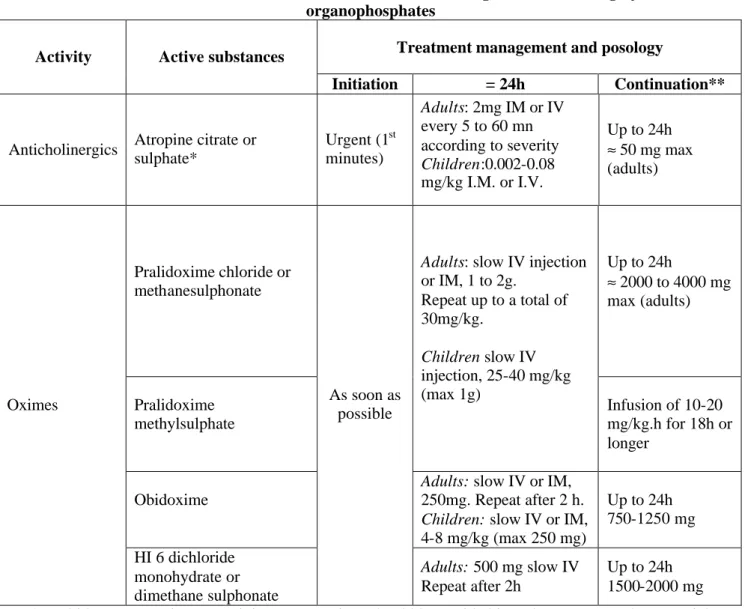
The European Agency for the Evaluation of Medicinal Products Pre-authorisation Evaluation of Medicines for Human Use
7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 8613
Texte intégral
7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 8613
Figure

Documents relatifs
Katarzyna Bandurska, Department of Microbiology and Biotechnology, Institute of Chemistry, Environmental Protection and Biotechnology, Jan Dlugosz University in
SUHVHQWVUHFHQWZRUNVFDUULHGRXWRQXVLQJ&DORQHDQG&FRXSOHGWRRWKHUWUHDWPHQWPHWKRGV DLPLQJDWWUHDWLQJZDVWHZDWHU
Considering the positive scientific opinion of artesunate-pyronaridine under the European Medicines Agency (EMA)’s Article 58, its inclusion in the WHO list of prequalified
This talk will provide an overview of this new challenging area of research, by analyzing empathy in the social relations established between humans and social agents (virtual
The agents that cause infant diar- rhoea have been largely identified, and vaccine candidates against most of the important pathogens (rotavirus, enterotoxigenic E.
LtdChinaChina othion WP (2 g/m2 active ingredient)Ki-Hara Chemicals LtdUnited KingdomNo information provided othion WP (2 g/m2 active ingredient)Sumitomo Chemical Co.
At lower resolutions of histological imagery, textural analysis is commonly used to capture tissue architecture, i.e., the overall pattern of glands, stroma and organ organization.
Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder.. Urology