Publisher’s version / Version de l'éditeur:
Technical Translation (National Research Council of Canada), 1963
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20331501
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Utilization of certain substances for the acceleration of the melting of
ice
Skorik, I. L.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=627a7ec2-344d-4614-bb0f-66c9aa17462e https://publications-cnrc.canada.ca/fra/voir/objet/?id=627a7ec2-344d-4614-bb0f-66c9aa17462e
A f a c t o r of s i g n i f i c a n t economic importance t o Canadian s h i p p i n g i s t h e time t h a t a harbour becomes i c e f r e e i n s p r i n g . In r e c e n t y e a r s , c o n s i d e r a b l e a t t e n t i o n has been given t o t h i s problem. Reports i n d i c a t e t h a t t h e Russians have s u c c e s s f u l l y advanced break-up i n many a r e a s , p a r t i c u l a r l y i n t h e f a r n o r t h , by t h e a p p l i c a t i o n of a s u i t a b l e d u s t . In response t o Canadian i n t e r - e s t i n t h e technique, t h e Snow and I c e S e c t i o n of t h e D i v l s i o n of B u i l d i n g Research has been g i v i n g a t t e n t i o n t o t h e p o s s i b l e
a p p l i c a t i o n of t h i s technique t o Canadian c o n d i t i o n s .
It i s one of' t h e r e s p o n s i b i l i t i e s of t h e Snow and I c e S e c t i o n t o c o l l e c t and make a v a i l a b l e i n f o r m a t i o n r e q u i r e d f o r t h e s o l u t i o n of snow and i c e problems encountered i n e n g i n e e r i n g p r a c t i c e . This t r a n s l a t i o n d e s c r i b e s r e s u l t s obtained from a Russian s t u d y on t h e e f f e c t i v e n e s s of v a r i o u s d u s t i n g m a t e r i a l s f o r advancing t h e break- up of an i c e cover. The paper was t r a n s l a t e d by M r . V.N. Pavloff of t h e T r a n s l a t i o n s S e c t i o n of t h e N a t i o n a l Research Council Library, t o whom t h e D i v i s i o n wishes t o record i t s thanks.
Ottawa R.F. Legget
Title :
Author :
NATIONAL RESEARCII COUNCIL OF CANADA
Technical Translation 1067
Utilization of certain substances for the acceleration of the melting of ice
(K
voprosu ob ispolfzovanii nekotorykh veshchestv dlya uskoreniyarazrusheniya l t d a ) I.L. Skorik
Reference: Trudy Arkticheskogo i Antarkticheskogo Nauchno Issledovatelfskogo
hstituta, 218: 200-208, 1960
UTILIZATION OF CERTAIN SUI3STANCES FOR TIE ACCELERATION OF TW NELTING OF ICE
It i s lcnown t h a t t h e t r a n s f o r m a t i o n p r o c e s s of w a t e r i n t o i c e and v i c e v e r s a i s a thermodynamic p r o c e s s involvine; t h e emission o r a b s o r p t i o n of thermal energy. The thawing of i c e which o c c u r s i n n a t u r e i s connected w i t h i n c r e a s e d i n t e n s i t y o f s o l a r r a d i a t i o n . Usually i t b e g i n s a t t h e g r a i n boundaries of i n d i v i d u a l c r y s t a l s of i c e , s i n c e i t i s h e r e t h a t t h e s a l t and b r i n e s o l u t i o n s of t h e s e a i c e a r e c o n c e n t r a t e d . Various s u b s t a n c e s embedded
i n t h e i c e become c e n t r e s of a b s o r p t i o n of s o l a r r a d i a n t energy, which l e a d s t o thawing of t h e i c e . The a c t i o n o f s o l a r r a d i a t i o n promotes m e l t i n g and enlargement o f t h e s e c e n t r e s i n t h e i c e mass and t h e r e b y speeds i t s
d e s t r u c t i o n .
I n o r d e r t o a c c e l e r a t e t h e d e s t r u c t i o n of i c e i t i s n e c e s s a r y t o i n t e n s i f y t h e a b s o r p t i o n of s o l a r r a d i a t i o n a t t h e s u r f a c e as w e l l a s a t c e n t r e s w i t h i n t h e i c e mass i t s e l f . The l a t t e r may be achieved by p a i n t i n g t h e i n t e r n a l m i c r o c a v i t i e s , s a l t n u c l e i , h o l e s and c a p i l l a r i e s w i t h i n t h e i c e w i t h t h e a i d of chemical dyes as w e l l as c e r t a i n n a t u r a l s u b s t a n c e s . A s u i t - a b l y chosen s u b s t a n c e p a i n t e d on t h e i c e s u r f a c e should r a p i d l y p e n e t r a t e t h e i c e mass through t h e s e numerous m i c r o c a v i t i e s w i t h i n t h e i c e s h e e t . If t h i s s u b s t a n c e i s w a t e r - i n s o l u b l e and i s c o l l o i d a l l y d i s p e r s e d , t h e n i t p o s s e s s e s a n e x t e n s i v e s p e c i f i c s u r f a c e which would p r o n o t e t h e a d h e r i n g o f i t p
p a r t i c l e s on t h e walls of t h e i c e p o r e s . T h i s would impede them from b e i n g washed o u t o f t h e i c e s h e e t i n t o , t h e w a t e r below.
A h i g h degree of d i s p e r s i t y o f s u b s t a n c e s s u i t a b l e f o r t h e d e s t r u c t i o n of i c e should d e c r e a s e t h e amount of m a t e r i a l needed f o r d u s t i n g , i n c r e a s e t h e i n t e n s i t y of t h e c o l o u r change produced a t t h e i c e s u r f a c e and promote t h e t r a n s f e r of a c o n s i d e r a b l e p o r t i o n of absorbed s o l a r energy from t h e upper s u r f a c e i n t o t h e i c e mass i t s e l f .
I n o r d e r t o check t h e s e assumptions, experiments were arranged on t h e i c e of Lake Ladoga i n 1952 and 1953. The f o l l o w i n g d u s t i n g a g e n t s were used on t h e i c e : ( 1 ) n a t u r a l sand, ( 2 ) sand dyed w i t h P r u s s i a n b l u e ,
( 3 )
P r u s s i a n b l u e . The experiments r e v e a l e d t h e marked e f f e c t i v e n e s s of sand dyed w i t h P r u s s i a n b l u e and of P r u s s i a n b l u e i t s e l f which i n v e r y minute q u a n t i t i e s( 0 . 5
-
1 . 0 d m 2 ) promoted v e r y r a p i d d e s t r u c t i o n o f t h e i c e c o v e r .Experiments on t h e u t i l i z a t i o n of dyes f o r t h e d e s t r u c t i o n of i c e on Lake Ladoga i n d i c a t e d t h e follor.ring:
and w i t h sand dyed w i t h P r u s s i a n b l u e , a s w e l l a s with P r u a s i a n b l u e i t s e l f , d e c r e a s e s r e f l e c t i o n o f s o l a r r a d i a t i o n from t h e i c e s u r f a c e and promotes i t s thawing.
2 . D u s t i n g with n a t u r a l sand l e a d s t o thawing of t h e i c e cover c h i e f l y from t h e s u r f a c e . P r u s s i a n b l u e , which p e n e t r a t e s w i t h i n t h e i c e mass, weakens t h e i c e c o v e r t o a much g r e a t e r e x t e n t .
3 .
For e q u a l d e n s i t y of d u s t i n g (400 d m 2 ) t h e e f f e c t i v e n e s s of d e s t r u c - t i o n of i c e depends on t h e i n t e n s i t y w i t h which t h e sand i s dyed w i t h P r u s s i a n b l u e : t h e more i n t e n s e t h e b l u e , t h e more e f f e c t i v e i s t h e d e s t r u c t i o n o f t h e i c e c o v e r .4 . On i n c r e a s i n g t h e i n t e n s i t y o f d y e i n g of t h e i c e c o v e r w i t h P r u s s i a n b l u e from 0.4 t o 1 . 6 d m 2 t h e d e g r e e of d e s t r u c t i o n of t h e i c e cover i n c r e a s e s o n l y s l i g h t l y .
5.
Dyeing t h e i c e cover w i t h P r u s s i a n b l u e ( 0 . 5 d m 2 ) i s more e f f e c t i v e i n t h e m e l t i n g o f i c e t h a n d u s t i n g t h e i c e c o v e r w i t h n a t u r a l sand (400 d m 2 ) .The r e s u l t s of t h e s e experiments on t h e a c c e l e r a t i o n o f t h e d e s t r u c t i o n o f i c e by d u s t i n g i t w i t h sand and d y e i n g w i t h P r u s s i a n b l u e have been con- firmed by t h e work of G.N. Yakovlev on t h e s e a i c e of V i l ' k i t s k i i S t r a i t dur- i n g May-June 1952.
In o r d e r t o s t u d y t h e e f f e c t of o t h e r s u b s t a n c e s on i c e , experiments were performed i n Dickson Bay d u r i n g t h e p e r i o d June 2-23, 1953. The a i m of t h e s e experiments was t o s t u d y t h e speed and t h e n a t u r e o f m e l t i n g o f s e a i c e on d u s t i n g w i t h a n i l i n e b l a c k , P r u s s i a n b l u e , dyed and n a t u r a l sand, Cambrian and Dickson c l a y s , c o a l d u s t , c i n d e r s , magnesium and aluminium d u s t , Dutch lamp- b l a c k , calcium c h l o r i d e and calcium phosphate. It was a l s o proposed t o check t h e s t a b i l i t y o f t h e a r t i f i c i a l d y e i n g of sand and i c e i n r e l a t i o n t o A r c t i c c o n d i t i o n s
-
s o l a r r a d i a t i o n , s e a and thaw water, a i r t e m p e r a t u r e , e t c .The l o c a t i o n o f e x p e r i m e n t a l a r e a s i s shown i n Table I. The d u s t e d a r e a s were arranged in s t a g g e r e d rows. C o n t r o l a r e a s which were n o t d u s t e d were l o c a t e d between t h e d u s t e d s e c t i o n s . In t h e t a b l e t h e d u s t e d a r e a s a r e
numbered, and t h e d e n s i t y of d u s t i n g with v a r i o u s s u b s t a n c e s i s c l e a r l y shown. The e x p e r i m e n t a l s e c t i o n s were
5
-
5
= 25 m2 i n a r e a . Each t e s t v a r i a n t was performed s i n u l t a n e o u s l y on two e x p e r i m e n t a l s e c t i o n s .The d e n s i t y of d u s t i n g o f t h e i c e w i t h sand (dyed and n a t u r a l ) was every- where t h e same and was e q u a l t o 400 g / m 2 of t h e i c e c o v e r . The sand used was l i g h t c o l o u r Dickson sand, which was dyed with:
( a ) P r u s s i a n b l u e
-
two d e g r e e s o f i n t e n s i t y ( 0 . 5 g and 1.0 g p e r 400 go f s a n d )
For dyeing of t h e sand with P r u s s i a n b l u e , 40 g of potassium f e r r o c y a n i d e were d i s s o l v e d i n 1 0 l i t r e s of cold water. 1 0 kg of sand were soaked with t h i s s o l u t i o n . Next, a s o l u t i o n of 20 g f e r r i c c h l o r i d e i n 1 l i t r e of water was added t o t h e r e s u l t i n g wet mass. The sand t h e r e b y became i n t e n s e l y b l u e i n c o l o u r . The dyed sand was scooped o u t of t h e c o n t a i n e r and poured on matting t o d r y .
For dyeing of t h e sand with a n i l i n e black, 23 g of a n i l i n e were d i s s o l v e d i n 100 m l c o n c e n t r a t e d s u l p h u r i c a c i d . The r e s u l t i n g s o l u t i o n was mixed with 500 m l of water, and while s t i l l h o t was poured i n t o a wooden tub a t t h e bottom of which l a y 10 kg of wet sand. A l l of t h e c o n t e n t s of t h e t u b were thoroughly mixed. The wet sand was cooled, and a s o l u t i o n of 3 0 g potassium bichromate i n 75 m l water was g r a d u a l l y poured i n t o i t w i t h mixing i n such a way t h a t t h e temperature of t h e mixture n e v e r r o s e above
l o 0 .
The r e s u l t i n g d a r k mixture was l e f t o v e r n i g h t i n a c o o l b u i l d i n g , and i n t h e morning as o l u t i o n of 40 g potassium bichromate i n 120 m l water was added t o i t by drops and with mixing. Next t h e c o n t e n t s of t h e tub were l e f t f o r 6 hours i n a c o o l p l a c e . The remaining l i q u i d was poured o u t , and t h e sand s e t t l e d a t t h e
bottom of t h e t u b was scooped o u t on m a t t i n g and l e f t t o d r y . The d r y dyed sand d a r k g r a y i n c o l o u r . I f i n s t e a d of 10 kg,
5
kg o f sand were used ( w i t h o u t any o t h e r changes i n procedure), t h e r e s u l t i n g sand was dyed d a r k b l u e.
The p r e p a r a t i o n of pure a n i l i n e b l a c k and p u r e P r u s s i a n b l u e f o r d i r e c t p a i n t i n g of t h e i c e was undertaken i n t h e same way a s f o r dyeing sand. The o n l y d i f f e r e n c e was t h a t t h e dye came o u t of t h e s o l u t i o n n o t on t h e sand s u r - f a c e b u t on t h e bottom of t h e c o n t a i n e r , whence i t was t a k e n f o r d i s t r i b u t i o n on t h e experimental i c e a r e a s .
Coal d u s t was o b t a i n e d by s i f t i n g f i n e N o r i l t s k c o a l through a s i e v e w i t h a p p e r t u r e s 0.5 mm.
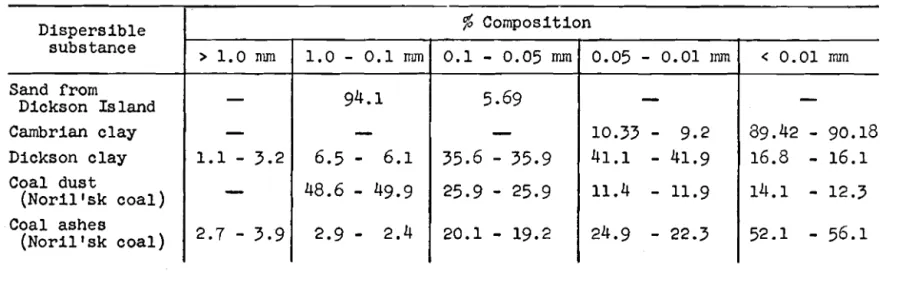
The g r a i n s i z e composition of t h e s u b s t a n c e s used f o r d u s t i n g i c e was determined a c c o r d i n g t o t h e method of Klenova and Avilov a s w e l l ao from s i e v e a n a l y s i s data ( ~ a b l e s I1 and 111).
The e f f e c t o f t h e dyes was determined a c c o r d i n g t o m e l t i n g of t h e i c e cover ( i n r e l a t i o n t o t h e d i f f e r e n c e between dyed and b a r e a r e a s ) . In o r d e r t o s t u d y t h e n a t u r e of t h e d e s t r u c t i o n of i c e caused by t h e v a r i o u s dyes and d u s t s , specimens of i c e were carved o u t of t h e experimental a r e a s and observed. I n t h e course o f t h e o b s e r v a t i o n s t h e a i r temperature v a r i e d from -lo t o +14OC.
A t t h e s t a r t of o b s e r v a t i o n s t h e i c e cover was 160 cm t h i c k .
D i f f e r e n c e s i n t h e m e l t i n g of i c e in t h e e x p e r i m e n t a l a r e a s became e v i d e n t a l r e a d y on t h e second day f o l l o w i n g t h e s t a r t of o b s e r v a t i o n s . D e s t r u c t i o n of t h e i c e was e s p e c i a l l y i n t e n s e i n t h e experimental a r e a s which were dyed w i t h
Prussian blue and a n i l d n e black and i n those dusted with dyed sand and
N o r i l f s k c o a l c i n d e r s . A t t h e end of observations, when due t o g e n e r a l warm- i n g t h e bare i c e a r e a s were melting j u s t a s i n t e n s e l y , t h e r e l a t i v e thawing of the dusted a r e a s did not i n c r e a s e but, i n a c t u a l f a c t , decreased. In t h i s case, melting of t h e bare i c e a r e a was more rapid due t o i t s higher l o c a t i o n than t h a t of t h e dusted a r e a .
Observed r e s u l t s of t h e melting process a r e l i s t e d in Table N.
It i s c l e a r from Table N t h a t f o r equal d e n s i t y of d u s t i n g , i n s p i t e of i t s l i g h t e r colour, t h e blue Cambrian c l a y promotes melting of i c e more
e f f e c t i v e l y than does t h e rust-brown Dickson c l a y .
Application of Cambrian c l a y with a d e n s i t y of 20
d m 2
i s j u s t a s e f f e c - t i v e on t h e i c e a s d u s t i n g with n a t u r a l sand with a d e n s i t y of 400 g / m 2 .Although d u r i n g l a t e r observations t h e n a t u r a l sand was found t o be more e f f e c t i v e than t h e Cambrian c l a y i n melting t h e i c e , t h i s was n o t due t o t h e n a t u r e of these m a t e r i a l s but was t h e r e s u l t of e x t e r n a l f a c t o r s (e.g. t h e e f f e c t of snow cover on the experimental a r e a s ) .
By June 23, t h e 21st day of t h e experimental s e r i e s , t h e t e s t a r e a s which were dyed with Prussian blue had melted t o a depth of 50
-
60 cm i n comparison t o t h e l e v e l of t h e c o n t r o l areas; t h e t e s t a r e a s dyed with a n i l i n e black-
t o a depth of 32-
34 cm; t h e t e s t a r e a s dusted with sand dyed with Prussianblue
-
t o a depth of 30-
45 cm; t h e t e s t a r e a s dusted with sand dyed with a n i l i n e black-
t o a depth of 50-
55
cm; t h e t e s t a r e a s dusted with N o r i l l s k c o a l c i n d e r s-
t o a depth of 54 cm. During t h e same period t h e experimental a r e a s dusted with n a t u r a l sand had melted t o a depth of 40 cm.The t e s t a r e a s dusted with c o a l d u s t with a d e n s i t y of
5
g / m 2 melted t o a depth of 23 cm, while f o r a d e n s i t y of 10d m 2
t h e thaw depth was 54 cm, and f o r 20 g/m2, i t was 60 cm.It should be i n d i c a t e d t h a t t h e l a t t e r f o u r experimental a r e a s were almost bare of snow. They were covered with a l a y e r of water 10
-
15
cm t h i c k ,through which one could s e e the weakened i c e , which resembled a s b e s t o s i n i t s s t r u c t u r e . On studying specimens which were carved o u t of t h i s i c e , i t was found t o c o n s i s t of long, v e r t i c a l c r y s t a l s . P a r t i c l e s of c o a l d u s t were
located on t h e boundary between an upper l a y e r of weakened gray-blue i c e and a lower l a y e r of l i g h t - b l u e f i r m i c e . Numerous small p a r t i c l e s of c o a l had
passed through t h e upper l a y e r of i c e , l e a v i n g i n i t s o many extremely t h i n v e r t i c a l pores ( t r a c e s of downward movement), t h a t the i c e developed a needle- l i k e s t r u c t u r e with a fkrmness somewhere between o r d i n a r y i c e and snow. Hard p a r t i c l e s of c o a l d u s t had p e n e t r a t e d t h e i c e t o a depth of 45
-
47 cm.S t u d i e s of t h e n a t u r e and speed of t h e d e s t r u c t i o n of i c e i n d i f f e r e n t experimental a r e a s showed t h a t the r a t e of t h e d e s t r u c t i o n of t h e i c e cover
depends n o t o n l y on t h e n a t u r e of t h e dyes and t h e d e n s i t y of d u s t i n g b u t a l s o on t h e d i s p e r s i t y . The e f f e c t of f i n e l y subdivided m a t e r i a l s i n t h e d e s t r u c - t i o n of i c e was noted by almost a l l of t h e s c i e n t i s t s s t u d y i n g t h e a c c e l e r a t e d thawing of i c e w i t h t h e a i d of fine-grade s u b s t a n c e s .
I f a dye i s by n a t u r e a poor a b s o r b e r of s o l a r r a d i a t i o n b u t e x i s t s i n a s t a b l e s t a t e w i t h a h i g h degree of d i s p e r s i o n , i t s o v e r a l l a b i l i t y t o a b s o r b r a d i a t i o n may be g r e a t e r t h a n t h a t of a dye w i t h g r e a t e r a b s o r p t i v e p r o p e r t y b u t low d i s p e r s i t y . Thus, Dickson c l a y i s more i n t e n s e l y coloured t h a n
Cambrian c l a y , b u t f o r e q u a l d e n s i t y of d u s t i n g i t w i l l absorb l e s s r a d i a t i o n . This phenomenon i s e x p l a i n e d by t h e g r a i n s i z e composition of t h e c l a y s : t h e d i s p e r s i t y of Cambrian c l a y i s c o n s i d e r a b l y h i g h e r t h a n t h a t of Dickson c l a y
a able
11).The lower r a d i a t i o n a b s o r b i n g a b i l i t y of a n i l i n e b l a c k , which i s d a r k e r i n c o l o u r t h a n P r u s s i a n b l u e , may be e x p l a i n e d from t h e f a c t t h a t p a r t i c l e s of a n i l i n e b l a c k ( a s a r e s i n o u s m a t e r i a l ) r a p i d l y adhere t o g e t h e r forming
l a r g e r masses and t h e r e b y d e c r e a s i n g t h e degree of d i s p e r s i o n . The d i s p e r s i t y of P r u s s i a n b l u e remained p r a c t i c a l l y c o n s t a n t in t h e course of t h e
o b s e r v a t i o n s .
N.T. Chernigovskii, who i n 1949 performed experiments on t h e d e s t r u c t i o n of i c e by t h e r a d i a t i o n method, c o n s i d e r s t h a t " t h e d e s t r u c t i v e e f f e c t of
d u s t i n g with c o a l d u s t and c i n d e r s i s almost t h e same". However, on cornparing t h e c o l o u r a t i o n of c o a l d u s t and c i n d e r l a y e r s , i t i s c l e a r t h a t t h e former has a more i n t e n s e c o l o u r . Consequently, one may e x p e c t t h e d e s t r u c t i v e
e f f e c t of c o a l d u s t t o be g r e a t e r t h a n t h a t of c i n d e r s . I f i n t h e experiments of
N.T.
Chernigovsl.ci1 t h e d e s t r u c t i v e e f f e c t s of b o t h d u s t s were observed t o be e q u a l , t h i s would probably be due t o t h e h i g h d i s p e r s i t y of t h e c i n d e r s compensating f o r t h e l i g h t e r c o l o u r .Experiments performed i n 1953 r e v e a l e d t h a t t h e m e l t i n g a c t i o n of c o a l d u s t f o r a d e n s i t y of d u s t i n g 20 d m 2 was o n l y s l i g h t l y g r e a t e r t h a n t h e melt- i n g a c t i o n of c i n d e r s f o r a d e n s i t y of d u s t i n g 100 d m 2 , .which i s e x p l a i n e d by t h e r e l a t i v e l y low d l s p e r s i t y of c i n d e r s . F o r e q u a l degree of d i s p e r s i o n and d e n s i t y of d u s t i n g , c o a l d u s t should, t h e r e f o r e , have a m e l t i n g a c t i o n many times g r e a t e r t h a n t h a t of c i n d e r s .
I n t h e s e experiments t h e g r e a t e s t m e l t i n g a c t i o n was observed i n t h e c a s e of dyeing i c e d i r e c t l y w i t h P r u s s i a n b l u e ( 1 d m 2 ) and d u s t l n g w i t h c o a l d u s t
(20 @;/m2). Dusting w i t h c o a l d u s t (10 d m 2 ) and sand dyed a n i l i n e b l a c k ( 1
-
2 g p e r 400 g s a n d ) f o r a d e n s i t y of d u s t i n g 400 d m 2 , a s w e l l as d u s t i n g w i t k
c i n d e r s (100 d m 2 ) and d y e i r ~ g w i t h P r u s s i a n b l u e (0.5 d m 2 ) had a s l i g h t l y weaker d e s t r u c t i v e e f f e c t on t h e i c e . An even l e s s s i g n i f i c a n t e f f e c t i n t h e d e s t r u c t i o n of t h e i c e cover was observed ln t h e c a s e of sand dyed P r u s s i a n
blue (1 g p e r 400 g sand), n a t u r a l sand and sand dyed a n i l i n e black (1
-
2 g p e r 400 g sand) f o r a d e n s i t y of d u s t i n g 400 d m 2 , a s w e l l a s Ca~nbrian c l a y( 2 0 d m 2 ) .
The high melting a c t i o n of Prussian blue and coal d u s t may be explained by t h e n a t u r e of these substances (dark c o l o u r ) a s well a s by t h e i r high d i s p e r s i t y . In a l l p r o b a b i l i t y t h e a c t i v e r o l e of c o a l d u s t i n t h e d e s t r u c - t i o n of t h e i c e cover i s a l s o due t o t h e good thermal c o n d u c t i v i t y of i t s p a r t i c l e s . These p r o p e r t i e s of c o a l d u s t d i d i n f a c t favour i t s p e n e t r a t i o n
i n t o t h e i c e t o a depth of 47 cm i n s p i t e of t h e 10
-
15
cm t h i c k l a y e r of thaw water on t h e s u r f a c e of t h e i c e s h e e t .Conclusions
1. Dusting of t h e i c e s u r f a c e with hard i n s o l u b l e f i n e l y - d i s p e r s e d m a t e r i a l s promotes t h e thawing and d e s t r u c t i o n of t h e i c e .
2. The r a t e of thawing and d e s t r u c t i o n of t h e i c e cover depends on t h e n a t u r e and degree of s u b d i v i s i o n of t h e d i s p e r s e d m a t e r i a l a s well a s on t h e d e n s i t y of d u s t i n g of the i c e s u r f a c e with t h i s substance,
3 . Dyeing of n a t u r a l sand with Prussian blue and a n i l i n e b l a c k (0.5, 1
and 2 g p e r 400 g sand) pronlotes i t s d e s t r u c t i v e e f f e c t on t h e i c e , although t o a l e s s e x t e n t than could be expected on t h e b a s i s of t h e s e p a r a t e e f f e c t s of sand and dye. The d e s t r u c t i v e e f f e c t of dyed sand on i c e Is n o t i n f a c t t h e sum e f f e c t of i t s components. On dyeing t h e sand, the dye s t i c k s t o t h e sand p a r t i c l e s ( t h e r e b y i n c r e a s i n g t h e colour I n t e n s i t y .and r a d i a t i o n absorbed) and a l s o forms dye f i l m s on t h e sand p a r t i c l e s which r e s u l t s i n d e c r e a s i n g t h e d i s p e r s i t y of t h e dye ( t h e r e b y reducing t h e e f f e c t i v e n e s s of t h e dye f o r i n c r e a s i n g absorption of s o l a r r a d i a t i o n of t h e d y e ) .
4. The a c t i o n o f , c o a l d u s t ( p a r t i c l e s i z e l e s s than 0.5 mm) on i c e has s e v e r a l f e a t u r e s d i s t i n g u i s h i n g i t from o t h e r d u s t - l i k e m a t e r i a l s :
( a ) 500 g and even 250 g of c o a l d u s t spread over an i c e s u r f a c e a r e a of 25 m2 destroyed t h e i c e cover more e f f e c t i v e l y than d i d 10 kg of sand.
( b ) t h e c o a l d u s t p e n e t r a t e d t h e i c e t o a depth of 47 cm i n s p i t e of t h e 10
-
15
cm t h i c k l a y e r of water.5.
I n t h e course of t h e experiment, t h e dye Prussian blue which was applied d i r e c t l y on t h e i c e , a s w e l l - a s - t h a t used f o r dyeing sand, g r a d u a l l y changed colour from blue t o green and even rust-.brown. The dye a n i l i n e black d i d not change i t s colour throughout t h e e n t i r e experiment. Prussian blue was twice as e f f e c t i v e in t h e d e s t r u c t i o n of i c e than was a n i l i n e black when each was applied d i r e c t l y t o t h e i c e s u r f a c e . The a c t i o n of P r u s s i a n blue i n dye-i n g sand was considerably weaker than t h a t of a n i l i n e black used f o r t h e same purpose.
6. Since t h e r a d i a t i o n a b s o r p t i v i t y of hard, opaque subdivided m a t e r i a l s depends on t h e i r degree of s u b d i v i s i o n a s much a s on t h e i r colour, i n o r d e r t o promote t h e d e s t r u c t i o n o f i c e i t i s more p r a c t i c a l t o use small q u a n t i t i e s ( s e v e r a l grams p e r s q u a r e metre ) of h i g h l y d i s p e r s i b l e s u b s t a n c e s r a t h e r t h a n l a r g e q u a n t i t i e s of s u b s t a n c e s w i t h low d i s p e r s i t y , e . g . sand. The e x i s t i n g s t a n d a r d s f o r d u s t i n g t h e i c e cover with c o a l d u s t i n o r d e r t o melt channels I n t h e i c e may be decreased up t o s e v e r a l hundred times i f t h e p a r t i c l e s s i z e i s correspondingly decreased.
7. Under A r c t i c c o n d i t i o n s d u s t i n g of t h e i c e s u r f a c e with sand, c o a l c i n d e r s a s w e l l as c o a l d u s t i s g e n e r a l l y performed i n o r d e r t o promote
d e s t r u c t i o n of t h e i c e cover with t h e a i d of s o l a r r a d i a t i o n . The r a d i a t i o n a c t i v i t y o f t h e s e s u b s t a n c e s may be i n c r e a s e d I n two ways:
( a ) By dyeing some of t h e s e m a t e r i a l s , e.g. n a t u r a l sand, with P r u s s i a n blue and a n i l i n e black ( 1
-
2 g p e r 400 g sand) and ( b ) by f u r t h e r f i n e sub- d i v i s i o n of t h e dark-coloured, e a s i l y crumbled n a t u r a l m a t e r i a l a , e.g. c o a l ,Table I
k c a t i o n of experimental a r e a s in Dickson Bay
P r u s s i a n blue, 1 . 0 g/m2 (exp. a r e a 5 ) Sand, 400 g / m 2 (exp. a r e a 1 0 ) Coal d u s t , 5 (exp. a r e a 15 Aniline black 2
g/m
(exp. a r e a 20) Cambrian c l a y , 2g/
m 2 (exp. a r e a 25) Calcium c h l o r i d e , 40 d m 2 ' ( e x p . a r e a 3 0 )I
Dickson c l a y , 2 g / m 2 (exp. a r e a 3 5 ) Coal d u s t , 10d m 2
(exp. a r e a 40)I
Magnesium powder, 1.0d m 2
(exp. a r e a 45) Coal d u s t , 1.0 g/m2 (exp. a r e a 50)I
Coal d u s t , 2 (exp. a r e a 55 Coal d u s t , 2 (exp. a r e a 60B/m2
Sand, 400 g / m 2+
Prussian blue, 0. g / m 2 (exp. a r e a 9 Aniline black, 1 . 0d m 2
(exp. a r e a 1 9 ) Cinders, 100 (exp. a r e a 29 Coal d u s t , 20d m 2
(exp. a r e a 3 9 ) Aluminium powder, 49 Coal d u s t ,5
(exp. a r e a 59 Prussian blue, 0.5 g / m 2 (exp. a r e a 4 ) Sand, 400 g / m 2+
a n i l i n e black, 1 . 0 g / m 2 (exp. a r e a 1 4 ) Cambrian c l a y , 1 0 g / m 2 (exp. a r e a 24) Dickson c l a y , 1 0d m 2
(exp. a r e a 3 4 ) Magnesium powder6.B/m2
(exp. a r e a 44 Coal d u s t , 0.5 g/m2 (exp. a r e a 5 4 ) Sand, 400 d m 2+
P r u s s i a n blue, 0.5d m 2
(exp. a r e a8 )
Sand, 400 g / m 2+
a n i l i n e black 2 g / m 2 (exp. a r e a 18) Cambrian c l a y , 20 Id m 2
(exp. a r e a 2 8 ) va
Dickson c l a y , 20 g/m2 ( e m . a r e a 3 8 ) Aluminium powder 1 . 0 g / m 2 (exp. a r e a48)
Calcium orthop5os- phate, 0.5 g / m (exp. a r e a58)
Continueda
B
d +,5
0 I H a, r-l P a (YL
cu OCU 0 r-l cd a, .k m cd 71 a5
5
0- (Y5
n OCU CUn .cd 4JO m k 5 6 71a
06
a, 0- n Ot-.
r-l n t- OCU t-i cd-
al22
r-l 0 a I= X cd a, d- k P N8 1
n t- tc\ 0 r-l cd a,-
k h d cd:
*
c
a, 0- m Xa21
n tc\ OCU (U cd .a, h k cd cd t-i"
3
P
a, d- k 9-s5
U r-l . h 4 r - l c dTable
I1Grain s i z e cornposition of several d i s p e r s i b l e substances,
a s determined
bythe method of Klenova and Avilov
Table
I11Dispersible
substance
Sand from
Dickson
Island
Cambrian clay
Dickson clay
Coal dust
(Noril
' s kc o a l )
Coal ashes
( N o r i l t s k c o a l )
Grain s i z e composition of several d i s p e r s i b l e substances,
accordlng t o s i e v e a n a l y s i s data
$Composition
> 1.0 nlm-
-
1.1-
3.2
-
2.7
-
3.9
Dispersible
substance
Coal cinders
(Noril
s kcoal
)Coal dust
Sand 1.0-
0.1 mm94.1
-
6 .
-
6.1
48.6
-
49.9
2.9
-
2.4
$Composition
0 . 1-
0.05
mrn5.69
-
35.6
-
35.9
25.9
-
25.9
20.1
-
19.2
>2.0
mm25.56
-
-
2.0-
1.0 mm13.19
-
-
0.05
-
0.01 Inrn-
10.33
-
9.2
41.1
-
41.9
11.4
-
1 . 9
24.9
-
22.3
1.0-
0.5
mm11.37
10.54
0.9
< 0.01 mrn-
8 9 . 4 2 - 9 0 . 1 8
16.8
-
16.1
14.1
-
12.3
52.1
-
56.1
0.25
-
0.10 mn4.86
45.04
92.4
0.5
-
0.25
rnm8.16
4.37
0.8
t 0 . 1 0 m i35.18
38.56
5.69
Table IV
R e l a t i v e
thawingof dyed and dusted i c e a r e a s
f o r June 9 and 23, 1953
Dye o r d u s t used
Natural sand
Prussian blue 0.5
g / m 2Prussian blue 1.0 g / m 2
Sand 400
g / m 2+
Prussian blue 0.5 g/rn2
Sand 400
g / m 2+
Prussian blue 1.0
g/m2Aniline black
1 g/m2Aniline black
2 e;/'m2Sand 400
d m 2+
a n i l i n e black
1 g / m 2Sand