Supplemental information
Carboxylated Chitosan Nanocrystals: A Synthetic Route and Application as
Superior Support for Gold-Catalyzed Reactions
Tony Jina, Davis Kurdylaa,b, Sabahudin Hrapovicb, Alfred C.W. Leungb, Sophie Régnierb, Yali
Liub, Audrey Mooresa,c* and Edmond Lamb*
aDepartment of Chemistry, McGill University, 801 Sherbrooke St. West, Montreal, Quebec, H3A 0B8 Canada bAquatic and Crop Resource Development Research Centre, National Research Council of Canada, 6100 Royalmount
Avenue, Montreal, Quebec, H4P 2R2 Canada
cDepartment of Materials Engineering, McGill University, 3610 University Street, Montreal, Quebec H3A 0C5,
Canada Experimental Section ... 2 Materials ... 2 ChNC/ChsNC synthesis ... 2 ChNC scale-up synthesis ... 2 ChsNC scale-up synthesis ... 2
Au@ChsNC by layer-by-layer method (LBL)... 3
Au@ChsNC by reduction under H2 pressure (HR) ... 3
Characterization ... 3
Catalytic reduction of 4-nitrophenol ... 4
A3-coupling reaction ... 4 Supplemental Figures ... 5 Figure S1 ... 5 Table S1 ... 6 Figure S2 ... 7 Figure S3 ... 8 Figure S4 ... 9
Experimental Section Materials
All of the reagents and solvents were of reagent grade and were used as received. The water used was Milli-Q 18 MΩ. Chitin (from shrimp shells), 4-nitrophenol, sodium borohydride, benzaldehyde (99%), piperidine (98%) and phenylacetylene (99%) were obtained from Sigma Aldrich. Ammonium persulfate was obtained from FMC Corp. (Philadelphia, PA). Hydrogen tetrachloroaurate was obtained from Strem Chemicals (Newburyport, MA).
ChNC/ChsNC synthesis
To prepare ChNCs, shrimp chitin (27.2 g) was added to ammonium persulfate (APS, 1 M, 540 mL) and refluxed at 60 °C for 16 h (Figure 1A). The suspension was centrifuged at 10,000 rpm (RCF = 15,300 g) for 10 min, and the pellet was washed six times with water under the same centrifugation conditions. ChNCs (14 g) were isolated via lyophilization as a white solid. ChsNCs
were prepared by mixing ChNCs (9 g) and NaBH4 (0.9 g) in NaOH (0.9 L, 40 wt% solution in
water) and refluxing at 117 °C for 18 h. The suspension was centrifuged at 10,000 rpm for 10 min, the supernatant was decanted, and the pellet was resuspended in NaOH (0.9 L, 40 wt% solution in water) and refluxed under the same conditions. This process was repeated two to three times, centrifuging the suspension followed by resuspending the pellet in fresh NaOH. A small sample of solid was taken after each reflux step and the DDA was determined by monitoring the
FTIR absorbance peaks at 1030 and 1560 cm-1 according to a characterization method outlined by
Shigemasa et al.1 Once the DA was below 20%, the ChsNC pellet was suspended in deionized
water and centrifuged in the same conditions as above. The centrifugation/washing cycles were
repeated eight times until the solution conductivity was below 400 µS⋅cm-1. The purified pellet
was resuspended in water, and HCl (1 M) was added until the solution became slightly acidic pH 5. The ChsNC product was lyophilized to yield a white powder (5.7 g, 63% yield).
ChNC scale-up synthesis
Scale-up synthesis of ChNC at the 200 L scale was performed using a 250 L jacketed stainless steel reactor equipped with overhead stirring. Shrimp chitin (5 wt.%) in 1 M ammonium persulfate (200 L) was heated at 60 ºC for 16 h with stirring. The product was purified from the by-products (mainly ammonium sulfate and sulfuric acid) by decantation several times until the conductivity
of the supernatant solution reached about 1 mS•cm-1 (~pH 3-4) to yield ChNC (4.9 kg, 49% yield).
ChsNC scale-up synthesis
Scale up synthesis of ChsNC at the 5 L scale was performed using a 5 L 3-neck round bottom flask
equipped with condenser and overhead stirrer. ChNCs (40 g) and NaBH4 (2 g) in NaOH (2 L, 40
wt.% solution in water) were refluxed at 117 °C for 18 h. Similar processing conditions were employed as described above to yield ChsNCs (24 g, 60% yield).
Au@ChsNC by layer-by-layer method (LBL)
ChsNCs (1 g) was stirred in 0.5 wt% polydiallyldimethylammonium chloride (PDDA) solution
(500 mL) for 24 h. To promote ionic interactions, KNO3 (2.5 g) was added and stirred for an
additional 24 h. NaOH (1 M, 1 mL) was then added to precipitate out the ChsNC/PDDA intermediate which was centrifuged and rinsed with deionized water three times, then isolated via
lyophilization. Au NPs (3 nm diameter) were prepared according to a literature method.2
ChsNC/PDDA (50 mg) was dispersed in 50 mL deionized water and mixed with Au NP solution (50 mL) and HCl (1 M, 1 mL). This solution was stirred for 24 h, after which NaOH (1 M, 1 mL) was added to precipitate the product. This product was centrifuged and rinsed with water three times, then lyophilized. The catalyst was isolated as a light purple solid (51.3 mg, 63% yield). A similar procedure was used to synthesize bulk chitosan, ChNC, and bulk chitin catalysts with immobilized Au NPs.
Au@ChsNC by reduction under H2 pressure (HR)
A ChsNC suspension (10 mg mL-1, 25 mL) was mixed with 0.01 M aqueous HAuCl4 solution
(3.75 mL). The reaction mixture was stirred for 10 min, before being placed under 4 bar H2
pressure in a PARR reactor system for 2 h. The solution was subsequently dried under vacuum and lyophilized further to obtain a yellow powder (84%, 0.22 g). An identical procedure was used to synthesize a bulk chitosan nanocomposite catalyst.
Characterization
The Transmission Electron Microscopy (TEM) images were taken on a Technai F-20 operating at the accelerating voltage of 200 kV. The TEM imaging was done on freshly glow-discharged grids using EMS GloQube-D, Dual chamber glow discharge system (Electron Microscopy Sciences, PA) operating in negative mode and applying the plasma current of 25 mA during 45 s. Afterwards, a 10 µL of sample solution was adsorbed to a glow-discharged carbon-coated copper TEM grid (Electron Microscopy Sciences, PA). The partially dried grid was washed three times with distilled water and excess water was removed by carefully bloating the grid with a piece of filter paper. The sample was subsequently stained twice for 14 and 45 s with 1.0 mM uranyl acetate, then dried at room temperature prior the TEM observation.
The Low Voltage Transmission Electron Microscopy (LV-TEM) images were obtained using a Delong LVEM (Soquelec, Montreal, QC, Canada) low-voltage TEM at 5 kV. A small amount of
X-ray diffraction spectra were acquired using a Bruker D8 Advance X-ray diffractometer equipped with a CuKα filament, scanned with a 2θ range between 5-60° with an increment of 0.02°. Atomic Force Microscopy (AFM) micrographs were obtained using a Nanoscope IV (Digital Instruments, Veeco, Santa Barbara, CA) with a silicon tip operated in tapping mode.
Particle analysis of the micrographs was performed using Scanning Probe Image ProcessorTM.
Dynamic light scattering and the zeta potential of the samples (2 mg mL-1) in water were
determined using a Zetasizer Nano-ZS (Malvern Instruments, Malvern, UK) in triplicate.
Fourier transform infrared (FTIR) spectra were collected from 4000 to 400 cm-1 for 100 scans at a
resolution of 4 cm-1 using a Bruker Tensor 27 FTIR spectrometer. Samples were run in the ATR
mode over a ZnSe crystal.
UV-Visible spectra were collected from 400 to 800 nm on a Cary5000 UV-Vis-NIR spectrometer.
Catalytic reduction of 4-nitrophenol
Catalytic species were suspended in water (1 mg mL-1) and added to a solution containing NaBH4
(56.7 mg) and 4-nitrophenol (0.05 mM, 30 mL). All reactions were performed in triplicate in a temperature-controlled ethylene glycol bath with continuous stirring. Reactions were monitored using UV-Vis spectrometry (Beckman DU 640 spectrophotometer) by measuring the absorbance peak at 400 nm corresponding to the 4-nitrophenolate ion. Following addition of the catalyst, measurements were taken at one-minute intervals from 0 to 10 min. For slower reactions,
measurements were taken at 2 min intervals from 0 to 20 min. ChsNCs (100 µL, 5 mg mL-1
solution in water), PDDA/ChsNCs (100 µL, 5 mg mL-1 solution in water), PDDA (900 µL 20 wt%
solution in water), bulk chitosan (100 µL, 5 mg mL-1 solution in water), and catalyst-free
conditions were tested as negative controls.
A3-coupling reaction
The vessel used in the A3-coupling reaction was a 2-5 mL microwave reaction vial (acquired from
VWR) equipped with an aluminum seal installed with blue PTFE-faced silica septa. The aldehyde was chosen as the limiting reagent, with the ratios of the reagents being 1, 1.2, and 1.5 equivalents
for the aldehyde, amine, and alkyne, respectively. In a typical reaction, benzaldehyde (102 𝜇𝜇L),
piperidine (119 𝜇𝜇L), phenylacetylene (165 𝜇𝜇L), catalyst, and diphenylmethane (as an internal
standard) were added to the vessel equipped with a magnetic stir bar and heated to 70 °C for 24 h.
After the reaction, the crude mixture was directly added to deuterated chloroform (CDCl3) and
filtered through Celite. 1H NMR was used to determine the absolute yield. The proton NMR
Supplemental Figures
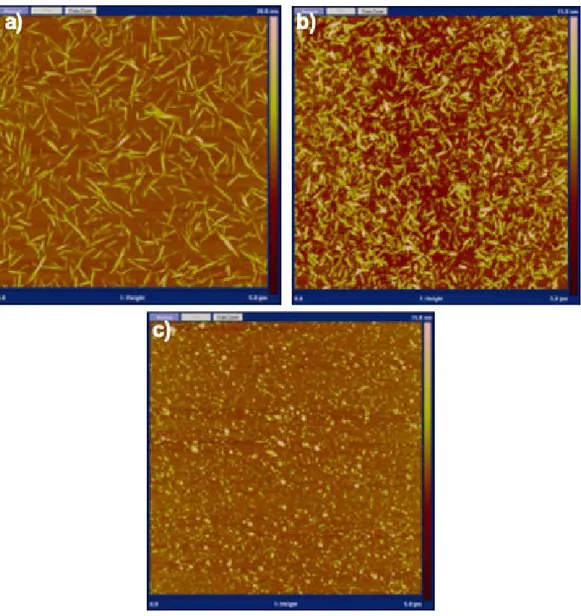
Figure S1: AFM micrographs of (a) ChNC, (b) ChsNC, and (c) ChsNC fabricated without the
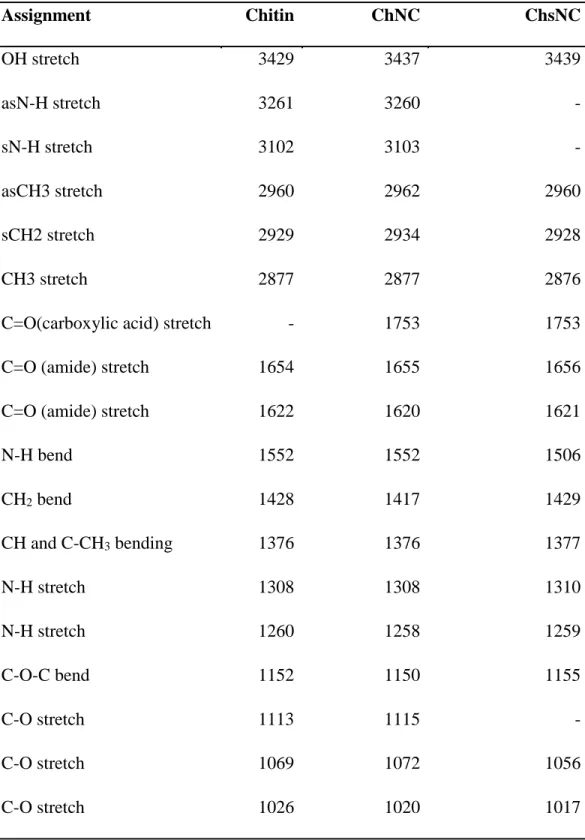
Table S1: Peak assignments for Figure 1c FTIR spectra. Assignment Chitin ChNC ChsNC OH stretch 3429 3437 3439 asN-H stretch 3261 3260 - sN-H stretch 3102 3103 - asCH3 stretch 2960 2962 2960 sCH2 stretch 2929 2934 2928 CH3 stretch 2877 2877 2876
C=O(carboxylic acid) stretch - 1753 1753
C=O (amide) stretch 1654 1655 1656
C=O (amide) stretch 1622 1620 1621
N-H bend 1552 1552 1506 CH2 bend 1428 1417 1429 CH and C-CH3 bending 1376 1376 1377 N-H stretch 1308 1308 1310 N-H stretch 1260 1258 1259 C-O-C bend 1152 1150 1155 C-O stretch 1113 1115 - C-O stretch 1069 1072 1056 C-O stretch 1026 1020 1017
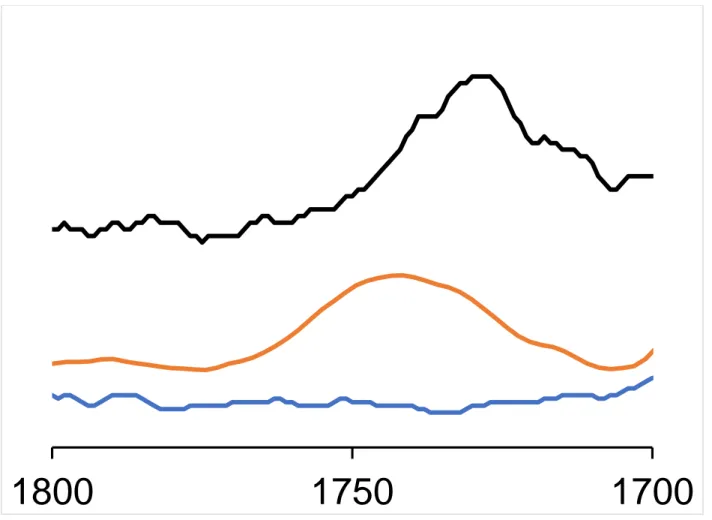
Figure S2: FTIR spectra of (orange) ChNC, (black) ChsNC and (blue) bulk chitin between
1800-1700 cm-1
1700
1750
Figure S3: ChNC after deacetylation procedure (45% NaOH, 80° C, 16 h) without NaBH4. No retention of the rod-like shape is observed, with small nanoparticulates appearing instead.
Figure S4: Plot showing change in 𝜁𝜁-potential (solid, left axis) and size (dotted, right axis) of
ChsNCs suspended in AcOH relative to pH at varying concentrations. A brief schematic is drawn below to illustrate the effect of pH on ChsNC in aqueous solution.
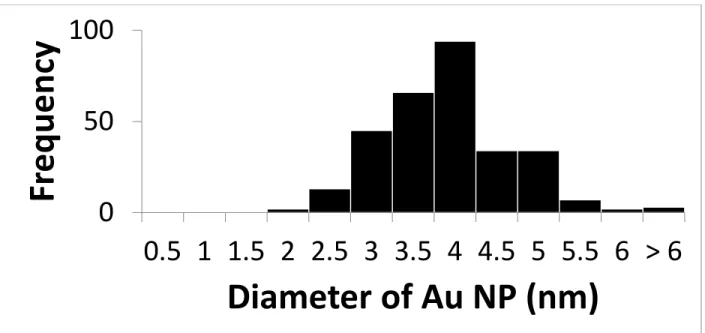
Figure S5: Histogram of size of Au-NPs for Au@ChsNC-LBL method (n=300)
0
50
100
0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 > 6
Fr
eq
uen
cy
Diameter of Au NP (nm)
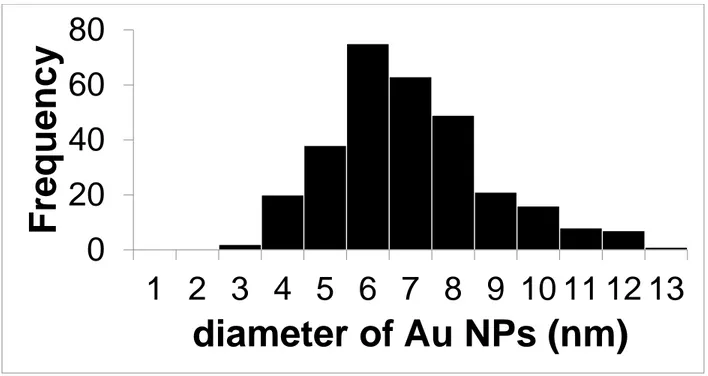
Figure S7: Histogram of size of Au-NPs for Au@ChsNC-HR method (n=300)
0
20
40
60
80
1 2 3 4 5 6 7 8 9 10 11 12 13
Fre
qu
e
nc
y
diameter of Au NPs (nm)
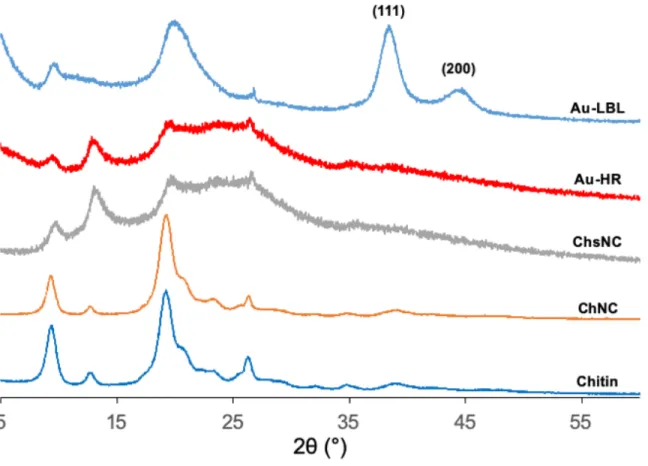
Figure S8: XRD spectra showing the powder XRD patterns of chitin, ChNC, and ChsNC
comparing with the two fabricated nanocatalysts Au@ChsNC-HR and Au@ChsNC-LBL. The 111 and 200 reflection planes correspond to the Au XRD pattern.
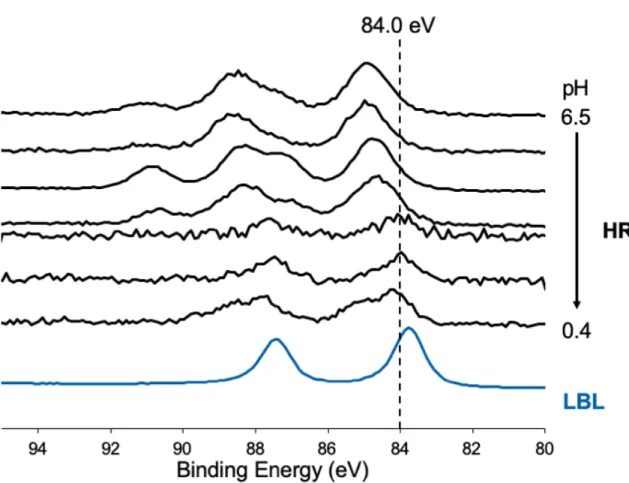
Figure S9: Au 4f XPS spectra of Au@ChsNC-HR in order of decreasing pH from top (pH = 6.5)
to bottom (pH = 0.4) and a representative spectrum of Au@ChsNC-LBL (blue line).
The impact of pH on the oxidation state of Au immobilized on ChsNC
The pH of the solution has an important role in altering the chemical environment of functional groups located on the surface of the ChsNC which we have seen in the earlier in Figure S4. By lowering the pH from 6.5 (the pH of the ChsNC suspension prior to pH adjustment) to around pH 0.5, more prominent reduction of Au occurs using the HR method (Figure 2d), which shows that the ChsNC has a significant role in the reduction mechanism. In a comprehensive study of Au(III)
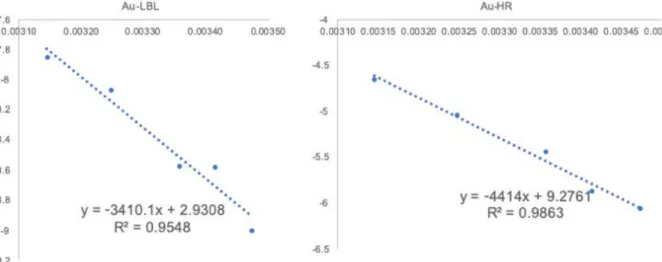
Figure S10: Arrhenius plots of the Au@ChsNC-LBL and Au@ChsNC-HR catalysts for the
Table S2: Rate constant and turnover frequency values for corresponding metal-supported
catalysts. All catalyst supports have Au as the active metal catalyst. Other active metal catalytic components other than Au are bolded.
Catalyst Support Rate constant, k, (s^-1) TOF (h^-1) ref.
Chitosan-Fe3O4 5.2 x 10^-3 528 4 MFCNC (Pd) 5.83 x 10^-3 3168 5 CNC 1.47 x 10^-2 641 6 Glucan 1.9 x 10^-3 5922 7 CSNF 5.9 x 10^-3 563 6 PDDA-CNC 5.1 x 10^-3 212 8 GO-Fe3O4 3.22 x 10^-2 484 9 PMMA 7.2 x 10^-3 89 10 α-cyclodextrin 4.7 x 10^-3 34 11 PCP (Au-Ag) 2.87 x 10^-3 130 12 PAM/PPY/GO (Ag) 2.1 x 10^-2 153 13 Graphene hydrogel 3.17 x 10^-3 12 14 HPGNP (Cu) 7.47 x 10^-3 94 15 PNIPAP-beta-P4VP 1.5 x 10^-3 16 16 Poly(DVP-co-AA) 6.0 x 10^-3 222 17 PDMAEMA-PS 3.2 x 10^-3 1 18 carbonate stabilized 3 nm Au NPs 4.4 x 10^-3 540 8 ChsNC 1.09 x 10^-2 8557 This work p(AAm-co-TMT) 2.3 x 10^-3 323 19 CA beads - 0.11 20 RGO@AC/Pd - 36 000 21
References
1. Shigemasa, Y.; Matsuura, H.; Sashiwa, H.; Saimoto, H., Evaluation of different
absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin.
Int. J. Biol. Macromol. 1996, 18, 237-242.
2. Hermanson, G. T., Bioconjugate techniques. Academic press: 2013.
3. Pestov, A.; Nazirov, A.; Modin, E.; Mironenko, A.; Bratskaya, S., Mechanism of
Au(III) reduction by chitosan: Comprehensive study with 13C and 1H NMR analysis of chitosan degradation products. Carbohydr. Polym. 2015, 117, 70-77.
4. Chang, Y.-C.; Chen, D.-H., Catalytic reduction of 4-nitrophenol by magnetically
recoverable Au nanocatalyst. J. Hazard. Mater. 2009, 165, 664-669.
5. Wu, X.; Shi, Z.; Fu, S.; Chen, J.; Berry, R. M.; Tam, K. C., Strategy for Synthesizing
Porous Cellulose Nanocrystal Supported Metal Nanocatalysts. ACS Sustain. Chem. Eng. 2016, 4, 5929-5935.
6. Yan, W.; Chen, C.; Wang, L.; Zhang, D.; Li, A.-J.; Yao, Z.; Shi, L.-Y., Facile and
green synthesis of cellulose nanocrystal-supported gold nanoparticles with superior catalytic activity. Carbohydr. Polym. 2016, 140, 66-73.
7. Sen, I. K.; Maity, K.; Islam, S. S., Green synthesis of gold nanoparticles using a glucan
of an edible mushroom and study of catalytic activity. Carbohydr. Polym. 2013, 91, 518-528.
8. Lam, E.; Hrapovic, S.; Majid, E.; Chong, J. H.; Luong, J. H. T., Catalysis using gold
nanoparticles decorated on nanocrystalline cellulose. Nanoscale 2012, 4, 997-1002.
9. Hu, J.; Dong, Y.-l.; Chen, X.-j.; Zhang, H.-j.; Zheng, J.-m.; Wang, Q.; Chen, X.-g., A
highly efficient catalyst: In situ growth of Au nanoparticles on graphene oxide–Fe3O4 nanocomposite support. Chemical Engineering Journal 2014, 236, 1-8.
10. Kuroda, K.; Ishida, T.; Haruta, M., Reduction of 4-nitrophenol to 4-aminophenol over
Au nanoparticles deposited on PMMA. J. Mol. Catal. A: Chem. 2009, 298, 7-11.
11. Huang, T.; Meng, F.; Qi, L., Facile Synthesis and One-Dimensional Assembly of
Cyclodextrin-Capped Gold Nanoparticles and Their Applications in Catalysis and Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2009, 113, 13636-13642.
12. Fu, J.; Wang, S.; Zhu, J.; Wang, K.; Gao, M.; Wang, X.; Xu, Q., Au-Ag bimetallic
nanoparticles decorated multi-amino cyclophosphazene hybrid microspheres as enhanced activity catalysts for the reduction of 4-nitrophenol. Mater. Chem. Phys. 2018, 207, 315-324.
13. Mao, H.; Ji, C.; Liu, M.; Cao, Z.; Sun, D.; Xing, Z.; Chen, X.; Zhang, Y.; Song,
X.-M., Enhanced catalytic activity of Ag nanoparticles supported on
polyacrylamide/polypyrrole/graphene oxide nanosheets for the reduction of 4-nitrophenol. Appl.
Surf. Sci. 2018, 434, 522-533.
14. Li, J.; Liu, C.-y.; Liu, Y., Au/graphene hydrogel: synthesis, characterization and its use
for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 8426-8430.
15. Jiang, J.; Gunasekar, G. H.; Park, S.; Kim, S.-H.; Yoon, S.; Piao, L., Hierarchical Cu
nanoparticle-aggregated cages with high catalytic activity for reduction of 4-nitrophenol and carbon dioxide. Mater. Res. Bull. 2018, 100, 184-190.
16. Wang, Y.; Wei, G.; Zhang, W.; Jiang, X.; Zheng, P.; Shi, L.; Dong, A., Responsive
17. Liu, W.; Yang, X.; Huang, W., Catalytic properties of carboxylic acid functionalized-polymer microsphere-stabilized gold metallic colloids. J. Colloid Interface Sci. 2006, 304, 160-165.
18. Zhang, M.; Liu, L.; Wu, C.; Fu, G.; Zhao, H.; He, B., Synthesis, characterization and
application of well-defined environmentally responsive polymer brushes on the surface of colloid particles. Polymer 2007, 48, 1989-1997.
19. Ilgin, P.; Ozay, O.; Ozay, H., A novel hydrogel containing thioether group as selective
support material for preparation of gold nanoparticles: Synthesis and catalytic applications. Appl.
Catal., B 2019, 241, 415-423.
20. Saha, S.; Pal, A.; Kundu, S.; Basu, S.; Pal, T., Photochemical Green Synthesis of
Calcium-Alginate-Stabilized Ag and Au Nanoparticles and Their Catalytic Application to 4-Nitrophenol Reduction. Langmuir 2010, 26, 2885-2893.
21. Xi, J.; Sun, H.; Wang, D.; Zhang, Z.; Duan, X.; Xiao, J.; Xiao, F.; Liu, L.; Wang, S.,
Confined-interface-directed synthesis of Palladium single-atom catalysts on graphene/amorphous carbon. Appl. Catal., B 2018, 225, 291-297.