Cyclic tensile strain triggers a sequence of
autocrine and paracrine signaling to regulate
angiogenic sprouting in human vascular cells
The MIT Faculty has made this article openly available.
Please share
how this access benefits you. Your story matters.
Citation
Yung, Yu Ching et al. “Cyclic tensile strain triggers a sequence of
autocrine and paracrine signaling to regulate angiogenic sprouting
in human vascular cells.” Proceedings of the National Academy of
Sciences 106.36 (2009): 15279-15284. © 2009 National Academy of
Sciences
As Published
http://dx.doi.org/10.1073/pnas.0905891106
Publisher
United States National Academy of Sciences
Version
Final published version
Citable link
http://hdl.handle.net/1721.1/55306
Terms of Use
Article is made available in accordance with the publisher's
policy and may be subject to US copyright law. Please refer to the
publisher's site for terms of use.
Cyclic tensile strain triggers a sequence of autocrine
and paracrine signaling to regulate angiogenic
sprouting in human vascular cells
Yu Ching Yunga,b, Jeiwook Chaec, Markus J. Buehlerd, Craig P. Hunterc, and David J. Mooneya,e,1
aHarvard School of Engineering and Applied Sciences andcDepartment of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138; bDepartment of Chemical Engineering, University of Michigan, Ann Arbor, MI 48109;dLaboratory for Atomistic and Molecular Mechanics, Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139; andeWyss Institute for Biologically Inspired Engineering, Cambridge, MA 02138
Edited by Robert Langer, Massachusetts Institute of Technology, Cambridge, MA, and approved June 30, 2009 (received for review May 29, 2009)
Mechanical signals regulate blood vessel development in vivo, and have been demonstrated to regulate signal transduction of endo-thelial cell (EC) and smooth muscle cell (SMC) phenotype in vitro. However, it is unclear how the complex process of angiogenesis, which involves multiple cell types and growth factors that act in a spatiotemporally regulated manner, is triggered by a mechanical input. Here, we describe a mechanism for modulating vascular cells during sequential stages of an in vitro model of early angiogenesis by applying cyclic tensile strain. Cyclic strain of human umbilical vein (HUV)ECs up-regulated the secretion of angiopoietin (Ang)-2 and PDGF-, and enhanced endothelial migration and sprout formation, whereas effects were eliminated with shRNA knock-down of endogenous Ang-2. Applying strain to colonies of HUVEC, cocultured on the same micropatterned substrate with nonstrained human aortic (HA)SMCs, led to a directed migration of the HASMC toward migrating HUVECs, with diminished recruitment when PDGF receptors were neutralized. These results demonstrate that a singular mechanical cue (cyclic tensile strain) can trigger a cascade of autocrine and paracrine signaling events between ECs and SMCs critical to the angiogenic process.
angiogenesis兩 angiopoietin-2 兩 endothelial cells 兩 shRNA 兩 strain gradient
A
ngiogenesis requires an orchestrated series of cell activities in a specific spatial and temporal sequence (1). Significant research in this field has focused on documenting cell response to exogenous biochemical cues, and a variety of growth factors have been identified to have key roles in angiogenesis (2). Among these biochemical cues, VEGF has been identified as a potent factor during the early stages of angiogenesis, activating migration, and sprout formation (3). Angiopoietin (Ang)-1, a cytokine that mediates the interactions between endothelial cells (ECs) and smooth muscle cells (SMCs), and Ang-2, an early angiogenic factor that inversely acts to disrupt and dissociate these bonds, are ligands expressed by vascular cells that com-petitively bind to the membrane receptor Tie-2, and act syner-gistically with VEGF to regulate angiogenesis (4). PDGF-, a chemotactant released by ECs, is a late stage cytokine that recruits SMCs to stabilize the nascent EC sprouts (5).Understanding of the effects of these soluble factors alone is, however, likely insufficient to fully understand the angiogenic process. Physiologically, ECs and SMCs are exposed to cyclic tensile strain resulting from blood hemodynamic forces, a cue important to vessel adaptation (6). Mechanical signals regulate the function of both cell types in vitro, including the alteration of EC proliferation (7, 8), alignment (9, 10), migration (11, 12), and in vitro sprout formations (13, 14), likely through activating various intracellular signaling pathways (15–21). Similarly, phys-iologically relevant hemodynamic forces have demonstrated to modulate SM phenotype (22), migration (23), and intracellular signaling (24). Altogether, these past studies suggest that the
angiogenic process is governed by an interplay between chemical and mechanical signals.
We hypothesized that cyclic tensile strain may provide a singular cue to regulate vascular cells by triggering EC secretion of angiogenic factors that mediate multiple stages in the angio-genic process. Human umbilical vein (HUV)ECs and human aortic (HA)SMCs were used here as model cell types represent-ing the vascular endothelium and stabilizrepresent-ing supportive layer, respectively. Vascular cells were cultured in 2D directly on elastomeric poly(dimethylsiloxane) (PDMS) substrates and in fibrin 3D cultures. In vitro sprouting was used as a model for the initial stages of angiogenesis, as previously described (25–28). Vascular cell cultures were exposed to cyclic tensile strain with an amplitude of 7%, representative of that experienced by vascular cells in large vessels, and also in the same range as previous calculations of capillary wall strain (29, 30). Cyclic tensile strain was demonstrated to alter vascular cell phenotype and the secretion of Ang-2 and PDGF by EC. Altered Ang-2 secretion mediated changes in early angiogenic processes, EC migration, and in vitro capillary formation, whereas strain-enhanced secretion of PDGF provided a directional cue for SMC recruitment toward EC colonies.
Results
Cyclic Tensile Strain Regulation of Vascular Cell Phenotype, Angio-genic Factor Secretion, and Migration. The previously reported effects of cyclic strain on EC migration and in vitro sprout formation were first confirmed. Cyclic tensile strain (7%, 1 Hz) enhanced, by 1.6-fold, the directional migration of HUVECs in 2D culture (Fig. S1 A), as expected (31). Two days of cyclic tensile strain also enhanced sprout formation by HUVECs in fibrin gels by 4-fold, compared with static culture (Fig. S1B), again in agreement with earlier investigations (13). Addition of recombinant human VEGF-165, a known stimulant to capillary formation (3, 5), also increased sprout formation both under static and strained conditions (Fig. S1B), confirming the ex-pected biological responsiveness of the cells used in these studies. The effect of varying the magnitude of cyclic tensile strain was first assessed by analyzing the fraction of cells that migrated out from the original cell culture region, the average cell velocity, and directionality of migratory cells. Cyclic tensile strain en-hanced the fraction of HUVECs that migrated out of the original culture region by 2-fold when an amplitude of 6% cyclic strain
Author contributions: Y.C.Y., M.J.B., and D.J.M. designed research; Y.C.Y. performed research; J.C. and C.P.H. contributed new reagents/analytic tools; Y.C.Y., M.J.B., and D.J.M. analyzed data; and Y.C.Y. and D.J.M. wrote the paper.
The authors declare no conflict of interest. This article is a PNAS Direct Submission.
1To whom correspondence should be addressed. E-mail: mooneyd@seas.harvard.edu. This article contains supporting information online atwww.pnas.org/cgi/content/full/
0905891106/DCSupplemental. APPLIED
BIOLOGICAL
was applied, whereas at an amplitude of 13% cyclic strain, the fraction of migrating cells was enhanced⬇4-fold (Fig. S1C). The average velocity of HUVEC migration at 13% cyclic strain was 15-fold higher, as compared with the static condition (Fig. S1D), resulting in an average velocity of 15m/h. Further, the migra-tion of HUVECs exhibited a clear direcmigra-tionality, perpendicular to the direction of strain application (Fig. S1E). Similarly, 2 days of cyclic tensile strain led to a 3-fold enhancement in the number of migrating HASMCs at 13% strain, as compared with the nonstrained condition, but little effect was noted at 6% strain amplitude (Fig. S1F). The average velocity of migrating HASMCs, at 13% strain, was⬇6-fold higher than cells in static
culture, but was notably slower than HUVEC migration rates (Fig. S1G). Strikingly, and in contrast to HUVECs, whereas HASMCs migration was increased with cyclic strain, the cells did not demonstrate directional migration under these conditions (Fig. S1H).
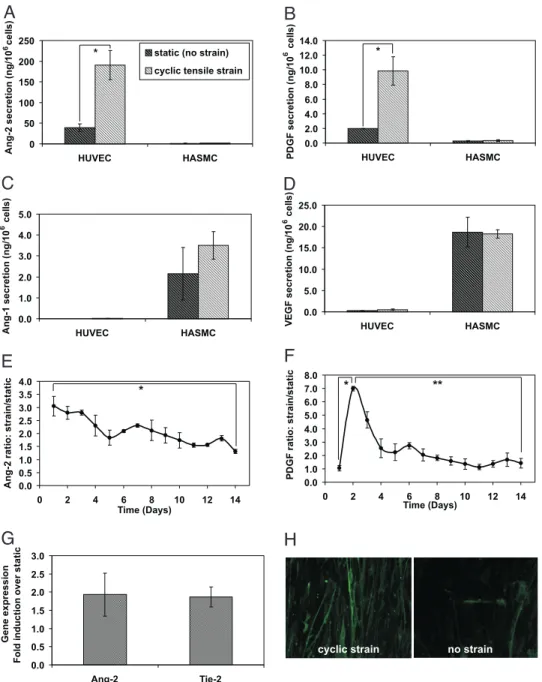
To examine whether cyclic strain up-regulated the expression of genes involved in angiogenesis, the levels of angiogenic proteins secreted by the vascular cells were quantified over a 5-day time-course of cyclic strain. Cyclic strain of HUVECs led to a 4.8-fold up-regulation of Ang-2 (Fig. 1A), and a 5-fold up-regulation in the secretion of PDGF- (Fig. 1B). In contrast, cyclic strain of HASMCs resulted only in a slight enhancement
A
B
C
D
F
H
G
E
Fig. 1. Cyclic tensile strain up-regulated secretion of angiogenic factors by vascular cells in a temporal manner. (A–D) Secretion of PDGF, Ang-2, Ang-1, and VEGF by HUVECs and HASMCs was quantified after exposure to 5 days of cyclic strain. Protein levels were quantified using enzyme immunoassays and values (n⫽ 3) were normalized to total cell number per well. (E and F) Expression profiles of HUVECs secretion of Ang-2 and PDGF in response to 14 days of cyclic strain. Values represent normalized levels (n⫽ 3) under the cyclic strain condition normalized to levels secreted under static (no strain) conditions. (G) Cyclic strain effect on gene expression of Ang-2 and Tie-2 mRNA levels, after a duration of 5 days, was quantified by real-time RT -PCR (n⫽ 6). Expression levels, with and without cyclic strain application, were normalized to GAPDH levels and the ratio of strained to nonstrained levels are presented in the graph. (H) Expression of PDGF-R was enhanced after 48 h of cyclic strain application to HASMCs, as documented using immunohistochemistry directed to PDGF-R (green fluorescence).*, P⬍ 0.05; **, P⬍ 0.005.
of Ang-1 (Fig. 1C), whereas secretion of VEGF did not appear to be effected by strain (Fig. 1D). In both strained and non-strained conditions, the secretion of PDGF- and Ang-2 by HASMC, and Ang-1 and VEGF by HUVECS, respectively, was minimal. The time course of up-regulation of PDGF- and Ang-2 secretion by HUVECs was next investigated. Ang-2 expression was increased 3-fold by strain at day 1, and then slowly subsided over the ongoing 13 days to control levels (Fig. 1E). PDGF- secretion, in contrast, did not rise until 2 days of cyclic stretch, and then quickly returned to baseline control levels (Fig. 1F). Because minimal effects of cyclic strain on angiogenic factor secretion by HASMC were noted, all subsequent studies of factor secretion focused on HUVECs. The gene expression levels of Ang-2, and its receptor (Tie-2) in HUVECs, was analyzed using real-time RT-PCR, and application of cyclic strain resulted in 1.5- and 2-fold increases in mRNA levels for Ang-2 and Tie-2, respectively (Fig. 1G). Last, cyclic tensile strain was also found to up-regulate surface availability of PDGF- receptors (R) on HASMCs (Fig. 1H).
Role of Ang-2 in EC Migration and Sprout Formation.To determine whether the levels of altered angiogenic factors resulting from cyclic strain were capable of altering EC phenotype, HUVECs in fibrin gels were exposed to exogenous recombinant human Ang-2, Ang-1, PDGF, and VEGF, at levels corresponding to those produced by cells under strained conditions. Ang-2 and VEGF enhanced the formation of sprouts (Fig. S2 A and B), whereas PDGF- (Fig. S2C) and Ang-1 (Fig. S2D) had no discernible effects. The effects of these factors on HUVEC migration across porous transwell membranes was next exam-ined, and VEGF was found to enhance HUVEC migration (Fig. S2 E), concurring with previous descriptions of the effects of this cytokine (32). Ang-2 similarly enhanced HUVEC migration, whereas Ang-1 had no effect (Fig. S2 E). Altogether, these data suggests new roles for Ang-2 in EC biology, and indicate the
increase in Ang-2 expression in ECs that result from cyclic strain is sufficient to increase the angiogenic activity of these cells.
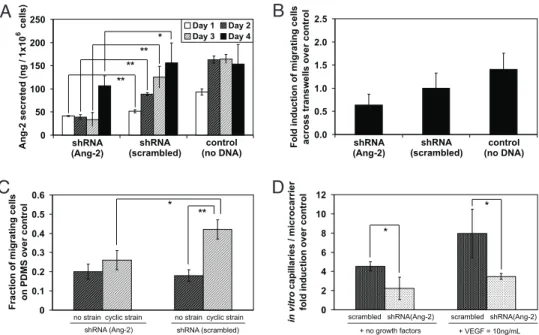
To test whether cyclic strain-induced up-regulation of Ang-2 was causative for the strain induced increase in EC migration and sprouting, RNAi was used to knockdown the endogenous ex-pression of Ang-2 in HUVECs. HUVECs were transfected with a plasmid that was designed and constructed to release a 63-mer shRNA that binds specifically to the intracellular mRNA of Ang-2 and blocks the translation of this protein. Examination of Ang-2 secretion by cells positively transfected with shRNA (Ang-2) confirmed a dramatic inhibition of Ang-2 expression for 4 days after treatment (Fig. 2A). The baseline (no cyclic strain) migration of ECs with shRNA (Ang-2), was decreased by ⬇1.6-fold (Fig. 2B), whereas cells subjected to strain exhibited a 2-fold decrease in migration with shRNA treatment (Fig. 2C). Inhibiting Ang-2 also resulted in a 2.2-fold decrease in sprouting with exposure to cyclic strain, in both the absence and presence of exogenous VEGF in the culture medium (Fig. 2D).
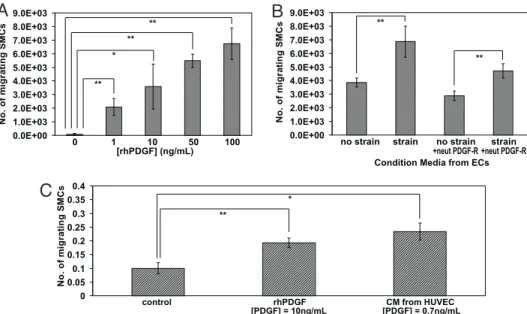
Cyclic Strain Directs SMC Recruitment Toward Migrating ECs.The next series of studies examined whether strain induced change in EC cytokine expression could alter HASMC function, and in particular whether PDGF- could serve to recruit SMCs to forming vessels. In the first experiment, HASMCs were exposed to increasing doses of exogenous recombinant human PDGF-, and migration was found to increase with PDGF- concentra-tion (Fig. 3A). Next, condiconcentra-tioned media was taken from strained ECs and added to HASMC culture, and this enhanced HASMC migration by ⬇2-fold, as compared with conditioned media taken from cells under nonstrained cultures. To determine whether the enhanced SM migration was a direct result of the strain-mediated increased PDGF- levels, we neutralized PDGF-R on HASMCs and reassessed their response to condi-tioned media taken from strained ECs. Neutralizing PDGF-Rs decreased migration by 45% (Fig. 3B). Next, to determine
A
B
C
D
Fig. 2. RNAi was used to determine the role of Ang-2 in HUVEC response to cyclic strain. (A) Effectiveness of shRNA knockdown of endogenous Ang-2 secretion by HUVECs transfected with shRNA to Ang-2, a control shRNA (scrambled sequence), and control untreated cells. Ang-2 was quantified using enzyme immunoassays, daily (n⫽ 5) over 4 days. Values represent mass of protein secreted, normalized to total cell number per well. (B) Level of static HUVEC migration, over 24 h, across transwell inserts, normalized to untreated control cells, with either shRNA to Ang-2 or scrambled shRNA control (scrambled treatment) (n⫽ 4). (C) Level of HUVEC migration in response to 48 h cyclic strain on PDMS. Values represent number of cells under cyclic strain that migrated out of original confined circular region, d⫽ 2 mm, normalized to static, nonstrained conditions (n ⫽ 3). (D) Formation of sprouts under application of strain was quantified using HUVECs with and without Ang-2 shRNA treatment, in culture medium with or without added VEGF. Values (n⫽ 3) are normalized to a nonstrained, no growth factors control.*, P⬍ 0.05;**, P⬍ 0.005.
APPLIED
BIOLOGICAL
whether PDGF- has the ability to direct HASMC migration over significant distances, a depot of either recombinant human (rh)PDGF or a depot of conditioned media from strained cells was placed 2,000m away from patterned cultures of HASMC. The migration of HASMCs toward both depots increased by 2-and 2.25-fold, respectively (Fig. 3C), as compared a blank depot with no stimulants.
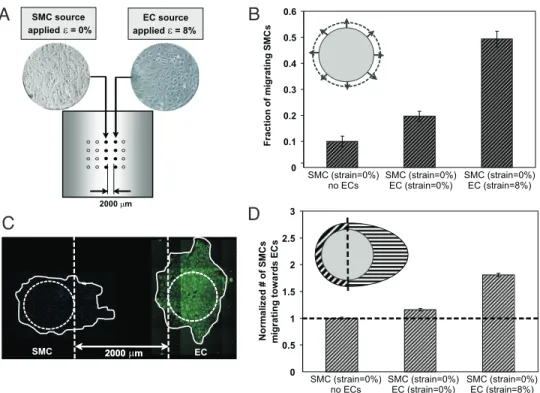
Finally, the ability of cyclic strain of ECs to induce not only directed EC migration, but also recruitment of nonstrained SMCs was assessed. Micropatterned cultures were developed (Fig. 4A), in which ECs were subjected to cyclic strain or maintained under static culture, whereas adjacent colonies of HASMC were subjected to minimal strain. Culture of SMCs adjacent to nonstrained ECs led to a 2-fold increase in SMC migration (Fig. 4B). Strikingly, when SMCs were cocultured adjacent to strained HUVECs, the migration of HASMCs was enhanced by 5-fold (Fig. 4B), as compared with a negative control (SMCs with no coculture, no strain). There was no notable directionality of HASMC migration when cultured adjacent to nonstrained HUVEC colonies. However, strained HUVECs not only increased HASMC migration, but also provided cues to direct the migration of HASMCs toward the migrating HUVEC colony (2-fold increase in directional migra-tion) (Fig. 4 C and D).
Discussion
The results of these studies demonstrate that cyclic strain can activate endogenous biochemical cues to direct vascular cells through sequential stages important to the angiogenic process. The angiogenic phenotype of ECs, characterized here by di-rected cell migration and in vitro sprout formation, was en-hanced by recombinant Ang-2 and in response to cyclic uniaxial strain. Cyclic strain increased the early expression of both Ang-2 and its receptor, Tie-2, in ECs, and this increased Ang-2 expression was found to mediate the cyclic strain induced alterations in EC angiogenic phenotype. Cyclic strain enhanced, at a later time point than Ang-2, the secretion of PDGF- by ECs, and the PDGF- modulated the directional migration of
HASMC toward EC colonies (Fig. S3). Although past studies have largely focused on vascular remodeling of precapillary structures (e.g., arterioles) in response to altered load, these results suggest that the capillaries themselves may also undergo significant remodeling in response to mechanical cues. Break-down or structural damage to capillary walls resulting from disease may provide one situation in which vascular cells expe-rience altered loading (33); exercise may also provide this type of stimulus, and previous studies have noted alterations in capillary densities with exercise (34). Previously calculated strain levels in capillary walls during systolic contraction and stable diastolic arrest range between 0 to 22% (30, 35), supporting the physiologic relevance of the 7% strain amplitude used in of our studies (29). Other investigations have used similar strain levels to study the effects of mechanical cue effects on ECs in vitro, specifically in the context of angiogenesis (36 –38).
Cyclic strain up-regulated EC secretion of angiogenic cyto-kines, specifically, PDGF- and Ang-2. Previous studies re-ported that cyclic strain enhanced the expression of PDGF-R (12, 39), and shear stress enhanced gene expression of PDGF- (40, 41) and Tie-2 (42). However, up-regulation of PDGF- and Ang-2 in response to cyclic strain has, to our knowledge, not been previously documented. Interestingly, VEGF, a potent factor in angiogenic activation, was not affected by strain, although regulation of this cytokine is governed by other local cues (43). The temporal profile of increased angiogenic cytokine secretion by ECs in response to cyclic strain was striking, because Ang-2, a factor important to the initiation of angiogenesis (44), was up-regulated early, followed by later expression of PDGF-, which plays important roles in later stages of angiogenesis (44, 45). These data suggests that cyclic strain modulates angio-genesis by altering the balance of angiogenic factors, and by temporally mediating the up-regulation of factors driving acti-vation versus those promoting subsequent vessel stabilization.
Ang-2 was found in this study, even in the absence of mechanical stimulation, to enhance sprout formation and mi-gration of ECs. The role of angiopoietins in vascular develop-ment has been the subject of active investigation, and until
B
A
C
Fig. 3. PDGF enhanced HASMC migration and recruitment. (A) Exogenous application of increasing concentrations of rhPDGF enhanced SM migration across transwells inserts after 24 h in static culture (n⫽ 4). (B) Application of conditioned medium from ECs cultured under static or cyclic strain (strain) conditions, with and without addition of neutralizing antibodies to PDGF-R, was added to HASMCs and their migration was quantified (n⫽ 4). (C) SM migration was enhanced when a depot source of PDGF: rhPDGF or conditioned media (CM) from strained ECs was placed in a depot located 2 mm away from the HASMC colon. Migration results are presented as the fraction of cells that migrated out of the original culture area as compared with a control (no growth factor) condition (n⫽ 4). *, P⬍ 0.05;**, P⬍ 0.005.
recently, it was believed that Ang-1 had solely a stabilizing role via activation of the tyrosine kinase receptor Tie-2 (46), whereas Ang-2, the antagonist to Ang-1, was believed to have more of a facilitative role (47). For example, expression of Ang-2 was identified primarily at sites of active vessel remodeling (47–50). However, recent studies demonstrate that there may exist a contextual role to the functions of Ang-2, because it serves in some instances to inhibit vascular leakage (51), whereas in other situations, it may function as a proinf lammatory cytokine (52). Increased secretion of Ang-2 in response to biochemical stim-ulants has also been documented, supporting the suggestion (53) that Ang-2 function is more complex than initially identified (54). Although VEGF-A and angiopoietins have distinct roles in vascular development, they also have complementary and co-ordinated roles. VEGF-A has been shown to modulate migration (32) and in vitro capillary formation (55) of ECs, and Ang-2, at levels secreted in response to cyclic strain signals, appears to have similar effects on ECs. Although the molecular mechanisms linking cyclic strain to Ang-2 expression are not clear, they likely involve the various intracellular signaling pathways previously documented to mediate mechanical effects on ECs (16, 56) that induce local differentiation and the formation of nascent blood vessels.
Cyclic tensile strain was found in this study and by others (57) to enhance the expression of PDGF-R on HASMCs. A result-ing enhanced responsiveness to PDGF signalresult-ing via the tyrosine kinase pathway (activated by ligand binding to PDGF-R) is likely involved in the HASMC migration in response to cyclic strain. Cyclic strain has been previously demonstrated to modulate ERK1/2 (58, 59), PI3K (60), p21 (19, 61), tyrosine kinase (59, 62), and RhoA signaling. Cyclic strain also likely enhances the expression of other receptors as well, all which may cooperatively regulate the HASMC responses to cyclic strain, including direct
and indirect responses (e.g., secondary to EC-induced alter-ations in gene expression resulting from cyclic strain). The finding that HASMCs cultured under static conditions can be actively and directionally recruited to strain-stimulated ECs supports the potential physiologic relevance of these findings.
The sequential regulation by cyclic tensile strain of early and late stages of vascular remodeling represents a previously un-recognized and potentially critical role for mechanical signaling in angiogenesis. Reciprocal signaling of EC-induced SMC re-cruitment via PDGF has been examined in vivo (63, 64), but not with events activated by external mechanical stimuli. More broadly, the findings of this study provide a specific example of how localized mechanical signals can be translated into biochem-ical cues (65) capable of signaling over physiologic relevant distances. This coupled mechanism may provide multiple points to intervene and regulate the angiogenic process, and may also improve the current understanding of various vascular diseases.
Materials and Methods
For cell culture, HUVECs (Cambrex) and human HASMCs (Cambrex) were cultured at 37 °C, 5% CO2in endothelial growth medium (EGM)-2 and smooth muscle cell growth medium (SmGm)-2, respectively (Cambrex), containing 2% FBS. HUVECs were used between passages 3 and 6, and HASMCs were used between passages 3 and 7. Cocultures of HUVECs and HASMCs were main-tained in culture medium constituted of 1:1, EGM-2 and SmGm-2.
Detailed information on quantification of migration in response to che-motactic gradients, quantification of migration in response to cyclic strain, in vitro angiogenesis, sprouting assay, quantification of angiogenic protein secretion, real-time RT-PCR, vector construction and synthesis, plasmid trans-fection and FACS, creation of an array of isolated cell cultures, and quantifi-cation of Vascular Cell Migration in response to cyclic tensile strain are included in theSI Materials and Methodssection.
ACKNOWLEDGMENTS. This work was supported by the National Science
Foun-dation Materials Research Science and Engineering Center Program Grant DMR 02-13805 and the National Institutes of Health Grant R01 HL069957.
A
B
C
D
Fig. 4. HASMC migration is enhanced and directed by cyclically strained HUVECs. (A) An array of cell colonies (either of HASMCs alone or cocultured with HUVECS) were cultured on PDMS well surfaces that presented a surface strain gradient (where under application of strain, white region experienced no strain, and gray regions 8% strain). HASMCs were seeded in the central column (white), whereas HUVECs (under cocultured conditions) were seeded in colonies to the right or left regions (gray). (B) Migration of statically cultured HASMC, alone and in response to coculturing with static and cyclically strained HUVECs, after 48 h (n⫽ 4). (C) Mosaic of 30⫹ images (at 100⫻) illustrating directed migration of HASMCs toward cyclically strained HUVECs. (D) Effect of HUVEC cocultures, both under static and strained conditions, on directing migration of HASMC colonies based on a hemisphere partition (n⫽ 4).
APPLIED
BIOLOGICAL
1. Risau W (1990) Angiogenic growth factors. Prog Growth Factor Res 2:71–79. 2. Jain RK (2005) Normalization of tumor vasculature: An emerging concept in
antian-giogenic therapy. Science 307:58 – 62.
3. Gerhardt H, et al. (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177.
4. Yancopoulos GD, et al. (2000) Vascular-specific growth factors and blood vessel for-mation. Nature 407:242–248.
5. Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389 – 395.
6. Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519 –560.
7. Upchurch GR, Jr, Leopold JA, Welch GN, Loscalzo J (1998) Nitric oxide alters human microvascular endothelial cell response to cyclic strain. J Cardiovasc Pharmacol Ther 3:135–142.
8. Woodell JE, LaBerge M, Langan EM, III, Hilderman RH (2003) In vitro strain-induced endothelial cell dysfunction determined by DNA synthesis. Proc Inst Mech Eng 217:13– 20.
9. Naruse K, Sai X, Yokoyama N, Sokabe M (1998) Uni-axial cyclic stretch induces c-src activation and translocation in human endothelial cells via SA channel activation. FEBS
Lett 441:111–115.
10. Girard PR, Nerem RM (1995) Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins.
J Cell Physiol 163:179 –193.
11. Li S (2005) Analysis of endothelial cell migration under flow. Methods Mol Biol 294:107–121.
12. Kakisis JD, Liapis CD, Sumpio BE (2004) Effects of cyclic strain on vascular cells.
Endothelium 11:17–28.
13. Von Offenberg Sweeney N, et al. (2005) Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem Biophys Res Commun 329:573–582.
14. Matsumoto T, et al. (2007) Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng 13:207–217.
15. Wang Y, et al. (2005) Visualizing the mechanical activation of Src. Nature 434:1040 – 1045.
16. Giancotti FG, Ruoslahti E (1999) Integrin signaling. Science 285:1028 –1032. 17. Shyy JY, Chien S (1997) Role of integrins in cellular responses to mechanical stress and
adhesion. Curr Opin Cell Biol 9:707–713.
18. Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260:1124 –1127.
19. Li C, Hu Y, Mayr M, Xu Q (1999) Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J Biol Chem 274:25273–25280.
20. Tzima E, et al. (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437:426 – 431.
21. Zheng W, Christensen LP, Tomanek RJ (2004) Stretch induces upregulation of key tyrosine kinase receptors in microvascular endothelial cells. Am J Physiol Heart Circ
Physiol 287:H2739 –2745.
22. Nikolovski J, Kim BS, Mooney DJ (2003) Cyclic strain inhibits switching of smooth muscle cells to an osteoblast-like phenotype. FASEB J 17:455– 457.
23. Li C, Wernig F, Leitges M, Hu Y, Xu Q (2003) Mechanical stress-activated PKCdelta regulates smooth muscle cell migration. FASEB J 17:2106 –2108.
24. Ingber DE (2002) Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91:877– 887.
25. Wilson BD, et al. (2006) Netrins promote developmental and therapeutic angiogenesis.
Science 313:640 – 644.
26. Kamei M, et al. (2006) Endothelial tubes assemble from intracellular vacuoles in vivo.
Nature 442:453– 456.
27. Schuksz M, et al. (2008) Surfen, a small molecule antagonist of heparan sulfate. Proc
Natl Acad Sci USA 105:13075–13080.
28. Tsujii M, et al. (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93:705–716.
29. Dobrin PB (1978) Mechanical properties of arteries. Physiol Rev 58:397– 460. 30. Abovsky M, Lanir Y, Nevo E (1996) Tethering affects the mechanics of coronary
capillaries. J Biomech 29:597– 607.
31. Wang JH, Goldschmidt-Clermont P, Wille J, Yin FC (2001) Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech 34:1563–1572. 32. Koch AE, et al. (1994) Vascular endothelial growth factor. A cytokine modulating
endothelial function in rheumatoid arthritis. J Immunol 152:4149 – 4156.
33. West JB, Mathieu-Costello O (1992) Stress failure of pulmonary capillaries: Role in lung and heart disease. Lancet 340:762–767.
34. Fibich G, Lanir Y, Liron N, Abovsky M (1993) Modeling of coronary capillary flow. Adv
Exp Med Biol 346:137–150.
35. Goto M, et al. (1991) Cardiac contraction affects deep myocardial vessels predomi-nantly. Am J Physiol 261:H1417–1429.
36. Sedding DG, et al. (2003) Mechanosensitive p27Kip1 regulation and cell cycle entry in vascular smooth muscle cells. Circulation 108:616 – 622.
37. Ghosh K, et al. (2008) Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci USA 105:11305–11310.
38. Fan J, Walsh KB (1999) Mechanical stimulation regulates voltage-gated potassium currents in cardiac microvascular endothelial cells. Circ Res 84:451– 457.
39. Resnick N, et al. (1993) Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci USA 90:4591– 4595. 40. Malek AM, Gibbons GH, Dzau VJ, Izumo S (1993) Fluid shear-stress differentially modulates expression of genes encoding basic fibroblast growth-factor and platelet-derived growth factor-B chain in vascular endothelium. J Clin Invest 92:2013–2021. 41. Hsieh HJ, Li NQ, Frangos JA (1991) Shear-stress increases endothelial platelet-derived
growth-factor messenger-Rna levels. Am J Physiol 260:H642–H646.
42. Lee HJ, Koh GY (2003) Shear stress activates Tie2 receptor tyrosine kinase in human endothelial cells. Biochem Biophys Res Commun 304:399 – 404.
43. Enholm B, et al. (1997) Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 14:2475– 2483.
44. Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9:685– 693. 45. Risau W (1997) Mechanisms of angiogenesis. Nature 386:671– 674.
46. Asahara T, et al. (1998) Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res 83:233–240. 47. Holash J, Wiegand SJ, Yancopoulos GD (1999) New model of tumor angiogenesis:
Dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 18:5356 –5362.
48. Maisonpierre PC, et al. (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55– 60.
49. Stratmann A, Risau W, Plate KH (1998) Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol 153:1459 –1466.
50. Fiedler U, Augustin HG (2006) Angiopoietins: A link between angiogenesis and in-flammation. Trends Immunol 27:552–558.
51. Daly C, et al. (2006) Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci USA 103:15491–15496.
52. Fiedler U, et al. (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12:235–239.
53. Fiedler U, et al. (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103:4150 – 4156. 54. Holash J, et al. (1999) Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science 284:1994 –1998.
55. Tonnesen MG, Feng X, Clark RA (2000) Angiogenesis in wound healing. J Investig
Dermatol Symp Proc 5:40 – 46.
56. Li C, Hu Y, Sturm G, Wick G, Xu Q (2000) Ras/Rac-Dependent activation of p38 mitogen-activated protein kinases in smooth muscle cells stimulated by cyclic strain stress. Arterioscler Thromb Vasc Biol 20:E1–9.
57. Ma YH, Ling S, Ives HE (1999) Mechanical strain increases PDGF-B and PDGF beta receptor expression in vascular smooth muscle cells. Biochem Biophys Res Commun 265:606 – 610.
58. Cheng TH, et al. (2001) Reactive oxygen species mediate cyclic strain-induced endo-thelin-1 gene expression via Ras/Raf/extracellular signal-regulated kinase pathway in endothelial cells. J Mol Cell Cardiol 33:1805–1814.
59. Ikeda M, Takei T, Mills I, Kito H, Sumpio BE (1999) Extracellular signal-regulated kinases 1 and 2 activation in endothelial cells exposed to cyclic strain. Am J Physiol 276:H614 – H622.
60. Ikeda M, Kito H, Sumpio BE (1999) Phosphatidylinositol-3 kinase dependent MAP kinase activation via p21ras in endothelial cells exposed to cyclic strain. Biochem
Biophys Res Commun 257:668 – 671.
61. Iwasaki H, Yoshimoto T, Sugiyama T, Hirata Y (2003) Activation of cell adhesion kinase beta by mechanical stretch in vascular smooth muscle cells. Endocrinology 144:2304 – 2310.
62. Lehoux S, Tedgui A (2003) Cellular mechanics and gene expression in blood vessels.
J Biomech 36:631– 643.
63. Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126:3047–3055. 64. Zeller PJ, Skalak TC, Ponce AM, Price RJ (2001) In vivo chemotactic properties and spatial
expression of PDGF in developing mesenteric microvascular networks. Am J Physiol
Heart Circ Physiol 280:H2116 –2125.
65. Buehler MJ, Yung YC (2009) Deformation and failure of protein materials in physio-logically extreme conditions and disease. Nat Mater 8:175–188.