last accessed). This isolate (submitted in 2010) originated from Poland and is described as methicillin susceptible. Multilocus sequence typing showed that the isolate belonged to the novel sequence type ST2497, a single-locus variant of ST1943 with one nucleotide difference in the glpF gene.
The isolate described in this study represents the first detec-tion of an mecC-containing MRSA from an animal host in Norway. The mecC gene has been detected recently in a total of eight MRSA isolates from humans in Norway7 (and K. W. Larssen, unpublished data). All eight mecC MRSAs, isolated during 2006–12, belonged to CC130. The genotype of the feline isolate represents a new mecC-positive genotype identified in our country; however, isolates within this clonal lineage with mecC have been described from other countries.1,4
The detection of mecC in an MRSA from a clinical sample from a cat submitted to our diagnostic bacteriological service unit demon-strates the importance of taking mecC into consideration in diag-nostic units that examine samples from companion animals. Our finding extends our knowledge of MRSA carrying mecC from animals and demonstrates that detection of mecC is not only a rare event when screening historical isolate collections.
Acknowledgements
We would like to thank Bjørn Bengtsson (National Veterinary Institute, Uppsala, Sweden) for donation of MRSA SVA-AB-773.
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
References
1 Garcı´a-Alvarez L, Holden MTG, Lindsay H et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis 2011; 11: 595–603.
2 Shore A, Deasy EC, Slickers P et al. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55: 3765–73.
3 Pichon B, Hill R, Laurent F et al. Development of a real-time quadruplex PCR assay for simultaneous detection of nuc, Panton-Valentine leucocidin (PVL),
mecA and homologue mecALGA251. J Antimicrob Chemother 2012; 67:
2338–41.
4 Stegger M, Andersen PS, Kearns A et al. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring
either mecA or the new mecA homologue mecALGA251. Clin Microbiol
Infect 2012; 18: 395–400.
5 Cuny C, Layer F, Strommenger B et al. Rare occurrence of methicillin-resistant Staphylococcus aureus CC130 with a novel mecA homologue in humans in Germany. PLoS One 2011; 6: e24360. 6 Laurent F, Chardon H, Haenni M et al. MRSA harbouring mecA variant gene mecC, France. Emerg Infect Dis 2012; 18: 1465– 7.
7 NORM/NORM-VET 2011. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. http://www.vetinst.no/Publikasjoner/ Norm-Vetrapporten/Norm-Norm-Vet-rapporten-2011 (7 November 2012, date last accessed).
8 SVARM 2012. Swedish Veterinary Antimicrobial Resistance Monitoring. http://www.sva.se/upload/Redesign2011/Pdf/Om_SVA/publikationer/ Trycksaker/Svarm2011.pdf (7 November 2012, date last accessed). 9 Paterson GK, Larsen AR, Robb A et al. The newly described mecA
homologue, mecALGA251, is present in methicillin-resistant Staphylococcus
aureus isolates from a diverse range of host species. J Antimicrob Chemother 2012; 67: 2809–13.
10 Walther B, Wieler LH, Vincze S et al. MRSA variant in companion animals. Emerg Infect Dis 2012; doi:10.3201/eid1812.120238.
11 Poulsen AB, Skov R, Pallesen LV. Detection of methicillin resistance in coagulase-negative staphylococci and in staphylococci directly from simulated blood cultures using the EVIGENE MRSA detection kit. J Antimicrob Chemother 2003; 51: 419–21.
J Antimicrob Chemother 2013
doi:10.1093/jac/dks474
Advance Access publication 25 November 2012
HIV-1 integrase variability and
relationship with drug resistance in
antiretroviral-naive and -experienced
patients with different HIV-1 subtypes
S. Reigadas
1–3*, A. G. Marcelin
4–6, A. Houssaı¨ni
5,6,
S. Yerly
7, D. Descamps
8, J. C. Plantier
9, A. Ruffault
10,
C. Amiel
6, M. A. Trabaud
11, Philippe Flandre
4–6,
H. Fleury
1–3and B. Masquelier
1–3on behalf of the
ANRS AC11 Resistance Study Group†
1
Univ. Bordeaux, Microbiologie fondamentale et Pathoge´nicite´, UMR 5234, F-33000 Bordeaux, France;2CNRS, Microbiologie
fondamentale et Pathoge´nicite´, UMR 5234, F-33000 Bordeaux, France;3Laboratoire de Virologie, CHU de Bordeaux, F-33000
Bordeaux, France;4AP-HP, Hoˆpital Pitie´-Salpe´trie`re, Service de Virologie, Paris, France;5INSERM, UMR-S 943, F-75013 Paris,
France;6UPMC Univ. Paris 06, ER1 DETIV, F-75013, Service de Virologie, Hoˆpital Tenon, AP-HP, F-75020 France;7Laboratory of
Virology, Geneva University Hospital, Geneva, Switzerland;8EA 4409 Universite´ Paris-Diderot, Paris 7 and AP-HP, HUPNVS, Hoˆpital Bichat-Claude Bernard, Service de Virologie, Paris, France;
9Laboratoire de Virologie, CHU Charles Nicolle, Rouen, France; 10
Laboratoire de Virologie, CHU de Rennes, Rennes, France;
11Laboratoire de Virologie, Hospices Civils de Lyon, F-69004 Lyon,
France
*Corresponding author. UMR 5234, Laboratoire de Microbiologie Fonda-mentale et Pathoge´nicite´ (MFP), CNRS, Laboratoire de Virologie, CHU Bordeaux, Hoˆpital Pellegrin, 1 place Ame´lie Raba Le´on, 33076 Bordeaux
cedex, France. Tel:+33-5-56-79-55-10; Fax: +33-5-56-79-56-73;
E-mail: sandrine.reigadas@chu-bordeaux.fr
†Members are listed in the Acknowledgements section.
Keywords:human, viruses, sequences, nucleotides
Research letters
969
Sir,
The prevalence of natural polymorphisms and mutations asso-ciated with integrase (IN) inhibitor (INI) resistance in the HIV-1 IN has already been analysed.1–5The aim of the study was to characterize the HIV-1 IN variability in antiretroviral (ARV)-naive and -experienced patients, never treated with INIs, in a panel of different HIV-1 subtypes, and the relationship with drug resist-ance. This multicentre study included 590 HIV-1-infected indivi-duals never treated with INIs (308 drug-naive and 282 ARV-experienced patients) who were enrolled in seven clinical centres in France and one centre in Switzerland. Nucleotide and amino acid sequences were compared with the HxB2 HIV-1 clade B consensus sequence (GenBank accession number K03455.1) using the Bioedit software program. The sequences of the samples have been submitted to GenBank and assigned accession numbers JX425421 to JX425885 and JX451875 to JX451963. Thirty-seven of the 590 sequences are not available in the NCBI database because we have only the description of the polymorphism for these sequences and not the nucleotide sequence. The median CD4+ T cell count at the time the samples were drawn was 280 CD4+ T cells/mm3
(range 2–1692) and the median log10viral load was 4.43 log10
copies/mL (1.92 –6.94). Drug-treated patients were exposed to an average of 1.96+2.65 nucleotide reverse transcriptase inhibi-tors (n¼ 526), 0.18+0.41 non-nucleoside reverse transcriptase inhibitors (n¼ 473) and 0.86+1.59 protease inhibitors (n¼ 481). The entire IN protein sequences (288 amino acids) derived from 308 drug-naive and 282 experienced patients infected with HIV-1 B or non-B subtype, all INI-naive, were analysed (Figure1). All important residues involved in catalytic activity or in binding to the human cellular cofactor LEDGF/p75 were conserved in both drug-naive and ARV-treated patients (variability ,0.5%). The presence of polymorphic substitutions at codon 124 was significantly associated with previous ARV exposure (P ¼ 0.03). Limited data are available on the preva-lence of specific polymorphisms in the IN gene of HIV-1 non-B subtypes. We investigated the diversity of the IN region of different HIV-1 subtypes in INI-naive patients. Among the 590 samples, 252 corresponded to non-B sub-types. No difference in reverse transcriptase and IN sequences was observed between subtypes. Twenty-six changes were found with significantly different prevalence between B iso-lates and at least one non-B group (prevalence of polymorph-ism .50%; P, 0.05). Polymorphpolymorph-isms at codons D10, K14, S17, A21, S24, D25, V31, V32, S39, V72, L101, T112, T125, G134, I135, K136, D167, V201, T206, I208, K215, T218, L234, D256, D278 and S283 could be related to specific non-B sub-types. Among the 26 analysed codons, we analysed the T125A mutations. T125A (specific GCA codon) was significantly more prevalent in non-B samples (P, 0.0001). A similar result was found in a recent study on the analysis of polymorphisms in the IN gene of ART-naive patients infected with HIV-1 non-B subtypes.6
Twenty-two out of 36 HIV-1 IN resistance mutations (H51Y, L68I/V, V72I, L74M, Q95K, T97A, S119G/R, A128T, T125K, V151I, M154I, K156N, E157Q, K160N, G163K/R, V165I, V201I, I203M, T206S, S230N, D232N and V249I) already associated with INI resistance were detected. Polymorphic changes included some known residues associated with INI resistance, such as V72I, L74M, T97A, V151I, E157Q and I203M, but were
not statistically different between ARV-naive and -experienced patients not including INIs. In contrast, the frequency of L101I and T124A mutations, but not the M154I mutation, selected in vitro by dolutegravir was higher in naive patients. Regarding viral subtypes, mutations L101I and T124A, either alone or in combination, were significantly more prevalent in non-B than B subtypes in ARV-naive patients (65.5% versus 34.6% for L101I, 74.4% versus 25.6% for T124A and 85.7% versus 14.3% for L101I+T124A; P,0.0001 in all cases), as recently described by Garrido et al.7 Except at position 157 (E157Q), none of the primary mutations detected in patients failing on raltegravir-containing regimens (Y143R/C, Q148H/K/R and N155H) or on elvitegravir-containing regimens (T66I, E92Q, E138K, S147G, Q148H/K/R and N155H) was detected. The E157Q mutation was observed among 2.9% (n¼ 17) of patient samples, including four of subtype B, one of subtype H, seven of subtype CRF02_AG, one of subtype A, two of subtype D, one of subtype CRF11_cpx and one of subtype G, without a significant difference in poly-morphism between ARV-naive and -experienced patients. The primary mutations detected in patients failing on dolutegravir-containing regimens (V151L, S153Y, T66K/L74M, E92Q/N155H, E138A/K+Q148H/K/R, G140C/S+Q148H/K/R and Q148R/N155H) were completely absent.
In our study, dolutegravir resistance-associated mutations, in particular R263K, were not found to be polymorphic. Only the mutations L101I and T124A, which were previously shown to be selected in vitro in the presence of dolutegravir8,9 either alone or in combination, were common in both naive and experi-enced patients. However, these mutations have shown little impact on virological response to dolutegravir. Recently, the HIV-1 CRF01_AE IN coding region of the pol gene was evaluated for the presence of natural polymorphisms in 87 ARV-naive indi-viduals from Cambodia, Thailand and Vietnam.10 Amino acid substitutions occurred in 60% of the subjects and none of these substitutions have been reported to be associated with re-sistance to INIs. Many polymorphisms in non-B viruses are con-sidered to be secondary resistance mutations since they emerge in B subtype viruses after drug exposure.11Nevertheless, the se-lection of resistance mutations could be influenced by the natur-ally occurring variations between the different non-B subtypes.
In conclusion, all patients in our study lacked previously described major resistance mutations to raltegravir, elvitegravir and dolutegravir. However, we found evidence of important var-iations regarding the IN polymorphisms according to the differ-ent HIV-1 subtypes. Further studies of INI-treated patidiffer-ents will be needed to fully elucidate the role of polymorphic IN muta-tions in the context of HIV-1 variability.
Acknowledgements
We thank all patients included in the study and the Agence Nationale de Recherche sur le SIDA et les He´patites virales (ANRS, France). We also acknowledge Professor Ray Cooke for editing the manuscript and all the members of the ANRS AC11 Resistance Study Group.
Members of the ANRS AC11 Resistance Study Group
C. Roussel (Amiens), C. Alloui (Avicennes), H. Leguillou-Guillemette (Angers), D. Bettinger (Besanc¸on), C. Pallier (Bice`tre), D. Descamps, F. Brun-Vezinet and G. Peytavin (Bichat, Paris), B. Masquelier, P. Pinson
Research letters
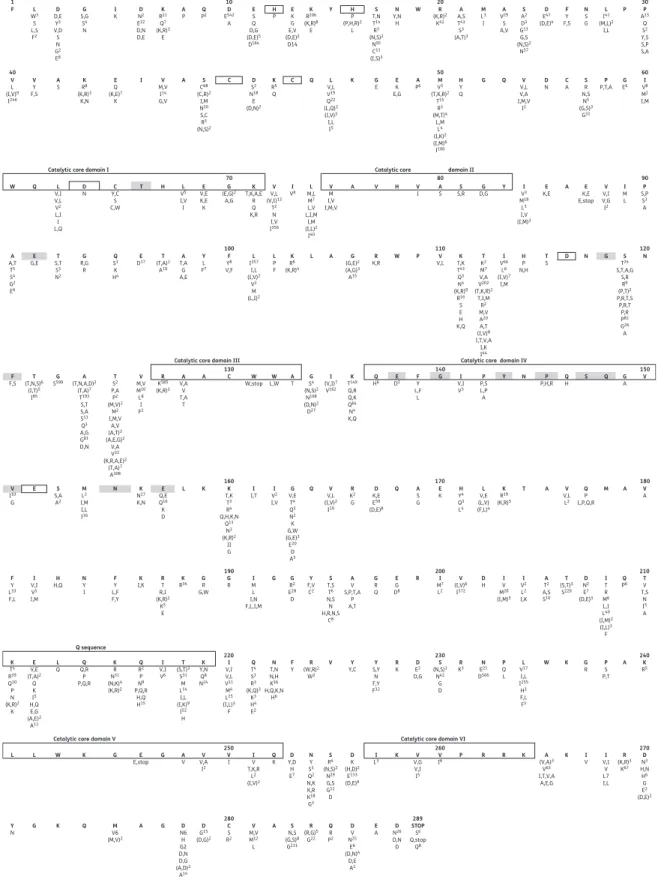
1 10 20 30 40 50 60 70 100 110 120 130 140 150 180 170 160 190 200 210 240 230 220 250 260 270 289 280 80 90 F L W3 S L,S F2 V L (I,V)9 I246 V Y F,S A S W Q L V,I V,L V2 L,I I L,Q A A,T T5 S4 G2 E6 E G,E F F,S V I10 G F Y L33 F,L K T4 R39 Q10 P N (K,R)2 K L L W K G E G E,stop Y N G K Q V6M (M,V)2 D N6 H G2 D,N D,G (A,D)2 A14 D G15 (D,G)2 C S R2 V M,V M12 L S N,S (G,S)8 G231 R (R,G)5 G22 Q R P2 D V N25 E6 (D,N)4 D,E A2 E A ND26 D,N D STOP S6 Q,stop Q8 A A G A V V,AV I2 V I IV T,K,R L2 (I,V)2 Q K Y,DD H E7 N Y S3 Q2 N,K K,R K18 G3 S R4 (N,S)2 N29 G,S G12 D D K (H,D)2 E133 (D,E)6 I L3 V,GV V,I I5 V I6 (V,A)A3 V63 I,T,V,A A,E,G I V (K,R)R3 K67 D N3 H,N H6 G E2 (D,E)2 I V,I V L7 I,L K P R R K K E V,E (T,A)2 Q K I5 H,Q E,G (A,E)2 A12 L Q Q,RQ P P,Q,R K R N31 (N,K)4 (K,R)2 Q R2 P N9 P,Q,R H,Q H15 I V,I V6 T (S,T)3 S31 M L14 I,L (I,K)9 I52 H K Y,N Q8 N24 I V,I V,L V11 M4 L25 (I,L)3 F Q T4 S3 R3 (K,Q)3 K3 H4 E2 N T,N N,H K36 H,Q,K,N H6 R (W,R)2 W2 V F Y Y,CY S,YY N F,Y F32 R K KR3 PQ L G R PS P,T K R5 A L V17 I,L I255 H3 F,L F3 W K N E21 D566 S (N,S)3 N42 G D D E2 D,G I V,I V5 I,M H H,Q NY I F Y L,F F,Y K I,K RT R,I (K,R)2 K5 E K R34 GR G,W I M L I,N F,L,I,M G R RG2 E28 D Y F,V C2 A V S,P,T,A P A,T G R Q E G D8 I M7 L2 V (I,V)6 I372 D H IT R M6 L,I L40 (I,M)2 (I,L)3 F Q P6 TV T,S N I5 A I V M20 (I,M)5 I V2 L2 I,K A T2 A,S S10 T (S,T)3 S220 D N2 E7 (D,E)3 R S T,S T6 N,S N H,R,N,S C8 G S S,A A2 M L2 I,M I,L I16 K N27 K,N E Q,E Q16 K D K T,K T3 R4 Q,H,K,N Q13 N3 (K,R)2 II G I I,T VI2 I,V V V,L (I,V)2 I29 R K2 G D K,E E59 (D,E)8 A S G H Y4 Q3 L4 E K V,EL (L,V) (F,L)4 V V,L L2 V A Q P L,P,Q,R K R19 (K,R)5 T A M A Q G V,E T4 Q5 N2 K G,W (G,E)3 E20 D A5 Q L K N E G S590 ST2 P,A P2 (M,V)2 M2 I,M,V A,V (A,T)3 (A,E,G)2 V,A V32 (K,R,A,E)2 (T,A)7 A308 V M,V M10 L8 I F2 R K585 (K,R)3 A V,A V T,A T A C W W,stop L,WW AT GS4 (N,S)2 N168 (D,N)2 D27 I (V,I)7 V162 K T149 Q,R Q,K Q64 N4 K,Q Q H6 DE3 FY L,F L I V,I V3 P P,S L,P A Y N P P,H,R QH S Q GA V G A (T,N,A,D)2 (T,A)7 T193 S,T S,A S11 Q3 A,G G81 D,N T (T,N,S)6 (I,T)5 I85 T S,T S3 N2 G R,G R Q S3 K H4 E D17 T,AA G A,E Y L F7 F Y8 V,F L I357 I,L (I,V)2 V2 M (L,I)2 L P F K R6 (K,R)4 G (G,E)2 (A,G)3 A15 R K,R W P V,LV T,KK T43 Q3 N4 (K,R)9 R16 S E H K,Q T K2 M7 V,A V202 (T,K,R)2 T,I,M R2 M,V A20 A,T (I,V)8 I,T,V,A I,K I44 I V66 L6 (I,V)7 I,M H P N,H T S TS24 S,T,A,G S,R R9 (P,T)2 P,R,T,S P,R,T P,R P82 G26 A N D N G L A T (T,A)2 A10
Catalytic core domain I Catalytic core domain II
Catalytic core domain III Catalytic core domain IV D N Y,CC S C,W T H L V5 I,V I E V,E K,E K G (E,G)2 A,G K T,K,A,E R Q K,R V V,L (V,I)13 T2 N I,V I356 L M,L M7 L,V L,I,M I,M (I,L)2 I40 I V3 M18 L3 I,V (I,M)3 E K,E K,EE E,stop V V,I V,G I2 I M L P S,P S3 A A V M I,V I,M,V A V H V I AS S,RS D,GG Y I V8 K R8 (K,R)3 K,N E Q (K,E)7 K V M,V I14 G,V A S C68 (C,R)2 I,M N20 S,C R3 (N,S)2 D S2 N18 E (D,N)2 L V,L V19 Q22 (L,Q)2 (I,V)3 I,L I5 K R5 Q C Q K G E KE E,G A P6 HY Q V V,L V,A I,M,V I2 C A P,T,AP EG6 VI8 M2 I,M S R N,S N5 (G,S)3 G31 D N G Q M V3 (T,K,R)2 T15 R3 (M,T)4 L,M L4 (I,K)2 (I,M)6 I100 C I D D,E Y3 V,D S N G2 E8 G S,G S4 N I K ND2 E22 D,N D,E K R21 Q7 (K,R)3 E A P PQ2 ED542 A E S Q D,G (D,E)5 D164 H P EK G E,V (D,E)2 D14 K R206 (K,R)8 E Y H P (P,H,R)2 L S T,N T14 R3 (N,S)2 N95 C11 (I,S)3 N Y,N H W R (K,R)2 K42 A A,S T43 S3 (A,T)3 M L3 I A V29 S A,V S A2 D3 G13 G,S (N,S)2 N57 D E47 (D,E)4 F Y F,S N S G P P A15 Q S2 Y,S S,P S,A L I41 (M,L)2 I,L
Catalytic core domain V Q sequence
Catalytic core domain VI
Figure 1. Distribution of variants among group M HIV-1 IN sequences. Amino acid polymorphism in HIV-1 IN from 308 plasma samples from drug-naive patients and 282 samples from experienced patients are reported. The consensus subtype B sequence is shown in bold at the top of each 30 amino acid section. Numbers given as superscripts below each position are the numbers of isolates with that specific polymorphism. Grey boxes signify positions associated with in vivo resistance defined according to the algorithms from the ANRS (update October 2012, v.22, http:// www.hivfrenchresistance.org/2012/Algo-sep-2012.pdf). Highly conserved motifs, the HHCC motif (coordinates zinc binding), the DDE motif, catalytic core domains I– VI and the Q sequence, are indicated by boxes.
Research letters
971
and S. Reigadas (Bordeaux), S. Vallet (Brest), J. D. Poveda (Cerba), A. Mirand (Clermont-Ferrand), A. Krivine (Cochin, Paris), C. Auvray and A. de Rougemont (Dijon), S. Yerly (Gene`ve), A. Signori-Schmuck (Gre-noble), L. Bocket (Lille), S. Rogez (Limoges), C. Tamalet (Marseille), V. Schneider and C. Amiel (Tenon), M. Bouvier-Alias (Mondor), B. Montes (Montpellier), E. Schvoerer (Nancy), V. Ferre´ (Nantes), M. L. Chaix (Necker, Paris), J. Guinard (Orleans), S. Haim-Boukobza (Paul Brousse), C. Soulie´, A. G. Marcelin, P. Flandre, L. Assoumou and V. Calvez (Pitie´-Salpe´trie`re, Paris), A. Maillard (Rennes), L. Morand-Joubert (St Antoine, Paris), C. Chaplain (St Denis), C. Delaugerre (St Louis, Paris), T. Bourlet (St Etienne), S. Bertsch (Strasbourg), J. C. Plantier (Rouen), S. Raymond (Toulouse) and S. Marque-Juillet (Versailles).
Funding
The research leading to these results received funding from the European Community’s Seventh Framework Program (FP7/2007-2013) under the project ‘Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN)’—grant agreement no. 223131 and the Agence Natio-nale de Recherche sur le SIDA et les He´patites virales (ANRS, France).
Transparency declarations
None to declare.
References
1 Lataillade M, Chiarella J, Kozal MJ. Natural polymorphism of the HIV-1 integrase gene and mutations associated with integrase inhibitor resistance. Antivir Ther 2007; 12: 563–70.
2 Myers RE, Pillay D. Analysis of natural sequence variation and covariation in human immunodeficiency virus type 1 integrase. J Virol 2008; 82: 9228– 35.
3 Ceccherini-Silberstein F, Malet I, D’Arrigo R et al. Characterization and structural analysis of HIV-1 integrase conservation. AIDS Rev 2009; 11: 17– 29.
4 Rhee SY, Liu TF, Kiuchi M et al. Natural variation of HIV-1 group M integrase: implications for a new class of antiretroviral inhibitors. Retrovirology 2008; 5: 74.
5 Low A, Prada N, Topper M et al. Natural polymorphisms of human immunodeficiency virus type 1 integrase and inherent susceptibilities to a panel of integrase inhibitors. Antimicrob Agents Chemother 2009; 53: 4275– 82.
6 Sierra S, Lubke N, Walter H et al. The SnoB study: frequency of baseline raltegravir resistance mutations prevalence in different non-B subtypes. Med Microbiol Immunol 2011; 200: 225– 32.
7 Garrido C, Soriano V, Geretti AM et al. Resistance associated mutations to dolutegravir in HIV-infected patients-impact of HIV subtypes and prior raltegravir experience. Antiviral Res 2011; 90: 164– 7.
8 Kobayashi M, Yoshinaga T, Seki T et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 2011; 55: 813– 21.
9 Sato A, Seki T, Kobayashi M et al. In vitro passage of drug resistant HIV-1 against a next generation integrase inhibitor (INI), S/GSK1349572. In: Abstracts of the Forty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2009. Abstract H-932. American Society for Microbiology, Washington, DC, USA. 10 Nouhin J, Donchai T, Hoang KT et al. Natural polymorphisms of HIV-1 CRF01_AE integrase coding region in ARV-naive individuals in Cambodia,
Thailand and Vietnam: an ANRS AC12 working group study. Infect Genet Evol 2011; 11: 38– 43.
11 Wainberg MA, Brenner BG. Role of HIV subtype diversity in the development of resistance to antiviral drugs. Viruses 2010; 2: 2493–508.
J Antimicrob Chemother 2013
doi:10.1093/jac/dks486
Advance Access publication 18 December 2012
Pharmacokinetic interaction of
maraviroc with tacrolimus in a patient
coinfected with HIV and hepatitis B
virus following hepatic transplant due
to hepatocellular carcinoma
Ngozi E. Dufty
1,2*, Gerry Gilleran
1, Daniel Hawkins
1,
Laura J. Else
3and Stephen Taylor
1,41
Birmingham Heartlands HIV Service, Directorate of Infection, Birmingham Heartlands Hospital, Birmingham, UK;2Department
of Military Medicine, Royal Centre for Defence Medicine, Birmingham, UK;3Department of Molecular and Clinical
Pharmacology, University of Liverpool, Liverpool, UK;4Division of Immunity and Infection, University of Birmingham,
Birmingham, UK
*Corresponding author. Birmingham Heartlands HIV Service, Directorate
of Infection, Birmingham Heartlands Hospital, Birmingham, UK. Tel:
+44-121-424-3361; Fax:+44-121-424-3211; E-mail: ngozi.dufty@nhs.net
Keywords:HIV antiviral pharmacology, hepatic transplantation, immunosuppression, HBV
Sir,
Limited data are available regarding interactions between tacro-limus and commonly used highly active antiretroviral therapies, such as first-line nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors (NNRTIs) and some protease inhibitors (PIs). When first-line combinations are con-traindicated and newer antiretroviral agents are required, there are even less data on the interactions between newer agents such as maraviroc (a CCR5 inhibitor) with immunosuppressants such as tacrolimus (a calcineurin inhibitor). There are some animal model data of the beneficial effects on cardiac allograft survival when using maraviroc alongside immunosuppressants, with the potential that CCR5 inhibition could improve long-term outcomes after transplantation.1,2 In our patient undergoing hepatic transplant, with limited antiretroviral therapy options and the necessity to be started on a newer agent, we set out to observe concentrations of the immunosuppressant tacrolimus before and after administration of maraviroc to ensure that effective and non-toxic concentrations of both drugs were achieved.
We describe a 49-year-old man from Sierra Leone, recently diagnosed with fully sensitive HIV clade C and chronic hepatitis B virus (HBV). After routine blood tests revealed abnormal liver