Publisher’s version / Version de l'éditeur:
Journal of Applied Chemistry, 13, 4, pp. 150-158, 1963-06-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Compacts of powdered material as porous bodies for use in sorption
studies
Sereda, P. J.; Feldman, R. F.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=1602b5b3-e74b-481c-984e-9dab9ee67dd6 https://publications-cnrc.canada.ca/fra/voir/objet/?id=1602b5b3-e74b-481c-984e-9dab9ee67dd6S e r TH1
N21r 2
no.
1 8 5 c . 2NATIONAL
RESEARCH
COUNCIL
CANADA
DIVISION O F BUILDING RESEARCH
COMPACTS O F POWDERED MATERIAL A S POROUS BODlES
FOR USE IN SORPTlON STUDIES
BY
P.
J .
SEREDA A N D R. F. FELDMAN
R E P R I N T E D FROM
JOURNAL O F APPLIED CHEMISTRY VOL. 13, N O . 4, APRIL 1963, P . 150
-
158R E S E A R C H P A P E R N O . 185 O F T H E
DIVISION O F BUILDING RESEARCH
#@==-*----
/
fCUILDf%G RE'SEJ~RCH-
L.iS?;c\Ry
-
OTTAWA
JUNE
1963 P R I C E 25 C E N T S N R C 7 3 3 0T h i s publication i s being d i s t r i b u t e d by the Division of Building R e s e a r c h of the National R e s e a r c h Council. It
should not be r e p r o d u c e d in whole o r in p a r t , without p e r m i s - sion of the o r i g i n a l publisher. The Division would be glad t o be of a s s i s t a n c e in obtaining such p e r m i s s i o n .
Publications of the Division of Building R e s e a r c h m a y be obtained by m a i l i n g the a p p r o p r i a t e r e m i t t a n c e , ( a Bank, E x p r e s s , o r P o s t Office M ~ n e y O r d e r o r a cheque m a d e p a y - able a t p a r in Ottawa, to the R e c e i v e r G e n e r a l of Canada, c r e d i t National R e s e a r c h Council) to the National R e s e a r c h Council, Ottawa. S t a m p s a r e not acceptable.
A coupon s y s t e m has been introduced to m a k e p a y - m e n t s for publications r e l a t i v e l y s i m p l e . C ~ u p o n s a r e a v a i l - able i n denominations of 5, 25 and 50 c e n t s , and m a y be ob- tained by making a r e m i t t a n c e a s indicated above. T h e s e coupons m a y be u s e d f o r the p u r c h a s e of a l l National R e s e a r c h Council publications including specifications of the Canadian Government Specifications Board.
Repritzted fro117 the Juur~lrtl oj Applied Clze~?zistt.y, 1963, Vol. 13, p p . 150-158
COMPACTS OF POWDERED MATERIAL AS POROUS BODIES FOR
USE IN SORPTION STUDIES
By P. J. SEREDA and R. F. FELDMAN
Details are given for the production of rigid porous bodies (compacts) ~nade by compacting ullder high pressure fine poxvdcrs in the particle range of 10-0.01y of silica, calcium carbonate, plaster of l'aris and molecular sicvcs. 'Shesc compacts xx7crc used as samples to study sorption and related dimen- sional changes. The resulls \\-ere used to conlpute spreading pressure by applying the Gibbs adsorption equation and alloxved derivation of the all1 vs 4 plots using the Dangham relation.
I17ith compacts nlade froin molecular sieves, it xvas possible to study the forces imposed on the particles by water entering or leaving the micropore system.
151 SEREDA 6 FELDMAN--COMPACTS I N SORPTION STUDIES
Introduction
There has been a great need for rigid porous bodies that might be used l o study the various phenomena, especially dimensional changes, associated with the sorption of water on internal surfaces of materials. Naturally occurring materials are usually non-l~omogeneous, non-isotropic and non-reproducible froin sample to sample, and contain impurities in varying amounts. Of those synthetically produced, the two classical materials that have been used for such studies are carbon rods and Vycor glass, both of ~vllicll represent an unusual range of porosity and internal surface area. To extend this range and to avoid some of the limitations of natural rigid porous materials, a technique has been developed to produce such porous bodies with a wider range of properties by compressing fine powders of different materials into compacts, as was done by Dollimore and co-worlters,l. and as is done in powder metallurgy and catalyst technology.
Powders of different materials in the particle range 10-0.01[~. were compressed in a mould a t pressures of 4000 to 46,400 p.s.i. to give rigid porous bodies. Each powdered material has optimum conditions of moisture content and pressure at which satisfactory compacts are made. Although all finely powdered material will produce a rigid body when compressed a t a suitable pressure, not all such compacts can withstand immersion in water without disintegration, nor do all have a sufficient mechanical strength when dry.
The formation of a rigid body by compression of a fine powder must involve, in the first place, the bringing together of enough of the surface a t distances where the van der Waals' attractive forces come into play. As in the case of po~vder metallurgy, much strength of the compact can be derived from primary bonds resulting from bridging between particles in contact, where the surfaces are under pressure or are deformed and will recrystallise, hydrate or react more readily than other surfaces. Solid-state reactions can be postulated for the formation of
bridge^.^
Where the powder can hydrate, only traces of water may be required to cause bridging by the formation of minute quantities of the hydrate.
Experimental
Teclznique for preparation of compacts
For the work described in this paper, compacts measuring 3.125 cin. in dia. and about 1.5 mnl. thick were made in a steel mould consisting of a cylinder and two closely fitting pistons. The cylinder was first mounted vertically, wit11 the bottom piston located in the cylinder about 1.2 cm. below the top (this spacing varied for different powders and different compacting pressures in order to malte the samples the same thicltness). The powder was placed in the mould by tamping wit11 the edge of a spatula against the top edge of the cyliilder while excess powder remained heaped over the mould. Tamping ~ v i t h equally spaced strokes in two directions, a t right angles to each other, was concluded b y striking off the excess powder level ~vith the top edge of the cylinder. The bottom piston was lowered wit11 the sample and the top piston placed in the cylinder; tlie assembly was then mounted in a 60,000-lb. testing machine. While the first increment of load was applied, the cylinder was rotated slightly and held up to allow both pistons t o float and to ensure that the compression of the sample was equal froin both sides.
Pressures of 4000--46,400 p.s.i. have been used in compressing samples; when inert materials were used, a small amount of water was added to improve compression.
All
powders in the particlesizes 10p and smaller had t o be agglomerated b y worlting them in a mortar with pestle (with or without the addition of water) until the powder formed coarse agglomerates, which could be packed more uniformly into the mould. This step proved importailt when compacting very fine powders such as silica (Cab-0-Sil), although in the case of plaster of Paris i t mas not necessary because the fine particles were already in a state of agglomeration. While some materials will form satisfactory compacts over a very wide range of pressures, others require a specific pressure to be satisfactory. If, after compression, the material tends to rebound or relas as if in a high state of strain, then the forming of a satisfactory compact above a certain pressure may be im- possible; any density variation will result in craclting along lines of maximum density gradient representing different magnitudes of recovery or relaxation between two adjacent sections of the compact.
Procedwe
Samples for determining both the sorption ancl expansioil isotherins were obtained from the
SEREDA & FBLDIVIAN-COAdPi4CTS IiIr SOPJ-"TION STUDIES 152
same compact. Each compact was inaclc in the lorill ol a disc 1.5 m m thick and was cut to givc two rectailgular prisms 7
x
2 s inm. to be inountecl on cstensomcters, ancl two segments weighing about 1 g. to be mouilted on the quartz spirals.The apparatus consistecl of sis tubcs illounting the &IcBain-Bakr type of quartz spirals, ~ v l ~ i c h were sensitive to 2.5 x 1 0 - 9 . in conjunction xilit11 the cathetometer reading to 0.001 cm., and sis cells equipped with optical windows colltaining the optical estensometei-s (modified Tuclternlan gauge) sensitive to 2 x 10-Vn./iu. The Tucl<crman optical cstensometcr system was obtained from thc American Instrument Co. The estcnsometer itself was modified to allow the mouilting of the sample finnly on it ancl the prism mas replaced by a polished metal mirror. These sis cells were part ol the high-vacuum systcn~ consisting of a thi-ee-stage oil diffusion pump baclted by a rotary vacuuin pump to obtain pressures less than 10-"m. I-Ig.
Water vapour or other adsorbates were introduced into the system fro111 a bulb in a bath controlled to any desired temperature in the range -17 to 21"
(A
0.05"). The temperature of the samples was also controlled at 21" by means of a bath surrounding the tubes and cells.Belore deterilliiliilg the soi-ptioil and expansion isotherms, all sainples were outgassed a t 150-200" to a vacuuin of less than 10-"nin. Hg until negligible weight loss had been recorded lor 15 11. For equilibrium to be attained between points along the isotherm, cliffereilt periods were required for different samples. 111 all cascs a period of about 15 11. of negligible weight change and negligible clinleilsional change was allowed belore the system was considered to be in equilibrium.
Chnmcteristics of con~jacts
Three types of systcms were represented in the coillpacts produccd lor this study:
Typc I : largc particles having no internal rnicropores and lox\. surfacc area. This system was represented by compacts of calciunl carbonate of particle size about 1-51~.
Typc 11: very small particlcs foriniilg \.cry small pores and giving a high surface area, represented b y silica (Cab-O-Sil-supplied b y Godfrcy L . Cabot, Inc.) having particlcs 0.01-0.02p.
Typc 111: large particlcs having a n internal micropore system. I n addition to giving a system such as Typc I, i t also gives a systenl such as Type 11, of special character. ~Iolccular sieves (Lindc 4A- supplied by Linclc Co.) having particlcs 2-5p and lnicroporcs consisting of 11.4.P cavities separated b y 4.211 opcnillgs reprcscntcd this system.
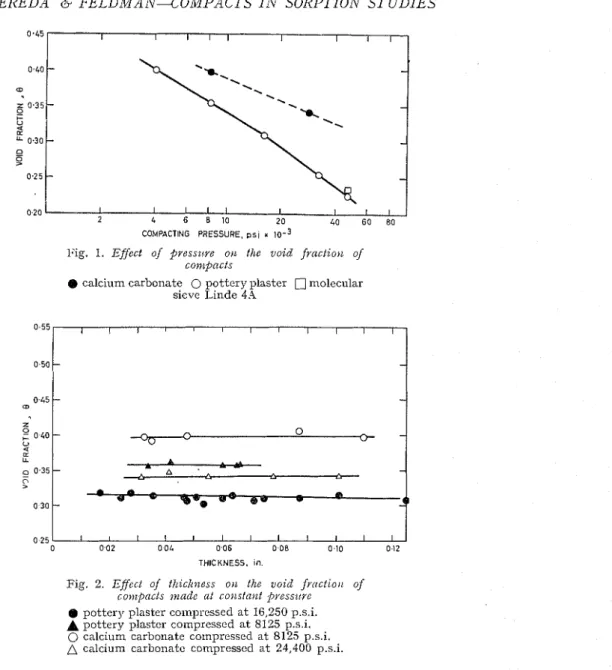
Onc of the most importailt properties of a compact to bc usecl for studies such as those described here is the void fractioil or porosity. I t can be changecl as much as two-fold by varying the pressure ol fornliilg lor such inaterials as plastcr
oI
Paris (see Fig. 1). The void fraction wasdeternliilecl from ineasureineilts of the clinlcnsioils of the compact, the dry \\;eight and the absolute clensity of the materials. I11 the case of lllisturcs ol inaterials in one compact, it was clifhcult to determine the void 11-action because the absolute cleilsity could not be ltnolvn accurately. These data can also be plottecl on tlie basis of axial stress as suggested by Amberg
h
Echigoya." This plot, however, docs not give a coillnloll line for the different materials. Values for compacts of calciuill carbonate a i d plaster of Paris clo not la11 on ally of the plots given by Amberg LG Echigoya.I t was considered of importance to determine the uniformity of the apparent density or porosity throughout the conlpact. To examine this property, compacts of different thicltnesses were macle at a series of constant pressure. Even though the thicl\rnesses were varied tenfolcl there was no significant cliffcrence in the voicl fraction for plaster of Paris at several pressures, and similar results were inclicatecl for other materials (see Fig. 2). From the work of Durvez
h
Zwell5 on metal powder compacts, it is evident that the pressure ratio shoulcl be very nearly unity for all sections of the compact, since all compacts, even thc thickest usecl in this study, have a very low thicltness-to-diameter ratio and are being coillpncted from both faces.In the use of compacts for the study of the cli~nensional changes of materials during sorption of water, the important question to be ailsivered is whether any strain remains in the compact as the result of compression of particles that 111aj7 be relased during wetting, thus exhibiting a diinensioilal change in no way related to sorption of water on the surfaces. Compacts of some materials, such as plaster of Paris and calciuill carbonate, will disintegrate \\?hen immersed in water, although they will not do so in lterosene or carbon tetrachloride. In fact, the dimensional change 01 these coinpacts during saturation from the dry state with these solvents is negligible. This would indicate that there is no residual strain in the compact.
153 SEREDA 6 FELDMAN-COLWPACTS I N SORPTION STUDIES
0.45 I I I I I I I I I
0.20 1 I I I I 1 I I I I 2 4 6 8 1 0 20 40 60 80
COMPACTING PRESSURE. nsi r 10-3
l'ig. 1. Efiect of pressz!re O I L the void f r a c t i o ) ~ of
conzpncts
calcium carbonate 0 pottery plaster C] molecular sicve Linde 4.q
025
o
0.02 0 04 0.06 0.08 0.10 0.12o
THICKNESS. in.
Fig. 2. Effect of thickness O I L tlze void fvnctioi~ of
compacts m a d e at cousfnrzt p ~ e s s f r r e
pottery plaster coinpressed a t 16,250 p.s.i.
A
pottery plaster compressed a t 8125 p.s i.0 calcium carbonate co~npressed a t 8125 p.s.i.
calcium carbonate con~pressed a t 21,400 p.s.i.
That residual strain is absent is further supported by the results obtained by an application of the Gibbs adsorption equation. This equation relates the surface-free energy, lowering D F as sorption occurs on a surface, to the pressure
9
dynes/cm.Qf the adsorbate, and the surface concentration s of the adsorbate on the adsorbent in g.-inol./cm3.A F = RT
S:
s/fi dfiWhen D F e r g s / ~ m . ~ , commonly caUed
4,
the spreading pressure, is calculated, a plot of All1 vs4
should, according to the Bangham r e l a t i ~ n , ~ Dl/l =A A F ,
yield a straight line through the origin. Dl/l is the expansion due to sorption, and A is a constant related to the elastic coefficient of the material.This plot did, in fact, produce acceptable straight lines through the origin for the Cab-0-51 and CaCO, samples in the region where adsorption without capillary condensation occurred. Similar plots for other materials, such as plaster of Paris, have been obtained and will be published separately.
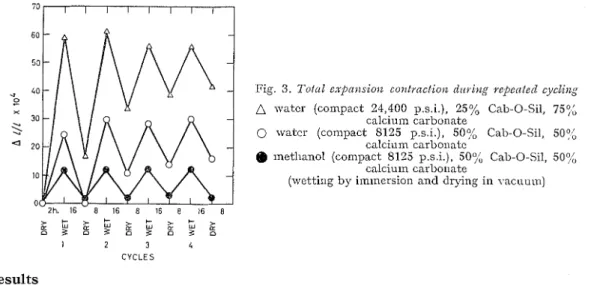
Most compacts of different materials do show a relaxation in the high-humidity region or on saturation, represented by an irreversible portion of the expansion. This same phenomenon occurs \vith methanol, but to a lesser degree. To examine the extent of this ill-eversibility,
S E R E D A & FELDJIAN-COIVPACTS I N SORPTION S T U D I E S 154
ii1ere exposed to repeated cycles of wetting, either in saturated water vapour condition
01- ~ J Timmersion, followed by drying over inagnesium perchlorate or by vacuum t o liquid air
trap. Fig. 3 shows this tendency, whicll is especially pronounced where a large proportion of material like calcium carbonate was used; it is attributed to a rearrangement of some of the
in the conlpact. Irreversible sivelling of the compact on repeated cycling from the saturated to the clry condition is the manifestation of the loss of strength or deterioration of many materials when subjected to such cyclings. This relasation or irreversible sii~elling pheno- menon is of no concern in the region of relative humidity below the point where this is pronounced; for many materials this may be just short of saturation.
Fig. 3. Toiccl e . ~ p a ~ z s i o n co?zt~nciio~z d l r i . i ~ ~ g repented e y e l i i z ~
watcr (compact 24,400 p.s.i.), 25% Cab-0-Sil, 75q{,
calciuin carbonate
0 ~vatcr (compact 8125 p.s.i.), 50% Call-0-Sil, 5001
calcium carboilatc
Q methanol (compact 8125 p.s.i.), 50% Cab-0-Sil, SOY;,
calciuin carbonate
(wetting by iininersion and drying in vacuum)
> + > + > + > +
a g a g a g a g g
I 2 3 4 C Y C L E S Results (1) Plaster conzfiactsCompacts have been prepared from several types of plaster of Paris and gypsum over a wide range of pressures (Fig. 1) to study the sorption-dimensional change relationship. Results of the study are reported separately. These compacts have also been used to study expansion during hydration at different tempciatures; this is an estension of the work previously reported7 ancl will be publislled elsewhere.
(2) Calciz~vz carbonate conzjacts
Compacts were fornled lrom precipitated reagent-grade calcium caiboilate at pressures of 8,000-46,400 p.s.i. The particles ol powder were estimated to be about 2 1 ~ in diameter.
Figs. 4(a) ancl 4(b) show the sorptioil and espansion isotherins respectively for calcium carbonate compacts formed a t 46,400 p.s.i. Both are Type I1 in Brunauer's classification, ancl the expansion isotherm sho~vs a continual expansion lor the estent of the isotherm. On desorp- tion for the sorption isotl~ei-m, hysteresis is observed in the region of a j/;ho value of 0.05-0-60. A value of 5.6 m.?/g. was obtained for the surface area by the B.E.T. method, and, because of this low value, high accuracy cannot be espccted.
For the espailsion isotherm, very little contraction was observed on desorption; apparently the sample had been exposed to humidities where rearrangement of the particles occurred. A plot of A W vs Aljl (Fig. 4c) illustrates this. The first portion of the plot, up to a fi/jo value o l 0.60 where sorption coverage is still low, can be consiclered linear through zeio, as was observed b y Amberg & M a c I n t ~ s l ~ ~ for Vycor glass. A slight increase instead of decrease in slope was observed on approaching a humidity of 70%, before capillary condensation at SO-S5% caused a decrease in slope. Fig. 5 illustrates this to advantage. The plot of Al/l vs
4
produces a straight line up to a ;h/fi, value of 0.60. Beyond this point an increase in slope may be observed, followed by a decrease after a ;h/fio value of 0.80. The esplanation for this, as nlentioned before, is the wealtening of the bonds between particles and their subsequent rearrangement. Whether or not the mecl~anism of healing postulated by Dollimore & Greggg occurs a t higher relative pressure was not studied. The clecrease in slope at ;h/;ho of 0.80 is due to the contractive force producecl b y capillary condensation. 011desorption, this contractive force disappears and an even greaterexpansion of the sanlple takes place. A significant contraction occurs only at very low relative pressures.
155 SEREDA 6 I;ELD:~IBIV-CO~VIP~~CTS IiV SORPTION STUDIES
p/p0 A WIW x l o 2 I:ig. 4. I?csztlts for c(1lcil17lz cnrboi2ntc coiilpcrcl
(a) sorption isothcr~ns (b) cspansion isotherms (c) expansion as related t o xxratcr sorbcd
@ adsorption 0 dcsorpiioil
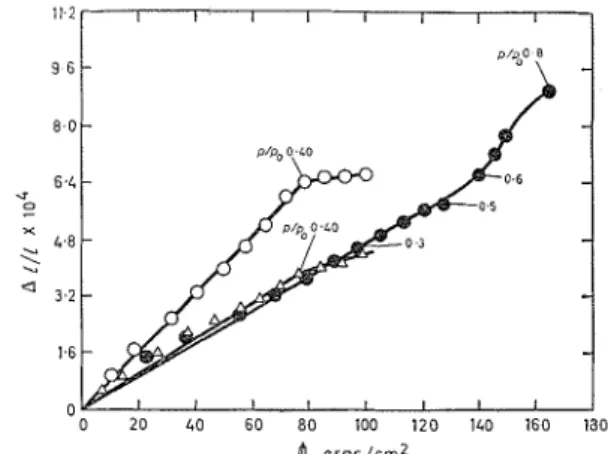
Fig. 5. E x p ( z ? ~ s i o i ~ of C O ~ I I P ~ C ~ S as n firq~clio?~ of the cnlctrlated spreadigzg force
@ calciunl carbonate
0 50% Cab-0-Sil
+
50% calciuill carbonatc 25% Cab-0-Sil i- 50% calcium carbonntc(fisures on curves arc p / p , valucs)
By calculating the Young's moclulus E for the material by a relation of Bangllanl from the
azjl vs
+
E = p d / h
lvllere p is the absolute clensity of thc material (g.1c.c.) ancl A the area ( ~ r n . ~ / g . ) , a value for E
of 6.97
x
1010 clynes/cm~vas obtained using a value of 2.93 g.1c.c. for p. This value is within the same orcler of magnitude for materials of this nature.Since compacts of calciunl carbonate disintegrate on immersion in water it is not surprising that they show relaxation when a partial pressure ol 0.6 is escceded, this being in the region of multi-molecular sorption.
(3) Cab-0-Sil with adtflixtzrre of calcilr,i~ carbo?zate
Compacts of the pure Cab-0-Sil were too brittle to nrorli with, and it n7as therelore diluted with various proportions of calciuln carbonate. The 25% Cab-0-Sil conlpacts \rere made a t a pressure of 46,400 11.s.i. and the 50% compacts a t 21,400 p.s.i. Sorption and expansion isotherms are given for both of these conlpacts (Figs. 6 and 7).
I'ig. 6. R e s ~ l l l s foi, co~gzpaci of 50% C a b - 0 - S i l
+
507; crrlciuii~ cnrbo~~clte
(a) sorption isothcrnis (11) cxpansion isotherms (c) cspailsion as rclated t o xvatcr sorbcd
@ adsorption 0 dcsorptioi~
SEREDA 6 FELDMAAT-COlWACTS I N SORPTION STUDIES
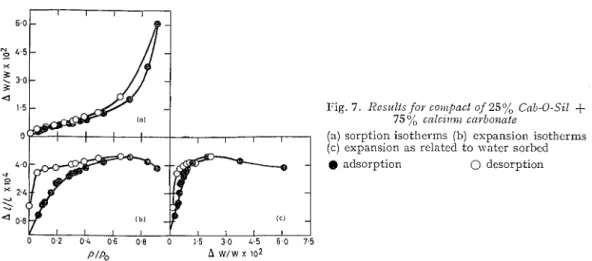
Fig. 7 . Results foior co)rlpnct of 357/, Cnb-0-Sil
+
75% cnlcz~c?)~ cnrbo+znle
( a ) s o r p t i o n i s o t h e r m s ( b ) e x p a n s i o n i s o t h e r m s ( c ) e x p a n s i o n a s related t o w a t e r sorbed
9 a d s o r p t i o n 0 dcsorption
The adsorption isotherm for the 50% Cab-0-Sil/50% CaCO, coinpact (Fig. 6a) is a normal smooth Type-I1 isotherm. A B.E.T. calculation yields 36.1 m.3/g. for the surface area. The adsorption and desoi-ption isotherms in the region of low $/$, vaues do not coincide and this separation is defined as secondary hysteresis. This is probably the result of the history of the preparation of the Cab-0-Sil silica and its resulting very small particle size.
The expansioil isother~n (Fig. 6b) shows a flattening of the curve at a $/$, value of about 0.40 ancl a contraction continuing from 0.50 to the end of the isotherin. This effect is also exhibited in the desorption part of the cycle where first an expansioil and then a contraction wei-e observed. The effect of the secondary hysteresis manifests itself also in this plot.
The Al/l vs A W plot (Fig. 6c) shows, as it did in a similar plot for CaCO,, a linear portion for low coverages, in this case up to a
$19,
value of approximately 0.35. A reduction of slope occurs a t higher humidities until a contraction occurs after 0.50$/$,. This contraction can be esplained by the formatioil of menisci in this region; the contractive force due to menisci forination in the case of this material is greater than the expansive force due to the lowering of surface free energy. The fact that the clesorption curve cloes not combine with the aclsorption curve, but ruils parallel to it, lnay be attributed to rearrangement a t the higher humiclities. The Al/l vs+
curve (Fig. 5) is a straight line through zero up to$I$,
0.40, after which the slope is almost zero. This, together ~ ~ i t h the other plots, indicates that capillary concleilsation occurrecl lor this coinpactecl system in the regioil of $/$, values of 0.40-0.45. This represents an equivalent pore radius of 15.7-ISA\. The value of E was calculatecl as 1.21x
1011 d y n e s / ~ m . ~ using a density of 2.7 g.1c.c.In the sample \vith 25% of Cab-0-51 and 75% of CaCO,, a similar result was obtained, although the effect of clilution of the Cab-0-Sil was noticed (Fig. 7). The area of the sainple nras 22.7 m.2/g. The contraction observed in the espansion isotherm as present, although it occurred a t a later
$I$,
value than for the more coilcentratccl Cab-0-Sil samples. Evidellce that more rearrangement had taken place than for the previous samples m7as observed in the clesorptioil parts of the All1 vs APT/ plot, although the adsorption part of this curve was again linear for low coverages. The Al/l vs+
plot in this case did not give as acceptable a straight line as for the previous samples, the points for lom7er pressure deviating somewhat. The increasing dilution, the effect of 1% hich had been considered negligible, and the errors in the graphical integration may have caused this. The value for E , however, was calculated as 1.35 x 1011 c l y n e s / ~ m . ~ (which is similar to the value obtained for those of higher concentrations). In thisAll1 vs
4
plot, the decrease in slope is again observed at$19,
values between 0.40 and 0.45, indicating, together with the other plots, capillary condensation at the same pressure as for the other samples.(4) Molecular sieves witlz adrnizt~~res of calcii~??~ cavbonnte
Compacts of the zeolite molecular sieve (Lincle 4A) formed a t various pressures remained whole, even on iminei-ison in water. In order to reduce the very large expansion on wetting, a calcium carbonate cliluent was used. For this study all compacts containing this molecular sieve were made a t a pressure of 46,400 p.s.i.
157 SEREDA
C
FELDiMA
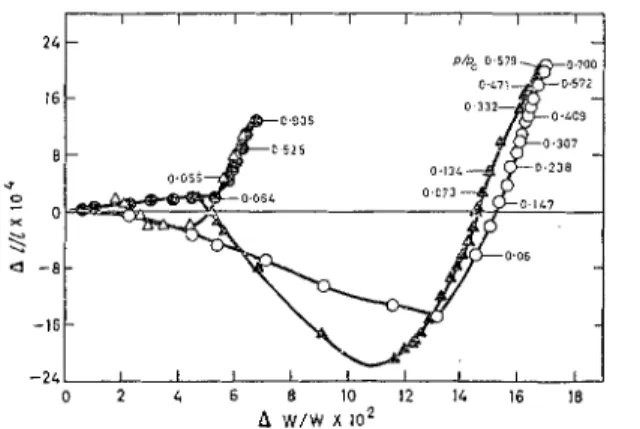
N-COIMPACTS I2V SORPTI Oh7 STUDIESThis unique material exhibited a specific sorption dimeilsional change relation coilsisting of a contraction on adsorption at relative pressures below 0.1 and even greater contraction during desorption as shown on AZ/l vs AW plots (Fig. 8).
Fig. 8. D l ~ ~ e l z s i o n n ~ chn?z~.e a s related to wnlev sovberlfov cor~rpacts of wzolec~rlav sleves n l ~ d c a l c i u ~ ~ l c a v b o ~ ~ a t e
25% molecular sieve
+
75% calciurn carbonate@ adsorption
a
desorption75% lnolecular sieve
+
25% calcium carbonate0 adsorption
A
desorptionAlong the adsorption loop it is postulated that there is 'molecular bridging' in the 4.2K openings; this type of bridging has originally been postulated by Lakhanpal LG Floodlo for sorption on carbon. On the desorptioil cycle, however, the large hysteresis which cannot be explained by bridging, seems to suggest a tension in the water in the 11.2A cavities. This tension is produced by the process of water molecules leaving the neclis of the pores and being replaced by water molecules from the cavity. Since this contraction is occurring in the region of $/Po below 0.05, which represents an equivalent tension far in excess of what the column of water in the cavity can withstand, then the water is in a non-equilibrium state. The low rate by which it proceeds to equilibrium is governed by the rate at which water leaves the narrow neclis of the molecular sieve and determines the extent of contraction. The contraction observed here is of the same order of inagnitude as that observed by Wiig
cG
Ju110la~~ for water on activated charcoal.As in previous cases where calcium carbonate was used, some relasation at high relative pressures must have occurred; as the concentratioil of the molecular sieves was increased, there was a greater tendency for the desorption curve to pass through zero. In addition, the effect of dilution is manifested in the plot for the molecular sieve coilceiltration of 25% where the contraction on adsorption observed for 75% was eliminated and the contraction on desorption was decreased.
Discussion and conclusions
I t has been shown that compacts co~npi-essed from fine powders will behave as rigid porous bodies within certain ranges of partial pressures, and this enables the study of the sorption and the related dimensional c h a n ~ e s of these materials. The lact that d o t s of All1 vs AW and AlIZ
- ,
vs4
are linear over the usefvul range for compacts of these matei'ials, as is 'the case for Vycor glass, confirms the validity of the above statement. The fact that samples of given mateiials can be made in a range of void ratios and in a range of surface areas malies these studies particularly significant. I n a compact composed of very s~nall particles, as in Cab-0-Sil, it was possible t o observe formation of menisci and the resulting contraction during the adsorption part of the loop at relatively low $/Po values.With compacts 111ade from molecular sieves, it was possible to study the forces imposed on the particles by water entering or leaving the micropore system.
Relaxation of the c o m ~ a c t s of some materials was observed a t high humidities above the u
region where capillary condensation is expected to begin, thus limiting the uselul range. Where hydrates are not involved, it should be possible to malie compacts useful over the entire range of
SEREDA & FELDMAAT-COMPACTS I N SORPTION STUDIES 158
relative pressures of water by follo~ving the procedures for sintering, as in the case of powder metallurgy and ceramics. Heating to temperatures of 0.5Tf (where Tf = melting temperature) should provide enough bridging between particles in close contact to prevent the relasation that was observed in this study when certain partial pressure was exceeded.
Acknowledgments
The authors grateiully ackno\vledge the valuable assistance of Messrs. H. F. Slade and S. E. Dods in setting up the apparatus and recording the information. This paper is published with the approval of the Director of the Division of Building Research, National Research Council, Canada.
National Research Council,
Division of Building Research, Ottawa 2,
Canada
Received 7 August, 1962; amcndccl n~anuscript 19 October, 1962
References
Dollimore, D., & Gregg, S. J., Trans. Brit. C c r a ~ r ~ . Sereda, P. J., iiature, Loud., 1960, 187, 929
Soc., 1955, 54, 262
2 Dollimorc, D., & Heal, G. P., J. apfil. Chem., 1961, a Amberg, C. H., & MacIntosll, R., Canad J . Clhenl.,
11. 459 1952, 30, 1012
liingery, W. D., 'Introduction t o Ceramics', 1960
(New Yorli: Wiley) Dollimore, D., & Gregg, S. J., Rescnrcl~, Londor~, ,I Amberg, C. H., & Echigoya, E., Canad. J. chenz. 1958, 11, 180
Engng, 1961, 39, 215 l o Lalchanpal, 34. L., & Flood, E. A., Cnnnd. J. Cllettl.,
"urvez, P., & Zwell, L., J. iWetals, 1949, 185, 137 1957,35, 887 "angham, D. H., & Maggs, F. A. P., Proc. Conf.
o n U l t r a - j i ~ e Str~~ctzsre of Coals and Cokes, 1943, l1 W i g , E. O., & Juhola, A. J., J. A m e r . cl~errr. Soc.,
p. 118 (British Coal Utilisation Res. Ass.) 1949, 71, 561