ZeitschriftfürPhysikalischeChemie, Bd. 212,S. 93-98 (1999) ©by Oldenbourg Wissenschaftsverlag, München
The Excitation
Wavelength
and
Solvent
Dépendance
of
the
Kinetics
of
Electron
Injection
in
Ru(dcbpy)2(NCS)2
Sensitized
Nanocrystalline
Ti02
Films
By
J. R.Durrant1*,
Y.Tachibana1,
I.Mercer1,
J. E.Moser2,
M. GrâtzelandD. R.
Klug1
1
CentreforPhotomolecularSciences, Departmentsof
Biochemistry
andChemistry, Imperial College,London,SW7 2AY2 Institut
deChimie
Physique,
École PolytechniqueFédéraledeLausanne,CH-1015Lausanne, Switzerland
(ReceivedAugust 14, 1998;
accepted
December 10, 1998)Electron
transfer
IDye
sensitisation INanocrystalline
I Solar cells IInterfacial
electrontransfer
Ultrafast transient
absorption
spectroscopy has been employed to monitor the kinetics ofphotoinduced
electroninjection
fromRu(2,2-bipyridyl-4,4'-dicarboxylate)2(NCS)2
(Ru(dcbpy)2(NCS)2)intonanocrystallineTi02films. This process foundtoexhibit
nonex-ponential
kineticsonthefemtosecond/picosecond
timescales. Amultiexponential analysisyielded
lifetimesof <100fs,1.3psand 13 ps with relativeamplitudesof0.35,0.22,0.43(±0.06)respectively. These kinetics were found to be independent of excitation wave-length,and towhether the filmwas immersed inorganicsolventor exposedto air.
Introduction
The
injection
of electrons from a sensitizerdye
intonanocrystalline
Ti02
[1—7]
is theprimary charge separation
step
ina newclass ofdye
sensitizedphotovoltaic
devices[8],
Anappreciation
of theparameters
controlling
the kinetics of this reaction islikely
to beimportant
fordevelopment
of thistechnology.
Several studies of the kinetics of electron
injection
haveemployed
or-ganic
sensitizerdyes
including
coumarin[1],
perylene
[2]
and fluorescein*
also focussed upon ruthenium
bipyridyl
dyes,
class of
dyes
hasyielded
the mostefficient sensitizerdyes
forphotovoltaic
cells to date[8],
Inparticular,
solar cellsemploying
the sensitizerdye
Ru(dcbpy)2(NCS)2
have achieved energy conversion efficiencies of —10%.Meyer
etal.[9],
employing
transientphotoluminescence
measurements tostudy
electroninjection
in aRu(dcbpy)2(NCS)2
sensitizedphotoelectro-chemical cell have
reported
distributionofinjection
rates with a maximumamplitude
at —109s~\Willig
et al.[4]
hasreported
aninjection
time of<25 fs from this
dye
intoTi02
electrodes underultrahigh
vacuum,although
we have
subsequently reported
that thisparticular study
may have beendistorted
by
degradation
of thesensitizerdye
[7].
Mostrecently,
Lian,
Nozik and co-workers[6]
havereported
a sub-50 fsrateofelectroninjection
from infrared transientabsorption
studies ofRu(dcbpy)2(NCS)2
sensitized filmsexposed
toair. Ourown studies[3]
havereported
that electroninjection
inRu(dcbpy)2(NCS)2
sensitized films covered with an inert solution(50/50
propylene carbonate/ethylene
carbonate)
is at leastbiphasic,
withinjection
times of <150fs and—1.2ps.
For thissystem,
thesubsequent charge
re-combination exhibits
nonexponential
kinetics which arestrongly
dependent
upon the
application
ofan externalbiaspotential
tothe film[10].
We have
recently
extended ourprevious
studies of electroninjection
toconsideration of the parameters whichcontrol therate of electron
injection.
Aspart
of thisstudy,
wepresent
here the results ofourconsideration of theinfluence of the
dye
solvent environment and excitationwavelength
upon theinjection
kinetics.Materials and methods
Nanocrystalline
Ti02
films coated with a 10%monolayer
ofRu(dcbpy),-(NCS)2
wereprepared
aspreviously
[3].
Transientabsorption
data werecollectedat room
temperature
using
apparatus
describedindetailelsewhere,
with a 100
—
250 fs instrument response, a 1 kHz
repetition
rate, excitationpulse energies
of10—35 nJ(0.35—0.7
mJcirr2)
depending
upon excitationwavelength,
a whitelight
probe
pulse,
andmultichannel detector. Transientspectra
werecollected between 700 and 800 nmon several differenttimes-cales. The
spectra
obtained wereindistinguishable
from thosereported
pre-viously
[3].
Forease ofpresentation,
transient dataareonly
shown here at asingle
wavelength
(760 nm).
Results and
discussion
We have
previously
demonstrated that electroninjection
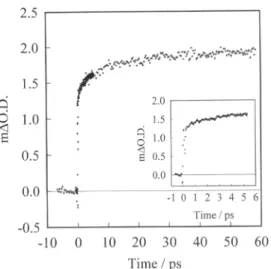
from theThe ExcitationWavelengthand Solvent Dépendanceof the KineticsofElectron... 95 2.5 2.0 1.5 O 1.0 < 0.5 0.0 -0.5
r
-10 12 3 4 5 6 Time/ ps j_ _ _ -10 0 10 20 30 40 50 60 Time /psFig. 1. Transient
absorption
data at 760nmfollowing
excitation ofRu(dcbpy)2(NCS)2
sensitized TiO,films at560nm.
absorption
maximum from —700nmto —800nm3.
This inducedabsorption
mostprobably
results from aligand-to-metal charge
transfer transitionas-sociated with the NCS groups
(oxidative
degradation
of thedye, causing
aloss of the NCS groups, results in loss of this induced
absorption
[7]).
InFig.
1 we show the kinetics of this redshift,
monitoredby
the appearanceof the induced
absorption
at760nm. These dataareinagreement
with thosewe have
reported
previously
[3].
However theimproved signal
to noiseand extension to
longer
timescales allow us to resolve additional kineticcomponents.
Amulti-exponential analysis
revealed at least threeex-ponential
components
with lifetimes of<100fs,
1.3 and 13 ps. It shouldalso be noted this
analysis
isonly
intended toprovide
asimple
quantifi-cation of the kinetics, which may result from an
underlying
distribution oflifetimes.
A full
spectral analysis
of thesekinetics,
allowing
consideration ofthecontribution of excitedstate
absorption
to thesignal
at 760nm(as
conduct-ed
previously
[3])
indicates that the relativeyields
electroninjection
associ-ated with these
components
are0.35, 0.22,
0.43(±0.06)
respectively.
Aslower —
lOOps
component
could also be resolved with lowamplitude
(<10%),
however theprobe wavelength dépendance
ofthiscomponent
sug-gests
it is notprimarily
associated with electroninjection.
These
nonexponential injection
kinetics are in contrast withmonoex-ponential
kineticsrecently reported
for theorganic
sensitizerdyes
coumarin[1],
perylene
[2]
andfluorescein[5].
Onepossible
reasonfor thisdifference2.0 1.5 Q O 1.0 < 0.5 0.0 -0.5 -10 0 10 20 30 40 50 60 Time / ps
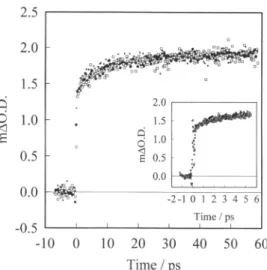
Fig.
2. Excitationwavelength dependence.
AsFig.
1 but with excitationwavelengths
of 520(+), 560(·)and 600( ) nm.different kinetics
resulting
from electroninjection
from differentelectronicstates
(recent
MO calculationssuggest
4 different states contribute to theground
stateabsorption
between 500 and 700nm[11]).
In orderto addressthis
possibility,
we monitored the electroninjection
kinetics as a functionof excitation
wavelength.
As shown inFig.
2,
we find that the kinetics ofelectron
injection
areindependent
of excitationwavelength.
We thuscon-clude that the
nonexponential
injection
kinetics are not related to thecom-plex photophysics
ofthisdye.
This conclusion is also consistent with ourrecent observation of
remarkably
similarnonexponential injection
kinetics with free base and Zinctetracarboxyphenyl porphyrins
(to
bepublished
elsewhere).
Alternatively
it ispossible
that thenonexponential
injection
kinetics could be associated with adynamic
solvation of thedye by
the solventenvironment.
Indeed,
the recentstudy by
Ellingson
et al.[6],
conducted with the same sensitizerdye
buton airexposed
films,
reported only
asingle
<50 fs
phase
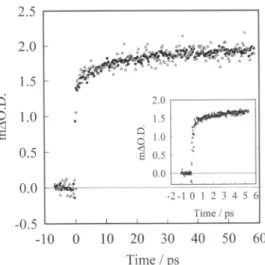
of electroninjection. Fig.
3 therefore compares the kineticswe obtain for
Ru(dcbpy),(NCS)2)
filmsexposed
to air and covered in 50/50propylene carbonate/ethylene
carbonate. Datawerealso obtainedwithethanolic and water free acetonitrile
electrolytes.
In all cases the transientkinetics are
indistinguishable
from eachother,
indicating,
solvation of thedye
does nothave asignificant
effect upon theinjection
kinetics. It shouldalso benoted that theethanol solution wasnot
dried,
and therefore contain-ed asignificant
water content.This watercontentevidently
has anegligible
effect upon the kinetics of electron
injection.
Time/ ps ._I_¡_L_
The ExcitationWavelengthand Solvent Dépendanceof the Kinetics of Electron 97 2.5 2.0
h
1.5h
Q O 1.0 -< S ö 101
0.5 1.5 0.5 o.o 0.0 -2-10 1 2 3 4 5 6 -0.5 l'ime / ps -10 o 10 20 30 40 50 60 Time /psFig.
3. Solventdependence.
AsFig.
1 butcomparison
of data collected for sensitized filmsunderapropylene carbonate/ethylene
carbonate solvent(·),ordried in air( ).We thus conclude that the kinetics of electron
injection
fromphotoexcit-ed
Ru(dcbpy)2(NCS)2
areindependent
oftheelectronic stateinitially
popu-lated,
and the solvent environment of the sensitizerdye.
Consideration of theparameters
which do have astrong
influence upon the rate of electroninjection
will bepresented
elsewhere.Acknowledgements
We would like to thank Dr. Chris Barnett for excellent technical
support.
J.R.D. and D.R.K.
acknowledge
support
from theEPSRC,
the EC Jouleprogrammeandthe British Council. J.R.D. isaBBSRC Advanced Research
Fellow. J.E.M. and M.G.
acknowledge
thesupport
of the Swiss National Science Foundation.References
1. J. M. Rehm,G. L.McLendon, Y.Nagasawa,K. Yoshihara,J. Moser andM. Gratzel,
J.
Phys.
Chem. 100(1996)2820.2. B. Burfeindt, T.
Hannappel,
W. Storck and F.Willig,
J. Phys. Chem. 100 (1996)16463.
3. Y.Tachibana,J.E.Moser, M.Gratzel,D. R.
Klug
and J. R.Durrant,J.Phys.
Chem. 100(1996)20056.4. T.
Hannappel,
B. Burfeindt,W. Storck and FWillig,
J.Phys.
Chem. 101(1997) 6799.7. J. E. Moser, D. Noukakis, U. Bach, Y. Tachibana, D. R. Klug, J. R. Durrant, R. Humphrey-Bakerand M.Gratzel,J.Phys. Chem. 102(1998)3649.
8. .
O'Regan
and M. Gratzel, Nature (London)353(1991)737. 9. T. A.HeimerandG.J.Meyer, J.Luminescence 70(1996)468.10. S. A. Haque, Y Tachibana, D. R. Klug and J. R. Durrant,J. Phys. Chem. 102 (1998)1745.