ANALYSIS OF A DIAMOND CVD PROCESS USING
COMPUTER SIMULATION
by Masaki Nagai
B.E. Nuclear Engineering Osaka University 1988 M.E. Nuclear Engineering
Osaka University 1990
Submitted to the
DEPARTMENT OF MATERIALS SCIENCE AND ENGINEERING
in partial fulfillment of the requirements for the degree ofMASTER OF SCIENCE IN MATERIALS SCIENCE AND ENGINEERING
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
September 1996© 1996 Massachusetts Institute of Technology All rights reserved
Signature of Author
Department of Materials Sc4Ence and Engineering August 9, 1996
Certified by
Profe sor Davia A. movyance Professor of Materials Engineering
Thesis Supervisor
Accepted by
Professor Linn W. Hobbs Chairman, Departmental Committee on Graduate Students
ANALYSIS OF A DIAMOND CVD PROCESS USING
COMPUTER SIMULATION
by
Masaki Nagai
Submitted to the Department of Materials Science and Engineering on August 9, 1996 in partial fulfillment of the requirements for the
degree of Master of Science in Materials Science and Engineering
Abstract
A mathematical model was customized to represent fluid flow, heat transfer, and chemical kinetics in a hot filament diamond CVD reactor. Computed results from a two dimensional system suggest that heterogeneous effects of the filament surface should be
included in the model for a more realistic representation of the system. An assumption was made to consider the effects of the filament surface as a catalytic factor for the hydrogen (H2)
dissociation reaction. The computational results for the gas species concentrations then gave a good agreements with
measurements reported in the literature. By examining the
characteristic diffusion lengths for the different species in the system, it was found that the concentration of methyl radicals (CH3), which are the precursors for diamond growth, as well as
methane (CH4) molecules are determined by chemical kinetics in
the gas phase. In contract, the concentration of atomic hydrogen (H) was not affected very much by chemical kinetics since atomic hydrogen created at the filament can diffuse very fast to the
substrate. The reaction, CH4 + H = CH3 + H2, was found to play a key role in determining the concentrations of CH4 and CH3 in the reactor. General trends involving the effects of several process parameters were identified in the analysis.
Thesis Supervisor: Professor David K. Roylance
Contents
Abstract .. ... 2 List of Symbols... 4 List of Figures... 7 List of Tables ... 9 Acknowledgements ...10 1. INTRODUCTION ... 111-1. Description of the diamond CVD process and applications of diamond films ... 12
1-2. Literature review ... 16
1-2-1. Hot filament diamond CVD ... 16
1-2-2. Experimental measurements on the hot filament CVD process ... 19
1-2-3. Modelling of the hot filament CVD process...28
2. DESCRIPTION OF THE MATHEMATICAL MODEL ... 37
2-1. General assumptions...37
2-2. Balance equations for mass, momentum, and energy...39
2-3. Gas species transport equations...40
2-4. Thermodynamic properties of gas mixtures...42
2-5. Transport properties of gas mixtures...42
2-6. Gas phase reactions ... 48
2-7. Surface reactions... 52
2-8. Boundary conditions ... ... 52
2-9. Numerical solution method (finite-volume method).. ..55
2-10. Dimensionless numbers ... 57
3. SIMULATION OF A TWO DIMENSIONAL AXISYMMETRIC REACTOR ... 60
3-1. Gas phase reaction mechanisms...60
3-2. Surface reaction mechanisms ... 63
3-3. Defect generation model...70
3-4. Simulation of the hot filament diamond CVD process...72
3-5. Results and discussion ...76
3-6. Predictions of the general trends in the hot filament diamond CVD process ... 92
4. CONCLUSIONS ... ... 95
Bibliography...98
Appendix A. Qualitative behavior of the hot filament diamond CVD process...103
Appendix B. Thermodynamic data and transport properties of the gas phase species...114
List of Symbols
Ain cross section area of inflow, m2
aj thermal diffusion factor for gas pair i-j C, molar concentration of gas species i, mole m-3
cp specific heat per unit mass, J-kg-1.K-1
Cp molar specific heat, J-mole-1.K-i
Dii binary ordinary diffusion coefficient, m2 "s-I D effective ordinary diffusion coefficient, m2.s- 1
D Wilke effective ordinary diffusion coefficient, m2.s-1
D T thermal diffusion coefficient, kg-m-1.s-'
EA activation energy, J0mole-1
f, species mole fraction for gas species i
Go standard Gibbs energy change of formation for species i, J mole-1
AGo standard Gibbs energy change for reaction k, J'mole-1
k
g gravity constant, m's-2
Hi molar enthalpy for species i, J-mole-1
Ho standard heat of formation, J'mole-1 I unit tensor
j diffusive mass flux, kg-m-2 "s-I
k Boltzmann's constant, 1.38x10-23 J.K-1
kk, forward reaction rate constant for kth gas reaction kk.b reverse reaction rate constant for kth gas reaction K number of gas reactions
Kk equilibrium constant for the kth gas reaction L number of surface reactions
id characteristic diffusion length, m m, molar mass, kg-mole-i
M average molar mass, kg-mole-i M number of surface species N number of gas species
N, Avogadro's number 6.023x1023 mole-'
n unit vector P pressure, Pa
P0 standard pressure, 1.0135x10 5 Pa
P, net mass production rate at the surface
Q
volumetric flow rate at standard conditions, sim Q total radiative heat sourcer radial distance, m
R gas constant, 8.314 Jimole-1.K-1
Rg, forward reaction rate for the kth gas reaction, mole m-3.s-'
R
gb reverse reaction rate for the kth gas reaction, mole m-3 .s-1Rs reaction rate for the lth surface reaction, molerm-2.s-'
Rd molar destruction rate,
mole-m-3 .s-1
Req partial equilibrium ratio So standard entropy, J'mole-1.K-1 t time, s
T absolute temperature, K T* reduced temperature = kT/E To standard temperature, 273.15 K V velocity vector, m's-1
Greek symbols
X, stoichiometric coefficient for the jth surface species in the ith surface reaction
6 Kronecker delta function
E/k ratio of maximum energy of attraction and Boltzmann constant, K
K thermal conductivity, W-m- I .K-1
S dynamic viscosity, Pa's
vik stoichiometric coefficient for ith gas species in kth gas reaction
p density, kg.m-3
0 collision diameter,
A
0 stoichiometric coefficient for ith gas species in Ith surface reaction
,e chemical destruction time, s
t viscous stress tensor, N.m-2
Q) mass fraction
Q1 tabulated function of T* Q tabulated function of T*
D
Subscripts
with respect to the ith species with respect to gas pair i-j
k
with respect to kth gas reaction
with respect to Ith surface reaction Superscripts
0
at standard temperature and pressure due to ordinary diffusion
LIST OF FIGURES
Figure 1 : A schematic diagram of the diamond CVD process ... 13
Figure 2 : Production process of a diamond film for cutting tools...15
Figure 3 : Basic setup of a hot filament CVD reactor...17
Figure 4 : Experimental setup of Martin and Hill [11,12] ... 24
Figure 5 : Experimental configurations of Debroy [22]...31
Figure 6 : Grid cells and staggered grids for finite volume method ... 56
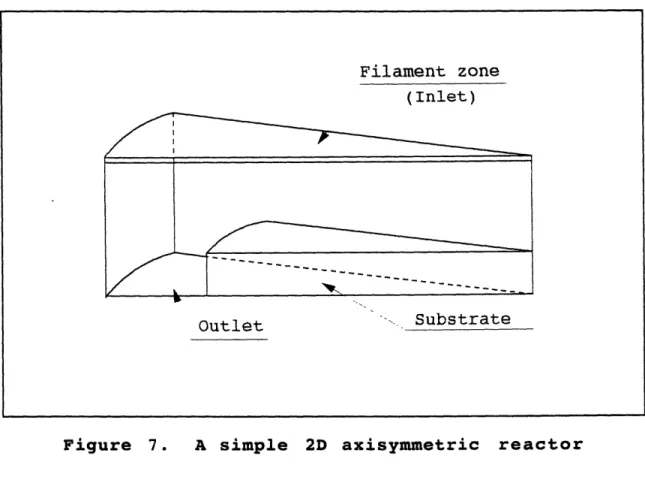
Figure 7 : A simple 2D axisymmetric reactor...61
Figure 8 : A schematic diagram of the surface of a diamond film ... 65
Figure 9 : Experimental setup of Hsu [9]...74
Figure 10: Computed gas flow in the region between the filament and the substrate in Hsu's reactor...77
Figure 11: A schematic diagram of the filament zone...79
Figure 12: Temperature gradient between the filament and the substrate in Hsu's reactor...82
Figure 13: Gas species mole fraction between the filament and the substrate in Hsu's reactor...83
Figure 14: Partial equilibrium ratio of the reaction CH4 + H = CH3 + H2 . . . 84
Figure 15: Relative concentrations of H, CH3, and CH4 between the filament and the substrate ... 86
Figure 16: Characteristic diffusion lengths for H, CH3, and CH4 . . . .88
Figure 17: Relative concentration of H and CH3 above the substrate ...90
Figure 18: Relative deposition rate and defect density above the substrate...91
Figure 19: Figure 20: Figure 21: Figure 22: Figure Figure Figure 23: 24:
25:
Effect of filament zone temperature on H and CH3
mole fractions ... 107 Effect of temperature in the filament zone
on the growth rate ...108 Effect of temperature in the filament zone
on the defect density...109 Effect of total pressure on H and CH3 mole
fractions ... 110 Effect of total pressure on the growth rate...111 Effect of total pressure on the defect density.... 112 Effect of total pressure on the uniformity of
LIST OF TABLES
Table 1 : Typical experimental conditions for the hot
filament diamond CVD process...19 Table 2 : Dimensionless numbers for the hot filament diamond
CVD process ...59
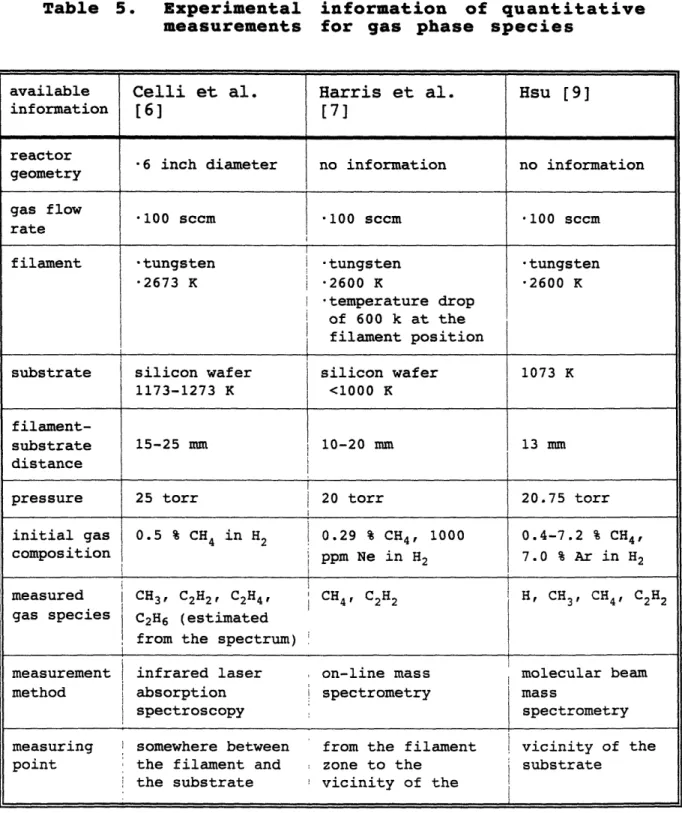
Table 3 : Gas phase reaction mechanisms...62 Table 4 : Surface reaction mechanisms ...69 Table 5 : Experimental information of quantitative
measurements for gas phase species ... ... 73
Table 6 : Experimental conditions of Hsu [9]...75 Table 7 : Computational conditions for Hsu's reactor...75
Table 8 : Comparison between measured and calculated gas
species mole fractions...78 Table 9 : Comparison between measured and calculated gas
species mole fractions assuming the heterogeneous
effects of the filament surface ... 79 Table 10: Predictions on the general trends of the hot
filament diamond CVD process...94 Table 11: Computational conditions for qualitative analysis..104 Table 12: Thermodynamic data of the gas species used in the
diamond CVD modeling
Table 13: Transport properties of the gas species used in the diamond CVD modeling
ACKNOWLEDGEMENTS
I would like to express my grateful appreciations to Professor Julian Szekely for his warm welcome to his research
group and for his advice on this work.
I would like to thank Professor David K. Roylance for being my advisor under short notice and for his encouragement and
discussion.
I am indebted to Dr. Gerardo Trapaga for many helpful discussions and his continuing support.
Special thanks are given to people in the Mathematical Modelling Group of the Department of Materials Science and
Engineering: Christy Choi, Robert Hyers, Nicole Lazo, Liping Li, Patricio Mendez, Adam Powell, Kris Schwenke, and Hirokazu Shima.
I owe much to my parents in Kobe, Japan for their great love and support in my life in U.S.A.
CHAPTER 1. INTRODUCTION
The ultimate objectives of this research are to develop a mathematical model for a filament assisted diamond chemical vapor deposition (CVD) reactor and use such model to investigate the
behavior of the reactor under various operating conditions and to understand the role of different process parameters, such as the filament temperature, substrate temperature, and inlet gas
composition. In this thesis, as a first step towards achieving the objectives, we developed a two dimensional mathematical model with detailed chemical kinetics to represent a typical
experimental reactor and to grasp the general behavior of the hot filament CVD process.
Detailed numerical modeling of diamond CVD process can work as an effective complement to the actual production operations, providing information of the fluid flow, heat transfer, and
chemical kinetics. A comprehensive computational model for the diamond CVD process requires solving for the temperature,
velocity, and species concentration fields, including both
convective and diffusive species transport, together with complex gas phase and surface reaction mechanisms. In addition, the
model should be capable of estimating the quality of the resulting diamond films.
Once the model is solved and understood, we can predict the various kinetics inside the reactor together with the gas phase
quality, and uniformity of the diamond films. We then are able to utilize it as the best tool to better understand the behavior of the system and to optimize the production conditions.
1-1 Description of the diamond CVD process and
applications of diamond films
Since the first publication by Matsumoto et al [1]
describing diamond CVD processes in detail at pressures and temperatures where diamond is metastable with respect to graphite, diamond CVD processes have been intensively
investigated and a multitude of diamond film production methods has been developed.
A diamond CVD process is schematically sketched in figure 1.
The general understanding of diamond CVD processes can be
described as follows: Gaseous reactants, typically methane (CH4)
and hydrogen (H2), flow into a reactor under reduced pressure (1
torr (133 Pa) - 200 torr (26600 Pa)). They are activated and
decomposed to carbon-containing reactive gas species by thermal or electromagnetic energy. Convection and diffusion mechanisms transport the reactive species to the substrate where they
decompose to diamond, together with other species (hydrogen, other hydrocarbons, graphite as an impurity), by means of
heterogeneous reactions. Atomic hydrogen is also generated and transported to the substrate, where it activates surface sites for the incorporation of reactive species, and promotes etching
REACTANTS
CH
4 ACTIVATION H 2H CH4 + H -CH3 + H2FLOW AND REACTION
DIFFUSIO
DIAM
C"r1- A -r1C
N
MOND
FILM
Figure 1. A schematic diagram of diamond CVD process
H
2of non-diamond species, such as graphite, from the deposition surface. This function of the atomic hydrogen is considered as a key process to produce a high quality diamond film in diamond CVD.
Diamond is currently grown by many different techniques, using hot filaments, microwave plasmas, combustion flames, and high speed direct current arc-jets, as the activation methods. Among these techniques, the hot filament diamond CVD method is the most widely used production technique because of its simple
setup, controllability of production parameters and flexibility for the scaling up of the reactor size.
Several companies commercialize diamond CVD products. The diamond film produced by CVD method has, of course, the typical properties of diamond, such as high hardness, high thermal
conductivity, and high degree of chemical inertness. It can be applied for heat sinks, cutting tools and wear resistant tools. A production process for cutting tools is sketched in figure 2
[2]. First, a diamond film is synthesized in a reactor (a). After the surface of the diamond on the substrate is polished to meet surface roughness required for cutting tools, the film is cut by laser (b). Next, the substrate is dissolved by acid (c) to get freestanding CVD diamond pieces (d). The freestanding CVD diamond pieces is brazed to cemented carbides to be the final products (e).
Figure 2. Production process of a diamond film for cutting tools [2]
the diamond film must be of high quality (low defect density). In terms of cost assessment, fast deposition of the film is desired. Since the diamond film surface has to be polished during the production process, good uniformity of the film thickness is also desired to reduce the polishing cost.
As a general rule, two properties, low defect density and high deposition rate, represent conflicting issues in diamond CVD operations. We believe that there should be the optimum
production condition and geometry that satisfies an optimal combination for both properties. Numerical simulations should help to find such conditions.
1-2 Literature review
In this section, important research efforts related to the hot filament diamond CVD will be reviewed. First, a typical
experimental setup for the hot filament method will be described. Next, we will focus on diagnostic techniques that intended to identify important gas species inside the reactor. Finally,
several modeling investigations on hot filament reactors will be discussed.
1-2-1 Hot filament diamond CVD
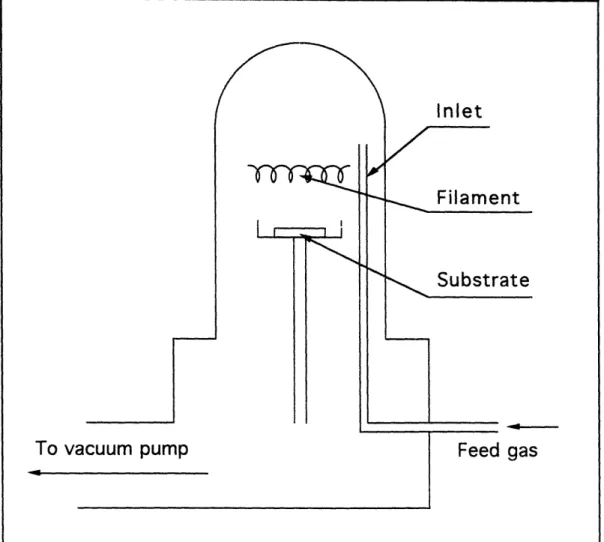
Figure 3 shows a typical hot filament diamond CVD reactor that was described by Matsumoto et al. in the first publication on diamond CVD in 1982 [1,3]. They used Raman spectroscopy and
To vacuum pump
Figure 3. Basic setup of a hot filament CVD reactor
scanning electron microscopy to identify the deposits as diamond. All major components necessary to achieve diamond deposition are included in figure 3. In a reduced pressured reactor, a
coiled or uncoiled refractory metal filament (usually tungsten or tantalum), which provides thermal energy to activate a gas
mixture of methane (CH4) and hydrogen (H2), is located at a distance of 5 - 20 mm from the substrate. The filament are resistively heated up to about 2000 - 2600 K and the substrate temperature is controlled at 1000 - 1300 K. Although the feed gases are typically Methane (CH4) diluted to about 1.0 % with
hydrogen, other species such as organic compounds + oxygen [4], acetylene (C2H2) + hydrogen + oxygen [5] have also been reported
as feed gas mixtures. Molybdenum, silicon, or silicon carbide are used as a substrate.
With this simple set up, diamond growth rates in the range 0.1- 1.0 !im/h can be achieved. Typical experimental data are listed in Table 1.
Table 1. Typical experimental
filament diamond CVD
conditions process for the hot1-2-2 Experimental measurements on the hot filament CVD
process
Many gas phase diagnostic studies have been carried out on the hot filament process intending to analyze the spatial
distribution of gas phase species, such as hydrogen (H2), atomic
hydrogen (H), methyl radical (CH3), acetylene (C2H2), methane
(CH4), existing in the region between the filament and the
substrate. These studies have been also aimed at understanding the mechanism of diamond formation under conditions of
metastability.
(a) Measurement of carbon containing species
Filament temperature 2000 K - 2600 K
Substrate temperature 1000 K - 1300 K
Filament-substrate distance 5 mm - 20 mm
Feed gas mixture 0.2 - 1.0 % CH
4 in H2
Pressure 1 - 200 torr
Filament material W , Ta
Substrate material Si, SiC, Mo
The first study of gas phase species in the region between the filament and substrate was performed by Celii et al. [8], employing infrared laser absorption spectroscopy to detect methane (CH4), methyl radical (CH3), acetylene (C2H2), and
ethylene (C2H4). During film growth, portions of the gas mixture
in the region above the substrate were scanned by the laser, and the species concentrations were estimated from the frequencies of the infrared absorption spectrum of the gas. Traces of acetylene
(C2H2) , methane (CH4), ethylene (C2H4), methyl radical (CH3) were
detected. Ethane (C2H6), various C3 hydrocarbon species, and
methylene (CH2) were below the sensitivity level.
Methane (CH4) and acetylene (C2H2) mole fractions in the
region immediately above the substrate were measured by Harris et al. [7] as a function of filament-substrate distance with the aid of a quartz sampling probe and on-line mass spectrometry. Feed gases were methane (CH4) and hydrogen (H2). They reported that the ratio of the concentration of CH4 and C2H2 reached at 1:1 in the vicinity of the filament. A temperature difference of 600 K was also measured between the filament temperature (2600 K) and that in the vicinity of the filament (2000 K).
Harris and Weiner [8] estimated methyl radical (CH3) and atomic hydrogen (H) concentrations at the surface of the diamond
film. Using a quartz sampling probe 3-5 mm from the hot tungsten filament and gas chromatography of the sampled gas, they measured ethylene (C2 H4) and ethane (C2 6). They assumed that these
species were formed by the following reactions involving the methyl radical (CH3) inside the probe:
CH3 + CH3 C2H 4+ H2 and CH3 + CH3 = C2H6
The mole fraction of methyl radicals in the reactor was therefore calculated from the sum of ethylene (C2H4) and ethane (C2H6) mole
fractions. From the methyl (CH3) mole fraction, atomic hydrogen (H) concentrations in the reactor were also calculated using the assumption of partial equilibrium for the reaction:
CH
4+
H
-
CH
3+
H
2The methyl radical (CH3) mole fraction increased from 2x10-4 to 7x10-4, when the methane (CH4) concentration was raised from 0.5 to 3.2 % volume. Simultaneously, the atomic hydrogen
concentration decreased slightly.
Hsu [9,10] used molecular beam mass spectrometry, which is the most direct measurement technique for the gas phase species, to determine the concentrations of atomic hydrogen (H), methyl
radical (CH3), acetylene (C2H2), and methane (CH4) in a hot filament reactor under diamond growth conditions. The gas
species were sampled through a 300 pm diameter orifice on the
center of the substrate and analyzed by the molecular beam
quadruple mass spectrometry. With a small amount of argon added to the methane/hydrogen mixture as the reference species, mole
fractions of H, H2, CH3, CH4, and C2H2 were determined with an
estimated error of 20 %.
With methane (CH4) fractions in the feed gas smaller than
1%, as usually employed in the hot filament diamond CVD,
acetylene (C2H2) was found to be the most dominant hydrocarbon
species at the substrate surface. With initial CH4 concentration
higher than 2%, CH4 became the dominant species over C2H2.
The atomic hydrogen mole fraction showed a sudden decrease
from about 2x10-3 at 2% methane concentration to 1x10- 4 at 7%
methane concentration.
(b) What are the "growth species" in diamond CVD?
From the above diagnostic studies, the question "What is the precursor that is responsible for diamond growth?" was left
unsolved. However, several groups have performed experiments to determine the growth species and now there appear to be
considerable evidences that the methyl radical (CH3) is the precursor for diamond growth. These studies include the "flow tube" experiments by Martin et al. [11-13], experiments with free and forced convective flow done by Schafer et al. [14], and
carbon-13 isotope studies performed at Rice university [15-17]. Martin and Hill [11] used microwave plasma only to generate a stream of atomic hydrogen in a flow tube system (figure 4). Then hydrocarbon gas species are injected to the downstream of the atomic hydrogen. After injecting a small amount about 2 % of methane (CH4) to the flowing 90% Ar/10% H2 mixture, within a
furnace at 1120 K downstream from the microwave plasma; the deposition of diamond was observed on the surface of the
substrates, which covered about 1-2 cm area, corresponding to 1 ms reaction time. In a subsequent publication [12], additions of methane (CH4) and acetylene (C2H2) were compared. Methane was found to be about an order of magnitude more effective for
diamond deposition than acetylene. In their third paper [13] the flow tube system was modelled including the gas phase reaction mechanism, and convective and diffusive mass transport.
According to their analysis, when methane (CH4) was added to the gas mixture, only methane (CH4) and methyl radical (CH3) were present in significant quantities that was able to account for a reasonable deposition rate. A reaction probability of 10-3 for
Injector
for
C-H species
ý F-
Tube
furnace
To pumpMicro
wave
Substrat s
H2/Ar
Flow
Experimental Setup of Martin and Hill [13,14]
_ ~ _ ~
-- I
- -- --- --- - - - -
diamond growth from methyl radical (CH3) was deduced from the
model, while 10-5
would apply to the growth from acetylene (C2H2).
This reactivity for acetylene is too small to account for the growth rate observed in hot filament CVD, implying that the
methyl radical (CH3) is the major growth species under such
conditions.
Schdfer et al.[14] performed hot filament growth experiments with four parallel uncoiled tantalum wires, 11.5 mm apart from
each other, at a filament substrate distance of 8 mm. Under the free convection conditions used in this study (filament
temperature = 2470 K, 0.5 % methane in hydrogen), they observed a
strong decrease of the diamond film growth rate at the center compared to the edges of the substrate. In order to explain this effect, they hypothesized that (1) a complete conversion of
carbon species to acetylene (C2H2) occurs in the filament region,
and (2) acetylene does not act as a "growth species" for diamond.
In the subsequent experiments, feed gas (CH4/H2 mixture or C2H2/H 2
mixture) was applied as a jet striking the substrate vertically
with velocities of several 1000 cm/s. In the CH4/H2 mixture
case, bell shaped growth rate profiles with maximum growth rates
up to 2 pm/h were found in the methane case. In the C2H2/H 2
was observed. These result showed that methyl radical (CH3) formed within the gas jet in CH4/H 2 mixture was responsible for
maximum growth rates.
Isotropic competition experiments using 13CH4/ 12C H2 feed gases in the hot filament environment were performed by Chu et al. [15, 16] and Evelyn et al. [17], for polyclystalline film growth, and for homoepitaxial growth on diamond (100), (111) and
(110) planes. The first order Raman peak frequency at 1332 cm-1 for 12C diamond was found to shift to 1282 cm-' for 13C diamond. Since this Raman peak shifts as a function of 13C fraction, it was able to be used to determine the isotopic composition of the diamond films grown. Irrespective of crystallographic
orientation, the 13C mole fractions in the films turned out to be equal to the corresponding mole fractions of methane (CH4), which
was sampled directly above the growth surface. Due to the reaction, CH4 - CH3 + H, methane (CH4) can be assumed to
represent the isotopic composition of the methyl radical (CH3). Similar experiments were performed in microwave plasma CVD, also showing the methyl radical to be about an order of magnitude more efficient in diamond formation than acetylene (C2H2).
The first direct and noninvasive H atom detection in the hot filament diamond CVD environment was the study of Celii and
Butler [18]. The dependence of atomic hydrogen concentration on
the filament temperature and CH4/H2 ratio was determined
employing the resonance enhanced multiphoton ionization (REMPI). They used one coiled tungsten filament and measured the gas
sample 8 mm away from the filament. At each CH4/H2 ratio the
REMPI signal increased with temperature, in agreement with
thermodynamics of hydrogen dissociation. At a fixed temperature,
the signal decreased with increasing CH4/H2 ratio, especially
pronounced at the ratio = 3%, where a sudden drop of the signal
of atomic hydrogen (H) was observed below 2200 K. The authors
attributed this observation to some sort of surface poisoning or phase change of the filament surface, rather than to gas phase reactions. From the result of the spatial distribution of atomic hydrogen, they concluded that atomic hydrogen was transported to the deposition region by diffusion.
Schafer and Klages measured the atomic hydrogen
concentration in the vicinity of hot filament by two photon
excited laser-induced fluorescence (LIF) [19,20]. Radial atomic hydrogen concentration profiles were measured from the filament
surface to a distance 25 mm beyond. At 1.5 mbar (1.125 torr), the near surface concentration measured was about 50% of the equilibrium concentration calculated for the measured filament
temperature (2640 K). Between 10 mbar (7.5 torr) and 100 mbar (75 torr) the atomic hydrogen concentrations were found to be pressure independent.
Frenklach and Wang [21] discussed in detail several roles of atomic hydrogen for diamond growth, namely: (1) preferential
gasification of graphite, (2) stabilization of sp3 hybridization
of surface carbon atoms against transformation sp2 or spi, and
(3) formation of gaseous diamond precursors, i.e., methyl radical (CH3).
Based on their own modeling studies, the authors concluded that the most important role of atomic hydrogen was to control the concentration of activated sites on the growing diamond surface. The high activation energy for the surface site activation explained the low temperature limit (< 700 K) of
diamond deposition. At such low temperatures, the concentration of the activated site was extremely small.
In addition, H atoms serve to transform sp2 carbon sites on
the surface to sp3 bonded carbon, thus preventing the formation of non-diamond phases. Preferential etching by H atoms, however,
although occurring at high temperatures, was a relatively slow process below 1000 K and can be neglected.
1-2-3 Modelling of the hot filament CVD process
determine steady state concentrations and reaction paths of
chemical species involved in diamond CVD processes [3,21-26]. In these previous models, heterogeneous reactions at the filament were completely neglected. As for the heterogeneous reactions on the substrate surface, although in early calculations they were also neglected, recent studies have successfully included both in
a simple and complex way [27-29, 33-36].
(a) Species transport
Species are transported by convection (i.e., natural and forced) and diffusion mechanisms. In natural convection, the driving force is provided by gravity due to density differences in the fluid, caused either by temperature or by concentration gradients within the system. In forced convection, the driving
force is provided externally or mechanically, for example by a pump. In a hot filament diamond CVD reactor, natural convection occurs to transport gas phase species. Diffusion also involves two types of mechanisms, one of which is ordinary diffusion
caused by concentration gradients in the fluid and the other is thermal diffusion, which is due to temperature gradients in the fluid.
In fluid dynamics, the dimensionless Peclet number
vxl
Pe
-D
coefficient of a species considered), which is a measure of the importance of convective mass transport relative to diffusive mass transport, is used to characterize CVD processes. The peclet number is usually much larger than 1 for the convection dominated high rate diamond CVD processes, such as dc arcjet diamond CVD, while for diffusion dominated CVD process, Peclet number is less than 1. In the hot filament diamond CVD process, the Peclet number in the region between the filament and the
substrate is well below 1, thus diffusion plays a more important role than convection in affecting gas species concentrations and
film growth.
Debroy et al. [22] showed this fact experimentally. Their set up was designed to have four configurations, which involved placing the substrate below or above the filament with two
different flow directions, upward and downward gas flow as sketched in figure 5. In any configuration, there was little difference in diamond growth rate, which showed the domination of diffusive mass transport, and hence, a less important role of natural convection in the hot filament CVD process.
Tube
f•urnace
configuration
1
configuration
3
configuration
2
configuration 4
Experimental configuration of Debroy [22]
_ ~I_ _ _ ~ II _ _1_1_ II I _ _ _ _ ~ _ I _ __II__ ___ ______ _ _ _ % -- 4:1....
7
Figure 5.I
(b) Modeling of gas phase reaction mechanism
Early kinetic calculations of the hot filament environment were usually zero dimensional approximations, in which the gas
phase composition was calculated, using an assumed temperature history [23,24].
More refined calculations are based on the assumption of one dimensional gas flow, including ordinary and thermal diffusion, with a prescribed temperature profile between the filament and substrate. Flow velocities of 1 cm/s were assumed around the filament region. The exact values of this velocity are not critical for the results of the calculation, as long as Peclet number is much less than 1 (diffusion dominated flow). Harris and Weiner [8] used a detailed gas phase chemistry model with 92 reactions, including oxygen containing species, in order to
develop a model to compare with their experiments. Heterogeneous chemistry at the diamond surface was completely ignored in their
calculations. The calculated values of methane (CH4), acetylene
(C2H2), methyl radical (CH3), and atomic hydrogen (H) showed very
good agreement with the measured value in their experiments. A detailed surface and gas phase kinetics model of diamond deposition was solved under an assumed temperature profile by Frenklach and Wang [21]. Results of the gas phase calculation including 158 reactions among 50 species were used as an input for their detailed surface kinetics model. Most dominant carbon
containing species (mole fractions > 10-4) in the gas phase were
methane (CH4), methyl radical (CH3), and acetylene (C2H2). The model predicted that both increasing methane concentration and
substrate temperature increased the growth rate and deteriorated the film quality.
A diamond CVD reactor simulation by Goodwin and Gavillet [25] used a two dimensional axisymmetric stagnation flow field at the substrate, produced by a uniform z-directional axial inflow. The resulting temperature and mole fraction distributions were radially independent, thus this was essentially a one dimensional calculation. The gas phase reaction mechanism took 25 species and 56 reactions into consideration. The temperature was
specified only at the gas inlet and the substrate (2000 K and 1000 K, respectively) and varied almost linearly between these boundaries. As in the model of Frenklach and Wang [21], CH4,
CH3, and C2H2 were the only carbon containing species with mole fractions over 10-4. Although several other species were found to have enough carbon concentration to meet typical growth rates, their gas phase production rates were not sufficient to
compensate the depletion of the species at the substrate surface. Reaction probabilities of 4x10- 3 and 4x10- 4 was required for
methyl radical (CH3) and acetylene (C2H2), respectively, to give a
Kondoh et al. [26] performed a study on a hot filament process employing a rapid gas flow toward the substrate. In their model, the Peclet number was between 1 and 10. Due to the slow reaction of methyl radical (CH3) to C2Hx species, the
acetylene (C2H2) mole fraction at the substrate could be
suppressed to below 10-5, more than an order of magnitude below
the methyl radical concentration. With increasing filament substrate distance the calculated methyl radical (CH3)
concentration decreased parallel to experimental growth rate, while the acetylene (C2H2) concentration increased. This
calculation also supported that the precursor for diamond growth was the methyl radical (CH3).
(c) Modeling of the surface reaction mechanism
Several elementary mechanisms have been proposed [15-17, 27-29] for diamond growth from methyl radical (CH3) and acetylene
(C2H2) which consider how the growth monomer (CH3 or CA2 2) can be
added to a particular site on a diamond surface through a series of elementary reactions. These models have assumed that
chemistry on the diamond surfaces could be understood in terms of well-known chemistry of alkanes since there is still little
information about the elementary mechanisms of diamond deposition themselves.
Coltrin et.al [30] used the SURFACE CHEMKIN package [31, 32], which was designed to handle the kinetics of a complex set of elementary reactions at the gas/surface interface for their modeling of dc plasma gun reactors. The surface mechanism was based on the dimer mechanism proposed by Garrison et al [33], which included the pathways for the incorporation of methyl
radical (CH3), acetylene (C2H2), and carbon atom (C) from the gas
phase.
Alternatively, several groups have proposed reduced mechanisms [34, 35], which do not attempt to describe each elementary step in detail, but seek to capture the correct qualitative behavior. In this way, Goodwin [36] proposed an
interesting reduced mechanism in which entire classes of elementary reactions are grouped into a single, approximate
overall reaction.
His reduced mechanism consists of four steps.
(1) Establishment of a steady state surface radical site coverage (2) Attachment of reactive hydrocarbon species to the surface at these sites
(3) Removal back to the gas phase of the surface adsorbates, either by thermal desorption or attack by atomic hydrogen
(4) Incorporation of the adsorbate into the diamond lattice By using this mechanism, the author was able to derive simple analytical expressions for the growth rate and defect
density, which may be compared to experiments.
As a defect generation mechanism, he proposed that defects were generated when an adsorbed hydrocarbon species reacted with
a nearby adsorbate before the species was fully incorporated into the lattice. According to his assumption, the defect density was simply expressed in the following equation:
Growth rate
Defect density Growth rate 2
(Atomic hydrogen concentration)
This defect generation mechanism will be adopted to the model used in this study.
A two dimensional axisymmetric model will be developed in this work. In chapter 2, mathematical background used for the model will be described. Based on the experimental and
theoretical investigations reviewed in this chapter, the model that includes ways to calculate the growth rate and the defect density will be developed in chapter 3.
CHAPTER 2. DESCRIPTION OF THE MATHEMATICAL MODEL
The objective of CVD reactor modeling is to relate operating parameters and reactor geometry parameters to measures of film quality (purity, uniformity) and to use the improved
understanding of the underlying physics and chemistry for
practical advantages, such as process optimization, performance prediction and reactor design.
In this study, a software package, PHOENICS, is used to model one of the experimental studies reviewed in chapter 1. The
program is designed to model the behavior of CVD reactors,
including fluid flow, heat transfer relating to a multi-component gas, and both gas-phase and surface chemical reactions. The
software can simulate up to 30 gas species undergoing multi-component diffusion in addition to the conventional convective and diffusive transport. Heat transfer is linked with the view factor surface radiation model.
A list of used symbols in this chapter is provided on page
4-6.
2-1 General assumptions
In order to reduce the complexity of the problem and the computational expense for the solution of CVD modeling equations, several general assumptions can be made within certain limits so
that the accuracy and applicability of the model are not affected.
1. The gas mixture can usually be treated as a continuum. The dimensionless Knudsen number
mean free path length (1)
K = (1)
characteristic length of the geometry (L)
is calculated to check the validity of this assumption. When K is small (<0.1), this assumption is good.
2. For the pressures and temperatures used in CVD techniques, the gases may be treated as ideal gases, behaving in accordance with the ideal-gas law and newton's law of viscosity.
3. The gas flow is assumed to be laminar. In general, a fluid flow becomes turbulent when either the Reynolds number (shear driven turbulence) or the Grashof number (buoyancy driven turbulence) of the flow becomes large. These dimensionless numbers and their underlying assumptions will be examined in section 2-11 in connection to hot filament diamond reactors.
4. Gas mixture in CVD reactors may generally be treated as
transparent for heat radiation from heated walls and substrates.
which is the irreversible energy conversion to internal energy for gases undergoing sudden expansion or compression, may be neglected since no large gradients of velocity and pressure appear in CVD reactors.
2-2 Balance equations for mass, momentum, and energy
With the assumptions outlined in section 2-1, the gas flow in CVD reactors is described by the conservation equations
ap
for mass
at
= - Vo(p0) (2)for momentum = -V(pVV) + V-. - VP + pg (3)
ct
(convective) (diffusive) (pressure) (gravity)
2
t =
p(VV
+(VV)f) -
y2(V0V)
(i
3 (4) * transposed vector for energy.aD
cP-t-(pT) = - c V(pT) + V(kVT) + V* RT M- V(Infi)(convective) (diffusive) (Dufour effect) (5)
N H N K
+ V. - HiVik(Rf n R-
R' )
1i-= i 1=1 k=1
(inter diffusion) (chemical reaction) The last term of equation (5) represents the heat
generation/consumption due to chemical reactions in the gas mixture.
2-3. Gas species transport equations
creation of gaseous species, we have to include a species balance term in the species balance equation. The balance equation for the ith gas species is
a
(pwo)
Kdt
=-V
*(p9i) - V*, + mi vik(Rp f R-b) (6)k=t
(convective) (diffusive) (chemical reaction) 2-3-1 Ordinary diffusion
In a binary gas mixture (N = 2), the ordinary diffusion fluxes are given by Fick's Law:
C
C
S= -I 2 = -pDJoD2 = pD 2 V1o, (7) A general expression for the ordinary diffusion fluxes in multicomponent gas mixtures is given by Stefan-Maxwell equation which relate the diffusive fluxes of all species to the
concentration gradients of all species. In terms of mass
fractions and mass fluxes, the Stefan-Maxwell equations [37] are: M 2 N -C -C
V(oM)- m D (i = 1, N) (8)
where M is the average mole mass of the mixture.
N
M = Z f,m, (9)
I=1
For easy implementation of the Stefan-Maxwell equations to the computer code, equation (7) should be rewritten as follows:
p
pm, D, M 'J
1, = -pD V- M + Mo)I vDC. (i = 1, N) (10)
where De is the effective diffusion coefficient
Di (i = 1, N) (11)
As an alternative to the Stefan-Maxwell equations, an approximate expression for the diffusive fluxes in a
multicomponent gas mixture has been derived by Wilke [38]. In this case, the diffusion for the ith species in a multicomponent mixture is written in the form of Fick's law of diffusion,
Ji = -pD , Vw i (i =1, N) (12)
with a mixture-composition-dependent effective-diffusion coefficient of the ith species:
D, = (1-f) (i = 1, N) (13)
2-3-2 Thermal Diffusion
The diffusive mass fluxes due to thermal diffusion are given
by
S-
=
-
D V(InT) (14)in which DT is the multicomponent thermal diffusion coefficient for the ith species.
2-4 Thermodynamic properties of gas mixtures
database [39], in PHOENICS, thermodynamic properties are defined as a function of the absolute temperature by seven polynomial
fitting constants, al to a7, and expressed as
C,(T) 4 R - a%+ a2T + a3T2 + a4T3 + a5T4 (15) RT - a+ 2T+ 3T+ 4T+ T + 6(16) 2 3 4 5 T So(T) a a a (T) a ln(T)+aT+ 3T2 + 4T + 5T +a (17) R 2 2 3 4
The thermodynamic data of ten gas species used in this study are extracted from the database and listed in Appendix B.
2-5 Transport properties of gas mixtures 2-5-1 Lennard-Jones potential
The transport properties of gas species and of gas mixtures may be calculated from kinetic theory with some assumptions for the intermolecular potential energy function of the gas molecules 'P(r). The most simple form is the so-called rigid elastic
sphere (r.e.s.) potential in which
W(r) = for r < Y,
I(r)
= 0 for r > o (18) A more accurate potential function, commonly used for non-polar molecules, is the Lennard-jones potentialThe Lennard-Jones parameters are taken from the CHEMKIN transport property database [40] which contains values of a and e/k for over 180 gas species. The transport properties of the gas species used in this study are summarized in Appendix B.
We need the integral functions, Wm(T*), WD(T*), A*(T*), B*(T*) and C*(T*) for the calculation of the ordinary and thermal
diffusion coefficients. In order to characterize their temperature dependence, a reduced temperature
* kT
T = (20)
is defined. For rigid elastic sphere potential, the value of these functions is identical to 1. For the Lennard-Jones potential, these functions vary slightly with temperature and their value is of order one. In PHOENICS, accurate polynomial
fits for their dependence on T* are adopted.
2-5-2 Density, viscosity and thermal conductivity
The density of an ideal gas species is simply given by the equation of state
Pm.
Pi RT (21)
RT
Similarly, the density of the gas mixture follows from PM
From the kinetic theory of gases, the dynamic viscosity of a single gas is given by
5 ;amiRT
i- 1•(23)
16 32 2(TNNA
Following the recommendations in ref. [41], in PHOENICS, the mixture viscosity is calculated from
N = Xil (24)
with
-05 0.0.5 2 =i--
+ +- {I + j (25)The thermal conductivity of a monatomic gas species predicted by kinetic theory is
15 R
m1 (26)
-4 mi (
For polyatomic gases, several semi empirical corrections predicted by kinetic theory have been proposed in order to account for the transfer of energy between internal degrees of freedom and translational motion. A reasonably accurate
expression is given by the modified Eucken correction [42]
S=15 + 1.32 _ 5 R( (27)
4 mR 2 m
N =i i (28) j-1 with +i mi-05 i0.s< 0.252
-
1 +
1 +
i
(29)
2-5-3 Ordinary diffusion coefficients
The binary diffusion coefficient of a gas pair i and j can be also calculated from kinetic theory. The value depends on the
temperature and the pressure, but it is virtually independent of the mixture composition. Defining Lennard Jones force parameters
for a gas pair i and j as
oi+ oj E kT
ij = 2 ' E ij = Ei1), Tij- (30)
the binary ordinary diffusion coefficient is obtained as
mi + 7 m )1/2 23R-T
3 m 2 2R3T
3
ia D, 16 mc mj pNAn oirciT (31)fc
Wile and Lee [43] suggested a correction factor for a fixed numerical constant in equation (31), the value of which varies
with the molecular weight of the diffusing gases.
3(mi+m 1/ 2 m + +m /2 2n3RT3
D
-16
1.15
-
0.00837
imi
j
PNnoT2(32)
2 mimT mimf PNfA ij D(Te)PHOENICS provides two ways of calculating the thermal diffusion coefficients. One is an exact model to get
multi-component thermal diffusion coefficients from kinetic theory, and the other is an accurate approximate model called Clark-Jones
approximate model, which adopted in this study. The latter model is explained in this section.
Clark-Jones approximate model
An approximate model for multicomponent thermal diffusion
coefficients was suggested by Clark-Jones [44]. This model is
based on an extension of the exact equation for binary mixtures to multicomponent mixtures. In a binary gas mixture, the thermal diffusion coefficients for each of the species is given by
T T P
D
= -D2 -T
m1m2D1 2a1 2f1f2 =pDA
a
2a1 21 2 2 (33)where a12 is the thermal diffusion factor. From kinetic theory,
a12 is given by 1 S M)fI - S(2)f2 12 12x - (6C12 - 5) (34) with S~m) m m, 12 15 m2-m 1
(35)
S, 2m ; - 1 (35) 2 X 4A*2 2mi 2) m, +m2
1215
m, - m
2 2m, %2 2 4A12 2, 2m22•1
(36)T I J2+ K12 = 0.00263 " 12 12, ,( ,(T12) 12
= 0.00263
-oalO (T*) K12 = 0.00263 f2 2f f2 f2 XX - + +-f2 2f, f2 Y= - Uc
(
+ U2(Y + f1 K fY 2ff 2 Y, - U(1) + U2_ + (38) (39) f2 2U (2) (40) (41) U 4 1 12 mI 1 (m, - m2) 2 S A- A* 12 i B *+ 1 (m -m 2 15 1 12 5 '2 M 2 2 m1 m2 m 4 (m +m2)2 2k 1 (12 5 12 (mm)2 U =1- A* 1• 2 -- B 1 B*,-5 m1 m 15 12 4mm2 , 12 5 12J
32A*, 5 ) I m,m X~, I k' 1 (42) (43)Using the binary thermal diffusion factors, an approximate
expression for the multicomponent thermal diffusion coefficients, suggested by Clark-Jones is given by
= ' m mjDiaififj =
j= 1,= 1 M T j= ,j I I
pD ja, oi (1)
(44)which is a multicomponent extension of equation (33). In the above equation, aij is calculated from equations (34)-(43),1J
(37)
°. A
-replacing f. with fi/(f+fj.) and f. with f./(fi+fj).
2-6 Gas phase reactions
If we assume that K reversible homogeneous chemical
reactions take place between N gaseous species, we can use the following general notation
N kk N
- Vikj
A
t
-vikV
Aij
1=k.b i =
(k = 1, K)
The stoichiometric coefficients, Vik, are taken positive for
reaction products and negative for reactants. The operator II
II
in equation (59) is defined asik - L vik ik
Viki2 (46)
When the forward reaction rate constant kk,f and the reverse reaction rate constant kk,b are known, the forward and reverse reaction rates Rk,f and Rk,b can be obtained from
R:,
= kk.f C" ,R
k.b = k .b ITI=1 l=1
(47)
Thus, the total reaction rate RO may then be obtained from
N R= R - .b kk.f IC - k I=1
N
N
f P i
k.bLIC
,= kk.f I Ti-1=1 l=1 I (45) - k l.b k. bi.•
1
RTp
V (48)Using mass fractions wm, equation (48) can be written as,
(wjV I (IVk N kI
R = kkp - k kb
'
(49)In general, the value of kk,f, kk,b depend strongly on the temperature and are independent of the pressure for sufficiently high pressures. In this high pressure region, the generalized Arrhenius expression can be combined with a pressure term, where
a pressure coefficient ck is fitted to experimental data to describe the pressure dependence.
kk,f = AkTexp
-
REAT P .P (50)At low pressures, however, kk,f and kk,b may enter the so called "pressure fall off regime", where the reaction rates depend linearly on pressure. Two methods of representing the rate expressions in the pressure fall-off regime have been included in PHOENICS. The simpler one was given by Lindemann
[45]. It requires three Arrhenius parameters for each of high pressure limit (k_) and low pressure limit (ko) dependent
expression for the rate constant
ko = AoT exp R k = AoT expl- (51)
The rate constant at any pressure is then taken to be
k = k rP F (52)
where the reduced pressure, Pr, is given by
P,= [m]kk (53)
with [m] representing the molar concentration of the mixture. The Lindemann form corresponds to F = 1. Therefore, six
parameters Ao, p0, EA, A , B and EA, are needed for the Lindemann form. When F is given by 0logP,+ c logF = 1+ log F (54) L n - d(log P,+ c) :_n cent with c = -0.4- 0.67log Fent (55) n= 0.75- 1.271og Fcent (56) d=0.14 (57)
Fcen cent (1 - a) exp + a expT- + exp (58)
T*"
TP
T
the Troe form [46] is obtained. Here four additional parameters a, T***, T*, and T** must be specified.
When either the forward reaction rate constant kk,f or the
reverse reaction rate constant kk,b is known, the reaction rate
equilibrium reaction. The equilibrium constant Kk for this reaction, defined as
KkN (f.eP (59)
may be calculated from
SAH 0(T) - TAS (T) IVk
Kk(T) = ex- RT (60)
where
N N
AH (T) = E vikH (T), and ASO(T) = v~kS(T) O (61)
l=1 ,=1
From equation (61) and (73), it may be deduced that kkf(P,T) and kk,b(P,T) are related through
kkf(P,T) RT I'
kk.b(P,T) = Kk(T) Po' (62)
Tabulated thermodynamic properties of specific heats, heats of formation, and standard entropies of the individual species were taken from the CHEMKIN database [39], as described in section
2-4.
Finally, we can combine the general equations (6) for species transport and chemical reactions, the expressions for ordinary diffusion (10,11), the expression for thermal diffusion
(14), and the expressions for the gas phase reactions (47,48) to solve the following equation for the species concentrations,
t
-•
* (p oi)-V * (pDiV mi)+V *(poiDiV(Inm)+V * m iDij.ji j(63)
+ V (D
V(In
T))+mvik
kkf
C- - kk.bk
[
C.kI
k-l I-1 i=l
2-7 Surface reactions
At the wafer surface, a total number of L surface reactions will take place, transforming solid and gaseous reactants into solid and gaseous reaction products
N M N M
5
- o jAj -I-xiJB
- V A -S
Xj Bj (1 =1, L) (64)i=1 j=1 1=1 j=I
The growth rate Gs of the bulk species s is given in m/s by,
ms
L
Gs
E Xs,R (65)Ps 1=1
2-8 Boundary Conditions
On non-reacting surfaces, the no-slip and impermeability conditions apply for the velocity
V = 0 (66)
Prescribed temperature boundary conditions and
zero-temperature gradients normal to the wall apply for isothermal and adiabatic walls:
T = Twal, (isothermal walls),
fi.VT
= 0 (adiabatic walls) (67)must be zero for each of the species:
.(Jc
+
=
0
(68)Due to the surface reactions (78), there will be a net mass production rate Pi of the ith gaseous species of the wafer
surface
Pi = m, Y o,R (69)
Thus, the velocity component normal to the wafer surface is expressed by
N L
(70)
p I=1 1=1
Assuming a no-slip condition, the tangential component is zero:
fi xV =O (71)
The reacting surface (substrate) are assumed to be isothermal, leading to
T = Tsubstrate (72)
The net total mass flux of the ith species normal to the substrate surface must be equal to P.:
1
n-(po9 + + j ) = m, cR,, (73) If an inflow of Qi slm (liters per minute at standard
in the inflow of the reactor for each species; the inflow velocity is given by 10-3
pO
Ti n1
N
V - 60 O P AiiE Qi (74) 60 T in ini-iThen, the boundary conditions for the velocity vector in the inflow are given by
v = vin,
fixV
= (75)The total mass flow of the ith gas species into the reactor must correspond to Qi according to
-e c T 10 3 PO Qimi
ni (po i V + i + ) = 60 TO RAin (76)
At the reactor exit, zero gradients for the total mass flux vector in the direction normal to the outflow opening may be
assumed, as well as zero heat and species diffusion fluxes. Furthermore, we assume that the direction of the velocity is
normal to the outflow opening
fi*(VpV) = 0, fixV = 0 (77)
fi.(X VT) = 0 (78)
C -T
fri(ji + JE) = 0 (79)
2-9 Numerical solution method (finite-volume method) PHOENICS applies the finite volume method to solve the differential equations described in equations (2)-(5). The differential equations contain similar terms, which are a
transient term, a convection term, a diffusion term and a "source" term, which contains additional contributions that cannot be included in the previous terms. We can rewrite the equations to the following general form:
at-(p)
= -V*(pV()
+ V(TFV ) + S.
(80)
transient convection diffusion "source"
here, is the variable to be solved, F is a diffusion coefficient and S is the source term. The solution domain, where the
differential equations apply, is divided into a number of adjoining rectangular control volumes, or grid cells, each of which are surrounded one grid point in which all scaler variables are calculated. The grid is structured in a sense that each
control volumes has a fixed number of neighbors. The vector quantities (velocity V, and species diffusion fluxes) are
calculated in points located at the cell walls, halfway between the scaler grid points, using a so-called staggered grid. Two dimensional grid is illustrated in figure 6. In figure 6, a control volume surrounding the grid point P has four neighboring grid points indicated by N (north), S (south), E (east), and W
O
W cell
o0w
0N cell
oE cell
" 0all
I-IUI I 0S cell
O
Control volume
Vector quantities are calculated at the walls. Scalar quantities are calculated at the center of the cell.
AY
AX
A X
cells and staggered grid for finite
The finite volume equations are obtained by integrating equation (80) over the control volume P.
fJf
[(p,)+V.(p, )+V.(FV)
dV
= fSJJSdV (81)By using the Gauss theorem, we have
(82)
Several discretization schemes, such as central scheme, up wind
56
Figure 6. Grid volume method (west).I
i I=
f
S
8
tdV
scheme, and hybrid scheme, can be used to get the value of the variable 41. After integration with one of the scheme, the
finite volume equation has the following form.
ap(,p = aN,(•N+ asp, + aE4(IE+ aW ~ +aT( T + source terms (83) with the subscripts P,E,W,N,S denoting the locations at which the variable is computed, and the subscript T denotes the time. The
a's are coefficients, temporarily treated as if they were
constants during each iteration. Those terms with subscripts N, S, E, W express the interactions between neighboring cells by way of diffusion and convection, while aT denotes the time dependence
effect. Equation (83) is obtained for each cell and each variables to be solved. Finally the variables are calculated
from
aNc)N+ as()S + aE(c)E+ aW 1 + aT( T+source ) terms
•
p
-
ap (=aN+as(84)
+ aE + a + aT)
2-10 Dimensionless numbers
Several dimensionless numbers describe the flow, heat and mass transfer regimes in CVD reactors. The key dimensionless
numbers are listed in table 2, together with typical values for the region between the filament and the substrate in hot filament diamond CVD reactors. The physical quantities represented are explained as follows.


![Figure 2. Production process of a diamond film for cutting tools [2]](https://thumb-eu.123doks.com/thumbv2/123doknet/13954794.452528/15.918.143.794.265.845/figure-production-process-diamond-film-cutting-tools.webp)



![Figure 9. Experimental setup of Hsu [9]](https://thumb-eu.123doks.com/thumbv2/123doknet/13954794.452528/74.918.200.733.637.976/figure-experimental-setup-hsu.webp)