COHORT PROFILE
Cohort Profile: The Bern Infant Lung
Development Cohort
Oliver Fuchs,1,2 Philipp Latzin,1,2 Claudia E Kuehni2 and Urs Frey1,3*
1
Division of Respiratory Medicine, Department of Paediatrics, Inselspital, University of Bern, Bern, Switzerland,2Institute of Social and Preventive Medicine, University of Bern, Bern, Switzerland and3University Children’s Hospital (UKBB), University of Basel, Basel, Switzerland
*Corresponding author. University Children’s Hospital (UKBB), University of Basel, 4056 Basel, Switzerland. E-mail: urs.frey@ukbb.ch
Accepted
8 November 2010
How did the study come about?
Childhood asthma resembles a complex syndrome rather than a single disease. Clinical manifestations include cough, shortness of breath and wheeze.1 Because of its high prevalence, it is of major public health relevance.2,3Presentation and disease course of childhood wheezing disorders vary,4 and its pheno-types are difficult to distinguish in early childhood despite the demand for diverse therapeutic strategies.5 Current treatments have no sustained effect on devel-opment of lung function and cannot prevent tracking of reduced lung function.6
Risk factors for childhood wheeze and subsequent asthma include genes, environmental factors and their interaction:1,7,8all these affect lung development directly or indirectly (Figure 1). Data on the associ-ation between lung development and respiratory symptoms9 in early childhood are mostly derived from small hospital-based studies. Larger epidemio-logical studies have been based on questionnaires and most studies have been conducted in older children,2 in high-risk populations10 or retrospective-ly.11 There is a lack of prospectively assessed lung function studies in unselected healthy infants that take into account genetic and environmental factors. However, in order to disentangle the complex inter-action of these predisposing factors on lung develop-ment and childhood wheeze, relatively large groups of infants need to be studied, with repeated lung func-tion measurements, assessment of environmental and hereditary factors and clinical outcomes. The ‘Bern Infant Lung Development’ (BILD) cohort aims to con-tribute towards closing this gap. It was set up in 1999 to study physiological properties of the respiratory system and environmental and genetic risk factors af-fecting lung development in healthy individuals from infancy through childhood in relation to wheeze.9
How is it funded?
Direct costs of the cohort are being paid by project grants from the Swiss National Science Foundation, the European Respiratory Society (ERS), the Austrian, German and Swiss Paediatric Respiratory Society and Swiss Governmental Anti-Tobacco Fund. The University Children’s Hospital in Bern, Switzerland, where the study centre is located, provides the infrastructure. All studies were approved by the local Ethics Research Committee.
What does it cover?
In a comprehensive approach, we search for early markers of common respiratory morbidity, including respiratory infections and asthma, by trying to disentangle predisposing and modifying factors of re-spiratory morbidity within the context of lung devel-opment. This includes the assessment of immune development and markers of airway inflammation in response to environmental triggers.
The strengths of the BILD cohort are its hallmarks: the prospective approach with standardized data col-lection starting before birth in unselected children; the accurate and standardized measurements of lung function and markers of airway inflammation; and the detailed assessment of incidence and determin-ants of respiratory symptoms especially during the first year of life.12
To explain developing properties of lung and airways during the vulnerable phases, lung function is mea-sured at the age of 1 month and at the age of 6 years, with further lung function measurements planned for the age of 12 years.
We study the complex reaction of lung and airways to environmental triggers, which is possibly
influenced both by inflammation and immune re-sponse. Here, we measure the association between exposure to environmental tobacco smoke (ETS), air pollution and lung function, markers of airway in-flammation and components of the immune system. In addition, we prospectively assess viral pathogens of lower respiratory tract infections during the first year of life, as well as related respiratory symptoms and their severity.
Data covering genetic risk factors for childhood asthma are rapidly increasing.13,14 However, little is known about their effect on lung development and childhood wheeze.14–16 This is particularly true for genes known, from animal models, to be involved in lung development. Therefore, we measure the effect of single-nucleotide polymorphisms in these genes in comparison with genes associated with clinical mani-festation of childhood allergy, asthma or wheeze.
Who is in the sample?
Recruitment for the BILD cohort is ongoing; about 35 unselected healthy neonates enter the cohort every year. Study participants are born after April 1999 in the agglomeration of Bern, the capital of Switzerland. One-third of the study population comes from the rural surroundings, and two-thirds from the urban area of Bern, which still offers rela-tively rural living conditions compared with other European cities.
Pregnant mothers are recruited at the four major maternity hospitals and practices of obstetricians in the agglomeration of Bern through advertisements and interviews. Interested families receive informative letters describing involved measurements and are contacted by the study centre. The following inclusion criteria apply: White ethnicity, term delivery (at least 37 weeks), ability of parents to speak one of the major Swiss languages (German, French), no severe maternal health problems, no maternal drug abuse other than nicotine, no known major birth defects or perinatal disease of the newborn, such as respira-tory distress, airway malformation or other major re-spiratory diseases diagnosed after birth.
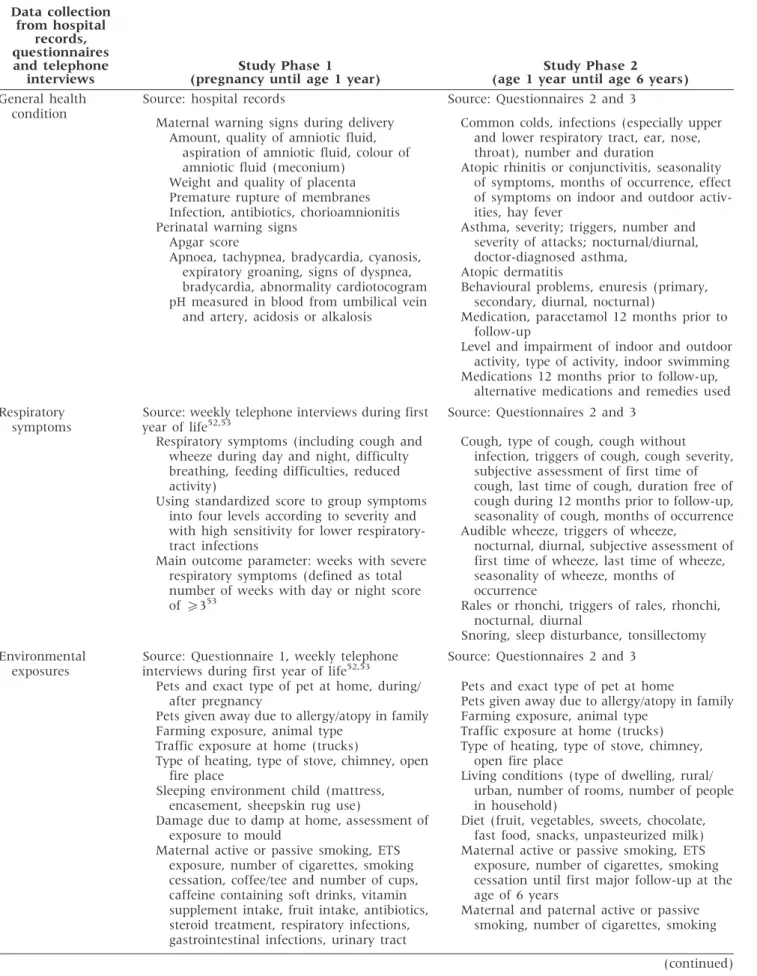
From 1999 until the end of 2009, a total of 42 110 mothers gave birth at the four major maternity hospitals in the agglomeration of Bern. By the end of 2009, we recruited 364 study participants (see Table 1 for an-thropometric data of infants with lung function data), and 107 have been followed-up so far at the age of 6 years (Table 2). The others have not yet reached this age.
Figure 1 Proposed effect of environmental and genetic risk factors and their interaction on lung development and evolution of wheeze phenotypes. The Figure illustrates how genetic background, environmental exposures (ETS, air pollution, infections, allergens, active smoking) and their interaction might influence lung growth and development from branching of the airways to alveolarization. We propose a direct effect of these exposures on lung develop-ment, and an indirect effect via changes in the balance between inflammation, injury and repair mechanisms, leading to remodelling. Differences in these pathways might lead to different phenotypes of wheezing disorders
Table 2 Number of participants initially recruited, number completing study Phases 1 and 2 and number of drop-outs during study Phases 1 and 2 from 1999–2009 in the BILD cohort
Year 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Total until 31/12/2009 Prenatally recruited 26 40 20 21 46 44 37 29 30 45 25 364 Completed study phase 1 23 37 19 20 45 44 36 28 29 44 25 350 Completed study phase 2 – – – – – – 28 14 11 18 36 107 Drop-outs during study phase 1 3 3 1 1 1 0 1 1 1 1 1 14 Drop-outs after study phase 1 – – – – – – 8 8 7 1 8 32
Table 1 Anthropometric data of study participants in the BILD cohort with information on lung function (N ¼ 344) at the age of 1 month
Anthropometric data Median
Interquartile
range Range Gestational age at birth
(weeks)
39.9 38.9–40.6 37.0–42.3 Birth weight (g) 3410 3060–3660 2170–4915 Age at study date (weeks) 5.0 4.6–5.4 3.6–8.3 Weight at study date (g) 4325 4010–4750 2890–6400 Length at study date (cm) 54.6 53.1–56.5 48.0–61.5
How often have they been
followed-up? What is attrition
like?
The study design of the BILD cohort is shown in Figure 2. It includes three study phases with three questionnaires, weekly telephone interviews during the first year of life, two visits to the study centre and two visits to the families’ homes at the time of the first lower respiratory infection.
Study Phase 1 starts with recruitment. After birth, mid wives extract perinatal history from medical re-cords, and take cord blood and first urine samples if eligibility is confirmed. At the age of 1 month, the infants visit the study centre. On this occasion, lung function measurements and maternal skin-prick tests are performed, and Questionnaire 1 is used to assess pre- and perinatal history. During the first year of life, study nurses perform regular weekly telephone calls with standardized interviews.
After the first year of life, Study Phase 2 starts. The first follow-up is performed at the age of 6 years, when participants visit the study centre again to undergo lung function measurements and skin-prick tests. Questionnaires 2 and 3 assess the history during Study Phase 2. A further follow-up as part of Study Phase 3, also including lung function measurements and skin-prick tests, is planned for the age of 12 years.
Attrition was low during Phase 1 and relatively low during Phase 2. We lost only 13 of 364 recruited in-fants (3.7%) during Phase 1 and only 31 of 138 chil-dren (22.5%) during Phase 2 (Table 2). Drop-outs were almost exclusively due to personal reasons (8 during Phase 1, 25 during Phase 2), and to families moving away (1 during Phase 1, 5 during Phase 2). Five children were excluded because of heart disease diagnosed after the neonatal period (four in Phase 1 and one in Phase 2).
What has been measured?
The data are derived from four sources: (i) hospital records; (ii) questionnaires; (iii) telephone interviews; and (iv) objective measurements. A detailed descrip-tion of data collected in the BILD cohort during Phases 1 and 2 is given in Table 3.
Hospital records (i): these are used to extract infor-mation about maternal warning signs during the delivery and perinatal data.
Questionnaires (ii): these assess information about health conditions, with an emphasis on respira-tory and atopic symptoms, infections and socio-demographic and environmental exposures. Question-naire 1 also collects information on pre- and perinatal risk factors including family and maternal past med-ical history. Questionnaires 2 and 3 assess the study participant’s history during Phase 2. While Figure 2 The BILD cohort: time-flow of recorded data, as well as of tests and procedures performed during the follow-up. Vt: tidal volume; FeNO: fraction of exhaled nitric oxide; MBW: multiple breath washout; FRC: functional residual capacity; LCI: lung clearance index; PCR: polymerase chain reaction; Rint: airway resistance by interrupter; Reff: effective airway resistance (measured by bodyplethysmography); FEV1: forced expiratory volume during the first second of expiration; MEF25-75: midexpiratory flow
Table 3 Detailed description of collected data and measurements in the BILD cohort during Phases 1 and 2 Data collection from hospital records, questionnaires and telephone interviews Study Phase 1 (pregnancy until age 1 year)
Study Phase 2
(age 1 year until age 6 years) General health
condition
Source: hospital records
Maternal warning signs during delivery Amount, quality of amniotic fluid,
aspiration of amniotic fluid, colour of amniotic fluid (meconium)
Weight and quality of placenta Premature rupture of membranes Infection, antibiotics, chorioamnionitis Perinatal warning signs
Apgar score
Apnoea, tachypnea, bradycardia, cyanosis, expiratory groaning, signs of dyspnea, bradycardia, abnormality cardiotocogram pH measured in blood from umbilical vein
and artery, acidosis or alkalosis
Source: Questionnaires 2 and 3
Common colds, infections (especially upper and lower respiratory tract, ear, nose, throat), number and duration
Atopic rhinitis or conjunctivitis, seasonality of symptoms, months of occurrence, effect of symptoms on indoor and outdoor activ-ities, hay fever
Asthma, severity; triggers, number and severity of attacks; nocturnal/diurnal, doctor-diagnosed asthma,
Atopic dermatitis
Behavioural problems, enuresis (primary, secondary, diurnal, nocturnal)
Medication, paracetamol 12 months prior to follow-up
Level and impairment of indoor and outdoor activity, type of activity, indoor swimming Medications 12 months prior to follow-up,
alternative medications and remedies used Respiratory
symptoms
Source: weekly telephone interviews during first year of life52,53
Source: Questionnaires 2 and 3 Respiratory symptoms (including cough and
wheeze during day and night, difficulty breathing, feeding difficulties, reduced activity)
Using standardized score to group symptoms into four levels according to severity and with high sensitivity for lower respiratory-tract infections
Main outcome parameter: weeks with severe respiratory symptoms (defined as total number of weeks with day or night score of5353
Cough, type of cough, cough without infection, triggers of cough, cough severity, subjective assessment of first time of cough, last time of cough, duration free of cough during 12 months prior to follow-up, seasonality of cough, months of occurrence Audible wheeze, triggers of wheeze,
nocturnal, diurnal, subjective assessment of first time of wheeze, last time of wheeze, seasonality of wheeze, months of
occurrence
Rales or rhonchi, triggers of rales, rhonchi, nocturnal, diurnal
Snoring, sleep disturbance, tonsillectomy Environmental
exposures
Source: Questionnaire 1, weekly telephone interviews during first year of life52,53
Source: Questionnaires 2 and 3 Pets and exact type of pet at home, during/
after pregnancy
Pets given away due to allergy/atopy in family Farming exposure, animal type
Traffic exposure at home (trucks)
Type of heating, type of stove, chimney, open fire place
Sleeping environment child (mattress, encasement, sheepskin rug use)
Damage due to damp at home, assessment of exposure to mould
Maternal active or passive smoking, ETS exposure, number of cigarettes, smoking cessation, coffee/tee and number of cups, caffeine containing soft drinks, vitamin supplement intake, fruit intake, antibiotics, steroid treatment, respiratory infections, gastrointestinal infections, urinary tract
Pets and exact type of pet at home
Pets given away due to allergy/atopy in family Farming exposure, animal type
Traffic exposure at home (trucks) Type of heating, type of stove, chimney,
open fire place
Living conditions (type of dwelling, rural/ urban, number of rooms, number of people in household)
Diet (fruit, vegetables, sweets, chocolate, fast food, snacks, unpasteurized milk) Maternal active or passive smoking, ETS
exposure, number of cigarettes, smoking cessation until first major follow-up at the age of 6 years
Maternal and paternal active or passive smoking, number of cigarettes, smoking
Table 3 Continued Data collection from hospital records, questionnaires and telephone interviews Study Phase 1 (pregnancy until age 1 year)
Study Phase 2
(age 1 year until age 6 years) infections, vaginitis, colpitis, worm
infections, other infections during pregnancy and during first year of life Paternal active smoking, number of
cigarettes, smoking cessation during pregnancy and first year of life
cessation until first major follow-up at the age of 6 years
Objective measurements
Study Phase 1 (pregnancy until age 1 year)
Study Phase 2
(age 1 year until age 6 years) Measurements
of lung function and inflammation of airways
Tidal breathing measurements12,50 Unsedated infants, during quiet natural sleep
Ultrasonic flowmeter (SpirosonÕ,
EcoMedics AG, Duernten, Switzerland) with infant face mask
Main outcome parameters: minute ventilation (tidal volume multiplied by respiratory rate) and expiratory flow Measurement of fraction of exhaled nitric oxide (FeNO)53
Rapid-response chemoluminescence analyzer (CLD 88, EcoMedics AG, Duernten, Switzerland), with a range of 0–100 parts per billion (ppb), infant face mask
Breath-by-breath measurement Main outcome parameter: mean FeNO Multiple Breath Washouts
(Sulfurhexafluoride)32
Ultrasonic flowmeter (see above), infant face mask
Main outcome parameters: lung volume (FRC) and ventilation inhomogeneity (LCI) Interrupter resistance measurement
Ultrasonic flowmeter (see above), shutter box, infant face mask
Main outcome parameter: Rint Anthropometric data at study date: body weight and length, head circumference
Tidal breathing measurements Quiet tidal breathing
Ultrasonic flowmeter (see left) with mouth-piece, filter and nasal clamp Main outcome parameters: minute
ventilation (tidal volume multiplied by respiratory rate) and expiratory flow Measurement of FeNO53
Rapid-response chemoluminescence analyzer (CLD 88, EcoMedics AG, Duernten, Switzerland), with a range of 0–100 ppb
Breath-by-breath measurement and single-breath manoeuvre, no nasal clamp Main outcome parameter: mean FeNO Multiple Breath Washouts (Helium)
Ultrasonic flowmeter (see above), nasal clamp
Main outcome parameters: lung volume (FRC) and ventilation inhomogeneity (LCI)
Interrupter resistance measurement Ultrasonic flowmeter (see above), nasal
clamp
Main outcome parameter: Rint Spirometry (bodyplethysmography)
MasterScreen Body, Jaeger, Germany; mouthpiece, filter, nasal clamp Main outcome parameters: lung volume
(FRC, intrathoracic gas volume), forced expiratory flows and volumes
Anthropometric data at study date: body weight and length
Skin-prick test Maternal skin-prick test Child skin-prick test Dog dander, cat dander, Dermatophagoides
pteronyssinus, mixed tree pollens, mixed grass pollens, Alternaria tenuis, positive control (histamine), negative control (NaCl), Allergomed, Switzerland
Dog dander, Cat dander, Dermatophagoides pteronyssinus, mixed tree pollens, mixed grass pollens, Alternaria tenuis, positive control (histamine), negative control (NaCl), Allergomed, Switzerland Immunology Specimen: cord blood
Haematocrite, haemoglobin count, white blood cell count, leucocyte subtypes Total IgE, eosinophil cationic protein,
eosinophil protein X, interleukin subtypes, interferon subtypes
Table 3 Continued Objective measurements
Study Phase 1 (pregnancy until age 1 year)
Study Phase 2
(age 1 year until age 6 years) Mannose-binding lectin (MBL) measured
as described previously with commer-cially available ELISA kits
(Antibodyshop, Gentofte, Denmark)60 Microbiology Specimen: anterior nasal swabs
Taken at any first acute respiratory infection, which is defined as42 days with cough or wheeze, together with fever4388C, acute rhinitis, otitis media or pharyngitis; specimens are analysed by polymerase chain reaction (PCR) based assays, e.g. Taqman real-time PCR, targeting 16 different respiratory viruses commonly infecting humans54,60 Genetics deoxyribonucleic acid (DNA) extraction from
white blood cells from cord blood
DNA extraction from mucosa cells retrieved per buccal swabs or from saliva collection Analysis of single nucleotide
polymorph-isms (SNPs)
Analysis of copy number variants in genes involved in lung growth per DNA chip analysis
Analysis of SNPs and
Analysis of copy number variants in genes involved in lung growth per DNA chip analysis
ETS and caffeine Specimen: first urine after birth and urine during first lower respiratory-tract infection
Nicotine and cotinine Caffeine
Air pollution at community level
Swiss National Air Pollution Monitoring Network (Nationales Beobachtungsnetz fu¨r Luftfremdstoffe, NABEL) measurement sites12
Daily mean levels of particulate matter with aerodynamic diameter of <10mm (PM10) Daily mean levels of nitrogen dioxide (NO2) Daily maximum of mean hourly levels of
ozone (O3)
Stationary passive samplers (Palmes Tubes) at measurement sites: daily mean levels of NO2
Swiss NABEL measurement sites12 Daily mean levels of PM10 Daily mean levels of NO2
Daily maximum of mean hourly levels of O3
Stationary passive samplers (Palmes Tubes) at measurement sites: daily mean levels of NO2
Air pollution at individual level
Mobile passive samplers: daily mean levels of PM2.5and PM10
Stationary passive samplers (Palmes Tubes) at study participants’ homes: daily mean levels of NO2
Mobile passive samplers: daily mean levels of PM2.5 and PM10
Stationary passive samplers (Palmes Tubes) at study participants’ homes: daily mean levels of NO2
Extraction of
routine data Study Phases 1–2 (until age 6 years)
Sources:
Questionnaires 1–3, telephone interviews
Study participant: name, gender, date of birth, maternity hospital, gestational age, birth weight, birth length, multiple birth, birth order, vaccinations
Feeding (breastfed, gastroesophageal reflux, hypoallergenic supplement), supplementary alimentation, later diet
Maternal and paternal hay fever, atopic dermatitis, asthma, therapy due to asthma or difficulty breathing
Demographic and socio-economic information: mother and father: names, birth dates, occupa-tion throughout study, naoccupa-tionality, religion, country of birth and language, all home addresses from pregnancy throughout study
Questionnaire 1 contains validated questions from a preschool cohort study,17,18 Questionnaires 2 and 3 include questions for school-age children from the International Study of Asthma and Allergies in Child-hood (ISAAC).19 All questionnaires have been vali-dated and used, with little variation since 1999. Questionnaire 1 is programmed as an online database for data entry during interview, Questionnaires 2 and 3 are distributed to parents in paper form (see avail-able as Supplementary Data at IJE online data A and B available as Supplementary Data at IJE online).
Weekly telephone interviews (iii): these interviews are carried out during the first year of life to collect information about respiratory symptoms. This in-cludes a standardized score with a high sensitivity for lower respiratory-tract infections (Table 4).20 Changes in socio-demographic and environmental ex-posures are also assessed (listed in Table 5 for the study population with data from Phase 1). In the event of a first lower respiratory-tract infection, the study nurses visit the families twice within 3 weeks and perform two anterior nasal swabs for virus diag-nostics by polymerase chain reaction (PCR).
Objective measurements (iv): these measurements during infancy include, besides the mentioned virus diagnostics, a lung function test at the age of 1 month in unsedated infants during quiet natural sleep (tidal breathing measurements, multiple breath washouts, interrupter resistance measurements). At the age of 6 years, the same lung function tests are performed, supplemented by spirometry and bodyplethysmogra-phy. The fraction of exhaled nitric oxide (FeNO), as a marker of airway inflammation, is measured at both time-points. All measurements are performed accord-ing to current standards by the ERS and the American Thoracic Society (ATS).21–24 Skin-prick tests are per-formed in mothers during the first visit and in infants at the age of 6 years.
In addition to data from questionnaires, ETS expos-ure during pregnancy is objectively assessed by the analysis of the first urine after birth. Exposure to out-door air pollution on the community level is measured by monitoring stations of the Swiss National Air Pollution Network (NABEL), including daily mean levels of particles with a 50% cut-off aerodynamic diameter of 10mm (PM10) and nitrogen dioxide (NO2), as well as the daily maximum of mean
hourly levels of ozone (O3). Air pollution at the indi-vidual level is measured in selected time periods by mobile passive samplers (daily mean levels of particles with a 50% cut-off aerodynamic diameter of 2.5mm (PM2.5) and PM10) and stationary passive samplers at the measurement sites (daily mean levels of NO2).
What has been found? Key
findings and publications
Development and standardization of
non-invasive lung function tests
To assess lung development in a large infant cohort, one needs non-invasive tests that can be performed in uncooperative study participants. In addition to the standardized data collection, we have significantly added to current ERS/ATS standards of lung function measurements in infants23,24 and to the development and standardization of additional non-invasive lung function techniques.9,25–48
Early programmers of lung development
Since the beginning of our prospective birth cohort, we have investigated physical properties of the air-ways in infants prone to wheeze.9 We showed that not only bronchoconstriction, but also impaired airway growth, plays a role for the development of wheeze.27,29 Even without current wheeze, infants with a past history of wheeze display altered airway wall mechanics. This might be due to remodelling or independent developmental differences already present at birth. This emphasizes theimportance of early programmers of lung
development.30,49,50
Outdoor air pollution might be such an early pro-grammer. By modelling outdoor air pollution expos-ure during pregnancy, we found that exposexpos-ure to PM10 was associated with altered lung function in newborns and that NO2 levels were associated with elevated FeNO levels after birth, indicating the induc-tion of inflammatory response in infants. This was enhanced if mothers smoked during pregnancy but independent of maternal atopy.12 Although atopy is a risk factor for allergic asthma, this adds to the hypothesis that lung development and evolution of Table 4 Symptom score used in weekly telephone interviews during the first year of life20
Symptom score
Day-time symptoms (cough, wheeze or breathing difficulties)
Night-time symptoms
(cough, wheeze or breathing difficulties)
0 None None
1 Slight; no treatment given Slight; sleep not disturbed
2 Required treatment but no help from outside Sleep disturbed once; no help required
3 Severe; required help from general practitioner (GP) Sleep disturbed more than once or child needed help 4 Very severe; admitted to hospital Sleep very disturbed or GP called
allergic asthma might be partly independent processes, which are both influenced by early programmers.25,51
Prevalence, risk factors and prognosis of
respiratory symptoms in early life
We were among the first to present prospective data on respiratory symptoms in a cohort of unselected infants. The incidence of symptoms was increased in
infants of asthmatic mothers, whereas maternal hay fever was inversely related to the number of symp-toms.52 High FeNO levels after birth were associated with severe symptoms during the first year of life in the offspring of atopic mothers, which was again pro-nounced if mothers smoked during pregnancy.53 Thus, FeNO after birth might be an important predict-or of later respiratpredict-ory symptoms and mpredict-orbidity.
The role of viral triggers for early
respiratory-tract infections
We analysed the pattern of viral pathogens causing respiratory infections during the first year of life and found that rhinoviruses were most common, fol-lowed by corona, parainfluenza and respiratory syn-cytial viruses. Patterns differed also with regard to the amount of viral shedding 3 weeks after incidence of symptoms.54–56 Additionally, we showed that Human Bocavirus (HBoV), a picornavirus isolated in children with lower respiratory-tract infections from several retrospective and hospital-based studies, is also asso-ciated with respiratory disease in healthy Swiss in-fants, and reported that HBoV circulates in an endemic fashion in the community, thus confirming the worldwide distribution.54
Early mechanisms and markers related to
airway inflammation, the development of
the immune system and childhood wheezing
disorders
We were able to add towards the knowledge about the effects of environmental triggers on airway in-flammation. We found that smoking during preg-nancy has an effect on components of the innate immune system of the offspring. Total leucocyte counts in cord blood were significantly lower if mothers smoked during pregnancy. The decrease was most prominent in neutrophils, monocytes, den-dritic cells and lymphocytes.57 Investigating the impact of outdoor air pollution on cytokine levels as of monocyte chemotactic protein-1 (MCP-1), interleukin-6 (IL-6), IL-10, for which large changes are seen after exposure to high pollution levels such as after bushfires, we found only small effects in cord blood of healthy term infants.58,59
Assessing the quantitative effect of the acute-phase plasma collection mannose-binding lectin (MBL) on respiratory morbidity, we found that low levels were only weakly associated, and high levels more strongly associated, with the incidence and severity of respiratory symptoms during the first year of life, particularly in infants with asthmatic parents.60
What are the main strengths
and weaknesses?
The BILD cohort, with its unique data set of com-prehensive lung function data, particularly during Table 5 Risk factors for respiratory disease of infants
during Phase 1 of the BILD cohort Number of infants Proportion of infants (%) History of maternal asthma
Negative 320 88.6
Positive 39 10.8
Unknown 2 0.6
History of paternal asthma
Negative 323 89.0
Positive 36 9.9
Unknown 4 1.1
History of maternal atopic diseasea
Negative 233 64.2
Positive 126 34.7
Unknown 4 1.1
Skin-pick test results in mothersb
Negative 197 65.0
Positive 106 35.0
Maternal smoking during pregnancy
Negative 316 87.5
Positive 42 11.6
Unknown 3 0.8
Paternal smoking during pregnancy
Negative 280 77.1 Positive 76 20.9 Unknown 7 1.9 Older siblings None 181 50.1 One 114 31.6
More than one 58 16.1
Unknown 8 2.2
This Table shows the distribution of risk factors for respiratory disease of study participants during Phase 1 of the BILD cohort, i.e. during the first year of life (n ¼ 364, information could not be retrieved for 3 out of 14 drop-outs during Phase 1).
a
Either maternal history of allergic rhinitis, allergic asthma or atopic dermatitis.
bSkin-prick tests were performed in a subgroup of 303 mothers.
Tests were positive in case of hives bigger than positive control (histamine) in any of the tested common allergens.
infancy, is an integrative and interdisciplinary frame-work to analyse how predisposing factors affect lung development from pregnancy through childhood and to predict later risk of respiratory disease. Especially with regard to the costly and time-consuming lung function measurements in infants, the BILD cohort has a number of methodological strengths, and normative data collected during these measurements in the BILD cohort have been published meanwhile.61 To ensure comparability, we measure lung function at any age in a standardized way, using the same tech-nique and equipment on every subsequent occasion in the same order. Measurements are performed according to the latest recommendations by the ERS and ATS; often we were even stricter. For instance, for the analysis of tidal breathing at the age of 1 month, we used 100 instead of 30 breaths as currently recommended, for better accuracy.21 There is still enough flexibility to develop new lung function testing methods.32,36 To validate exposure to air pol-lutants, we have started to assess individual exposure to air pollutants using passive samplers, which is expensive and has yet been rarely used in studies assessing the effect of long-term exposure to air pollution on lung function.
The BILD cohort has a prospective design. It in-cludes data assessments before and after birth, close follow-up with weekly standardized telephonic inter-views until the age of 1 year and a detailed reassess-ment at the age of 6 years. Lung function tests are performed at several time points covering the vulner-able phases of lung development (growth of airways and alveoli) during gestation and childhood. This fa-cilitates disentangling effects of pre- and perinatal risk factors from those of post-natal risk factors, such as exposure to airway pollutants.
With all included infants being healthy and of a narrow age range and all measurements in infants performed during quiet natural sleep, comparability within the cohort is given. Confounders such as physical activity, obesity and hypoxia important for older children are negligible in the studied infants. Nevertheless, these influences are prospectively assessed and will be included in the analysis of meas-urements at school age.
We have only minimal attrition during the study, especially during Phase 1, comparing favourably with other studies.62 This is probably due to the regular follow-up, particularly the weekly telephone interviews during the first year of life. The draw-back of such a detailed time-consuming study is that it can only be done on a limited number of participants.
Selection bias is not a major issue, as all participants are recruited prenatally without knowledge of expos-ures and outcomes. These are assessed and analysed independently from each other. So far, we have been able to adjust for known biological, time-variant and further possible confounders.
All involved families were recruited in maternity hospitals and only a minority of children born during the study period in the region was recruited. Although the main reason for non-participation was lacking information about the study, participants are likely to be biased towards a well-educated middle-class population. This is the case with most studies involving detailed measurements. In our ana-lyses, social class has so far neither been a risk factor nor a risk modifier for outcome measures. Therefore, although we believe that most results are fairly rep-resentative for the general population, it must be kept in mind that not all findings can be extrapolated to all Swiss or European children. Replication of the findings in independent cohorts is desirable.
Can I get hold of the data?
Where can I find out more?
The Bern cohort studies are carried out at the Department of Paediatric Pulmonology at Children’s University Hospital Bern, Switzerland. The depart-ment’s homepage provides information regarding personal contact data, team members and publica-tions ( http://www.kinderkliniken.insel.ch/kiheil-pneu-mologie.html). The principal investigator is Prof. Dr Urs Frey, MD PhD, head of the University Children’s Hospital (UKBB), Basel, Switzerland. Researchers interested in collaborative work or further information are invited to contact the principal inves-tigator, Urs Frey, urs.frey@ukbb.ch.
Supplementary Data
Supplementary Data are available at IJE online.
Funding
Swiss National Science Foundation (32003B_124654/ 1, B0-12099, to O.F.; B0-12099, 3200-052197.97/1 to P.L.; 3200-B0-12099, 3200-052197.97/ 1, 3233-069348, 3200-069349 to C.K.; and 32003B_ 124654/1, B0-12099, 052197.97/1, 3200-052197.97/2, 3200-068025 and 32-68025.02 to U.F.); European Respiratory Society (long-term research fel-lowship 675 to O.F.); Austrian, German and Swiss Paediatric Respiratory Society (training scholarship 2009 to O.F.).
Acknowledgements
We thank all study participants and their families in the canton of Bern for participating in the study and the staff of the four major maternity hospitals in the Bernese region (Klinik Engeried-Sonnenhof, Lindenhofspital, Salem-Spital, Universita¨tsklinik fu¨r Frauenheilkunde Bern) for support and recruitment.
We also thank the study nurses Monika Graf, Barbara Hofer and Christine Becher and the lung function technicians Gisela Wirz and Sandra Luescher for their invaluable assistance and support.
Conflict of interest: None declared.
References
1
Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995;332:133–38.
2Braun-Fahrlander C, Gassner M, Grize L et al. No further increase in asthma, hay fever and atopic sensitisation in adolescents living in Switzerland. Eur Respir J 2004;23: 407–13.
3
Kuehni CE, Davis A, Brooke AM, Silverman M. Are all wheezing disorders in very young (preschool) children increasing in prevalence? Lancet 2001;357:1821–25. 4Bush A, Menzies-Gow A. Phenotypic differences between
pediatric and adult asthma. Proc Am Thorac Soc 2009;6: 712–19.
5
Spycher BD, Silverman M, Brooke AM, Minder CE, Kuehni CE. Distinguishing phenotypes of childhood wheeze and cough using latent class analysis. Eur Respir J 2008;31:974–81.
6
Baraldi E, Filippone M. Chronic lung disease after prema-ture birth. N Engl J Med 2007;357:1946–55.
7
von Mutius E, Le Souef PN. Early gene-environment interactions: can they inform primary preventive strate-gies for asthma? Semin Respir Crit Care Med 2007;28: 255–63.
8Morgan WJ, Stern DA, Sherrill DL et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005;172:1253–58.
9
Frey U. Why are infants prone to wheeze? Physiological aspects of wheezing disorders in infants. Swiss Med Wkly 2001;131:400–6.
10Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J 2006;25:680–86.
11Lopez Bravo IM, Sepulveda H, Valdes I. Acute respiratory illnesses in the first 18 months of life. Rev Panam Salud Publica 1997;1:9–17.
12
Latzin P, Roosli M, Huss A, Kuehni CE, Frey U. Air pol-lution during pregnancy and lung function in newborns: a birth cohort study. Eur Respir J 2008;33:594–603. 13
Moffatt MF, Kabesch M, Liang L et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 2007;448:470–73.
14
Ober C, Hoffjan S. Asthma genetics (2006): the long and winding road to gene discovery. Genes Immun 2006;7: 95–100.
15
Guerra S, Martinez FD. Asthma genetics: from linear to multifactorial approaches. Annu Rev Med 2008;59: 327–41.
16
Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol 2008;8:169–82.
17Kuehni CE, Brooke AM, Strippoli MP, Spycher BD, Davis A, Silverman M. Cohort profile: the Leicester re-spiratory cohorts. Int J Epidemiol 2007;36:977–85. 18
Strippoli MP, Silverman M, Michel G, Kuehni CE. A parent-completed respiratory questionnaire for 1-year-old children: repeatability. Arch Dis Child 2007;92:861–65. 19
Asher MI, Keil U, Anderson HR et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995;8:483–91.
20Silverman M, Wang M, Hunter G, Taub N. Episodic viral wheeze in preschool children: effect of topical nasal corticosteroid prophylaxis. Thorax 2003;58:431–34. 21
Bates JH, Schmalisch G, Filbrun D, Stocks J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J 2000;16:1180–92.
22
Beydon N, Davis SD, Lombardi E et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool chil-dren. Am J Respir Crit Care Med 2007;175:1304–45. 23
Frey U, Stocks J, Coates A, Sly P, Bates J. Specifications for equipment used for infant pulmonary function test-ing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J 2000;16: 731–40.
24
Frey U, Stocks J, Sly P, Bates J. Specification for signal processing and data handling used for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J 2000;16:1016–22.
25
Frey U. The high speed interrupter technique (HIT) and its role in wheezing disorders in infants. Pediatr Pulmonol Suppl 1997;16:242–44.
26Frey U, Suki B, Kraemer R, Jackson AC. Human respira-tory input impedance between 32 and 800 Hz, measured by interrupter technique and forced oscillations. J Appl Physiol 1997;82:1018–23.
27
Frey U, Jackson AC, Silverman M. Differences in airway wall compliance as a possible mechanism for wheezing disorders in infants. Eur Respir J 1998;12:136–42. 28Frey U, Silverman M, Kraemer R, Jackson AC.
High-frequency respiratory impedance measured by forced-oscillation technique in infants. Am J Respir Crit Care Med 1998;158:363–70.
29
Frey U, Makkonen K, Wellman T, Beardsmore C, Silverman M. Alterations in airway wall properties in in-fants with a history of wheezing disorders. Am J Respir Crit Care Med 2000;161:1825–29.
30
Henschen M, Stocks J, Brookes I, Frey U. New aspects of airway mechanics in pre-term infants. Eur Respir J 2006; 27:913–20.
31
Jackson AC, Tennhoff W, Kraemer R, Frey U. Airway and tissue resistance in wheezy infants: effects of albuterol. Am J Respir Crit Care Med 1999;160:557–63.
32
Latzin P, Sauteur L, Thamrin C et al. Optimized temperature and deadspace correction improve ana-lysis of multiple breath washout measurements by
ultrasonic flowmeter in infants. Pediatr Pulmonol 2007;42: 888–97.
33
Schibler A, Hall GL, Businger F et al. Measurement of lung volume and ventilation distribution with an ultra-sonic flow meter in healthy infants. Eur Respir J 2002;20: 912–18.
34
Thamrin C, Latzin P, Sauteur L, Riedel T, Hall GL, Frey U. Deadspace estimation from CO2 versus molar mass meas-urements in infants. Pediatr Pulmonol 2007;42:920–27. 35
Thamrin C, Frey U. Effect of bacterial filter on meas-urement of interrupter resistance in preschool and school-aged children. Pediatr Pulmonol 2008;43:781–87. 36
Hutten GJ, van Eykern LA, Latzin P, Kyburz M, van Aalderen WM, Frey U. Relative impact of respiratory muscle activity on tidal flow and end expiratory vol-ume in healthy neonates. Pediatr Pulmonol 2008;43:882–91. 37
Frey U, Silverman M, Barabasi AL, Suki B. Irregularities and power law distributions in the breathing pattern in preterm and term infants. J Appl Physiol 1998;85:789–97. 38
Schibler A, Schneider M, Frey U, Kraemer R. Moment ratio analysis of multiple breath nitrogen washout in infants with lung disease. Eur Respir J 2000;15:1094–101. 39
Frey U, Silverman M, Suki B. Analysis of the harmonic content of the tidal flow waveforms in infants. J Appl Physiol 2001;91:1687–93.
40
Cernelc M, Suki B, Reinmann B, Hall GL, Frey U. Correlation properties of tidal volume and end-tidal O2 and CO2 concentrations in healthy infants. J Appl Physiol 2002;92:1817–27.
41
Schibler A, Frey U. Role of lung function testing in the management of mechanically ventilated infants. Arch Dis Child Fetal Neonatal Ed 2002;87:F7–F10.
42Frey U. Clinical applications of infant lung function test-ing: does it contribute to clinical decision making? Paediatr Respir Rev 2001;2:126–30.
43
Frey U, Reinmann B, Stocks J. The infant lung function model: a mechanical analogue to test infant lung func-tion equipment. Eur Respir J 2001;17:755–64.
44
Frey U, Kuehni C, Roiha H et al. Maternal atopic disease modifies effects of prenatal risk factors on exhaled nitric oxide in infants. Am J Respir Crit Care Med 2004;170: 260–65.
45
Baldwin DN, Suki B, Pillow JJ, Roiha HL, Minocchieri S, Frey U. Effect of sighs on breathing memory and dynam-ics in healthy infants. J Appl Physiol 2004;97:1830–39. 46
Baldwin DN, Pillow JJ, Stocks J, Frey U. Lung-function tests in neonates and infants with chronic lung disease: tidal breathing and respiratory control. Pediatr Pulmonol 2006;41:391–419.
47Kondo T, Minocchieri S, Baldwin DN, Nelle M, Frey U. Noninvasive monitoring of chest wall movement in infants using laser. Pediatr Pulmonol 2006;41:985–92. 48
Hall GL, Reinmann B, Wildhaber JH, Frey U. Tidal exhaled nitric oxide in healthy, unsedated newborn
infants with prenatal tobacco exposure. J Appl Physiol 2002;92:59–66.
49
Riedel T, Kyburz M, Latzin P, Thamrin C, Frey U. Regional and overall ventilation inhomogeneities in pre-term and pre-term-born infants. Intensive Care Med 2009;35: 144–51.
50
Latzin P, Roth S, Thamrin C et al. Lung volume, breathing pattern and ventilation inhomogeneity in preterm and term infants. PLoS One 2009;4:e4635.
51
Martinez FD. Asthma treatment and asthma prevention: a tale of 2 parallel pathways. J Allergy Clin Immunol 2007; 119:30–33.
52
Latzin P, Frey U, Roiha HL et al. Prospectively assessed incidence, severity, and determinants of respiratory symp-toms in the first year of life. Pediatr Pulmonol 2007;42: 41–50.
53
Latzin P, Kuehni CE, Baldwin DN, Roiha HL, Casaulta C, Frey U. Elevated exhaled nitric oxide in newborns of atopic mothers precedes respiratory symptoms. Am J Respir Crit Care Med 2006;174:1292–98.
54
Regamey N, Frey U, Deffernez C, Latzin P, Kaiser L. Isolation of human bocavirus from Swiss infants with respiratory infections. Pediatr Infect Dis J 2007;26:177–79. 55Regamey N, Kaiser L, Roiha HL et al. Viral etiology of
acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J 2008;27:100–5.
56
Kaiser L, Regamey N, Roiha H, Deffernez C, Frey U. Human coronavirus NL63 associated with lower respira-tory tract symptoms in early life. Pediatr Infect Dis J 2005; 24:1015–17.
57
Pachlopnik Schmid JM, Kuehni CE, Strippoli MP et al. Maternal tobacco smoking and decreased leukocytes, including dendritic cells, in neonates. Pediatr Res 2007; 61:462–66.
58
van Eeden SF, Tan WC, Suwa T et al. Cytokines involved in the systemic inflammatory response induced by expos-ure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med 2001;164:826–30.
59
Schaub B, Frey U, Roosli M, Latzin P. Air pollution during pregnancy and coord blood cytokine secretion. European Respir J 2008;32:246s.
60Schlapbach LJ, Latzin P, Regamey N et al. Mannose-binding lectin cord blood levels and respiratory symptoms during infancy: a prospective birth cohort study. Pediatr Allergy Immunol 2009;20:219–26.
61
Fuchs O, Latzin P, Thamrin C et al. Normative data for lung function and exhaled nitric oxide in unsedated healthy infants. Eur Resp J 2010. published ahead of print, doi:10.1183/09031936.00125510.
62
Douglas RM, Woodward A, Miles H, Buetow S, Morris D. A prospective study of proneness to acute respiratory illness in the first two years of life. Int J Epidemiol 1994; 23:818–26.