Publisher’s version / Version de l'éditeur:
Technical Translation (National Research Council of Canada. Division of Information Services), 1949-11-02
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20331569
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at Investigation of Raw Materials for the Production of Mineral Wool
Zhuravlev, V. F.; Sychev, M. M.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=7911fdbb-c7f1-4910-bc73-aa305c29a582 https://publications-cnrc.canada.ca/fra/voir/objet/?id=7911fdbb-c7f1-4910-bc73-aa305c29a582
NATIONAL RESEARCH COUNCIL OF CANADA DIVISION OF INFORMATI ON SERVICES
Technf c a l T r a n s l a t f on No, 13-10
IhWSTIGATION OF KAIV MATERIALS FOR THE
PRODUCTION OF FAINEHAL WOOL
( I s s l e d o v a n f e S y r f i a d l f a P r o f z v o d s t v a Mfneralnof S h e r s t f )
by
V,F, Zhuravlev and
MOM,
Sychev,OTTAWA
2 Nsvenber, 1949,
T h i s r e p o r t may n o t be p u b l i s h e d f n whole o r i n p a r t w i t h o u t t h e w r i t t e n c o n s e n t of t h e N a t i o n a l Research Council,
NATIONAL RESEARCH LABORATORIES Ottawa, Canada
TEZHNICAL TRANSLATION NO0 IS-10 0
E f v f sion o f Informati on Servf c e s
T i t l e : ! I n v e s t i g a t i o n of Raw Materials f o r the ProductLon
0 % Mineral ?Noolo
By:: V,F,
Zhuravlev
and MOM, SychsvoReference J. Zhurnal PrfkUadnof Khimff ( J o u r n a l of Applied
C h s m f a t r y ) Academy of Sef ences,
U,
So S6R,VcS,
21,No,
3, March, 194B0 T r a n s l a t e d by: Esther RabkfnJOURNAL O F APPLIED CHEMISTRY, ACADZJrTY OF SCIENCES, U, S o S,R, VOLo 21, N O e 3, MARCH, 19480
PnvestfgatiLon a-a-. of Raw - M a t e r i a l s f o r t h e P r o d u c t f o n of Mineral
-
Woof, bY=VoFo Zhuravlev and IdoMo Sychev,
Eenfngraz Tsv:hnoEogf c a l I n s t i t u t e Trar1.~kate<3. by E s t h e r Rabki
n
The most i m p o r t a n t p r o p e r t i e s of m f n e r a l wool a r e s smal: weight p e r u n i t volume (from 30 t o 160 kgo p e r 1 hI3) and
a v e r y s m a l l c o e f f i c i e n t of t h e r m a l c o n d u c t i v i t y (from 0 0 0 5 t o 0,07 k aal/M.hro "C). The f a c t t h a t m f n e r a l wool does n o t Pose
i t s heat i n a u f a t f n g p r o p e r t f o s a t f a f r l y h f g h t s m p e r a t u r e s , and
i t does n o t d e t e r i o r a t e from mofsture i s a l s o of c o n s i d e r a b l e impostarlce.
Mineral wool f i n d s wide a p p l f c a t f o n because of t h e s e p p o p e ~ ~ t i e s , Thus, f o r example, m i n e r a l wool i s u s e d a s a h e a t i n s u l a t o r i n b o i l e r i n s t a l l a t i o n s , a s a h e a t f n s u l a t o r and F i l t r a t i n g m a t e r i a l i n chemical a p p a r a t u s and i n r e f r f g e r n t f o n , b u t the bu%%df ng i n d u a t r y i s t h e mai n consumer of m i n e r a l wool a n d its p r o d u c t s o
The c u r r e n t methods f n t h e buf Pding i n d u s t r y , whi ch u t f l f z e p r e f a b r i c a t e d wooden c o n s t r u c t i ons on a l a r g e s c a l e , demand c o n s i d e r a b l e q u a n t i t i e s of h e a t i n s u l a t i n g r n a t e r f a l s such
as m i n e s a l wool, Because of t h i s , i t f a n e c e s s a r y t o e n s u r e t h a t t h e developing i n d u s t r y of m i n e r a l wool p r o d u c t i o n h a s a
The low c o s t of m i n e r a l wool and t h e p o s s f b f l f t y of utf.11 z i n g raw m a t e r i a l s of varf ous and abundant t y p e s permf t t h e
o r g a n i z a t f on of t h e i n d u s t r y i n regf ons of g r e a t e s t demando
L i t e r a t u r e s o u r c e s f n d f c a t e t h a t a wfde v a r f e t y of raw m a t e r i a l s can be u s e d f o r t h e p r o d u c t f o n of m i n e r a l wool, The use of t h e f o l l o w i n g m a t e r i a l s i s w e l l known8
1, S c h i s t dolomite e s n t a i n f n g c l a y ( P I
2, Minerals of t h e pyroxene group, p a r t i c u l a r i y w o l l a n s t o n i t e ( 2 ) 3, By-products of b a u x f t e (3)
4, Mixtures of c l a y e y s c h i s t , sandstone, c l a y and l i m e ( 4 ) 5, Lime marl w i t h a n admfxture of dolomite ( 5 )
An a n a l y s i s OF v a r i o u s samples of m i n e r a l wool gave t h e f o l l o w i n g v a r i a t i o n s f n t h e composftfon of t h e b a s i c com- ponents (6,7,3) Sf02 from 35 t o 46% A 1 2 0 3 from 1 0 t o 30$ Fe203 Prom 0 t o 35% CaO from 4 t o 43% MgO froro 0 t o 18% I n t h e United S t a t e s q u i t e o f t e n m i n e r a l wool f a c t o r f e a a r e b u i
Pt
b e s i d e P o r t l a n d cement f a c t o r i e s , so t h a t t h e y c a n u t i l i z e t h e by-products of t h e raw m a t e r f a l s u s e d i n t h e P o r t l a n d cement i n d u s t r y (9,lO). I n t h e Unfted S t a t e s m i n e r a l s whfch a r e s u i t a b l e f o r t h e p r o d u c t i o n of m i n e r a l wool 1vPthout i n t r o d u c f n g , a n y a d d i t i o n s i n t o t h e m i x t u r e a r e c a l l e d wwoolrockn, b u t t h e r o c k s whfchr e q u i r e t h e a d d f t f o n of a substance with e i t h e r a c i d i c o r b a s i c p r o p e r t i e s a r e c a l l e d "subwoolrockn,
The production of mineral wool i s p o s s i b l e from v a r i o u s t y p e s of raw m a t e r i a l and t h e r e f o r e t h e i n d u s t r y can be d i s -
t r i b u t e d throughout wide r e g i o n s of t h e S o v i e t Union where such m a t e r i a l s a r e a v a i l a b l e o The main problem i n choosing t h e raw m a t e r i a l i s t h e s e l e c t i o n of t h e proper mixture which w f l l pro-
duce mineral woo% of t h e n e c e s s a r y q u a l i t y . The s o l u t i on of
t h i s problem I s i n i t s t u r n dependent on t h e methods of e v a l u a t i n g t h e raw m a t e r i a l s and t h e methods of s e l e c t i n g t h e mixtureo
A s i s known, t h e production of m i n e r a l wool i s based on t h e obtainment of a s i l i c a t e f u s i o n and t h e a t o m i z a t i o n of t h e stream of t h i s f u s i o n i n t o very f i n e f i b r e s , Hence, f i r s t of a l l , i t i s n e c e s s a r y t o e s t a b l i s h s p e c i f i c a t i o n s f o r t h e p r o p e r t i e s and composition of s i l i c a t e f u s i ons s u i t a b l e f o r t h e production of mineral wool,
The hollowing c o n d i t i o n s can be e s t a b l i s h e d on t h e b a s i s of t h e d a t a e x i s t i n g i n l i t e r a t u r e s
P o The chemf e a l compositi on of t h e mixture s u i t a b l e f o r t h e production of mineral wool must be such a s t o bnsure t h a t t h e f u s i o n upon cooling w f l l become a g l a s s l i k e s t r u c t u r e without any tendency t o become b r i t t l e ,
2, The chemical composition of t h e mixture must e n s u r e a s u f - f i c i e n t l y low v f s c o s f t y of t h e f u s i o n i n t h e i n t e r v a l of 1250- 135OoC0 The v i u e o s i t y of t h e f u s i on during t h e I n i t i a l moment
of t h e f i b r e formation determines t o a g r e a t degree t h e q u a l i t y of t h e wool, and i s Gainly r e s p o n s i b l e f o r t h e f i n e n e s s of t h e f i b r e ,
3, The chemical composition of t h e mixture must be such a s t o
allow t h e u t i l i z a t i o n of t h e p r e s e n t l y e x i s t i n g melting a p p a r a t u s , I n cupola and b a t h f u r n a c e s , m a t e r f a l s can be h e a t e d up t o
temperatures of 1500-1600°C, I f we t a k e i n t o account t h a t an overheating of t h e f u s i o n up t o 200-300°C (12) i s r e q u i r e d durfng t h e production, t h e n temperatures of 1200-1250°C w i l l
be t h e optimum l i m f t s f o r t h e melting of t h e mfxtureo
When planning t h e mixture, i t i s good p r a c t i c e t o
c o r r e l a t e t h e e a s e of f u s i b i l i t y of t h e mixture with t h e minimum v f s c o s l t y of t h e f u s i o n ,
The work of Pavlov (13) proves t h a t t h e degree of l i q u e f i c a t i o n of b l a s t - s l a g i s d i r e c t l y p r o p o r t i o n a l t o t h e r a t i o of t h e q u a n t f t i e s of lime and s i l i c a t o c l a y p r e s e n t i n
themc A predomination of Si02
+
A1203 over lime makes t h e s l a gvf scous, t h i c k and slow-cooling, g r a d u a l l y becoming ( p a s s i n g through a doughy s t a t e ) a g l a s s - l i k e mass, S l a g s i n which t h e r a t i o Si02 + AP203 i s c l o s e t o u n i t y l i q u e f y more easil-y, cool
CaO
more qufckly, becoming a s t o n e - l i k e mass, which g i v e s an ochreous f r a c t u r e , I f t h e q u a n t i t y of lime i s i n c r e a s e d , t h e s e s l a g s
qui c k l y l o s e t h e f r vf s c o s i t y , become thf ck ( " s h o r t " s l a g s ) , and upon coolf ng df s i n t e g r a t e i n t o powder.
TABLF:
1
Lfme content
9n
s l a g-
30%TABLE 2
TABLE
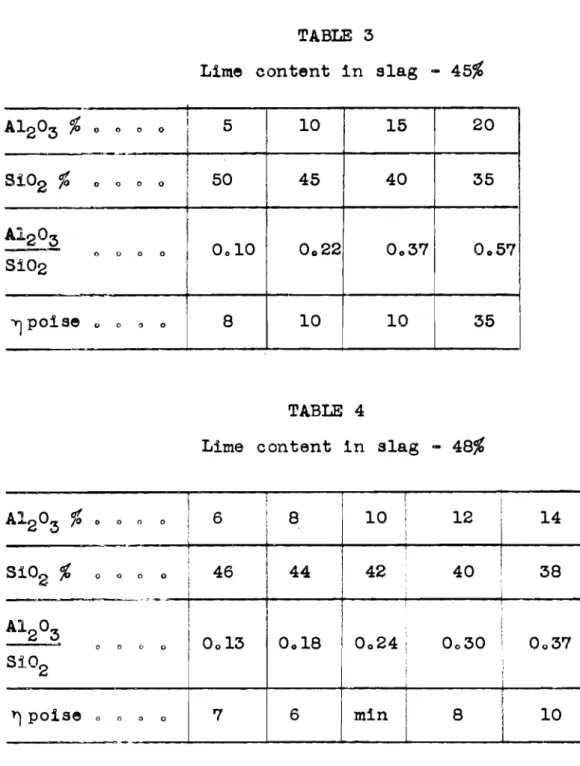
3 Lime c o n t e n t i n s l a g-
45%TABLE
4 Lfme c o n t e n t i n s l a g-
48% I t i s a l s o n e c e s s a r y t o e x p l a i n t h e e f f e c t of t h e A1203 r a t i o-
on t h e p r o p e r t i e s of t h e f u s i o n o Sf O2From a s t u d y of McCaffrey dfagrams on t h e v i s c o s f t y of s l a g 3 i t i s p o s s i b l e t o e x p l a l n t h e e f f e c t of t h e r a t t o I A'2°3
1
0.13 1 I I ? p o i s e.
o o o--
0.18 7 ' 0.24 0,30I
0037I
6-
I i min 8 ' 1 0on the v i s c o s i t y of the f u s i o n f o r a given lime content.
Sf O2
Data expressing t h e e f f e c t of the r a t i o A1203 on t h e Sf O2
vf s c o s f t y f o r f u s f ons with various contents of CaO a t a temperature of 1500°C a r e compf l e d i n Tables 1, 2, 3 and 4.
The following conclusions can be made on the a n a l y s f s o f t a b l e s 1, 2, 3 and 41
1. For low contents of CaO ( &30$) t h e r e i s a s u f f i c i e n t l y wide f n t e r v a l i n which a change i n the r a t i o A1203 (from 0.5
Sf 02
t o 1,O) has l f t t l e e f f e c t on the v f s c o s f t y of t h e fusfona, 2, By f ncreasfng the content of CaO, t h f s f n t e r v a l becomes considerably narrower ( a t CaO
=
40$,, "--
- -
from 0,2 t o 0,33,a t 45%
- - - -
from 0.1 t o 0.37); a t a content of CaO cor- reapondfng t o t h e mfnimum vf s c o s i t y (CaO=
48) thf s f n t e r v a l becomes a p o i n t , I t must be noted, t h a t although a t t h e optimumo f
content / ~ a 0 , t h f s f n t e r v a l becomes a point the quantf tat1 ve change f n the r a t i o *'z03 is very small,
Sf O2
3, A t a content of lfme l e s s than 30$, and a t an optfmum content of lfme (optfmum from the point of view of obtafnfng minimum
v f s c o s f t y ) a f a f r l y r i d e deviation i n the r a t i o "2O3 i s per- Sf
o,,
6,
mf s e f b l e ,
It i s possible t o c o n t r o l the p r o p e r t i e s of a fusion, obtained from any raw m a t e r i a l , f n order t o lower i t s vfscosfty. Thus a t 1400
-
1600° the i n t r o d u c t i o n of MgO up t o 5% n o t i c e a b l ylowers t h e v i s c o s i t y o A g r e a t e r I n c r e a s e I n t h e c o n t e n t of MgO makes t h e f u s i o n even more f l u i d , s o t h a t a t 15-20s of MgO t h e v f a c o s i t y of s l a g s i s 6
--
8 tfmes l e s s t h a n t h e v i s c o s i t y of s l a g s not c o n t a i n i n g MgO, I t i s a l s o e s s e n t f a l t o note t h a t w f t h t h e i n t r o d u c t i o n of I Q O , t h e r e g i o n of f a i r l y f l u i d s l a g s i s widened ( 1 4 ) , Therefore, i n a d d f t i o n t o t h e l f q u e f y f n g a c t i o n , t h e presence of I Q O makes i t p o s s i b l e t o widen t h e l i m i t s of t h e r a t i o *1203 without any e s s e n t i a lSf O2
change i n t h e v i s c o s i t y of t h e f u s i o n s ,
There a r e I n d f c a t i o n s t h a t the prejence of Fe203 i n t h e f u s i o n h a s even a g r e a t e r e f f e c t on t h e v ' i s c o s i t y t h a n t h e presence of MgO, p a r t i c u l a r l y i f FeZ03 i s p r e s e n t f n consider- a b l e q u a n t i t i e s,
I n order t o lower t h e m e l t i n g temperature of t h e mix- t u r e , 202% of a l k a l i ( r e c a l c u l a t e d on t h e oxide) a r e added, Sodium s u l p h a t e i a g e n e r a l l y used,
For t h e same purpose BaO may be i n t r o d u c e d up t o 6% (heavy s p a r may be used) a t t h e expense of lowerfng t h e content of CaO, I n t h i a case t h e temperatures of f u s i o n have been
Bswered t o t h e order of 100Oo0 I t must be p o i n t e d out t h a t t h e temperature of f u s i on w i l l a g a i n i n c r e a s e i f t h e percent content of BaO f s i n c r e a s e d ( 1 4 ) ,
I n o r d e r t o r e g u l a t e t h e p r o p e r t i e s of t h e f u s i on, a,
f l u x of t h e type CaF2 can be i n t r o d u c e d i n t o t h e mixture which s ~ m u l t a n e c u s l y lowers t h e temperature and t h e v i s c o s f t y of t h e f u s i on (141,
According t o Levinson
--
Lessfng (15) t h e halogen s a l t s produce e u t e c t f c s w i t h sf l f e a t e systems, and t h a t t h e s e e u t e c % i c s a r e d i s p l a c e d i n t h e d i r e c t i o n of t h e n o n - s f l f c a t eeomponen%s, From t h f s p o i n t of vfew, sodfum c h l o r i d e can be used a s a f l u , The p o s s i b i l i t y of u s i n g t h i s f s explafned f n t h e work by .Dflaktorskf (16),
The above s t a t e d can be summarfzed a s followsx
l o The r a t f o Si02 + A1203 should be g r e a t e r t h a n unf t y , which
CaO
w i l l ensure a g l a s s - l f k e s t r u c t u r e upon coolfng, Such a f u s f o n w f l l be a 'slowly coolfng f u s i o n , passfng through a doughy s t a t e , 2, The value of t h e r a t i o c o n s i d e r a b l y a f f e c t s t h e
Sf O2
v f s c o s f t y of t h e f u s i o n a t a d e f f n f t e lime content,
3, Fluxes should be used (dolomite, heavy s p a r , f l u o r s p a r , sodfum s u l p h a t e , sodfum c h l o r f d e ) f o r t h e c o r r e c t 1 on of t h e fusf on,
4, The tendency should be t o obtaf n eomposftf s n s which p o s s e s s m i nfmum f u s f on %emperatweso The f u s i on temperature of t h e mixture should not exceed 1200-1250°C,
S i l i c a t e f u s i o n s must p o s s e s s a number of d e f i n i t e propertf e s whf ch determf ne thef r suf tabf l f t y f o r t h e product1 on of mineral. wool, S p e e f f f e a t i o n s f o r t h e raw m a t e r f a l can be e s t a b l i s h e d by technologPca1 t e s t s , The method of t e s t i n g worked o u t by us i n c l u d e s t h e P o l l o w f n g ~
( a ) The d e t e r m i n a t i o n of t h e chemical compositi on of t h e r a w
m a t e r i a l s and f l u x e s ,
( b ) The p l a n n i n g of m i x t u r e s on t h e b a s i s of above s t a t e d s p e c i f i c a t i o n s r e g a r d i n g t h e chemical composi
ti
on of f u s f ons,( e l
The d e t e r m i n a t i o n of t h e f u s i o n t e m p e r a t u r e s f o r t h e s e l e c t e d m i x t w e s ,The F i b r e q u a l i t y of m f n e r a l wool i s determined by t h e v i s c o s i t y , t h e r a t e of cooling. " d u c t i l i t y " , and, f i n a l l y , by t h e value of t h e s u r f a c e t e n s i o n of t h e f u s i o n , The t o t a l e f f e c t of' t h e vf s c o s i t y , r a t e of c o o l i n g , " d u c t i l f
tyn
ands u r f a c e t e n s i o n can be a s c e r t a i n e d by a s t u d y of t h e phenomena of t h e s o c a l l e d f l o w a b i l i t y of t h e f u s f ono
Becuause of t h i s , t h e t e s t i n g method should a l s o
i nclude s
( d ) The d e t e r m i n a t i o n of t h e f l o w a b i l i t y of t h e f u s i o n s from t h e s e l e c t e d m i x t u r e s o
( e ) The p r o d u c t i o n of m i n e r a l wool from t h e sample f i s i onso ( f ) The s t u d y of t h e p r o p e r t i e s of t h e o b t a i n e d m i n e r a l wool (composition of t h e f i b r e s , weight p e r u n i t volume, c o e f f i c i e n t of t h e r m a l c o n d u c t i v i t y , h y g r o s c o p i c i t y ) , (g) C o r r e c t i r ~ g , when n e c e s s a r y , t h e p r o p e r t i e s of f u s i o n by i n t r o d u c i n g f l u x e s and by r e p e a t i n g t h e i n v e s t i g a t i o n s of t h e p r o p e r t i e s of t h e f u s i o n and t h e wool, ( h ) To e s t a b l i s h t h e p o s s i b i l i t y of" u t i l i z i n g t h e raw m a t e r i a l s under f n v e s t i g a t i on,
THE
EXSERINIENTAL SECT1 ONMethod e f i n v e s t i g a t i o n
The temperatuxe of f u s i o n of t h e m i x t u r e was d e t e r - mined by t h e Seger cone i n a KryptoP f u r n a c e ,
The d e t e r m i n a t i o n of t h e f l o w a b i l i t y of t h e f u s i on8
i s c a r r i e d out b y t h e S e l i v a n o v method (17, 181, The method c o n s i s t s of t h e f o l l o w i n g o t h e f u s i o n a t some d e f i n i t e
temperatuke i s poured out o n t o a pouring g a t e l o c a t e d a t a n a n g l e t o She h o r i z o n t a l , The c r o s s s e c t i o n o r t h e p o u r i n g g a t e is i ~ n i f s m Rhroughoue i t s l e n g t h , The P e r g t h of t h e
s t r e a m a f t e r c o o l i n g , and t h e weight of t h e c o o l e d m a t e r i a l a r e measured and t h e r a t 1 o of t h e l e n g t h of t h e stream t o t h e
weigh* of the m a t e r i a l i s c a l c u l a t e d , T h i s r a t i o seTves a s a a t a c d a r d f o r t h e f i o w a b i l i t y o
An i r o n a n g l e 25
x
25 p l a n e d a l o n g t h e i n n e r s u r f a c e s was iased a s t h e pouring g a t e , The a n g l e of i n c l i n a was 16'"I n l a b o r a t o r y i n v e s t i g a t f ons t h e s e l e c t i o n of t h e method f o r t h e productfon of m i n e r a l wool i s v e r y i m p o r t a n t and q u i t e comp9ex0 The main Tiff f e u h t y f s( t h e f a c t t h a t i t
i s n o t p o s s i b l e t o o b t a i n f u s i ons of consf d e r a b l e quantf t i e s ,
FOP t h e l a b o r a t o r y i n v e s t i g a t f o n s we have s e l e c t e d t h e mechanical method f o r a t o m i z a t i o n ( E n g l i s h p a t e n t Buss (131, w i t h modf-
f i c a t i ons by PerkaY a n d E p s t e i n (12) )
,
The i a b ~ r a t o r y a p p a r a t u s f o r t h e p r o d u c t i o n of m i n e r a l woo% was c o n s t r u c t e d , Theaoa%terlng!, and t h e n e c e s s a r y equf pment f o r i t s i n s t a l P a t 1 on and e l e c t p i eal conneetf onso
The ~ ~ t a t X n g element consi s t s of a m e t a l l i c p r o p e l l e r t o which a r e a t t a c h e d m e t a l l i c apokes of a s p e c i a l c r o s s -
aec$i on ( s e e photograph), Two such r o t a t i n g elements were pre- paredo One had e i g h t spokes and t h e o t h e r a i x t e e n , The pro- p e l l e r f s mounted on a v e r t i c a l a h a f t a t t a c h e d by two b a l l
bearlnga, The s h a f t i s a l s o s u p p l i e d wf t h a p u l l e y , The whole system f s i n s t a l l e d on a frame on whfch thm electric motor i s moua%edo An A o C o motor of 0 0 5
Kwo
and 1500 spm i s used, The r a t i o between the motor and the p u l l e y cn %he vertica'a a h a f t f a such t h a t t h e element r o t a t e a a % tiipproxi-mately 4030 spm,
The wool i s obtained i n t h e followf ng manner; a f i n e j e t cf t h e f u s i o n a t a d e f i n i t e temperature i s poured onto the apokes cf the r ~ t a t i n g element, The spokes due to the h i g h ~ s t a t i o n a P v e l o c i t y of t h e element (4600 x L6
-
64,000 blows on t h e j e t pe% minute) break up t h e j e t i n t o miriute drops whfch during t h e f l i g h t a r e d r a m out i n t o f i n e f i b r e s ,I n order t o p r o t e c t t h e worker from t h e s p a t t e r i n g 9 of t h o fusf on, a s p e c i a l coat w a s made from aheet i r o n ,
Thia 5. n s t a l l a t f on proved v e r y convenf e n t f o r t h e experiment
,
It i s p a p t i e u l a r l y valuable because e x p e r i men$ s w i t h small q u a n t i t i e s of f u s f on can be c a r r i e d o u t oName of M a t e r i a l 1, Marl 1.96 2, Dolomite 3, Speckled I 4. Sand 0,26 100.63
For our experiments we used a K r y p t o l f u r n a c e ,
The raw materialS t o be used f n a new f a c t o r y f o r t h e p r o d u c t i o n of m i n e r a l wool have been i n v e s t i g a t e d by t h f s method.
Marl, dolomite, dfatomf t e and sand were s u b j e c t e d t o t h e s e i n v e u t i g a t i ons, The chemical composi t f on of t h e components of t h e raw m a t e r i a l 1.3 gfven i n Table 5,
S i x mfxtures were compounded, A l l m i x t u r e s contaf ned a p p r o x i m a t e i y uniform amounts of A1203 and Fe2O3, whf ch p e r m i t t e d t o e s t a b l i s h t h e e f f e c t s produced by CaO and NO on t h e pro-
p e r t i e s of t h e f u s i o n o
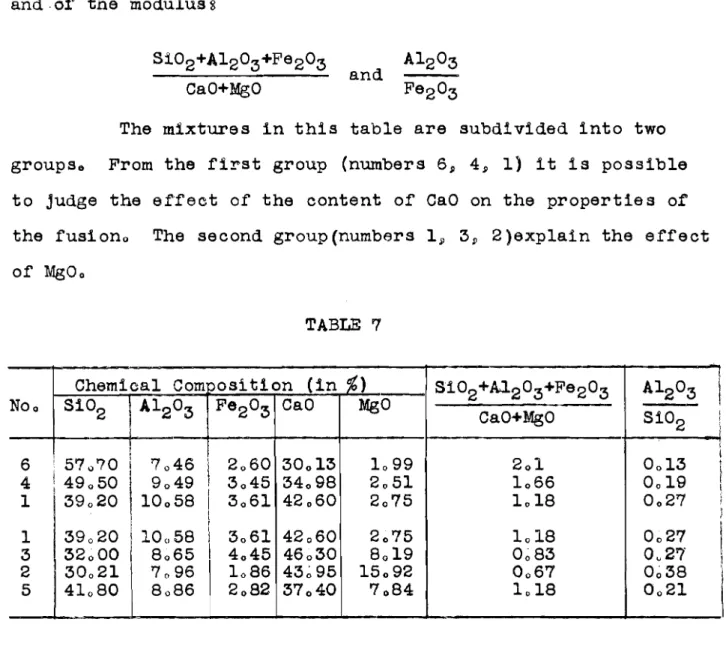
The chemical composition of t h e m i x t u r e s a r e compiled i n Table 6,
Table 7 g i v e s t h e chemical compositions of t h e f u s i o n s and
.of'
t h e modulus sThe mixtures i n t h f s t a b l e a r e subdf vided i n t o two groups, From t h e f f ~ s t group (numbers 6, 4 , 1 ) i t i s p o s s i b l e t o judge t h e e f f e c t of t h e content of CaO on t h e p r o p e r t i e s of t h e fusf on, The second group(nurnbers 1, 3, 2 ) e x p l a f n t h e e f f e c t
TABLE 4
The r e s u l t s of the d e t e r m i n a t i o n of t h e m e l t i n g temper- a t u r e of t h e mfxturea and t h e f l o w a b i l i t y of t h e f u s i o n a r e given i n t a b l e 80
From a comparison of t h e d a t a given i n t a b l e s 7 and 8, i t
i s e v i d e n t t h a t a n i n c r e a s e I n t h e s o n t e n t of CaO from 30 t o 40$
makes t h e kusi on more f l u i d ; an i n c r e a s e f n t h e content of MgO a l s o makes t h e f u s f on more f l u i d ; however, t h e i n c r e a s e i n t h e q u a n t i t y of MgO i n c r e a s e s t h e temperature of melting, Mixture No, 5 gave t h e most f l u i d f u s i o n with a s u f f i c i e n t l y low tempes- a t u r e of melting,
A mineral wool with t h e f o l l o w f n g p r o p e r t i e s was o b t a i n e d
from mixture NO, 5,
B,
Thickness of f i b r e x maxfmum --lo6p,
minimum - - l o 2 5p,
2, The compositi on of the wool by t h e f i b r e t h i ckness ( i n p e r c e n t ) z from 1,25 t o 9,4 JI --30, from g 0 4 t o 19 p--60, from 19 t o PO6 )1
--lo,
3, Length of f i b r e from 0 , s t o
l o o
cm,A eomparYson of t h e q u a l i t y of t h e o b t a i ned wool wPth t h e s t a n d a r d s p e c i f i c a t f o n s f o r s l a g c o t t o n ( s t a n d a r d 14--3915) shows t h a t t h e o b t a i n e d wool does not s a t f s f y t h i s standard,
From observatfons during t h e experiment, it i s p o s s i b l e t o con- clude t h a t t h e poor q u a l i t y of t h e wool i s due t o t h e v e r y h i g h v i s c o s i t y of t h e f u s f o n d u r i n g t h e time of i t s atomfzatfon,
The vf s c o s f t y of t h e fusf on can be lowered by t h r e e
met hods :
1, By i n c r e a s i n g t h e r e h e a t i n g temperature of t h e f u s i o n , T h i s could not be done s i n c e t h e experiments were c a r r i e d o u t a t a very hfgh temperatureo
2, By an i n c r e a s e i n t h e content of MgO; t h i s i n c r e a s e was un- d e s i r a b l e s i n c e the fusf on a l r e a d y contained about 8% of MgO and a f u r t h e r a d d i t i o n of MgO would l e a d t o a h i g h e r temperature of melting,
3 , By t h e i n t r o d u c t i o n of f l u x e s which would lower t h e v i s c o s i t y , but which would not i n c r e a s e t h e malting temperature of t h e
mixtureo
Sodium s u l p h a t e i n t h e amount of 6,58$ ( c a l c u l a t e d so a s t o i n t r o d u c e up t o 2% of NaaO i n t o t h e f u s i o n ) was used a s t h e f l u , The changed composition of t h e f u s i o n s due t o t h e addl ti ons of t h e sodfum s u l p h a t e a r e compiled i n Table 9,
The r e s u l t s of t h e d e t e r m i n a t i o n of t h e m e l t f n g t e m p e r a t u r e s of t h e m i x t u r e s c o r r e c t e d due t o t h e a d d i t i o n of sodium s u l p h a t e , and t h e f l o w a b i l i t i e s of t h e new s e r i e s of f u s f o n s a r e compfled i n t a b l e 10,
TABLE 1 0
b
Noo of t h e f u s f on
The i n t r o d u c t i o n up t o 2% of Na20 i n t o t h e composition of t h e f u s f ons lowers t h e v i s c o s i t y t o a c o n s i d e r a b l e degree, Moreover,
Chemical Compdsition of t h e F u s i o n s ( i n %) Number of t h e Mixture 1 29 3 9 4 ' 50 69
t h e value of t h f s d e c r e a s e i s n o t uniform f o r a l l f u s i o n s . The Si02 i
1'
38,85 2'
29054 31,34 48060 5 ' 40060 6 56073 Melting Temperature ( i n OC)-
1390 1200 1100 1160 1100 A1203-
10046 7010 8,50 9035 8 , 7 0 7035 F l o w a b i l i t y of F u s i o n ( k ) CaO 42,48 43010 45,80 34.39 37.00 29068 Fe203 3,58 lo87 4,40 3040 4,15 2056 Temperature of t h e experimentNo
2,73 15060 7,99 2046 7 , 6 5 l o 9 6 Length (1) of Stream 3 Na2 0 l o g o 2,06 l 0 9 7 l O 8 O l o g o 1 0 7 2 ( i n OC) ( i n m 0 ) c o o l e d m a t e r i a l7
k=
l / gj
Weight (g) of t h e 1400-
1400 1400 1400 1400 I ( i n g o )1
90-
17 2 89 118 52 1 l 0 2 5 8,OO-
i
,
-
I 14,40 11, 95 I 1 l O 7 O 7 , 6 0 9 0 97 11,84i
8029 6,27I n t r o d u c t i o n of Na20 produces t h e g r e a t e s t e f f e c t on f u s i o n s r i c h i n MgO, s i n c e t h e i n t r o d u c t i o n of Na20 i n c r e a s e s t h e l i q u e f y i n g a c t i o n of MgO. If t h e a d d i t i o n of 1.9% Na20 i n t o t h e composition of t h e f u s i o n number 1 i n c r e a s e s i t s f l u i d i t y by 26$, t h e n t h e a d d i t i o n of 1 ~ 9 7 % NaZO i n t o t h e composition of t h e f u s i o n number
3 ( r i c h i n MgO) i n c r e a s e s i t s f l u i d i t y by 73g0 Analogous r e s u l t s a r e observed f o r m i x t u r e number 5 ( c o n t a i n i n g a p p r o x i m a t e l y t h e same amount of MgO a s mixture number 3 ) t h e f l u i d i t y of which was I n c r e a s e d by 7 0 % ~
The m i x t u r e s N o . 1 ~ 5 and 3 p o s s e s s i n g t h e g r e a t e s t f l u i d i t y , were used t o o b t a i n m i n e r a l wool,
The c h a r a c t e r i s t i c s of t h e p r o p e r t i e s of t h i s m i n e r a l wool a r e compiled i n t a b l e llo
The l i n e a r v e l o c i t y of t h e atomizing element a t t h e p o i n t s of c o n t a c t of t h e f u s i o n j e t w i t h t h e spokes does n o t exceed 7 0 ~ / s e c . I n i n d u s t r y atomization i s c a r r i e d out by steam, which has a v e l o c i t y a t t h e time of e x i t from t h e b o i l e r of 300 t o 800 1vI/seo, By atom- i z i n g mixture No, 5 w i t h steam, i t i s p o s s i b l e t o o b t a i n
a
wool c o n t a i n i n g a l a r g e r p e r c e n t of f i b r s s having a diameter from 1 t oWe can recommend mixture Noo 5 t o i n d u s t r y , on t h e b a o i s of t h e experiments which we have c a r r i e d o u t o
I n s p i t e of t h e f a c t t h a t mixture NO. 3 produces a c o t t o n which f s more uniform i n q u a l i t y t h a n mixture NO, 5, we can n o t recommend mixture Noo 3 t o i n d u s t r y , s i n c e the f u s i o n has t h e
tendency t o break up i n t o powder upon cooling, This tendency i s
Si02
+
A1203+
Fe203q u i t e understandable, s i n c e t h e r a t i o
CaO
+
MgO f s l e s s t h a n u n i t y f o r mixture No, 3,,Conclusions
A method f o r t h e i n v e s t i g a t i o n of raw m a t e r i a l s t o be used
4n t h e production of mineral wool has been worked out, It was
a l s o p o s s i b l e t o o b t a i n a mineral wool from t h e raw m a t e r i a l s under i n v e s t i g a t i o n and recommend a composition of t h e mixture, Bibliography
l o Canado Min, Metallurg, Bull,, 2, 93 ( 1 9 3 6 ) ~
2, J O T o Thorndyke, UoSoP., 2, 193, 982*
30 UoSoPog 2, 022, 8110
Ro Rach, T o n f n d u s t r i e Zeitung, 46 (1937),
Do
Lo
Kaufman, Keramfka, 9, 1939,Khrapkovskf, American Technique and I n d u s t r y , 7, (1945).
Ch, Fo Ramseyer, U,S.P., 2, 193, 9820
Rock P r o d u c t s , 49, 1, 97 (1946)0 P f t and Quarry, 38 1, 107 (1946)0
Mo
G o Cherniak and M o C o Aslanova, Keramika and G l a s s , 4, (1938).Z o Eo P e r k a l a n d A o C, E p s t e i n , The I n d u s t r y of B u i l d i n g M a t e r f a l s , 48, (1940), M o A o Pavlov, S o v f e t Metallurgy, 3, (1932).
L.
V o Zverev, V i s c o s i t y of S l a g s , U n i t e d T e c h n o l o g i c a l Publf s h e r s (1935), Levinson--Le a s i n g , P e t r o g r a p h y o D i l a k % o r s U , Notes of t h e Union M i n e r a l o g f c a l S o c f e t y , 2nd S e r l e s , P t o 68, 1.Vo Po Selfvanov, The M e t a l l u r g y of Cast I r o n o
No No
Kurnakov and o t h e r s , Dokladi Akademli Nauk, New S e r i e s ,111, 30
J, Buss, B r , Po 435, 064,