Publisher’s version / Version de l'éditeur:
Wood Science, 6, 4, pp. 368-374, 1974-04
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Removal of solvent from swollen wood
Ashton, H. E.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=540cb543-d01d-4842-bae1-72c34949f084 https://publications-cnrc.canada.ca/fra/voir/objet/?id=540cb543-d01d-4842-bae1-72c34949f084
a,\yj;y;:
%Removal of Solvent From Swollen Wood
ABSTRACT. The removal of polar solvents previously proposed a s swelling agents for wood was followed by means of weight and volume changes. The degree of swelling and quantities of dimethyl sulfoxide, dimethylformamide, N-methyl pyrrolidone, and pyridine left in yellow birch, beech, white pine, and Douglas-fir after exposure for various times to evapora- tion a t 100 a n d 50 percent relative humidities, ovendrying, a n d vacuuin were determined. For the most strongly held solvent, water extraction was also used. Pine was found t o release solvents most quickly a n d fir most slowly. Pyridine evaporated rapidly and dimethylfor- mamide fairly rapidly a t 50 percent RH, but even after ovendrying, 2 to 4 percent by weight of these solvents w a s retained except in pine. Eleven to 19 percent dimethylsulfoxide a n d methyl pyrrolidone w a s retained in birch and beech after ovendrying, a n d application of moderate vacuum had little effect on the retention. Preliminary evaporation a t 100 percent R H i s un- necessary, and evaporation a t 50 percent RH is not practical with t h e slower solvents. Beech, which swells rapidly, i s prone to cracking. Sufficient material i s extracted from birch t o cause volumetric shrinkage. Treatment of white pine with dimethylformamide appears t o be the most practical system.
Chemical reaction with cellulose hydroxyl groups is one way of dimensionally stabilizing wood. Because the hydroxyl groups occur on polymer molecules which are part of larger micelles, they are not readily available for reaction. As a result, t h e conditions required to carry out a chemical reaction with t h e hydrox- yl groups are, in m a n y cases, so severe t h a t the wood structure is damaged. T h e author pro- posed swelling wood with polar organic sol- vents to make t h e hydroxyls more readily available to reactants and to catalyze the de- sired reactions.'
I n t h a t previous paper, the effectiveness of polar organic solvents in swelling wood was studied. I t was found t h a t the selected solvents caused greater swelling t h a n would be ex- pected from water; consequently, they could be used a s media i n which to carry out chemical reactions with the cellulose hydroxyl groups of wood. Following treatment i t would be necessary to remove whichever solvent was
be reduced through reaction, a n d physical en- trapment would be less in the swollen state.
P r o c e d u r e
The woods studied were yellow birch, beech, white pine, and Douglas-fir. T h e solvents used to swell them were dimethyl sul- foxide (DMSO), dimethylformamide (DMF), N-
methyl pyrrolidone (MP), and pyridine. T h e preparation of t h e wood blocks and solvents, t h e immersion process, and t h e measurement of the dimensions have all been described previously.
After m a x i m u m swelling had been reached, the excess solvent w a s blotted from t h e surface a n d t h e blocks were weighed. T h e
'Ashton, H. E. 1973. The swelling of wood in polar
organic solvents. Wood Sci. 6(2):159-166.
used and recover i t to make t h e process
The author i s Research Officer, Materials Sec-
economically feasible a n d to render t h e reacted tion, ~ i ~of iBuilding Research, National ~ i ~ ~ wood suitable for handling. This present paper Research Council of Canada, Ottawa, Canada. T h e
reports studies on the removal of the polar author acknowledges the assistance of R. C. Seeley
and L. R. Dubois who weighed a n d measured the
solvents from swollen but unreacted wood. I t is wood blocks. This p a er is a contribution from the expected t h a t solvent would be more readily Division of Building kesearch. National Research ublished with the ap-
removed from treated wood for two reasons:
~ ~ ~ ~ ~ f " ~ h , " ~ ~ " , ~ b ~ , " f " , h ~ ~ i v i s ~ o n .
It was received hydrogen bonding to hydroxyl groups would for publication in J a n u a r y 1973.solvents were then allowed to evaporate from the blocks under several drying conditions; the block weights a n d dimensions were measured a t intervals. Because all the samples h a d not been immersed in solvent for the s a m e length of time, the drying schedule was not t h e same in all cases. Evaporation first took place a t 100 percent and then a t 50 percent relative humidi- ty (RH). The intention was to prevent rapid sol- vent evaporation from the surface, which might cause checking because the interior would still be swollen.
To allow evaporation a t 100 percent RH, the blocks were placed in a desiccator con- taining distilled water i n the bottom. T h e desic- cator w a s kept i n a room maintained a t 2352°C (73.5 53.5"F). Since the water was not stirred, the humidity was probably somewhat below saturation. For drying a t 50 percent RH the blocks were stood on end on the bench i n a con- ditioned room which was designed for a n RH of 50 +2 percent RH. A s the room w a s large and well ventilated, evaporated solvent was dispersed rapidly.
Following the 50 percent RH conditioning, the blocks were ovendried for 24 hours i n a com- mon laboratory oven set a t 105 52°C (221 +3.5"F). After heating, the samples were cooled i n a desiccator containing phosphorous pentoxide. Generally the blocks were weighed within a few hours, but in one case they were left i n the desiccator over a weekend. The blocks were evacuated in the same desiccator b y connecting i t to a vacuum pump a n d evacuating the system to 28 inches when t h e pump was s h u t off. When t h e vacuum
decreased to 25 inches after 1 or 2 days, t h e pump was turned over again until the higher vacuum was restored. The vacuum referred to later a s 25 inches actually fluctuated between 25 and 28 inches.
The first group of samples to attain equilibrium swelling was left in t h e solvents for a week before weighing. When they were subse- quently dried according to the schedule, it w a s found t h a t more t h a n half t h e M P originally ab- sorbed was retained in the wood after a total of 16 days' gradual drying. Similarly, yellow birch contained more t h a n 20 percent DMF a n d pyridine, and beech more t h a n 20 percent DMF of the weight absorbed. Only white pine t h a t h a d been immersed in these l a s t two solvents contained less t h a n 10 percent solvent. Oven- drying twice a n d storing over P, 0 , reduced t h e weight pickup to below 4 percent i n most cases. It did not, however, remove the last 10 to 20 percent of MP except from the white pine.
The blocks were then subjected to avacuum of 25 inches mercury for various times, but t h i s treatment h a d little effect on retained weight. Finally the birch, beech, and pine blocks t h a t h a d been immersed in MP a n d still contained, respectively, 18, 12, and 4 percent of theamount originally absorbed were soaked i n water for a week. They were then redried a t 100 percent a n d 50 percent RH a n d i n a vacuum. Other samples i n the first a n d second groups were not sub- jected to this procedure.
Theimmersion period required for Douglas- fir to reach maximum swelling i n DMSO, MP, a n d pyridine h a d been considerably longer t h a n for the other wood-solvent combinations.
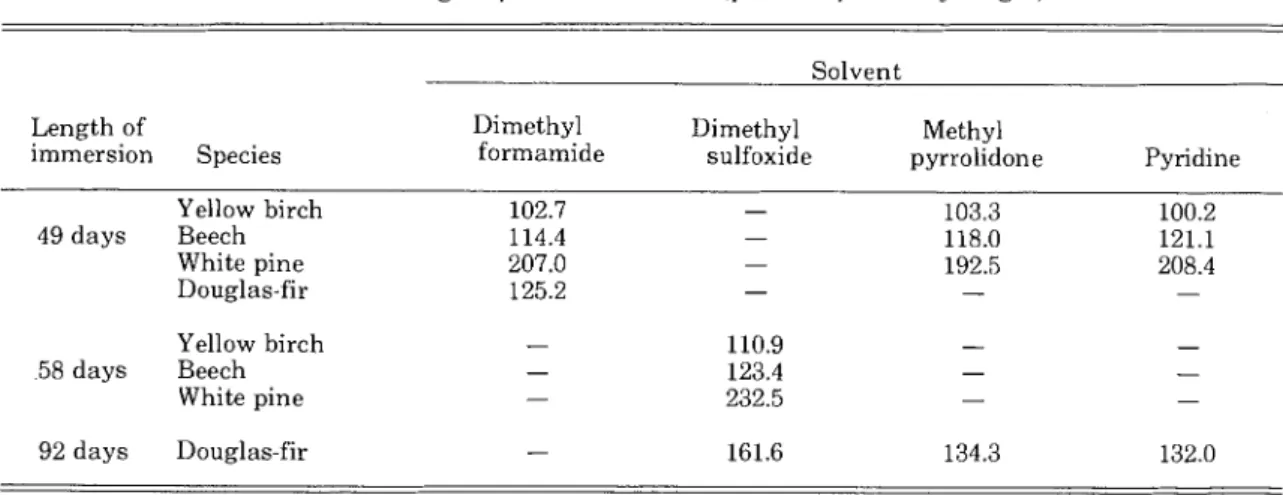
TABLE 1. - Weight of solvent absorbed (percent of ovendry weight).
Solvent Length of
immersion Species
Dimethyl Dimethyl Methyl
formamide sulfoxide pyrrolidone Pyridine
Yellow birch 102.7 - 103.3 100.2 49 days Beech 114.4 - 118.0 121.1 White pine 207.0 - 192.5 208.4 Douglas-fir 125.2 - - - Yellow birch 58 days Beech White pine 92 days Douglas-fir - 161.6 134.3 132.0 W O O D S C I E N C E Vo1.6.No.4 369
Consequently by the time the fir samples were removed from these solvents, it was known t h a t two ovendryingperiods a n d possibly soakingin water were necessary to remove most of the DMSO and MP. As a result they were only sub- jected to drying a t 50 percent RH.
R e s u l t s and D i s c u s s i o n
T h e dimensional a n d weight changes of the wood blocks during the various drying stages were calculated a s mean percent volumetric change a n d mean percent of original weight increase. The latter w a s used because calculating weight increase a s percent of the ovendry weight reflects mainly the specific gravity of the wood. For example, in 49 days the 2- by 1- by 1- inch blocks of pine ab- sorbed about 23.9 g of MP and the same size birch blocks about 0.3 g more. On percent of ovendry weight, the values are 192 percent for pine a n d 103 percent for birch; this suggests a greater difference between them t h a n i n fact ex- ists. T h e percent solvent originally absorbed i s given i n Table 1.
T h e volumetric and percent weight changes are plotted in Figures 1 to 5. It can be seen that when t h e wood blocks wereexposed to near 100 percent RH after solvent immersion, they all picked up some additional weight ex- cept for those containing pyridine. The volume, however, tended to decrease, in most cases only slightly. The added water resulted in slight shrinkage, confirming the conclusion of t h e first paper t h a t the three solvents have a greater effectiveness than water in swelling wood.
I t is also evident that evaporation of t h e solvents a t 50 percent RH was a relatively slow process other t h a n where the solvent w a s pyridine or the wood was pine. A t this drying condition, decrease in weight occurred faster t h a n volumetric shrinkage. Most of the blocks remained close to their final maximum volume for the first 3 to 4 days, while their weight decreased during this period. Volumetric shrinkage only became significant after 13 to 14 days' drying a t 50 percent R H , apart from all woods in pyridine, birch in DMF, and fir in DMSO. T h e slowest solvent to evaporate a t 50
TIME W A l t R 5 0 %
3;
:
5 O I R . H . 1 0 5 ' F 2 5 I N . I M M E R S I O N lWSbR.H. R.H. 25 I N . 3 DAYS I V A C U U M1
7 D A Y SI
B D A Y Sd
s
bAy5
I I l l I I I IFigure 1. - Volume a n d weight changes during evaporation of methyl pyrrolidone.
- - 3
370 A P R I L 1 9 7 4
0 Y E L L O V l B I R C H O BEECH O-Cu V O L U M E T R I C . _ _ _ o m O F W E I G H T TlME 10
Figure 2 . - Volume a n d weight changes during
evaporation of dimethyl formamide.
I l l I I 11 11 - I 1 0 D O U G L A S F I R - I I - I I A W H I T E P I N E 0- % V O L U h 4 E T I I I C -
.---
I t 9, O F W E I G H T - - - - I I -,
-',
- - -~'.-__._-
- -_ -- - --.
- A-A- - 1 - - - -1 - -1 (XPA - R.H. 5 0 % R . H . _ l O 5 ' C 25 I N . 3 DAYS 2 1 D a Y S I I I I I I I S 13 7 3 D A Y 5 '00" R.H. 5 0 5 , R . H . 1 0 5 ~ C 2 5 I N . 3 V A C U U M 1 DAYS I 21 D A Y S I i l l I - - 3Figure 4 . - Volume and weight changes during
evaporation of pyridine.
- 1 0
TIME TIME
Figure 3. - Volume a n d weight changes during Figure 5. - Volume and weight changes of wood dur-
evaporation of dimethyl formamide. ing evaporation of dimethyl sulfoxide.
3 5 1 3 1 2 3 D A Y S TlME M J % l O i a C \o-o R.H 5 O I R . H . 25 I N . - I 1 DAYS
--iT
1 1 1 I V A C U U U 21 D A Y S I I - 3percent RH w a s M P when considered on a volume basis. but DMSO w a s slowest on a weight basis.
T h e figures also show t h a t ovendrying caused a considerable drop i n both weight a n d volume. Pine t h a t h a d been immersed i n DMF a n d pyridine returned to its original weight although there w a s still some slight swelling. Birch a n d beech retained from 2.5 to 4 percent of these two solvents, but the volumes were less t h a n the original. Pine i n DMSO a n d M P a s well a s fir i n DMF retained from 2 to 4 percent excess weight with corresponding swelling after ovendrying. Birch retained 17 t o 19 per- cent DMSO a n d MP, a n d beech about 12
percent of these solvents. After two periods of ovendrying, MP w a s retained i n the respective samples to a greater extent t h a n DMSO both on a weight a n d a volume basis.
Continued heating might h a v e removed the l a s t of t h e retained solvent. However, i t w a s considered t h a t 3 days' heating a t 105OC
(221°F) might not be a practical process s o two
other methods were investigated.
First, a vacuum of 25 inches of mercury w a s applied. T h i s method did not significantly change t h e weights or volumes of t h e blocks even over longer periods.
Second, because M P h a d been the solvent most resistant to removal by ovendrying, t h e birch, beech, a n d pine samples containing i t were soaked i n water for a week to see if the material could be extracted. I t c a n be seen i n Figure
-
1 t h a t the woods did not swell a s muchin water a s i n t h e solvents. This difference m a y be due to the lower swelling effect of water, the much shorter immersion period, or a combina- tion of both factors. T h e weight gain w a s also less, r a n g i n g from 60 to 80 percent of t h e weightof solvent previously absorbed. On subsequent exposure to n e a r 100 percent RH, t h e blocks lost weight instead of gaining a s they h a d done when saturated with solvent. Again t h e volume decreased slightly a t t h i s condition. A t 50 per- cent RH, drying also occurred much faster t h a n previously with both weights a n d volumes con- siderably lower after 2 d a y s t h a n they were after 13 d a y s when solvent w a s being removed. T h e first application of vacuum to the water-soaked blocks reduced the weights to less t h a n those measured immediately before im- mersion. I t i s difficult to judge whether con- tinued drying a t 50 percent RH m a y have been just a s effective in producing the losses i n
weight. Volumetrically the beech a n d pine also decreased below t h e presoaked condition, but t h e birch was more swollen t h a n before immer- sion i n water. P e r h a p s a mixture of water a n d M P h a s a greater swelling effect on birch. Ex- posure to t h e vacuum for a n additional 10 d a y s resulted i n only minor gravimetric a n d volumetric decreases. At the end of the test t h e beech still retained about 2.5 percent of t h e weight a n d about 5 percent of t h e volume at- tained under equilibrium swelling in MP. P i n e h a d slightly decreased a n d birch moderately in- creased compared with the original ovendried weights a n d dimensions.
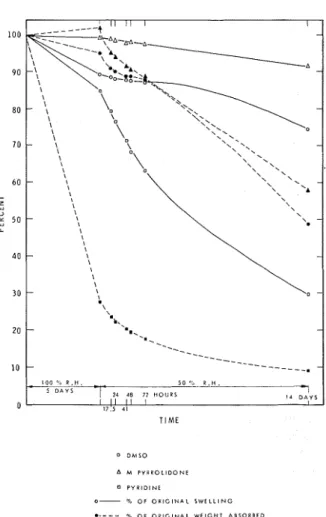
T h e results from the Douglas-fir samples t h a t had been measured more frequently while drying a t 50 percent RH are s h o w n inFig. 6. F o r ease of comparison t h e volumetric changes a r e
100 il R H , . 5 D A Y S - 5 0 -i LL-- 2L 4 8 12 HOURS I / II I l I I d D A Y S 0 1 7 ' 5 e l T I M E 0 Dh1SO A M P I P R O L I D O N E 0 P Y R l D l N E 0- 9 OF O l i l G l N A L S W E L L I N G a - - - - 4, OF O R I G I N A L W E I G H T A 8 1 0 R B E D
Figure 6. - Solvent removal from Douglas-fir. A P R I L 1 9 7 4
plotted here a s percent of the values reached a t maximum swelling. Again it i s obvious t h a t pyridine evaporated much more quickly t h a n the other solvents a n d t h a t high humidity did not noticeably retard its evaporation.
MP e v a ~ o r a t e d from fir a t a uniform rate bv weight after the first overnight period a t 50 per- cent RH. By contrast, t h e volumetric swelling remained fairlv constant for the first 2 to 3 davs and showed a significant decrease only over the last 11-day period. Unexpectedly, the fir blocks containing DMSO decreased volumetri- cally a s well a s gravimetrically during the 5 days a t 100 percent RH.
During the swelling tests it h a d been noted that most of the beech blocks developed checks, especially on the tangential faces. After the first set of blocks h a d been ovendried for 24 hours, they were examined visually to see whether a n y cracks or checks remained. Neither the pine nor birch showed a n y noticeable effects of the swelling and shrinking processes. Although beech h a d swollen volumetrically less t h a n birch, blocks t h a t had been immersed in DMSO had deep cracks on the tangential faces. The samples t h a t h a d cracked on swelling in DMF showed only a few small end grain checks on drying. One beech block had cracked in pyridine and one in MP but neither showed a n y evidence of this o n drying. Nevertheless, the application of solvent swell- ing to dried beech m a y not be possible since it appears prone to develop cracks t h a t would be visible if the wood were permanently distended. If chemical treatment of beech is considered desirable, it would probably be necessary to start with green wood a n d remove the water by solvent drying.2
Conclusions
Pyridine evaporated relatively quickly from wood when exposed to atmospheres of 100 a n d 5 0 percent RH. Dimethylformamide (DMF), which swells wood quickly, evaporated fairly rapidly a t 50 percent RH. About 30 per- cent of the DMF originally absorbed remained after 13 days, except for white pine where only 10 percent was retained. Methyl pyrrolidone (MP) a n d dimethyl sulfoxide (DMSO) were strongly held, and it i s questionable whether
'Seth, K. K., and A. B. Anderson. 1965. Solvent drying of Callfornla redwood with methanol. Forest Prod. J.
15(7):297-301.
permanent swelling of the wood by chemical reaction would have much effect on their retention.
Judging by weight losses, pine released solvents more quickly than the otherwoods a n d i n most cases w a s swollen less after drying a t 50 percent RH. T h i s agrees with the generally low swelling resistance coefficients obtained i n the previous work. Evaporation was usually a little faster from beech than from birch, but this was probably due to the cracks i n t h e former. A s with swelling, fir was the slowest to release sol- vent in the one comparable case.
Ovendrying was required to remove most of the remainder of the two solvents, which evaporated quickly, a n d the bulk of the more strongly held solvents. Between 11 and 19 per- cent of M P a n d DMSO was left in birch a n d beech after two 24-hour heating periods. From 2 to 4 percent of these solvents w a s left in pine, a n d the same quantity of DMF and pyridine remained in all woods except pine, which returned essentially to original weight when
heated.
Extraction would be required to remove strongly heldsolvents, and water was generally effective with MP. Although no tests were run, it is suggested t h a t a low-boiling water-soluble solvent such a s acetone or methanol might be more effective t h a n water in removing the l a s t of t h e retained polar compounds. The proposed solvents have been used to decrease markedly the time to remove water from green timber'
a n d should act similarly with polar solvents. Preliminary exposure to 100 percent RH i s a n unnecessary step in removing solvents from swollen wood. Evaporation a t 50 percent RH i s not practical with DMSO or MP. Application of a moderate vacuum was not effective in remov- i n g the last of a n y of the solvents.
The conclusions of the previous paper a r e reinforced by t h i s study a s to t h e best solvent, the ease of treating white pine and the dif- ficulties in treating Douglas-fir. As well a s swelling wood most rapidly, D M F was the sec- ond fastest to e v a ~ o r a t e and i s not a s ob- noxious to handle a s pyridine. T h i s study also a d d s to the previous adverse conclusion t h a t DMSO, while causing the greatest equilibrium swelling, requires pressure or vacuum im- pregnation to bring it about i n a reasonable time. I t w a s found here t h a t a n extraction
process would be needed to remove i t from the wood.
One result of this study t h a t adversely affects t h e previous work is the observation t h a t beech, which swells most rapidly, checks most readily. The rapid swelling i s probably enhanced by checking. Additional work would be needed to show whether beech can be swollen satisfactorily with polar solvents.
After swelling in solvent and subsequent drying, the radial and tangential dimensions of
birch were generally smaller t h a n originally. Either sufficient solvent-soluble material was extracted or there was partial collapse of t h e cells causing permanent shrinkage.
The greatest chance for successful treat- ment of wood appears to be with a process com- prising swelling white pine with DMF, reaction of the hydroxyl groups with the desired chemical reagent, washing with additional DMF to recover unreacted reagent, and subse- quent heating to remove the solvent.