HAL Id: hal-03088560
https://hal.archives-ouvertes.fr/hal-03088560
Submitted on 26 Dec 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Hedgehog signaling modulates glial proteostasis and
lifespan
Andrew Rallis, Juan Navarro, Mathias Rass, Amélie Hu, Serge Birman,
Stephan Schneuwly, Pascal Thérond
To cite this version:
Andrew Rallis, Juan Navarro, Mathias Rass, Amélie Hu, Serge Birman, et al.. Hedgehog signaling
modulates glial proteostasis and lifespan. Cell Reports, Elsevier Inc, 2020, 30 (8), pp.2627-2643.e5.
�10.1016/j.celrep.2020.02.006�. �hal-03088560�
Article
Hedgehog Signaling Modulates Glial Proteostasis
and Lifespan
Graphical Abstract
Highlights
d
Impaired Hedgehog (Hh) signaling in adulthood induces glial
proteostasis defects
d
Defective Hh signaling in glia leads to reduced lifespan and
neuronal integrity
d
Activation of Hh signaling in glia controls the expression of
chaperones
d
The protective function of Hh is achieved through the
regulation of proteostasis
Authors
Andrew Rallis, Juan A. Navarro,
Mathias Rass, Ame´lie Hu, Serge Birman,
Stephan Schneuwly, Pascal P. The´rond
Correspondence
therond@unice.fr
In Brief
Glial dysfunctions affect organismal
lifespan and neuron integrity. Rallis et al.
show that Hedgehog signaling
coordinates lifespan determination and
neuroprotection through the regulation of
glial proteostasis during adult life in
Drosophila. This process is mediated by
the regulation of chaperones upon
activation of Hedgehog signaling in glia.
Cell Reports
Article
Hedgehog Signaling Modulates
Glial Proteostasis and Lifespan
Andrew Rallis,1Juan A. Navarro,2Mathias Rass,2Ame´lie Hu,3Serge Birman,3Stephan Schneuwly,2
and Pascal P. The´rond1,4,*
1Universite´ C^ote d’Azur, CNRS, INSERM, iBV, Nice, France
2Department of Developmental Biology, Institute of Zoology, Universitaetsstr. 31, University of Regensburg, 93040 Regensburg, Germany 3Genes Circuits Rhythms and Neuropathology, Brain Plasticity Unit, ESPCI Paris, CNRS, Labex MemoLife, PSL Research University, 10 rue
Vauquelin, 75005 Paris, France
4Lead Contact
*Correspondence:therond@unice.fr https://doi.org/10.1016/j.celrep.2020.02.006
SUMMARY
The conserved Hedgehog signaling pathway has
well-established roles in development. However, its
function during adulthood remains largely unknown.
Here, we investigated whether the Hedgehog
signaling pathway is active during adult life in
Drosophila melanogaster, and we uncovered a
protective function for Hedgehog signaling in
coordi-nating correct proteostasis in glial cells.
Adult-spe-cific depletion of Hedgehog reduces lifespan,
locomotor activity, and dopaminergic neuron
integ-rity. Conversely, increased expression of Hedgehog
extends lifespan and improves fitness. Moreover,
Hedgehog pathway activation in glia rescues the
life-span and age-associated defects of hedgehog
mu-tants. The Hedgehog pathway regulates downstream
chaperones, whose overexpression in glial cells was
sufficient to rescue the shortened lifespan and
pro-teostasis defects of hedgehog mutants. Finally, we
demonstrate the protective ability of Hedgehog
signaling in a Drosophila Alzheimer’s disease model
expressing human amyloid beta in the glia. Overall,
we propose that Hedgehog signaling is requisite for
lifespan determination and correct proteostasis in
glial cells.
INTRODUCTION
The Hedgehog (Hh) signaling pathway is an evolutionarily conserved module that organizes animal patterning and is essential for determining cell identity and the final body plan (Briscoe and The´rond., 2013). Hh and signaling components of its pathway were initially discovered in Drosophila, and highly conserved members are present in the vast majority of metazoa including in mammals, which possess three Hh paralogs, the most well studied being Sonic Hedgehog (Shh) (Ingham 2018). In vertebrates, Shh signaling also plays an important role in stem cell homeostasis in adult organisms (Petrova and Joyner, 2014), and abnormally sustained activation in the adult is critical in the initiation and growth of many human tumors (Beachy et al.,
2004; Curran 2018). Although the developmental facets of Hh signaling have been well established, the role of Hh signaling in adult homeostasis has been difficult to evaluate due to the vital function of the Hh pathway during development.
Emerging lines of evidence suggest that Shh has adult specific functions in the nervous system (Petrova and Joyner, 2014). For instance, in mice Shh regulates the proliferation of neural stem cells throughout adult life (Lai et al., 2003; Palma et al., 2005; Ahn and Joyner., 2005; Ferent et al., 2014; Yao et al., 2016). Shh signaling has also been demonstrated to act as a modulator of brain plasticity and nerve regeneration (Yao et al., 2016). Furthermore, Shh signaling is elevated in different CNS injury models, such as stroke, spinal cord injury, and stab wound (Jin et al., 2015; Amankulor et al., 2009; Bambakidis et al., 2010). In line with the above, treatment with Shh or Shh agonists has shown to improve neurological function and promote neurore-generation (Chechneva et al., 2014; Huang et al., 2013; Bamba-kidis et al., 2012; Thomas et al., 2018) whereas depletion of Shh signaling components exacerbated the neuropathology following cerebral ischemia and nerve injury (Ji et al., 2012; Mar-tinez et al., 2015).
There is growing evidence that Shh signaling utilizes a neuron-glia communication mode to coordinate homeostasis in the post-natal brain. Under physiological conditions, Shh seems to be predominantly released by neurons (Gonzalez Reyes et al., 2012; Eitan et al., 2016; Okuda et al., 2016), and glial cells have been shown to be highly responsive to Shh signaling in vitro (Ugbode et al., 2017; Okuda et al., 2016). Furthermore, Shh signaling is active in vivo in the adult mouse forebrain (Garcia et al., 2010) in which signaling is activated in a discrete popula-tion of glial cells that respond to Shh secreted from neurons ( Gar-cia et al., 2010; Farmer et al., 2016). Importantly, activating Shh signaling in glial cells has been demonstrated to be neuroprotec-tive. For example, in vitro, Shh-stimulated mouse cortical astro-cytes protect co-cultured neurons from kainite (KA)-induced cell death (Ugbode et al., 2017). Furthermore, in neurodegeneration mouse models induced by intraperitoneal injection of KA, or sur-gical brain injury, Shh signaling in glia regulates proliferation of astrocytes and microglia (Sirko et al., 2013; Pitter et al., 2014).
Due to its role as a neuroprotective factor, it is hypothesized that Shh may be implicated in alleviating age-related neurode-generative disease (Zhang et al., 2014; Yao et al., 2017). For instance, in cultured rat hippocampal neurons, Shh signaling
A B B’ B” C C’ D D’ E E’ E” E”’ F F’ F” G G’ G” H H’ H” I J O K L M N
confers protection against neurotoxins such as the amyloid beta peptide (Ab 1-42) (Yao et al., 2017). However, the mechanism by which Shh exerts its protective function in this experimental paradigm has not yet been elucidated. In Alzheimer’s disease, accumulation of Ab 1-42 has been demonstrated to precede the formation of amyloid plaques, and the deposition of amyloid plaques correlates with an increase in age-related cognitive dysfunction (D’Andrea et al., 2001; Rodrigue et al., 2009). Ab 1-42 has a high propensity to aggregate, and there is a large body of evidence that chaperones prevent deleterious misfold-ing and aggregation of amyloid (Evans et al., 2006; Magrane´ et al., 2004). In fact, the Hsp70 chaperone and its partner Hsp40 have been demonstrated to act synergistically to inhibit the early stages of Ab 1-42 aggregation in vitro (Evans et al., 2006). Moreover, in rat primary neuronal cultures seeded with Ab 1-42, hsp70 overexpression improves cell viability (Magrane´ et al., 2004). Based on previous studies, it is possible that Shh prevents accumulation of Ab 1-42 aggregates; however, this has yet to be evaluated.
Here, we utilize Drosophila melanogaster to evaluate the func-tion of the Hh pathway at the physiological and cellular level during adult life. This model enables us to address the tissue-specific functional role of the Hh signaling pathway in the adult brain. We identify a new role for Hh signaling in adult lifespan and glial proteostasis and uncover the mechanism by which Hh prevents accumulation of protein aggregates. We show that increasing Hh levels promotes lifespan extension, whereas depletion of Hh activity during adult life reduces lifespan. We further demonstrate that specific activation of the Hh pathway in adult glia potently rescues the shortened lifespan and locomo-tor and dopaminergic neuron defects observed in hedgehog (hh) mutants. We found that proteostasis in glial cells is affected in the absence of Hh activity and identified two highly conserved chaperones, hsp40 and hsp68 (a member of the Hsp70 protein family), that are regulated by the Hh pathway. Their overexpres-sion potently rescues proteostasis defects in the glia of hh mutant animals and rescues hh mutant reduced lifespan. As proof of concept, we also demonstrate that activation of the
Hh pathway and one of its downstream chaperone targets in the glia is able to rescue the deleterious effects of expression of human Ab 1-42 in an in vivo Alzheimer’s disease model. The work described here makes a significant contribution to our un-derstanding of the role of the Hh morphogen during adulthood: it identifies for the first time a protective role for Hh on glia proteo-stasis and dissects its mechanism of action, showing that Hh op-erates through the regulation of highly conserved chaperones.
RESULTS
The Hh Signaling Pathway Is Present in Glial Cells of the Adult Brain
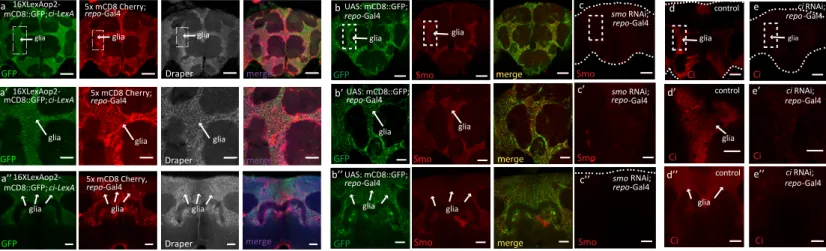
Here, we address the role of Hh signaling in the adult brain of Drosophila melanogaster that has not yet been studied. To do so, we first investigated the pattern of expression of Hh signaling components, in particular the Smoothened (Smo) serpentine protein and the transcription factor Ci/Gli that is able to activate the expression of target genes (Figures 1A–1D). For this, we uti-lized the CRISPR/Cas9 genome editing and generated a new Ci-Trojan Gal4 line that represents the full Ci expression pattern and, together with a second independent system (Ci-LexA line), found that Ci is predominantly expressed in glial cells in the adult fly brain (Figures 1A, 1B, andS1A). Immunofluorescent analysis using antibodies against Smo and Ci was also per-formed and revealed a strong localization of both components in glia (Figures 1C and 1D). RNAi-depletion of Smo and Ci in glia reduced immunoreactivity for both proteins, supporting specificity of the staining (Figures S1B–S1E). These findings are consistent with a recent single-cell transcriptome analysis of the Drosophila and human adult brain identifying Smo and Ci/Gli expression in glia (Davie et al., 2018; Lake et al., 2018).
Knowing that the Hh pathway is present in adult glial cells, we further examined the pattern of expression of Hh in the adult brain. Using two different hh-Gal4 reporter lines, inserted at two different positions in the hh genomic locus, we found that in the adult brain, hh is expressed in ~10–15 cells per hemisphere which are positive for the pan-neuronal marker Elav and negative
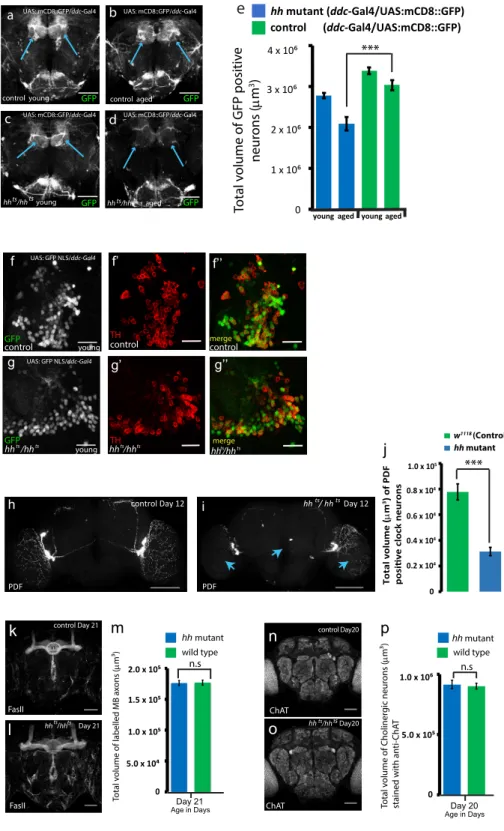
Figure 1. Location of Hh Signaling Components in the Drosophila Adult Brain and DA Neuron Loss in hh Mutants
(A) Schematic representation of the Ci-T2A-Gal4 Trojan line.
(B–B00) Cells positive for ci-T2A-Gal4 (Trojan line) expressing GFP NLS (B, green channel) are immunostained with the glial marker Repo (B0, red channel). The merged image (B00) reveals that almost all the cells expressing Ci are glial cells. Scale bars, 50 mm.
(C and D) Endogenous Smo and Ci are localized in the glial cells. Anterior 1 mM section of a whole mount adult brain in which glial cells are specifically labeled with (C) mCD8::GFP (green channel) or with (D) anti-Draper (a glial receptor, green) and immunostained for Smo or Ci (red channel). Note that Smo and Ci staining are present in glial cells (dashed boxes). Scale bars, 50 mm.
(C0and D0) Magnification of the region of the glia indicated by the dashed boxes. Scale bars, 20 mm.
(E–F00) hh-expressing cells in the adult brain have neuronal identity. Projection pattern for Hh expressing cells (E–E000). Cells positive for hh-Gal4 express membrane marker mCD8::GFP, (E–E000) images of 1 mm sections for the forebrain, midbrain, and hindbrain. (F) Expression of a nuclear GFP in hh-expressing cells
of adult brain are positive for Elav (red channel), but not Repo (blue channel). Scale Bar: 50 mm. (F0and F00) 53 magnification of the boxed regions shown in (F). The
merge between hh-expressing cells (green) and anti-Elav immunostaining (red channel) is shown in the right panel. hh-expressing cells are indicated by white arrows. Scale bars, 5 mm. Images consist of a 1-mm section.
(G–H00) Representative confocal images of the PAM cluster cell bodies of control (G and G00) and hh mutant (H and H00) of aged adult fly brains, in which DA neurons are labeled with a nuclear GFP marker (GFP NLS in gray) and with anti-TH immunostaining (in red). Scale bars, 10 mm.
(I and J) Quantifications of DA neuron number in the PAM cluster of hh mutant and control in both young (I) and aged flies (J).
(K–N) hh mutant and control brains with DA neurons immunostained with TH (in gray) in young (K and M) and aged flies (L and N). Arrows label the PAM DA neurons.
(O) Quantifications of DA neuron volume in control and hh mutant adult fly brains with TH immunostaining, in young (Y), middle (M), and aged flies (A). For DA neuron number statistics, the two-tailed t test was used, and for DA neuron volume quantification, two-way analysis of variance (ANOVA) test was used to compare the specified data points for hh mutant and control samples (***p < 0.001; **p < 0.01; *p < 0.05).
A B
C D
for the glia-specific marker Repo (Figures 1E, 1F,S1F, and S1G). Using a third reporter line for hh-expressing cells—the hh dsRed enhancer trap (Akimoto et al., 2005)—we identified glutamater-gic and GABAerglutamater-gic neurons that are positive for the hh-express-ing cells (Figures S1H–S1J). This shows that both subtypes of neurons produce Hh, in agreement with recent single-cell tran-scriptome analysis of the Drosophila adult brain (Croset et al., 2018; Davie et al., 2018) and with an equivalent study on Shh in the adult human brain (Lake et al., 2018).
Hh Signaling Is Needed to Maintain Dopaminergic Neuron Viability in the Adult Brain
To investigate the functional relevance of Hh signaling in adult, we specifically depleted Hh in the entire organism using a hh temperature-sensitive allele (hhts). This is a null allele for hh at non-permissive temperature (29!C) and, after 24 h at this
tem-perature, no more Hh staining or activity can be detected in ho-mozygote mutant animals (Ranieri et al., 2012; Palm et al., 2013). To avoid any patterning defect due to Hh developmental func-tion, hhts flies were raised at permissive temperature (18!C)
and transferred to non-permissive temperature upon eclosure (STAR Methods). Indeed, these flies do not exhibit any notice-able patterning defect (Strigini and Cohen, 1997). With this set up, we investigated whether Hh is required to maintain neuronal homeostasis in the adult brain. To explore this, we focused on dopaminergic (DA) neurons known to be highly susceptible to brain homeostasis defects and whose cell number, neuronal projection, and secretion of dopamine can be measured and quantified accurately (Friggi-Grelin et al., 2003; Navarro et al., 2014).
Nine DA clusters are present in the Drosophila adult brain; among these, the protocerebral anterior medial (PAM) cluster is part of the circuit regulating climbing activity (Riemensperger et al., 2013). Strikingly, using anti-tyrosine hydroxylase (TH, a functional marker for DA neurons) immunostaining (Friggi-Grelin et al., 2003), we observed a reduced number of DA neurons in the PAM cluster of aged hh mutant adult brains (Figures 1G, 1H, and 1J), whereas no change in DA neuron number was de-tected in young hh mutant flies (Figures 1I,S2F, and S2G). These defects suggest that overall DA neuron volume in the adult brain might be affected in absence of Hh. Indeed, we found that during adulthood, hh mutant brains globally showed an abnormally low level of TH staining (Figures 1K–1O;Table S1) as well as an over-all reduction of the mRNA levels of the neuronal-specific form of TH (nTH) and a decrease in brain dopamine levels (Figures 3N and 3O, compare green and blue bars). In an alternative
approach, we quantified DA neuron volume networks by labeling their cell membranes with the mCD8-GFP reporter transgene and found similar results (Figures S2A–S2E). To do so, we used the Ddc-Gal4 driver that targets a subset of DA neurons including the entire PAM cluster (Riemensperger et al., 2013). Indeed, DOPA decarboxylase (Ddc) is the enzyme that catalyzes the transformation of L-dopa into dopamine.
To investigate whether there is a wider neurodegenerative ef-fect in the brain as a consequence of adult-specific hh loss of function, other types of neurons were analyzed in the adult brain, including clock neurons, mushroom body neurons, and cholin-ergic neurons. Interestingly we found that small (s-LNv) and large lateral ventral (l-LNv) clock neurons (that are specifically immu-nostained with pigment dispersing factor [PDF]) also exhibited severe neurodegeneration (Figures S2H and S2I), with a drastic decrease in neuronal volume in hh mutants compared to age-matched wild-type samples (Figure S2J;Table S1). We did not find significant defects in other neuronal cell types (cholin-ergic and mushroom body neurons) in hh mutants at the time point at which we observed severe DA neuron defects (Figures S2K–S2P).
Taken together, these results show that Hh is necessary to maintain DA neuron homeostasis in the adult brain and the integ-rity of other populations of neurons, such as clock neurons that are known to be vulnerable to aging (Liao et al., 2017). This is illustrated by the substantial reduction of DA neuron functional integrity as well as the neurodegeneration observed in clock neu-rons in hh mutant flies.
Adult Expression of Hh Is Necessary for Correct Lifespan Determination
We next decided to investigate whether the hh mutant flies exhibit any survival or behavioral deficits, because defects in the dopaminergic neuron network have been demonstrated to have a detrimental effect upon lifespan and locomotor activity in both mammals and invertebrates (Thanos et al., 2016; Liao et al., 2017; Feany and Bender, 2000). Strikingly, we found that both female and male adult hhtsflies shifted to 29!C exhibit a
drastically shortened lifespan (80%–85% decrease in the me-dian lifespan when compared to control flies) (Figures 2A and
S3C;Table S2). When maintained at 25!C, but not at 18!C, the
hhtsmutant flies also exhibited a strongly decreased lifespan (Figure S3K;Table S2).
To confirm that the loss of Hh is responsible for this defect, we analyzed the heteroallelic combination of hhtsnull flies with hh15,
another strong allele of hh. The hhts/hh15mutant combination Figure 2. Hh Signaling Affects Adult Lifespan Determination and Fitness
(A) Overexpression of hh in hh-expressing cells—hh OE (hh Gal4)—extends survival rate, whereas hh depletion drastically reduces it. Both combinations of hh mutant alleles (hhts/hhtsand hhts/hh15) decrease the survival rate.
(B) hh overexpression in adult flies shows improved negative geotaxis ability during adult life compared to control flies, whereas aged hhts/hhts mutant flies exhibit strong climbing defects.
(C and D) The hh-dependent lifespan extension phenotype (C) and improved fitness (D)—hh OE (hh Gal4)—is abolished upon repression of hh expression in neurons (C and D)—hh OE (elav-Gal80)—but not in glia (Table S2).
(E and F) Expression of hh in glutamatergic neurons of hh mutant—hh mutant + hh OE (vGlut-Gal4)—efficiently rescues the short-lived phenotype of hh mutants (E) and the impaired locomotion phenotype (F). p values refer to the degree of difference between the hh mutant and control/rescue samples as well as hh OE and equivalent control at median survival. Detailed genotypes, % of changes in median survival, and climbing activity, as well as corresponding p values, can be found for all figures inTables S2,S3, andS4. For all lifespan studies, the log-rank test was utilized to compare median lifespans of two specified datasets, and for all climbing activity assays (Tables S2andS4) the two-way ANOVA test was used to compare the specified data points (***p < 0.001; **p < 0.01; *p < 0.05).
A B C D E F M N O G H I J K L
reduced lifespan by a similar degree as hhts(Figure 2A;Table S2). In addition, hhts adult mutant flies exhibited diminished
climbing ability when compared to age-matched controls ( Fig-ure 2B;Table S2). To further confirm that this longevity defect is specifically due to hh loss of function, re-expressing hh exclu-sively into glutamatergic neurons in hhtsmutant flies potently rescued the median lifespan (Figure 2E, red curve), reaching 68% of the control one (Figure 2E, green curve), whereas expres-sion in GABAergic neurons was sufficient to rescue median life-span in hhtsmutant flies up to 41% (Figure S3A;Table S2). The
hhtsclimbing defects were also significantly rescued even in
advanced-age flies (Figures 2F andS3B;Table S2). The individ-ual UAS and Gal4 transgenes alone had no significant effect on survival rate compared to control flies (Figures S3D and S3E; Ta-ble S2). These results suggest that Hh expression in the adult fly brain is essential for correct lifespan determination and locomo-tion activity.
Increased Level of Hh Results in Lifespan Extension
We tested whether increasing Hh level at adult stage extends lifespan. When hh was overexpressed (hh OE) in an adult-spe-cific manner using a driver under the control of the hh promoter (Table S2;STAR Methods), we observed a considerable expan-sion (21%) of the median lifespan accompanied with a signifi-cantly improved climbing ability (Figures 2A and 2B;Table S2). Interestingly, the hh OE-dependent phenotypes were almost completely abolished upon inhibition of neuronal hh OE (using elav-Gal80 repressor of Gal4) (seeSTAR Methods) (Figures 2C and 2D;Table S2). This effect was not observed upon expression of Gal80 in glial cells (using repo-Gal80) (Table S2). Altogether, these data suggest that the hh-dependent lifespan extension and fitness relates to hh expression in adult neurons, and poten-tially, a sub-population of both GABAergic and glutamatergic hh-expressing neurons contribute to this process.
Hh Signaling in Glial Cells Regulates Lifespan Determination
In order to confirm the function of the Hh signaling module in glia, we analyzed the consequence of smo or ci depletion in these cells. Glia-specific RNAi for smo or ci (using repo-Gal4) led to a strong reduction in survival rates, with a decrease of 43% and 48% in the median lifespan, respectively (Figure 3A;Table S2). In contrast, ci and smo depletion in neurons (using elav-Gal4) (Figure 3B;Table
S2) only had a minor effect on survival rates. To confirm the glial-specific effect of ci RNAi knockdown, we have generated a ci CRISPR line (see STAR Methods) which, upon co-expression with Cas9 in the glia, induces a reduction of 31% in the median life-span (Figures S3l–S3l00;Table S2), taken from an average of three
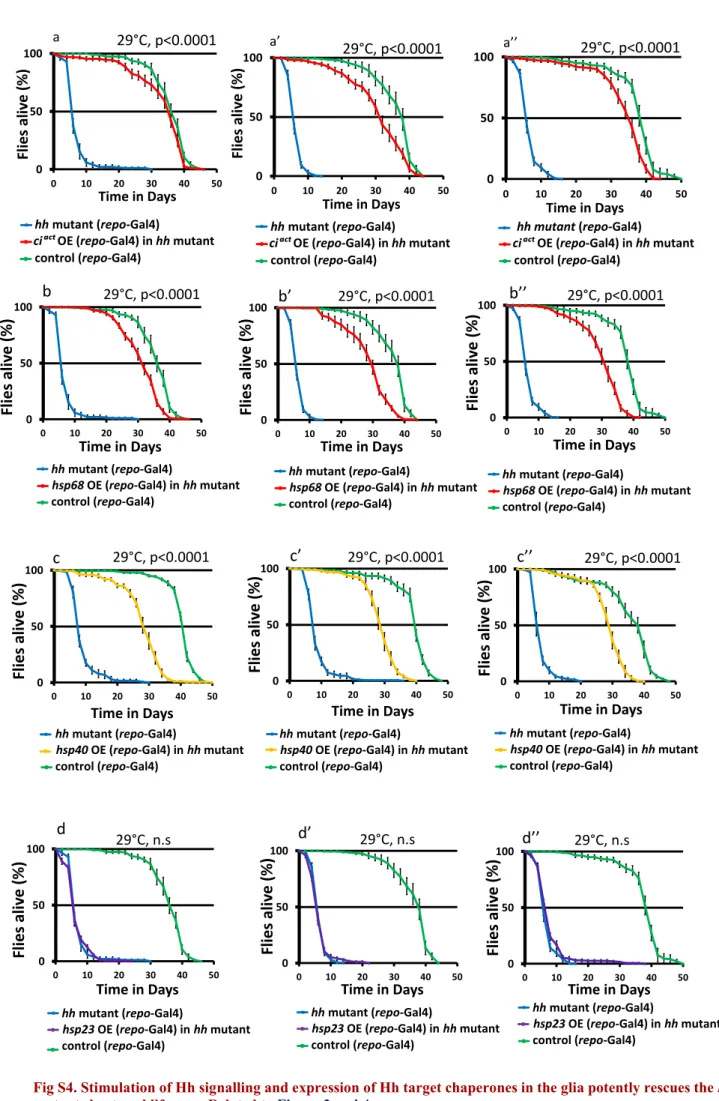
experimental triplicates (Table S2). Furthermore, expressing an activated form of Ci (Ciact) (Wang et al., 1999) in glial cells at
29!C or 25!C strongly rescued the hhts
mutant shortened median lifespan and defective climbing ability (Figures 3C, 3D,S3K, and
S4A–S4A00;Table S2). Additionally, overexpression of Ciactor an
activated form of Smo (Smoact) (Zhang et al., 2004) in glia was
suf-ficient to extend median survival time by 13% and 16%, respec-tively (Figures 3E and 3F). In all these experiments, the presence of each individual UAS transgene had a negligible effect on survival time (Figures S3F–S3J;Table S2).
To confirm that the DA neuron defects we observed in hh mutant brains (described in Figure 1) depends on the Hh signaling cascade in glial cells, we analyzed the integrity of DA neurons in brain of animal depleted for Ci in glia. Upon glial-spe-cific knockdown of ci with CRISPR, we observed a strong reduc-tion in DA neuron volume (Figures S3M–S3O;Table S1). To vali-date the importance of Ci activity in this phenotype, we analyzed whether expressing Ciactin glial cells of a hh adult mutant
res-cues the integrity of DA neurons. Indeed, expression of Ciactin glia in a hh mutant background strongly recovered the DA neuron volume (Figures 3G–3M) as well as the low neuronal TH mRNA expression and reduced dopamine content (Figures 3N and 3O). Overall, our data demonstrate that transduction of Hh signal through Smo and Ci activity in glial cells is necessary and suffi-cient for hh-dependent lifespan and fitness. It also shows that Ci-dependent activity in the glia is essential in protecting DA neu-rons during adult life.
Hh Signaling Regulates Proteostasis in Glial Cells
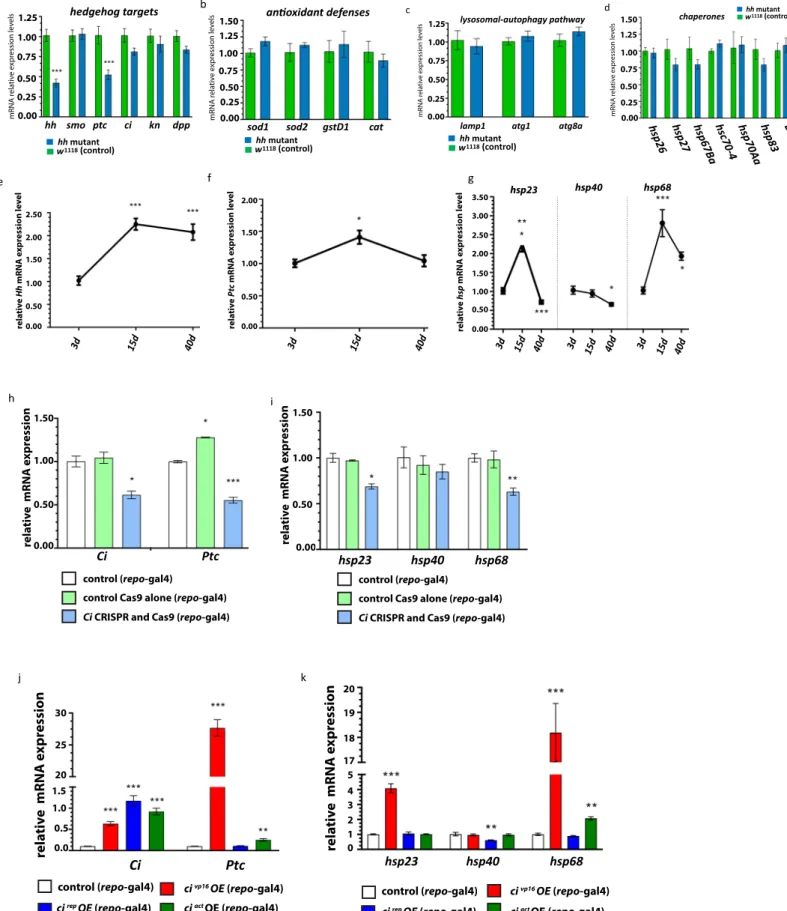
To identify the Hh targets involved in neuroprotection and lifespan determination, we searched for potential protective genes downstream of Hh-dependent transcription in the adult brain. We performed a selective qRT-PCR screen for anti-oxidant defense molecules, autophagy components, as well as chaperones (Figures 4A andS5B–S5D), that have been demon-strated to participate in lifespan regulation (Sun et al., 2012; Si-monsen et al., 2008; Tower, 2011). As proof of principle, we examined well-established Hh-specific targets regulated at the
Figure 3. Hh Signaling in Glial Cells Determines Hh-Mediated Lifespan and Is Protective
(A and B) Adult-specific depletion of Smo (RNAi smo) or Ci (RNAi ci) in glia (repo-Gal4), but not in neurons, (elav-Gal4) strongly reduces survival compared to the control.
(B) Cox-regressioncrstatistical analysis was used to compare control and RNAi lines and revealed no significant differences.
(C and D) Expression of an activated form of Ci in the glia (ciactOE, repo-Gal4) rescues the short survival time of hh mutant (C), at 29!C well as (D) the impaired locomotion.
(E and F) Overexpression of ciact(E) or smoact(F) in glial cells extends lifespan.
(G–I) Images of newly eclosed day 0 fly adult brains with TH immunostaining. (G) Control, (H) hh mutant, and (I) hh mutant expressing ciactin glia.
(J–M) The expression of ciact(L) in the glia of hh mutant flies, partially restores the volume of TH neurons exhibited in hh mutant flies (K) towards the control level (J, quantified in M). Scale bars, 50 mm, Z projections of entire adult brain.
(N and O) Neuronal TH (nTH) mRNA levels and dopamine brain concentration are significantly reduced in hh mutant flies. Expression of ciactin the glia potently restores both (N) nTH mRNA expression and (O) brain dopamine to control levels. For lifespan studies, the log-rank test was utilized, and for climbing activity assays and DA neuron volume, the two-way ANOVA test was used (***p < 0.001; **p < 0.01; *p < 0.05; seeTables S1,S2, andS4). Analysis of TH mRNA and dopamine levels differences between samples were determined by a two-tailed t test and one-way ANOVA test, respectively (***p < 0.001; **p < 0.01; *p < 0.05; seeSTAR Methods).
A B C D E E’ F F’ G G’ H I J K L L’ M N O P Q
transcriptional level, such as patched (ptc, the canonical Hh re-ceptor), decapentaplegic (dpp, of the transforming growth factor b [TGF-b] family), and knot/collier (of the EBF transcription factor family) as well as other members of the Hh module that are post-translationally regulated (ci and smo) (Figure S5A). In agreement with reports analyzing Hh targets during development (Biehs et al., 2010), we found that ptc was specifically downregulated in brains of flies depleted for adult Hh activity. Out of the protec-tive factors tested in aged hh mutant adult brain samples, a distinct set of three chaperones, the heat shock proteins hsp23, hsp40, and hsp68, displayed a significant mRNA level decrease when compared to controls (Figure 4B). Importantly, the expression of Ciact in glial cells of hh mutants potently rescued the mRNA levels of hsp23, hsp40, and hsp68 (Figure 4B), supporting the notion that hsp transcriptional downregulation is a consequence of the loss of Hh signaling. Furthermore, mRNA levels of both hsp40 and hsp68 are similar in aged-matched con-trol flies at both 25!C and 29!C, whereas both chaperone mRNA
levels are drastically elevated at 37!C heat shock conditions
(Figures 4C and 4D). This shows that lifespan condition run at 29!C exhibits no significant difference in regard to the mRNA
levels of hsp40 and hsp68 compared to 25!C conditions.
To confirm the downregulation of hsp expression in the absence of Hh activity, we analyzed their expression in animals depleted for Ci, using the Ci CRISPR line. In the case of glia-spe-cific (repo-Gal4) driven Ci-CRISPR/Cas9, we detected a signifi-cant reduction in the expression of both hsp23 and hsp68, but, interestingly, not hsp40 in adult brain samples (Figure S5I). Note that in this condition, we also found that mRNA levels of both ci and its direct target ptc were significantly decreased (Figure S5H). To further analyze the relationship between the Hh signaling pathway and the group of hsp genes downregulated in hh mutant adult brains, we induced the expression of different forms of Ci in the glia and monitored its consequence on the expression of hsp23, hsp40, and hsp68. We overexpressed different variants of Ci–the activated form Ciact, the repressor form CiRep, and
CiVP16(a fusion variant that promotes the nuclear translocation
of Ci) and found that all Ci transcripts analyzed by qPCR are up-regulated to similar levels (Figure S5J). Next, we tested the effi-ciency of our approach by measuring the expression levels of patched (ptc). Both active forms upregulated ptc expression, with CiVP16 having a much stronger effect than Ciact. The
repressor form has no influence on ptc (Figure S5K). Both CiVP16 and Ciact forms upregulated hsp68, the former to a
much larger degree, while CiVP16also elevated hsp23 mRNA levels (Figure S5K). In contrast, hsp40 mRNA level is only decreased upon expression of CiRep in the glia, which is not
the case for either hsp23 or hsp68. These results suggest that, in the hhtsbackground, in which only the repressor form of Ci
is present, the reduction of hsp40 expression is due to the increased amounts of Ci-Rep, whereas the downregulation of hsp23 and hsp68 is clearly due to the lack of an activated form of Ci. This is not surprising because several reports suggest that active Ci and Ci-Rep control different groups of genes (Biehs et al., 2010). This would also explain the lack of effect of the glia-specific Ci knockout on hsp40, because in this fly line, we also abolished the formation of the Ci-Rep form.
Interestingly, when examining the mRNA levels of these chaper-ones during aging in adult brain samples, both hsp23 and hsp68 exhibited an increase of over 2-fold (Figure S5G) from young (day 3) to middle-aged (day 15), which also correlated with the in-crease observed with hh (Figure S5E) and, to a lesser extent, ptc (Figure S5F). Contrastingly, from middle age (day 15) to advanced age (day 40) flies, there was a significant decline in mRNA levels of hsp23, hsp40, and hsp68, which resembled the trend observed for both hh and ptc (Figures S5E and S5F). Overall this suggests that Hh signaling in the brain may contribute to controlling chap-erone levels on a causal and temporal basis with age.
Because of the well-accepted role of Hsps in regulating pro-teostasis (Lindquist and Craig, 1988), we hypothesized that the decrease in hsp40 and hsp68 expression levels that we observed in hh mutant adult flies may promote accumulation of protein aggregates. We tested this hypothesis using a specific antibody detecting ubiquitin-positive aggregates in the
Figure 4. hh Target Chaperones Maintain Glial Homeostasis, Survival, and Fitness
(A) List of protective factors tested in a qRT-PCR screen. mRNA levels significantly decreased in adult hh mutant heads are highlighted in blue, whereas mRNA levels of other chaperones and genes implicated in antioxidant defenses and in lysosomal autophagy pathway (Figure S4) show no significant difference. (B) The mRNA levels of hsp23, hsp40, and hsp68 chaperones are all significantly decreased in 10-day-old hh mutants (two-way t test, p < 0.001***) and restored to close to or above control levels in hh mutant flies expressing ciactin the glia.
(C and D) Relative mRNA expression level of hsp40 (C) and hsp68 (D) at 25!C, 29!C, and 37!C for day 12 control flies. No significant difference (ns, by one-way ANOVA) was observed in mRNA levels of hsp40 and hsp68 between 25!C and 29!C.
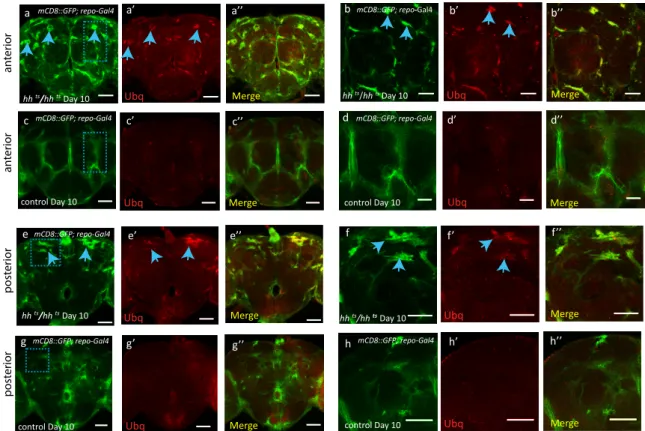
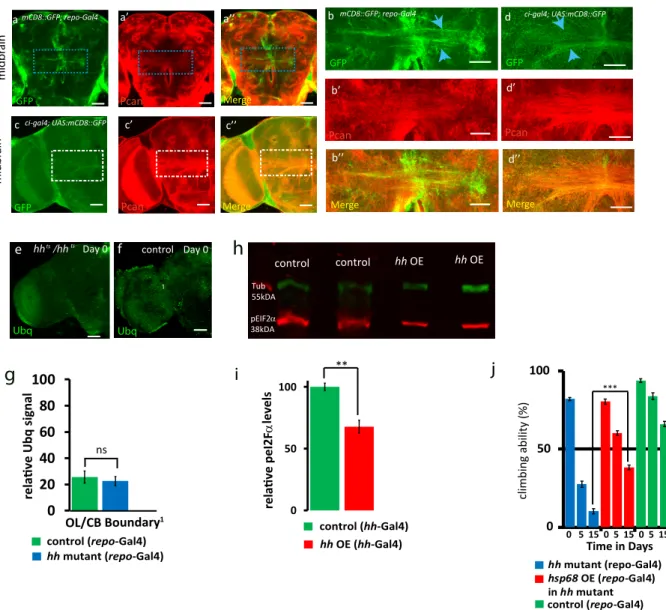
(E–G0) Day 15 hh mutant and control brains immunostained for ubiquitin (Ubq, green) and perlecan (Pcan, red in F and G). Scale bars, 50 mm. (E0and G0) Zooms of
squared areas labeled in (E)–(G). Scale bars, 30 mm, 1-mM section. Ubiquitin-positive aggregates (blue arrows) are predominantly located in midbrain glial tracts immunostained with Pcan (F0).
(H–L0) Day 15 hh mutant (H) and control (I) brains and (J and K) hh mutant flies expressing hsp40 (J) or hsp68 (K) in glia, immunostained for anti-ubiquitin. Ubiquitin aggregates (blue arrows) are observed in hh mutant adult brains (H). Expression of hsp40 (J) or hsp68 (K) in the glia of hh mutants reduces the occurrence of aggregates. Quantification of Ubq signal intensity (L and L0) were acquired from the equivalent boxed areas (I) in all samples. Box 1: CB/OL boundary. Box 2:
midline of central brain (H–K) (Figures S5Q and S5R). Scale bars, 50 mm. Area quantified for Ubq signal intensity: 31.62 mm 3 31.62 mm (1,000 mm2). Two-way
ANOVA test used to analyze differences in Ubq signal intensity between hh mutant and control samples in (L) and (L0), p < 0.001***. (H) and (K) consist of a stack of 25 mM (1-mM per section).
(M and N) Immunoblots (M) showing pEIF2a levels in hh mutant and hh mutant flies expressing hsp68 in the glia in day 12 adult head samples. (N) hh mutant flies exhibit a 2-fold increase in pEIF2a levels, which is rescued to control levels in hh mutant samples with glial expression of hsp68, (p < 0.0001****, one-way ANOVA with post hoc Tukey).
(O and P) Overexpression of hsp68 (O) and hsp40 (P) in the glia strongly rescues the hh mutant short-lived phenotype, log-rank test, p < 0.0001.
(Q) Reduced dopamine concentration in the brain of hh mutant flies is rescued to control levels in hh mutant brain samples with glial expression of hsp40 (one-way ANOVA test, ***p < 0.001).
A B C
D D’ D” D”’ D””
E’ E” E”’ E””
E
F F’ F” F”’
G G’ G” G”’
H H’ H” H”’
I I’ I” I”’
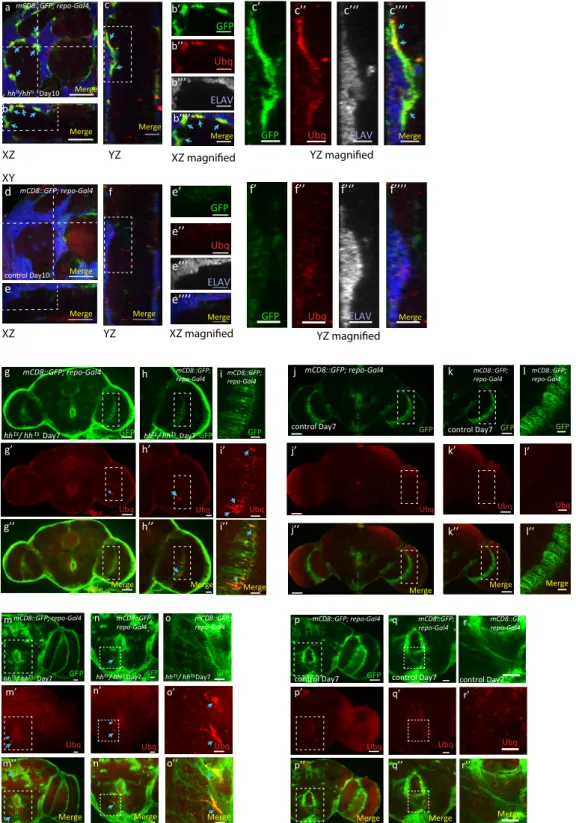
Drosophila brain (Nezis et al., 2008). Accordingly, we observed that old hh mutant flies exhibited a strikingly elevated level of ubiquitinated aggregates when compared to age-matched con-trol flies (Figures 4E–4G, 4L, and 4L0). Moreover, these
aggre-gates are predominantly localized in midbrain glia tracts, which are enriched for the proteoglycan perlecan (Pcan) (Davie et al., 2018), and also express Ci (Figures 4G,S7C, and S7D). In addi-tion, ubiquitinated aggregates were also detected in glial cells of the anterior and posterior brain regions of the hh mutant fly brain (Figures S6A, S6B, S6E, and S6F). The majority of these ubiqui-tin-positive aggregates colocalize with the glial membrane marker mCD8-GFP. Cross-section analysis of anterior brain re-gions exhibits defective glial membrane morphology (Figures S8A–S8C, blue arrows), confirming that the abnormal accumula-tion of ubiquitinated aggregates was almost exclusively present in glia cells and not neurons (Figures S8A–S8F). Note that glial aggregates are next to Elav-positive cells that do not show obvious nuclear defects or aggregates at this time point (Figures S8A–S8C). To confirm that glia is the initial site of aggregation, we analyzed the presence of ubiquinated aggregates at earlier time points. The earliest ubiquitinated aggregates we observed in 7-day-old hh mutant flies in the optic lobe region (Figures S8G–S8I) and central brain (Figures S8M–S8O) were also pre-sent exclusively in glial cells (Figures S8H00–S8I00 and S8N00–
S8O00). These observations strongly suggest that a reduction in
hsp23, hsp40, or hsp68 in hh mutants may cause the formation of ubiquitin-positive protein aggregates glial cells. Accordingly, when either hsp40 or hsp68 were overexpressed in glial cells of aged adult hh mutants, they strongly reduced ubiquitinated aggregates in glial cells (Figures 4H–4L0).
An additional readout for proteostasis function is the measure of pEIF2a levels that is indicative of the presence of misfolded proteins. The unfolded protein response (UPR) in the endo-plasmic reticulum (ER) activates a signaling cascade that ele-vates pEIF2a levels (Halliday et al., 2017). We tested this using the pEIF2a antibody that has been used to quantify pEIF2a in Drosophila (Edenharter et al., 2018). Interestingly, we found that hh mutant adult brains exhibited a 2-fold increase in pEIF2a levels, which are strongly reduced to wild-type control levels in hh mutant adult flies expressing hsp68 in the glia (Figures 4M and 4N). Conversely, Hh-overexpressing flies exhibit a 32% reduction in pEIF2a levels compared to age-matched control flies (Figures S7H and S7I).
Finally, to ascertain whether hsp40 or hsp68 contribute to hh-dependent survival and fitness, we specifically expressed each of them in glial cells of hh mutant flies (Figures 4O, 4P, and
S4B–S4D). Remarkably, we found that glial expression of either hsp68 or hsp40 potently increases the reduced lifespan observed in hh mutant animals (Figures 4O, 4P, and S4B– S4C00). In contrast, hsp23 alone was ineffective (Figures S4D–
S4D00). We also observed that glial expression of hsp68 rescued
the defective climbing ability of hh mutants by 57% (Figure S7J), and hsp40 was able to rescue the reduced dopamine level observed in hh mutant flies (Figure 4Q). Overall, our data demon-strate that the Hh signaling in glial cells promotes expression of protective chaperones required for maintaining correct proteo-stasis during adult life.
Hh Signaling in the Glia Has a Protective Function in a Human Amyloid Beta Disease Model
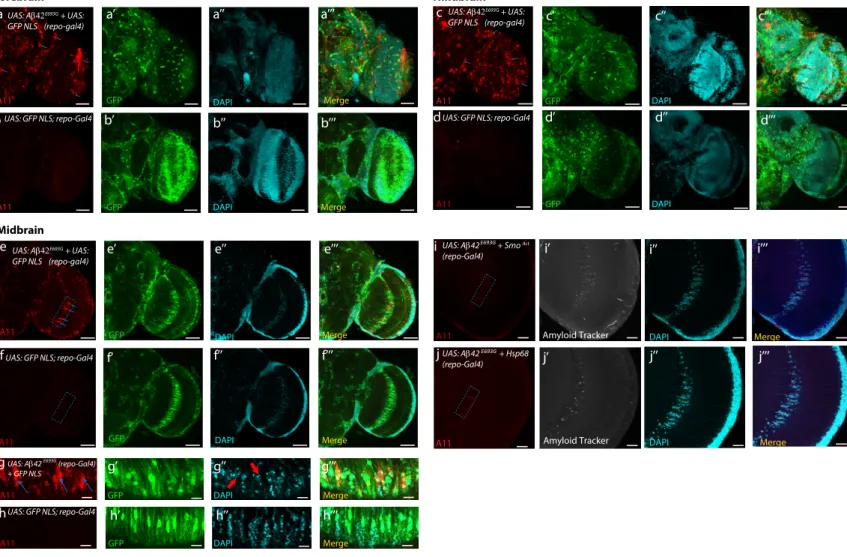
Defects in glial proteostasis observed upon Hh depletion and their rescue via expression of Hh target chaperones in the glia, raises the possibility that stimulating Hh signaling in the glia may reduce aggregate formation and its concomitant toxicity. In Drosophila, expression of the human Ab 1-42 in the glia induces abnormal aggregate formation, reduced life-span, and defective locomotor activity (Jonson et al., 2018). Consistent with this study, we expressed, in the adult Drosophila glia, the human arctic variant of Ab 42 (Ab
1-42E693G) which is known to have a high propensity to aggregate
and form fibrils in vitro (Nilsberth et al., 2001). We observed the formation of widespread amyloid aggregates throughout the adult brain (Figures S9A–S9H). This phenomenon is also notice-able in the giant glial cells of the optic chiasm (Figures S9E and S9G, dashed box) in the midbrain sections. The DAPI-positive cells exhibit an abnormal morphology with a markedly reduced cell number in this region (Figure S9G00) compared to
age-matched wild-type control, which exhibit no detectable amyloid aggregates (Figures S9F and S9H–S9H00). Expression of human
Ab 1-42E693Gin the glia also leads to reduction in lifespan as well as an age-dependent decline in locomotor activity (Figures 5A–5C).
Stimulation of the Hh pathway in glial cells expressing human Ab 1-42E693Gvia expression of Smoactreduced the presence of
amyloid aggregates in the giant glial cells of the optic chiasm, rescued DAPI-positive cells in this region (Figures 5G–5G000, 5J,
Figure 5. Hh Signaling in the Glia Has a Protective Function in a Human Amyloid Beta Disease Model
(A–C) The shortened lifespan and debilitated locomotion that results from glial expression of human Ab42E693Gcan be partially rescued by expression of (A and C)
an activated form of Smo (SmoAct) or (B and C) hsp68 expression in the glia. The rescues are more potent in the human Ab42E693Gmiddle-age animals at the 20%
mortality rate (Table S2). For lifespan analysis, log-rank test was used (p < 0.0001). For climbing activity, two-way ANOVA test was used, p < 0.001***. (D–E0000) Confocal section (5-mm stack) of the optical lobe of 15-day-old flies expressing human Ab42E693Gin the glia, (D) showing the presence of amyloid
aggregates (demarcated by the dashed box), in the giant glial cells of the optic chiasm (glial cells labeled with GFP NLS in the merged image); in control specimens the amyloid aggregates are not discernable (E). Scale bars, 20 mm.
(F–F000) In order to quantify the presence of aggregates and to count cell numbers in the region of interest (ROI) (dashed box), an area of approximately 43.14 mm 3
91.96 mm was selected for the ROI (F). Blue arrows indicate the presence of A11 positively stained amyloid aggregates in flies overexpressing human Ab42E693Gin
the glia (red channel) (F). The gray channel displays the presence of the pre-fibrillar form of amyloid (F0), as detected by the amyloid probe (AmyTracker 680), and the cyan channel displays DAPI-positive cells (F00), with the merge of all three channels also shown (F000). Scale bars, 10 mm. The equivalent ROIs in control samples
are shown in (I–I000).
(G–K) Overexpression of either SmoAct(G) or hsp68 (H) is able to reduce the presence of amyloid aggregates (J), (seeSTAR Methodsfor amyloid aggregate quantification) and rescue the decreased cell number (G00and H00) observed in flies overexpressing human Ab42E693G(K). For both amyloid aggregate
5K, andS9I–S9I000), and significantly rescued shortened lifespan
and locomotion defects (Figures 5A and 5C). The rescues were more effective in the Ab 1-42E693Gin young animals (80%
sur-vival rate) expressing SmoActwith a rescue of 40% and a 69%
rescue of debilitated locomotor activity at day 14 (Figures 5A and 5C;Table S2). Similarly, the expression of Hsp68 in the glia of Ab 1-42E693G-expressing animals improved longevity and locomotion by 43% and 88%, respectively (Figures 5B and 5C;Table S2), as well as a reduction of amyloid aggregates
DISCUSSION
Our findings provide evidence for a previously unidentified func-tion for the Hh morphogen during adulthood, distinct from its role as a patterning organizer and a tumorigenic factor (Briscoe and The´rond., 2013). Our results suggest that Hh signaling in the glia of the adult brain controls the expression of chaperone mol-ecules, including Hsp40 and Hsp68, which repress the formation of age-associated protein aggregates. We propose a model in which Hh morphogen signaling, interpreted by glial cells, regu-lates lifespan determination and associated fitness, while main-taining adult brain homeostasis, including DA neuron protection (Figure 6).
Hh Signaling in Glial Cells as a Lifespan Determinant
There is an emerging body of evidence that diverse glial cell dysfunction has an impact on neuroprotection and organismal lifespan. For instance, disturbance of mitochondrial function by frataxin silencing (Navarro et al., 2010) or impairment of glycol-ysis via depletion of glycolytic enzymes in the glial cells reduces lifespan and induces neurodegeneration in Drosophila ( Volkenh-off et al., 2015; Miller et al., 2012). Additionally, activation of the innate immunity response, namely the immune deficiency (IMD)/ nuclear factor kB (NF-kB) pathway, in glial cells resulted in an increased expression of antimicrobial peptides, inducing neuro-degeneration and diminishing lifespan (Kounatidis et al., 2017). Conversely, glial-specific suppression of the IMD/NF-kB signaling pathway culminated in an increased presence of the AKH hormone and lifespan extension (Kounatidis et al., 2017). Furthermore, it has been proposed that the glial neuropeptide RGBA-1 is able to modulate the rate of aging in C. elegans (Yin et al., 2017). Overall, our study reinforces the above notion that glia have an important function in the modulation of lifespan and neuroprotection.
As mentioned in the introduction, in vitro stimulation of mouse cortical astrocytes with Shh protected co-cultured neurons from kainate (KA)-induced cell death (Ugbode et al., 2017), which provides evidence that Shh-activated glial cells confer effective protection using a glia to neuron communication system. The present study revealed that the Hh signaling pathway is a novel regulator of glial proteostasis. The Hh targets in this cell type, Hsp40 and Hsp68, are highly conserved chaperones, (for which the Hsp40 and Hsp70 chaperone family members are the conserved homologs in mammals). These chaperones form part of a major protein complex, which recognizes and binds ubiquitinated misfolded proteins, preventing their aggregation and facilitating their refolding or degradation (Lindquist and Craig, 1988; Jackrel and Shorter, 2017; Zarouchlioti et al., 2018). Interestingly, Hsp40 and Hsp70 act cooperatively as safe-guards to prevent both the misfolding of newly synthesized pro-teins and protein aggregation upon cellular stress. A third Hh
1. Activation of Hh signalling
2. Hh receiving module in glial cells
3. Chaperone mRNA levels retained. (Glial proteostasis maintained)
4. DA neuron integrity/Dopamine levels maintained
1. Inhibition of Hh signalling 2. Hh receiving module in glial cells
3. Chaperone mRNA levels decrease. (Glial proteostasis declines).
pEIF2A Ubq lifespan mobility 4. DA neuron degeneration/loss
Dopamine Tyrosine Hydroxylase l
l
ll
ll ll
ll
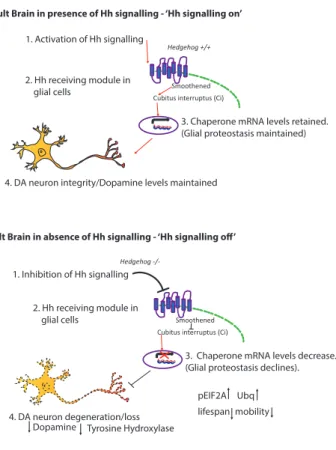
Figure 6. Proposed Model by which Hh Signaling Mediates Glial Proteostasis and Neuroprotection
We put forward the following models.
Upper scheme: (1) The presence of the Hh signal (2) induces activation of the canonical Hh signaling pathway in the glia via Ci activation. (3) This regulates mRNA levels of downstream target chaperones in the glia, which maintain proteostasis and (4) DA neuron integrity.
Lower scheme: (1) Conversely, in the absence of Hh signaling, (2) Ci remains inactive in glia, and (3) mRNA levels of downstream chaperones are decreased. Subsequently, glial proteostasis declines, leading to an increase in abnormal protein aggregation, characterized by an elevated level of Ubq-positive aggregates. This proteotoxic environment in the glia is deleterious to lifespan and mobility of the organism and leads to (4) degeneration of DA neurons and a decrease in dopamine levels, potentially due to a reduction in neuroprotective factors produced in the glia.
Hsp68 is able to rescue proteostasis defects present in the hh mutant glial cells, characterized by an elevated level of ubiquitin. Interestingly, hh mutant flies also exhibit increased levels of pEIF2a, indicative of activation of the unfolded proteins response (Halliday et al., 2017), which can be restored to wild-type levels through glial expression of Hsp68. Conversely, Hh-overexpressing flies display reduced levels of pEIF2a, suggest-ing that stimulatsuggest-ing Hh signalsuggest-ing is protective against protein misfolding.
There are compelling studies suggesting that there is a global decline in cellular proteostasis during aging in the fly (Nezis et al., 2008; Demontis and Perrimon 2010). The decreased hsp mRNA levels observed in hh mutant is likely to accelerate the age-asso-ciated proteostasis defects as is corroborated in a recent vivo study that depletes Hsp40 family members in C. elegans (Kirstein et al., 2017).
Failure in Glia Proteostasis Has Dramatic Consequences at the Organismal Level
Our data support the model in which failure of proteostasis in glia (as opposed to neurons) has dramatic consequences at the cellular and organismal levels. Several examples in the liter-ature already suggest that compromising glial proteostasis can induce neurotoxicity and pathology. For example, in Drosophila, glial-specific expression of human glial fibrillary acidic protein (GFAP) results in protein aggregates. This also in-duces a non-cell-autonomous response in neurons and results in loss of neurons in the lamina and loss of GluRIIB neurons in the adult brain (Wang et al., 2011). Moreover, astrocyte-specific depletion of the mitochondrial protease in mice (mAAA)—a pro-tein involved in the propro-tein quality control in mitochondria— causes neurological defects (motor impairment), neurodegen-eration of Purkinje cells in the cerebellum, as well as a decline in synaptic function (Murru et al., 2019). All this suggests that glial proteostasis defects will hinder the supporting roles of glia in the nervous system (and therefore can have a direct consequence on neuronal viability), and chaperones expressed in glia may confer neuroprotection. We propose that the defec-tive glial proteostasis affect specific neuronal populations sus-ceptible to the aging process, such as DA and clock neurons (Figures 3G–3O andS2H–S2J) (Liao et al., 2017), and not other neuronal cell types, such as cholinergic and mushroom body neurons, which were not affected at the time we observed se-vere DA and clock neurons defects in hh mutant (Figures S2K– S2P).
Some unanswered questions remain, for instance, what subtype of glial cells are responsible for mediating Hh coordi-nated adult survival and neuronal integrity. One possibility is that the cortex glia that envelopes neuronal cell bodies could perform this function. It has been suggested that this subtype can provide trophic support to neurons (Stork et al., 2012). Alternatively, the ensheathing glia or the surface glia forming the glia of the blood brain barrier may be the location of Hh-mediated neuroprotection. Several lines of evidence in our study suggest that the cortex and ensheathing glia are responsive to Hh. Glial cells positive for Smo and Ci immuno-staining have a morphology characteristic of cortex and en-sheathing glia and are also positive for Draper, which is a
spe-cific marker of these glial cells (Figures 1C–1D0). We also
found ubiquitin aggregates in ensheathing glia (enriched for Pcan) in hh mutant, confirming that these glial cells are sensi-tive to Hh activity (Figures 4E–4G andS5E–S5I00). Ci staining in
neuropile glia is very weak, suggesting that these glial cells might not be responsive to Hh signaling (Figures S1B–S1D00).
This has been confirmed in a single-cell transcriptome anal-ysis (Davie et al., 2018), showing that Ci is primarily expressed in both cortex and ensheathing glia and is weakly expressed in astrocyte-like/neuropile glia. Further investiga-tion is required to address the contribuinvestiga-tion of each glial sub-type to Hh-mediated survival and fitness.
A further line of inquiry to address would be the degree to which protective Hh signaling in the glia is acting in a cell/non-cell autonomous manner. Given that both, Hsp40 and Hsp68, were demonstrated in our study to reduce the presence of ubiq-uitin-positive aggregates in the glia as well as rescue survival rates of hh mutant flies, this suggests that they act in an auton-omous manner, maintaining glial proteostasis. A recent study, however, demonstrated that both Hsp40 and Hsp70 can be secreted on exosomes and exert a paracrine neuroprotective ef-fect in an in vitro co-culture experiment (Takeuchi et al., 2015). This suggests that the chaperone targets of Hh signaling may be secreted from the glia and thereby contribute toward Hh-mediated neuroprotection and lifespan determination in a non-cell autonomous manner.
Ci Transcriptional Regulation of Downstream Chaperones
Our study showed that a distinct set of three chaperones, Hsp23, Hsp40, and Hsp68, exhibit reduced mRNA levels in a hh null con-dition in the adult brain. Importantly, the expression of Ciactin
glial cells of hh mutants potently rescued the mRNA levels of all three chaperones supporting the notion that hsp transcrip-tional downregulation is a consequence of the loss of Hh signaling. Interestingly, expression of Ci-activated forms was only able to elevate both Hsp23 and Hsp68 but not Hsp40. How-ever, expression of the Ci-Rep form was only able to significantly reduce Hsp40 and not Hsp23 or Hsp68, suggesting that Hsp40 is under the transcriptional control of Ci-Rep. Taken together, these results demonstrate the ability of Ci to control the expres-sion of these genes.
The Potential of Hh Signaling in Alleviating Degenerative Disease
As proof of principle, we tested components and targets of Hh signaling in an in vivo Alzheimer’s disease (AD) model in Drosophila expressing the human arctic variant of Ab 1-42 (Ab 1-42E693G) in the glia. In agreement with a recent study (Jonson et al., 2018), we show that glial expression of the Ab 1-42E693G
variant shortened lifespan, induced debilitated locomotion, and increased the presence of amyloid aggregates. Remarkably, we found that induction of Hh signaling in the glia was able to considerably rescue the Ab 1-42E693G-dependent phenotype, both at the molecular and physiological level, providing the first evidence, to our knowledge, that confirms the efficacy of Hh signaling in an in vivo AD model. We also show that increasing expression of Hsp68 reduces the level of Ab 1-42E693G
aggregates in vivo, which is consistent with a study showing that expression of Hsp70 reduces the level of amyloid b aggregates in transgenic mice brains expressing a variant of the amyloid pre-cursor protein (Hoshino et al., 2011). Overall, we propose that targets under the regulation of Hh signaling in the glia have the potential to ameliorate proteinopathies, such as those caused by Ab 1-42 in AD. It is now proposed that the glia play an impor-tant role in various proteinopathies and could function as carriers for the initiation and propagation of aggregates in the adult brain. In fact, it has been demonstrated that glial cells can replicate and seed Ab amyloid fibrils in the mammalian brain independent of neuronal expression (Veeraraghavalu et al., 2014). Thus, activa-tion of the Hh signaling pathway in the glia may have beneficial effects in combating a wide-range of proteinopathies which manifest themselves through aberrant protein aggregation and misfolding as observed in Parkinson’s disease, AD, and Hunting-ton’s disease.
A Conserved Role for Hh Signaling in Healthspan and Neuroprotection
We propose that this new function for Hh signaling as a lifespan determinant may be conserved in mammals. All molecular players described here are conserved; the vertebrate counter-part of Drosophila Hh, Shh, is also expressed in mouse adult neurons, and glial cells respond to this signal (Garcia et al., 2010; Farmer et al., 2016). Because the activation of Shh signaling protects against diverse neurotoxins such as Ab pep-tide in vitro, (Ab 1-42) (Yao et al., 2017), our study suggests that Hh signaling in glia may ameliorate age-related neurodegen-erative diseases caused by aberrant protein aggregation as well as prolong healthspan and fitness.
STAR+METHODS
Detailed methods are provided in the online version of this paper and include the following:
d KEY RESOURCES TABLE
d LEAD CONTACT AND MATERIALS AVAILABILITY
d EXPERIMENTAL MODEL AND SUBJECT DETAILS
B Fly Stocks and genetics
B Adult specific inactivation of the Hh mutants
d METHOD DETAILS
B Lifespan/Locomotion Assays B Immunohistochemistry
d QUANTIFICATIONS AND STATISTICAL ANALYSIS
B DA neuron volume quantification in the adult brain with Imaris software
B DA neuron cell counting B Dopamine quantifications
B Ubiquitin signal intensity quantifications
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online athttps://doi.org/10.1016/j. celrep.2020.02.006.
ACKNOWLEDGMENTS
We thank all ‘‘fly’’ members of the iBV Institute, Tamas Matusek, Laurence Staccini-Lavenant, and Caterina Novelli, and Luc Martin from the iBV Bioinfor-matics facility, for additional help. We thank Julien Marcetteau, Pierre Le´opold, Florence Besse, Caroline Me´dioni, Herve´ Tricoire, Thomas Pre´at, Pauline Spe´der, Laure Bally-Cuif, and Catherine Rabouille for critical analysis of the manuscript. We also thank Julien Marcetteau for the generation of the Graph-ical Abstract. A.R. held a Ligue Nationale Contre le Cancer fellowship. This work was supported by grants from Ligue National and Contre le Cancer Equipe labellise´e 2016; the Labex SIGNALIFE (ANR-11-LABX-0028-01 to P.P.T.) and MemoLife (ANR-10-LABX-54 MEMO LIFE to SB); the French Na-tional Research Agency (ANR) (ANR-15-CE13-0002-01 to P.P.T.); and by joint Research Funding Projects from the ANR and the German Research Founda-tion (DFG) (ANR-17-C814-0041-01 to P.P.T. and DFG-SCHN 558/9-1 to S.S.) and from the Fe´de´ration de la Recherche sur le Cerveau (to S.B. and P.P.T.).
AUTHOR CONTRIBUTIONS
A.R. and P.P.T. conceived the experiments and wrote the manuscript. J.A.N. and S.S. conceived expression profile analysis and the ER stress experiments. J.A.N. and M.R. generated the Ci-T2A-GAL4 line, the UAS-Ci CRISPR line. J.A.N. and M.R. conducted the experiments onFigures 1A, 1B,3N,4A–4D, 4M, 4N,S1F–S1G000,S5A–S5K,S7H, and S7I. S.B. and A.H. conducted the ex-periments onFigures 3O and4Q. A.R. conducted all other experiments. All au-thors participated in the critical analysis of the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests. Received: July 26, 2019
Revised: November 11, 2019 Accepted: January 31, 2020 Published: February 25, 2020
REFERENCES
Ahn, S., and Joyner, A.L. (2005). In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437, 894–897.
Akimoto, A., Wada, H., and Hayashi, S. (2005). Enhancer trapping with a red fluorescent protein reporter in Drosophila. Dev. Dyn. 233, 993–997.
Amankulor, N.M., Hambardzumyan, D., Pyonteck, S.M., Becher, O.J., Joyce, J.A., and Holland, E.C. (2009). Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J. Neurosci. 29, 10299–10308.
Awasaki, T., and Lee, T. (2011). New tools for the analysis of glial cell biology in Drosophila. Glia 59, 1377–1386.
Bambakidis, N.C., Petrullis, M., Kui, X., Rothstein, B., Karampelas, I., Kuang, Y., Selman, W.R., LaManna, J.C., and Miller, R.H. (2012). Improvement of neurological recovery and stimulation of neural progenitor cell proliferation by intrathecal administration of Sonic hedgehog. J. Neurosurg. 116, 1114–
Biehs, B., Kechris, K., Liu, S., and Kornberg, T.B. (2010). Hedgehog targets in the Drosophila embryo and the mechanisms that generate tissue-specific out-puts of Hedgehog signaling. Development 137, 3887–3898.
Brand, A., and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415.
Briscoe, J., and The´rond, P.P. (2013). The mechanisms of Hedgehog sig-nalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416–429.
Chechneva, O.V., Mayrhofer, F., Daugherty, D.J., Krishnamurty, R.G., Banner-man, P., Pleasure, D.E., and Deng, W. (2014). A Smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis. 5, e1481.
Croset, V., Treiber, C.D., and Waddell, S. (2018). Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. eLife 7, e34550.
Curran, T. (2018). Reproducibility of academic preclinical translational research: lessons from the development of Hedgehog pathway inhibitors to treat cancer. Open Biol. 8, 180098.
D’Andrea, M.R., Nagele, R.G., Wang, H.Y., Peterson, P.A., and Lee, D.H. (2001). Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology 38, 120–134.
Davie, K., Janssens, J., Koldere, D., De Waegeneer, M., Pech, U., Kreft,q., Aibar, S., Makhzami, S., Christiaens, V., Bravo Gonza´lez-Blas, C., et al. (2018). A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Single-Cell 174, 982–998.
De Luca, M., Roshina, N.V., Geiger-Thornsberry, G.L., Lyman, R.F., Pasyu-kova, E.G., and Mackay, T.F. (2003). Dopa decarboxylase (Ddc) affects varia-tion in Drosophila longevity. Nat. Genet. 34, 429–433.
Demontis, F., and Perrimon, N. (2010). FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 143, 813–825.
Diao, F., Ironfield, H., Luan, H., Diao, F., Shropshire, W.C., Ewer, J., Marr, E., Potter, C.J., Landgraf, M., and White, B.H. (2015). Plug-and-play genetic ac-cess to Drosophila cell types using exchangeable exon cassettes. Cell Rep. 10, 1410–1421.
Edenharter, O., Schneuwly, S., and Navarro, J.A. (2018). Mitofusin-Dependent ER Stress Triggers Glial Dysfunction and Nervous System Degeneration in a Drosophila Model of Friedreich’s Ataxia. Front. Mol. Neurosci. 11, 38.
Eitan, E., Petralia, R.S., Wang, Y.X., Indig, F.E., Mattson, M.P., and Yao, P.J. (2016). Probing extracellular Sonic hedgehog in neurons. Biol. Open 5, 1086–1092.
Evans, C.G., Wise´n, S., and Gestwicki, J.E. (2006). Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J. Biol. Chem. 281, 33182–33191.
Farmer, W.T., Abrahamsson, T., Chierzi, S., Lui, C., Zaelzer, C., Jones, E.V., Bally, B.P., Chen, G.G., The´roux, J.F., Peng, J., et al. (2016). Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351, 849–854.
Feany, M.B., and Bender, W.W. (2000). A Drosophila model of Parkinson’s dis-ease. Nature 404, 394–398.
Ferent, J., Cochard, L., Faure, H., Taddei, M., Hahn, H., Ruat, M., and Traiffort, E. (2014). Genetic activation of Hedgehog signaling unbalances the rate of neural stem cell renewal by increasing symmetric divisions. Stem Cell Reports 3, 312–323.
Friedrich, M.V., Schneider, M., Timpl, R., and Baumgartner, S. (2000). Perlecan domain V of Drosophila melanogaster. Sequence, recombinant analysis and tissue expression. Eur. J. Biochem. 267, 3149–3159.
Friggi-Grelin, F., Iche´, M., and Birman, S. (2003). Tissue-specific develop-mental requirements of Drosophila tyrosine hydroxylase isoforms. Genesis 35, 175–184.
Garcia, A.D., Petrova, R., Eng, L., and Joyner, A.L. (2010). Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J. Neurosci. 30, 13597–13608.
Gonzalez-Reyes, L.E., Verbitsky, M., Blesa, J., Jackson-Lewis, V., Paredes, D., Tillack, K., Phani, S., Kramer, E.R., Przedborski, S., and Kottmann, A.H. (2012). Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron 75, 306–319.
Halliday, M., Hughes, D., and Mallucci, G.R. (2017). Fine-tuning PERK signaling for neuroprotection. J. Neurochem. 142, 812–826.
Hardie, S.L., and Hirsh, J. (2006). An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. J. Neurosci. Methods 153, 243–249.
Hoshino, T., Murao, N., Namba, T., Takehara, M., Adachi, H., Katsuno, M., So-bue, G., Matsushima, T., Suzuki, T., and Mizushima, T. (2011). Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J. Neurosci. 31, 5225–5234.
Huang, S.S., Cheng, H., Tang, C.M., Nien, M.W., Huang, Y.S., Lee, I.H., Yin, J.H., Kuo, T.B., Yang, C.C., Tsai, S.K., and Yang, D.I. (2013). Anti-oxidative, anti-apoptotic, and pro-angiogenic effects mediate functional improvement by sonic hedgehog against focal cerebral ischemia in rats. Exp. Neurol. 247, 680–688.
Ingham, P.W. (2018). From Drosophila segmentation to human cancer therapy. Development 145, dev168898.
Jackrel, M.E., and Shorter, J. (2017). Protein-Remodeling Factors As Potential Therapeutics for Neurodegenerative Disease. Front. Neurosci. 11, 99.
Ji, H., Miao, J., Zhang, X., Du, Y., Liu, H., Li, S., and Li, L. (2012). Inhibition of sonic hedgehog signaling aggravates brain damage associated with the down-regulation of Gli1, Ptch1 and SOD1 expression in acute ischemic stroke. Neurosci. Lett. 506, 1–6.
Jin, Y., Raviv, N., Barnett, A., Bambakidis, N.C., Filichia, E., and Luo, Y. (2015). The shh signaling pathway is upregulated in multiple cell types in cortical ischemia and influences the outcome of stroke in an animal model. PLoS ONE 10, e0124657.
Jonson, M., Nystro¨m, S., Sandberg, A., Carlback, M., Michno, W., Hanrieder, J., Starkenberg, A., Nilsson, K.P.R., Thor, S., and Hammarstro¨m, P. (2018). Aggregated Ab1-42 Is Selectively Toxic for Neurons, Whereas Glial Cells Pro-duce Mature Fibrils with Low Toxicity in Drosophila. Cell Chem. Biol. 25, 595–610.
Kirstein, J., Arnsburg, K., Scior, A., Szlachcic, A., Guilbride, D.L., Morimoto, R.I., Bukau, B., and Nillegoda, N.B. (2017). In vivo properties of the disaggre-gase function of J-proteins and Hsc70 in Caenorhabditis elegans stress and aging. Aging Cell 16, 1414–1424.
Kounatidis, I., Chtarbanova, S., Cao, Y., Hayne, M., Jayanth, D., Ganetzky, B., and Ligoxygakis, P. (2017). NF-kB Immunity in the Brain Determines Fly Life-span in Healthy Aging and Age-Related Neurodegeneration. Cell Rep. 19, 836–848.
Lai, K., Kaspar, B.K., Gage, F.H., and Schaffer, D.V. (2003). Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neuro-sci. 6, 21–27.
Lake, B.B., Chen, S., Sos, B.C., Fan, J., Kaeser, G.E., Yung, Y.C., Duong, T.E., Gao, D., Chun, J., Kharchenko, P.V., and Zhang, K. (2018). Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 36, 70–80.
Larsen, C.W., Hirst, E., Alexandre, C., and Vincent, J.P. (2003). Segment boundary formation in Drosophila embryos. Development 130, 5625–5635.
Liao, S., Broughton, S., and Na¨ssel, D.R. (2017). Behavioral Senescence and Aging-Related Changes in Motor Neurons and Brain Neuromodulator Levels Are Ameliorated by Lifespan-Extending Reproductive Dormancy in Drosophila. Front. Cell. Neurosci. 11, 111.
Lindquist, S., and Craig, E.A. (1988). The heat-shock proteins. Annu. Rev. Genet. 22, 631–677.