Publisher’s version / Version de l'éditeur:
Transaction of the ASME Journal of Engineering for Gas Turbines and Power,
120, January, pp. 225-231, 1998-01-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Examination of the ignition delay period in gas-fueled diesel engines
Liu, Z. G.; Karim, G. A.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=f6123423-b365-40ff-ab86-d23c70b09ba6
https://publications-cnrc.canada.ca/fra/voir/objet/?id=f6123423-b365-40ff-ab86-d23c70b09ba6
http://www.nrc-cnrc.gc.ca/irc
Ex a m ina t ion of t he ignit ion de la y pe riod in ga s-fue le d die se l e ngine s
N R C C - 4 2 0 2 4
L i u , Z . G . ; K a r i m , G . A .
J a n u a r y 1 9 8 8
A version of this document is published in / Une version de ce document se trouve dans:
Transaction of the ASME Journal of Engineering for Gas Turbines and Power,
120, January, pp. 225-231, January 01, 1998
The material in this document is covered by the provisions of the Copyright Act, by Canadian laws, policies, regulations and international agreements. Such provisions serve to identify the information source and, in specific instances, to prohibit reproduction of materials without written permission. For more information visit http://laws.justice.gc.ca/en/showtdm/cs/C-42
Les renseignements dans ce document sont protégés par la Loi sur le droit d'auteur, par les lois, les politiques et les règlements du Canada et des accords internationaux. Ces dispositions permettent d'identifier la source de l'information et, dans certains cas, d'interdire la copie de documents sans permission écrite. Pour obtenir de plus amples renseignements : http://lois.justice.gc.ca/fr/showtdm/cs/C-42
Z.
Liu
G. A. Karim
Department of Mechanical Engineering, The University of Calgary, Calgary, Alberta, Canada T2N 1N4
An Examination of the Ignition
Delay Period in Gas-Fueled
Diesel Engines
Changes in the physical and chemical processes during the ignition delay period of
a gasjueled diesel engine (dual-fuel engine) due to the increased admission of the
gaseous fuels and diluents are examined. The extension to the chemical aspects of the ignition delay with the added gaseous fuels and the diluents into the cylinder
charge is evaluated using detailed reaction kinetics for the oxidation of dual-fuel
mixtures at an adiabatic constant volume process while employing n-heptane as a
representative of the main components of the diesel fuel. In the examination of the physical aspects of the delay period, the relative contributions of changes in charge temperature, pressure, physical properties, pre-ignition energy release. heat transfer. and the residual gas effects due to the admission of the gaseous fuels are discussed and evaluated. It is shown that the introduction of gaseous fuels and diluents into the diesel engine can substantially affect both the physical and chemical processes within the ignition delay period. The major extension of the delay is due to the
chemical factors, which strongly depend on the type of gaseous fuel used and its
concentration in the cylinder charge.
Introduction
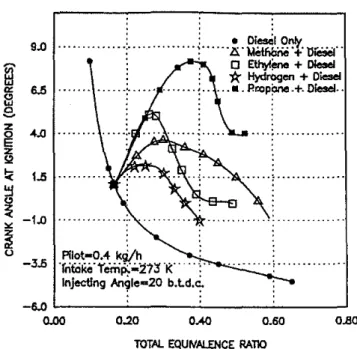
The ignition delay period in a compression ignition engine is a very important performance parameter that influences sub-sequent combustion processes, engine perfonnance. and exhaust emissions. In gas-fueled diesel engines (dual-fuel engines), the control of the ignition delay period is equally important and can display trends significantly different from those observed in the corresponding diesel engine operation. The injection of a small quantity of liquid diesel fuel is maintained in dual-fuel engine operation to ensure ignition of the charge. The length of the ignition delay, however. increases significantly with the increased admission of the gaseous fuel up to a detectable maxi-mum value and then drops gradually, as shown typically in Fig. 1. Changes in the type of gaseous fuel used in a dual-fuel engine for the same injected quantities of liquid pilot fuel and total equivalence ratio also produce significant changes in ignition delay. For example, as shown in Fig. 1, the admission of pro-pane increases the length of the ignition delay in dual-fuel en-gine operation to an extent much longer than the corresponding values observed with methane or with other gaseous fuels. For the admission of the diluents nitrogen and carbon dioxide into the intake air, however, the observed experimental values of エィセゥァiャゥエゥqョ、・ャ。ケ in the operation of a diesel engine display yet different trends from those observed for the admission of gaseous fuel in the cylinder charge [1, 2]. Neither nitrogen nor carbon dioxide addition could produce comparable trends in increasing the ignition delay of the diesel fuel.
Some research has been carried out in the past to examine the changes in the ignition delay period during the operation of dual fuel engines. Moore and Mitchel [3] indicated that the increase in ignition delay observed in dual-fuel engines was caused by the presence of the gaseous fuels. The length of the ignition delay could be improved by increasing the pilot quan-tity and preheating the intake charge. Karim and Burn [2] indi-cated that the observed variations of the ignition delay in dual-fuel engines cannot be explained wholly on the basis of the reduction of the partial pressure of oxygen in the intake charge
Contributed by the Internal Combustion Division for publication in the JOURNAL OF ENGINEERING FORGAS TURBINES AND POWER.Manuscriptreceived atASME Headquarters May 1997. Associate Technical Editor: W. K. Cheng.
as a result of gaseous fuel admission. The gaseous fuel must affect in an unknown manner the pre-ignition processes of the diesel pilot fuel to bring about the observed specific variations in the length of the ignition delay. In our previous work [5], the effects of changes due to gaseous fuel admission, in mean charge temperature, external heat transfer to the surroundings, and the extent ofーイ・セゥァョゥエゥッョ energy release during the com-pression process on the length of the ignition delay of a dual-fuel engine have been examined. while using detailed reaction kinetics for the oxidation of the gaseous fuel and air and em-ploying an experimentally based fonnulation for the ignition delay of the liquid pilot. The effects of any chemical interaction between the pilot and gaseous fuels during the preignition pre-cesses could not be accounted for.
The present contribution examines further the observed changes in the physical and chemical processes during the igni-tion delay period of a gas-fueled diesel engine (dual-fuel en-gine) due to the increased admission of the gaseous fuels and diluents. In the examination of the chemical aspects of theゥァョゥセ tion delay, the environment of the mixture of diesel pilot with the gaseous fuel is simulated by an adiabatic constant volume process while employing nセィ・ーエ。ョ・ as a representative of the main components of the diesel fuel. The effects of changes in the concentrations of the added gaseous fuels, such as methane, propane, and hydrogen, and the diluent'. carbon dioxide and nitrogen to the cylinder charge as well as the changes in intake charge temperature on the ignition delay of the fuel mixture in air are evaluated through using detailed reaction kinetics for the oxidation of the n-heptane and gaseous fuels mixture.
In the examination of the physical aspects of the delay period, the relative contributions to the ignition delay, resulting from changes in charge temperature. pressure, physical properties. pre-ignition energy release, heat transfer, and the effects of the residual gases due to the admission of gaseous fuels are dis-cussed and evaluated. It is shown that the introduction of gas-eous fuels and diluents into the diesel engine will change both the physical and chemical processes of the ignition delay period. The extension to the chemical process of the-delay period with the admission of the gaseous fuel is the main rate controlling processes. Its extent. which depends on the type of the gaseous fuel used and its concentration in the cylinder charge,
will
be decreased with the improvement of combustion perfonnance. Journal of Engineering for Gas Turbines and PowerCopyright C> 1998 by ASME
whereP and T8represent, respectively, the mean cylinder
pres-sure (atm) and charge temperawre (K) during the ignition delay
penod.
4>
ISthe eqUivalence ratIo of the fuel vapor-air mixture.A, a, b, and c are constants to be established experimentally
for the relevant operating conditions. The values of these con.
stants as obtained, for example, by Hiroyasu [6, 1] in a constant
volume vessel are:
nonnally bring about a decrease in the charge temperature While other changes, such as the contribution of the residual gases could result in an increase in the charge temperature. The・ヲヲ・」エセ of any chemical interaction between the diesel and gaseous fuels during the preignition reactions can be accounted for・ュセ
piricaIly through a modification to the effective overall activa-tion energy tenn of the mixture, which will be strongly depen-dent on the types and concentration of gaseous fuels involved. hセョ」・N when 。」」ッオョセゥョセ .for the ・ヲセ・」エウ of these changes, a
modified value for the IgnItIOn delay10dual fuel engines can
be produced [5]. This modified value,T
+
D.
T(s), in order tobe compared to the corresponding value under diesel operation
[6, 7], can be written as:
T
+
D.T = A(P+
D.P)"(¢+
D.¢)' exp (c
+
D.c)
(2) T,+
D.TA = 4.0 X 10-3
, a = -2.5, b = -1.04, C= 6000 (3)
In the present work, the equivalent modifications to the charge temperature in accordance with Eq. ( 1) can be correlated from the results of analysis and experiment. The corresponding
chemical effects on the ignition delay period of the
dual-fuel-air mixture are examined on the basis of adiabatic constant volume reaction conditions while employing detailed reaction kinetics for the oxidation of the dual-fuel-air mixture. In these kinetic considerations,n-heptane is chosen to represent the oxi-dation of the diesel fuel since the chemical kinetics of the diesel fuel oxidation are currently unavailable. Moreover, the adiabatic constant volume conditions tend to be close to the environment
of the diesel pilot fuel in dual-fuel engines during the delay period (for example, a charge mean temperature of 780 K and
a charge pressure of 28 atm). The concentrations of the mixtures of n-heptane vapor and the gaseous fuel are considered in tenns of thdr corresponding stoichiometric value, which tends to be associated with the peak value of the reaction rates for the oxidation of the mixture. The chemical reaction mechanism of the mixtures ofn-heptane and the other gaseous fuels was based
on those schemes reported by Westbrook et aI. [8]. This
de-tailed kinetic scheme consisted of 1966 elementary reaction steps and 380 chemical species. The corresponding
thermo-chemical data were obtained mainly from JANAF tables [10].
The residual gases from the previous cycle, which were as-sumed to fill the clearance volume when a new cycle began, were considered to have the same composition as that for the exhaust gases at the end of expansion to atmospheric pressure. These residual gases, when the inlet valve was opened, mixed
completeiy and adiabatically with the fresh charge at the same
pressure. For heat transfer, the mean wall temperatureTwwas
assumed to remain constant over the cycle, while convective and radiative heat transfer was accounted for by a fomulation
recommended by Annand[11]following his analysis of a wide
range of experimental data in engines.
Results and Discussion
When the pilot diesel fuel is injected into the combustion chamber under high injection pressures, the pilot fuel is atom-ized and distributed within its spray cone. The resulting axial and radial fuel concentration profiles could be calculated ap-proximately by employing established theoretical procedures
for the flow of jets [12]. Increasingly more surrounding fuei
along with the air are entrained into the spray cone as the jet
0.60 0.80 0.Ml 0.20 1.5 . 6.5 . 4.0 . -3.5 ·rnt'Qko·r..riip;;.;.:t1:l' K'I'Ilot-0.4 kWh . Injectingaョセャ・NRP 「Nャ、Nセ -6.0 L..-_ _-'-._ _-.:...._ _--'-_ _---l 0.00 z
セ
!<
セ
-1.0セ
oFig.1 Variations ofignition delay with various gaseous fuels admission
over a range of equivalence ratio In a dual fuel engine operation
9.0 ....
TOTAl. EQUWALENCE RAllO
Analytical Considerations
The ignition delay in a diesel engine is defined as the time
interval between the start of fuel injection and the
commence-セ・ョエ of rapid energy release due to combustion. The
pre-igni-tion processes can be viewed as being initiallypredominantly
physical, followed by overlapping somewhat later chemical pro-cesses. In the physical part of the delay process, the fuel is
Injected, atomized, vaporized, and mixed with the air. The aSso-ciated chemical processes are controlled by the precombustion reactionsセヲエィ・ local mixtures of the fuel, air, and residual gases. A convement approach based on experimental observation of セ・N セ・ャ。ケ in 、ゥ・セ・ャ engines is to consider the length of the Igm.tIon delay p.enod to be a function of the charge pressure and eqUIvalence ratIO of the fuel vapour and air, and exponentially dependent on the inverse of the mean charge temperature
[6, 1).
In dual-fuel engine operation, the admission of a gaseous fuel
with the air will influence profoundly both the physical and
chemical ignition processes of the mixture. This admission will bring about variations in the physical properties of the mixture. such as the specific heat ratio and heat transfer parameters. These can lead to significant changes in the charge temperature and pressure levels at the time of fuel injection and extend the
physical ignition delay period of the mixture. The presence of
the gaseous fuel-air charge during the pre-ignition reaction results in changes to the energy release and the associated pro-duction of active species. Moreover, any chemical interactions
エィセエ may take place between the diesel vapor and gaseous fuel Will produce further changes in the chemical ignition processes of the mixture.
Some of the major effects of the introduction of the gaseous fuel on the ignition period of the dual-fuel-air engine can be expressed conveniently as a modification to the compression mean charge temperature,6.T, and can be written as:
D.T
=
D.T"+
D.T..+
D.T"+
D.Tn+ . . .
(I)where ATrhis the temperature change due to any variations in
the thermodynamic and physical properties of the mixture, D.T..
IS the change due to the contribution of residual gases, 6.TItlis
the change due to the heat transfer, andATnis the change due
to the contribution of pre-ignition energy release. Changes in the thermodynamic and physical properties of the mixture could
(a)
Upstream Region of the Jet
250,0 PUot.;oM kg/ft
...
ᄋᄋcZrZセQTNWゥヲ:
.
:tNMRセS
K : ...セ...GセGNMNQ[[N[[B ᄋァェZエエエXᄋセᄋᄋᄋᄋᄋ:
- -:- - CH4i-i35
: - ...- - CH4t-4.6...;
:···f···
50,0 100,0 150.0 200.0 RelativeOiotQI1C<lAlong the Jet,w"(X/D)...
ᄋᄋᄋᄋᄋᄋᄋᄋᄋᄋ^ᄋ\ᄋセᄋセᄋLᄋᄋᄋヲᄋᄋᄋᄋᄋᄋᄋᄋᄋᄋᄋ
/ ....
-
: :...
NヲZセZ
_
セセ
.\.l.
.
A
:
'.:\
\
:\'
\:,
...;...:
···\"\···..i· ,
.
:
:
::',
セ \\
: \
セ.
j
\ \
セ
\
0.0 1.-_ _...:. -'-. ..1...:·L-'-...:.'_..._...J 0.0 6.0 2.0 8.0 10.0€:
14.0e.
'l;セ
4.0i
Fig. 3 Calculated relative width ofthestoichiometric ratio envelopes In jet fuel mixture with changes In methane concentration in the ウオイセ
rounding charge
-
-diesel pilot fuel
100.0
eo,o
""'poi><
···t··· .... L4c1:hanc 60,0 Qᄋᄋᄋᄋᄋᄋᄋᄋᄋᄋᄋセᄋᄋᄋᄋᄋᄋ⦅ᄋ⦅M セ hy、セ·
.
·
.
·
.
-40,0 20,0 0.0 l-_-'-_ _-'-_ _-'-_--''-_...J
0.0 6.0 セ . 15.0 20.0,..---:----.,..--...,---r-,.---,
Adtabattci:onstantVolume
stclchl.m.trlo
RatIo:
Temperolli..-aoo
K:pイ・ウウオイ・セRX otm セ .
...,
!
Fig. 4 Calculated Ignition times of the stoichiometric、オ。ャセヲオ・ャ mixture withchanges In the percentage of gaseous fuel in the total mixture for an adiabatic constant volume process
PercerMgeof GaseousFUe{InHeptane and GaseousFuel-AirMbdure
·
.
.
.
·
- -- セ.
.
-.-.
.
·
.
.
,·
.
,.
·
.
.
.
·
,,.
.
.
.
.
.
.
.
.
·
·
.
,·
.
.
.
.
,.
,.
.
of the fuel mixture tends to be initially very slow until the percentage of the methane in the fuel mixture is substantially increased to beyond around 70 percent. The increased percent-age of hydrogen in the mixture does not increase the ignition delay significantly. However, for propane admission, the igni-tion delay extends more rapidly with increasing the percentage of propane in the fuel mixture. These changes in the ignition delay period, which depend on the type of the gaseous fuel and its concentration in the cylinder charge, are consistent with those observed experimentally in Fig. I for dual-fuel engine operation. Figure 5 shows similarly the corresponding variations of the ignition delay period with increasing concentrations of gaseous fuel and the diluents carbon dioxide and nitrogen in
the local stoichiometric mixture for n-heptane and the added gas. It can be seen that unlike with the admission of gaseous fuels. neither the presence of small amounts of nitrogen nor of diesel fuel moves downstream. The downstream radial fuel
concentration profiles become increasingly flatter. Conse-quently, for the same overall fuel-air ratio in the cylinder charge, the relative concentration of the gaseous fuel at a down-stream jet location, as shown schematically in Fig. 2(0) and
2(b), has a much higher value than that at an upstream jet
location due to the entrainment of the surrounding gaseous fuel. The induction of more gaseous fuel into the engine cylinder will further change the local fuel composition and increase the concentration of the total fuel at any point while the concentra-tion of the air reduced by the same amount. However, this change in the local fuel composition is not unifonn for the mixture within the jet spray cone. As shown in Fig. 3, the overall size of the stoichiometric region at every section downstream of the nozzle is increased. both in width and length, as the concentration of the gaseous fuels in the surroundings is in-creased. However, the increase of gaseous fuel in the local fuel composition for the same stoichiometric zone tends to be prominent only at regions away from the discharge point of the jet. These changes in local fuel compositionwi th the admission of the gaseous fuels play an important role in modifying the ignition and combustion characteristics of the engine from those of normal diesel· operation.
It is expected that the most probable location for first ignition encountered· will be along a stoichiometric mixture envelope of the fuel jet for the same temperature level. In the operation of a dual-fuel engine, the ignition delay period will depend not only on the concentration of the mixture but also on the local fuel composition for the same values of the stoichiometric mix-ture, since the admission of the gaseous fuel changes the local fuel composition, as shown in Figs. 2 and 3. It has been 」ッョセ
finned in Fig. 4 that for stoichiometric mixtures at mean temper-ature and pressure values similar to those during the delayー・セ
riod in engines, the calculated ignition delay period of heptane and gaseous fuel mixtures in air is gradually extended as the percentage of the gaseous fuel in the mixture is increased. For methane admission, the extension to the ignition delay period
Fig.2 Schematlc representation offuelconcentration profile withina
diesel fuel jet issuing in an atmosphere of gaseous fuel and air mixture
(b)
Downstream Region of the Jet
O.llO5 0.015 620.0
r---,---,.---,---,.--....,
: : Nitrogen: . 8OQ.O{|セ
••••ZイZ[ZZセZZBZBᄋGエMセセョMBBBセNMセセャNGNB
g
B|セG-....;.
.
.
.
-, ...eci-ben
llIoldile
:
l:!
\ :
,,:.;..:
:
セ
78<J.O .. •.. · \ ..( ...tZBL[セセH
··
セ
78<J.OxセNセNセャNNN
..L
>:+,""' ..
I
\\: :
i ·....
7.a.o ...·f··..\;. ··..
j..··..··..·:···· ..
I
NtセゥAセセ
..
セZ
\
;
l .
720Jl c.R.-14.2:1 : : : . ALOT-o'f KG/H \ : : 700..0l..-._-'-_ _,-_-,-_ _'-_...J
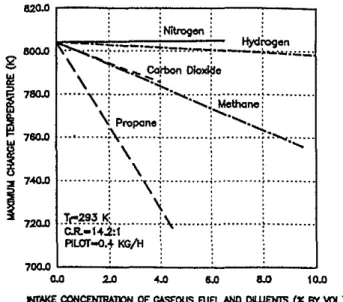
0.0 2.0 .... 6.0 8.0 10.0 fI1llj(E COfolCENlRAllON OF OOEOUSf\JE!.ANO OWEHTS (X BY VOL)Flg.7 Variations of calculated maximum temperatures with equivalence ratios for propane, methane, hydrogen, carbon dioxide, and nitrogen In a motored engine operation
0.020
r--r--:-;-;-:;-::-""7"':7":":-:---'
1
MlQbatic ConotaalVolume " • TemPeratuMl-aooI(:
"'"'ioure-28
atrl>
•: Stok:hlomolricRcllIoI:
:
:
...
;
;
.
lciJHa
:
1
j ji :
;
.
···.."1 ; ···
1·
··
[
..
i
j : :i
j
.:
j
..../
-r
;
セNセNI.
/_t---:
·
' セM Nセセ H2.
N2
g
セ
0.010i
Fig. 5 Variations of. calculated Ignition times with changes in the con-centration of gaseous fuel and diluent In the mixture for an adiabatic constant-volume process
CO2 :
Nッッ
'::-_-'-_=-=--_---'_ _
...J0.0 2.0 4.0 6.0 8.0
The COllcellbatlonofGaaeous Fuels and ORuenta In t.MxtuMl (VollI)
pilot jet where sufficient amounts of diesel fuel vapor and air but with small concentration of gaseous fuel form the most reactive ignition elements. Those regions that are close to or far away from the discharge point of the fuel jet
will
tend to produce a later ignition due to either the lack of vaporized diesel fuel or a relatively high concentrations of the gaseous fuel in the local mixture.Another major effect of the introduction of a gaseous fuel or a diluent in the engine cylinder with the intake air is the resulting variations in the charge temperature during compression. These are due to the changes in the physical properties of the mixture, such as the specific heat ratio and heat transfer parameters, as well as any energy release arising from the pre' ignition reactions of the gaseous fuels. As shown in Fig. 7. the addition of hydro-gen to the engine intake air reduces the maximum temperature level of the charge at the end of compression- only relatively litt!(,l. However, the corresponding values of temperature ッ「セ served with the addition of methane or propane decreaseュ。イォセ cdly. With propane addition. for the typical case shown, a drop in temperature of around 90 K was observed for the stoichiomet-ric mixture. Similarly. the mean value of the charge temperature at the end of compression decreases essentially linearly with increasing the concentrations of carbon dioxide in the cylinder charge. The addition of nitrogen to the-intake air hardly changes the charge temperature.
The effect of changes in the physical properties of the mixture and heat transfer arising from the admission of the gaseous fuel on the charge temperature at the end of compression can be correlated on the basis of the type and concentration of the gaseous fuel. As shown in Fig. 8, this temperature is markedly reduced WIth the increased addition of methane into the air while following similar trends for wide working conditions. The values predicted by the correlated fonnulation agree very well with the corresponding individually calculated values according to the following relationship:
エNtLセ
セ
(T,mu - T,mu) = T,mu(1-exp (MセII
(4) 60.0 60.0 '!<l.0 20.0 0.0 '----..:...._ _...:..._ _---'...;_--l
0.0 8.0e.o
r - - - - ; - - - - , - - - , - - - ,
hliabcllcセョ。エ。ョエ
Vclurf,e SliochiomelHc Volume :Heptone an\! Methane Mixture
i
pイ・ウウオイ・MRセ
abn:
:
.
...:
,
,
.f. ..
:
:
:
/'
:
:
:
- - !Temperaturei-eoo
I( : /:
:
:/
..
MᄋMᄋ⦅ᄋGtセgkᄋ.. ···:·· .•...
:
:
:
:
: /
..
·
·
.
/.
, / ' : , / '.
..."
:
:
---".
..- ·..·F:-···;····
2.0carbon dioxide with n-heptane can produce significant increases
in the ignition delay period. The small changes observed are
セウッ」ゥ。エ・、 mainly with the reduction of the partial pressure of
oxygen in the surroundings. Figure 6 shows that the decrease in the mixture temperature extends the ignition delay period of the mixture without significantly changing the trends of ignition delay period with the increased admission of gaseous fuels.
Accordingly, on the basis of these trends, the admission of エィセ gaseous fuel with the intake air in 、オ。ャセヲオ・ャ applications wIll result in an extension to the ignition delay period due to the chemical interactions between the diesel and gaseous fuels.
セウ extension will be further increased, but not proportionally, With the increase of the concentration of the gaseous fuel in the cylinder charge. It can be anticipated that ignition in the dual-fuel engine will first take place within the region of the diesel
p・イ\Z・ョャ。セ・ ofMethane In Mixture ell;)
Fig. 6 Variaflons of calculated Ignition times with changes In Intake temperatures for methane admission for an adiabatic constant volume )rocess
where Tgmuis the charge temperature at the end of compression
with the admission of gaseous fuel, TlImuis the chargeエ・ュー・イ。セ
ture at the end of compression with pure air admission, I.{J is the equivalence ratio of the gaseous fuel, a is a constant having the following values 16.61.8.90.55.31. and 13.56 for the
g
-760 . セ 470g
410I
I
...•• 350 o.o . .-,..-,.--,,...---,---,---,+-.
M£T1io'J£ADMIsSIoN PIlOT..;o.226 KQ:/H セMGQᄋWZQ : INTN<E;エエmpNMセYS K....
セ ;.
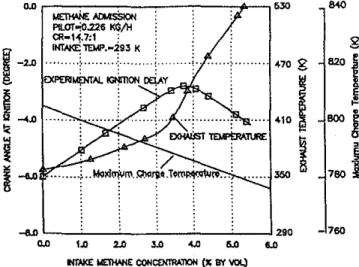
-8Jl ' - _ - - ' -_ _' - _ - - ' - _ - - ' - ' - _ - ' - _ - '290 0.0 1.0 2.0 3J) 4.0 5.0 6.0Fig. 10 Variations of experimentallgnltlon delay, exhaust temperature and calculated maximum charge temperature with methane admission
1.0 0.6 0.6 0.+ 0.2
·
.
·
.
,.
...
セi···i'···"r
·
.
·
.
·
.
Symbol:Calculatod 1hdMduolly . Gウッセ、
.
ョョセᄋiGゥo、ヲcヲNゥ、',bY',c;rmu"itloii
·
.
·
.
·
.
·
.
·
.
.
310 QHLZ」NrNMャKセ N-l000i"m
.
.
.
.
.
.
.-
-
セN·
.
·
.
·
.
.
:RYセ
I(,c.R.-i+.2.N-100l7""",>--g
セ 650.0t
セ • 800.0X
J
750.0 900.0r--...,..--....,...--..,.---,-.---,
: T.-315 1(,; C.R.-19.2; N-l500f1'"'
Intake Equlwlonoo Ratio with l.Iothone Nlmlsslon Fig. 8 Comparison of maximum charge temperatures predicted by full calculation and by the correlated fannulatlon for methane admission of
Eq. (4)
Fig.9 Variations of experimental and modified ignition delay valueswith
the Intake concenb'atlon of carbon dioxide and nitrogen for two intake
temperatures ; .
ignition delay period with the admission of carbon dioxide is mainly attributable to the decrease in the charge temperature due to the variations of the physical properties of the charge and the heat transfer during compression. The addition of a small amount of carbon dioxide does not significantly affect the ーイ・セゥァョゥエゥッョ reaction processes. Meanwhile, as shown in Fig. 5, the admission of a small amount of carbon dioxide into the intake air does not produce a significant extension to the chemical part of the ignition delay period of the mixture. Hence, when these factors that affect the ignition delay period due to the addition of carbon dioxide have been excluded, the modified ignition delay calculated byEq. (2) remains, as shown in Fig. 9, at almost the same value as observed in pure diesel operation and will not change with the increased carbon dioxide concen-trations.
As shown in Fig. 6, the addition of nitrogen will not change the mean charge temperature and no extension to the physical process of the ignition delay period is expected with the admis-sion of small amounts of nitrogen. The corresponding changes in the ignition delay for nitrogen admission, as show in Fig. 9, are far less evident than those observed with carbon dioxide admission. The very small increase observed in the value of the ignition delay resulted from the small extension to the chemical' process of the delay period.
Unlike with the admission of the diluents, Fig. 10 shows that the ignition delay period, measured in a direct-injectionウゥョァャ・セ cylinder dual-fuel engine, initially increases significantly up to a maximum value as the methane concentration in the cylinder charge is increased. The admission of the gaseous fuel influ-ences the length of the ignition delay not only through a possible modification of the nature of the chemical reactions of the mix-ture but also through changes in the charge temperamix-ture levels, heat transfer, the extent of pre-ignition energy release during compression, and the associated changes in the contribution of residual gases from previous cycles. As shown typically in Fig. 10, the increased admission of methane reduces the calculated values of the maximum charge temperature at the end of com-pression. The corresponding measured exhaust temperature, which is consistent with the state of the residual gases, is ini-tially increased slowly but with a continued increase in methane concentration in the cylinder charge its value is enhanced very significantly. The marked reduction in the length of the ignition delay is accompanied by the significant increase in the exhaust temperature.
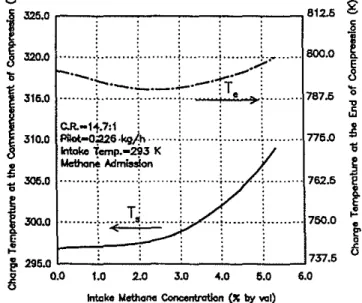
Figure 11 shows typically the variations of the charge temper-atures at the commencement and end of the compression stroke with methane admission, when the effects of the changes in physical properties of the mixture, heat transfer, the extent of
e.o
·
.
...セ...•..•.... ! .·
.
·
.
- . Ii. "Z>- : ••••••••••••••;••••••••••••••セ•••••••••••••• I- •••••••••••••.
.
Ol..etFueI.lo.+kgjh :'lnjectlnQ' "",nle-2Ctセ t>.tet.e:··· .. ···;··· .. ·
.
.
セ⦅[[Z[⦅[[[Z⦅セ⦅fNZZ⦅ZZZa⦅セ⦅セ⦅ZᄆaイMMMエMeiMᄋᄋᄋ
..··.
···T...セBkᄋᄋᄋᄋᄋᄋᄋᄋ[....···
·
.
セ '----.:...--.:..._ _セ _ _--l 0..0 2.0 <4..0e.o
OIWENTS AOt.4ISSlON (x BY\'Qt.)tion of methane, propane, hydrogen, and ethylene, respectively. On this basis when the charge temperature at the end of
com-pression for pure diesel operation is known, the charge
tempera-tures at the end of compressionwiththe admission of、ゥヲヲ・イセョエ gaseous fuels can be evaluated.
With the consideration of both the physical and chemical effects of the admission of the gaseous fuels or the presence of the diluents on the ignition delay period of the mixture. the observed changes in the experimental values of the ignition delay during the operation of dual fuel engines can be diag-nosed. As shown in Fig. 9. lhe observed experimental values of the ignition delay increase almost linearly with the extent of carbon dioxide admission to the cylinder charge. A higher intake temperature results in a relatively shorter ignition delay. while an essentially linear trend is maintained. The increase in the
e.o
e.o
E'
ffi
<4..0e
%セ
2.0 % 52!<
% 0.0セ
セ -2.0"
セ
-4.0Fig. 12 Variations of experimental and modified Ignition delay values
withIntake concentration for methane admission
-a.o
L-_-'--_-'--_-'-_-'-_----'_---'0.0 t.o 2.0 3.0 '.0 5.0 6.ll
IIIfAKE lIElWoNE CONCENTRATION (XB'(VOL)
..
; ;.: .NNMNセNBBB
.
: / :..
.y<: : ...•... :. •セfQed.
セュon.
o$.AY ,.
.
OlcrnTcal Extension·
.
·
.
セi」\ャャ eクエ」セウャッョ·
.
, セ セNend of compression continues to increase. The corresponding extension to the chemical processes of the ignition delay is also reduced under the increased charge temperatures. Thus, the ignition delay period at relatively high load is decreased with
the increased methane concentration in the cylinder charge. These changes in the ignition delay period of the dual-fuel engine show that the extension to the chemical processes of the ignition delay with the admission of the gaseous fuel is the main rate controlling processes during the delay period of the dual fuel engine. The extent of this extension, which depends on the type of gaseous fuel used andits concentration' in the cylinder charge, will be decreased with the improvement of combustion perfonnance.
These analytical results can be used further to explain other changes in the ignition delay period observed in dual fuel engine operation. For example, the admission of propane into the dual-fuel engine results in a significant decrease in the charge temper-ature and a longer extension to the chemical part of the ignition delay period. The value of the delay for propane admission is higher than tbose values observed for methane or hydrogen admission. Moreover. an increase in the size of the pilot fuel, which improves the combustion characteristics of the dual fuel engine, reduces the relative concentration of the gaseous fuel in the fuel mixture for the same overall equivalence ratio, re-sulting in higher charge temperatures at the end of the」ッューイ・ウセ
sion and a shorter chemical extension to the ignition delay period. Hence, the observed values of the ignition delay are lower than those observed for small pilots[I, 2].
Conclusion
The introduction of gaseous fuels or diluents withthe intake air into a diesel engine will change both the physical and chemi-cal processes of the ignition delay period. The corresponding extension to the physical process of the delay period is related to changes in the charge temperature, pressure, pre-ignition energy release, heat transfer, and the effects of the residual gases. The extension to the chemical process of the ignilion delay, which results from the chemical interactions between the diesel and gaseous fuels, is the main rate-controlling process during the delay period of the dual-fuel engine. The extent of the extension to tho ignition delay period depends strongly on the type of the gaseous fuel used and its concentration in the cylinder charge.
0.0..---:---;---,.----;---...,.---,
I.4E1K'oNE
ADMISSION PlLOT..;o.226i<G!H
CR-19:1 : iエitセ·
ュNエpNMセYS.
K.
.
..
...;·
;. セ ! セ . ,.
,.
,.
.
.
.
experiセヲZntaャN IGIlfTlON OE\AY : :£:
セ 305.0t
300.0 セ..
セ 295.0l-_-'-_ _'-_--'-_ _"'-_--'-_--'737.56
0.0 1.0 2.0 3.0 '.0 5.0 6.0Fig.11 Variations of calculated charge temperatures at the commenc"," mont and end of the compression stroke for methane admission
pre-ignition energy release during compression. and the。ウウッ」ゥセ ated contribution of residual gases from previous cycles on the charge temperature have been considered on the basis of the results in Fig. 10. It can be seen that at lightload, which involves very lean gaseous fuel-air mixtures, the charge temperature at the commencement of the compression stroke is not increased significantly with the consideration of the contribution of the residual gases, since the combustion of the mixture at light load is confined to around the pilot fuel and cannot produce sufficiently hot residual gases. The values of the corresponding charge temperature at the end of the compression stroke are lower than the' value observed for pure diesel operation due to the variations of the physical properties of the charge and exter-nal heat transfer effects. With the continued increase in the gaseous fuel concentration, the combustion process can extend gradually further into regions of the mixture made up of the gaseous fuel and air to produce higher residual gas temperatures. The values of the charge temperature at the commencement of the compression stroke, as shown in Fig. 11, is then significantly elevated through the increased contribution of the hotter residual gases. These increases in the charge mean temperature at the conunencement of compression will contribute to counteracting the corresponding reduction in the charge temperature due to the admission of methane to produce eventually a higher charge temperature at the end of compression.
These changes in the charge temperature with the admission of methane influence significantly the ignition characteristics of the pilot fuel. As shown in Fig. 12, the changes in the ignition delay period of the dual-fu.,1 engine canbe considered in terms of physical and chemical extensions calculated in accordance with Eqs. (I) and(2).At light load, the decrease in the charge temperature, as shown in Fig. 11, extends the physical process of the ignition delay period. Meanwhile, the increased presence of methane with the diesel fuel chargewillresult in some exten-sion to the chemical processes of the delay, as displayed in Figs. 4 and 5. Both of these extensions to the ignition period lead to the observed increase in the delay period in dual-fuel engines as the methane concentrations in the cylinder mixture are increased.
At relatively high loads. the charge temperature at the end of compression can reach high values mainly due to the increased contribution of the hotter residual gases. Hence, as shown in Fig. 12, the extension to the physical process of the ignition delay is reduced gradually as the charge temperature at the
Intake11_..eonce-tlon (Xbywi)
Acknowledgments
:11e
financial support of Natural Science and Engineering Research Council of Canada (NSERC) and Alternative Fuel System, Inc., is gratefully acknowledged. The contributions of Mr. K. S. Burn, Mr. E. Khalil, and Dr. C. K. Westbrook are also acknowledged.References
1 Nielsen, O. B., Qvaie, B.• and Sorenson. S.
c..
"Ignition Delay in the Dual Fuel Engine," SAE Paper No. 870589, 1987.2 Karim, G. A., and Bum, K. S.• "The Combustion of Gaseous Fuels in
Dual Fuel Engine of the Compression IgnitionType With Particular Reference
to Cold Intake Temperature Conditions," SAE Paper No. 800263, 1980.
3 Moore, N. P. W., and Mitchell, R. W. S•• "Combustion in Dual Fuel En· gines,"Proc. FounhCombustion ConferenceofASME and I. Mech. E.,London, 1955.
セ
The American Society of
®Mechanical Engineers
4 Karim, G. A., Jones, W" and Raine,R. R., "An Examination of the Ign tion Delay Period in Dual Fuel Engines," SAE Paper No. 892140, 1989.
S Liu.Z.•and Karim,G.A., "Ignilion Delay Period in Dual Fuel Engines, SAE Paper No. 95950466. 1995.
6 Hiroyasu,H., "Diesel Engine Combustion and Its Modelling,"Diagno. tics and Modelling ofCombusrion in Reciprocation Engine,Tokyo, 1985, pp. 20
45.
7 Hiroyasu, H., "Fuel Spray Trajectory and Dispersion in aD.I. Dies Combustion Chamber," SAE Paper No. 890462, 1989.
8 Westbrook, C. K., Wamaze,1.. and Pitz, W.J.. "A Detailed Chemic Kinetic Reaction Mechanism for the Oxidation of Iso-Octane and n-Heptal OveranExtended Temperature Range Bnd Its Application to Analysis of Engil Knock," Proc. 22nd Symp. (/nt.)onCombustion, 1988, pp. 893-901.
9' Pitz, W.I., Westbrook, C., and Leppard, W. R.• "Autoignition Chemist of C4 Olefins Under Motored Engine Condilions: A Comparison of Experiment and Modelling Results," SAE Paper No. 912315.1991.
10 JANAF,Thernwchemical Tables,3rd ed., ACS&Aip for Nation, Burel of Standards, 1985.
II Annand, W.J.D., "Heat Transfer in the Cylinders of Reciprocating Inu nal Combustion Engines," froc. /. Mech. E.,Vol. 177, 1963, pp. 973-980.
12 Abramovich, G. N.,The Theory of Turbulent Jets,M.I.T. Press, 1963.
ASME COUPON BOOKS
Use coupons to purchase
all
ASME publications
-
including special publications, codes and
standards, and
エセ」ィョゥ」。ャpapers (preprints).
Use coupons to save money. Technical papers
cost
less
when you purchase them with
coupons! One coupon may be redeemed for one
technical paper. That's a savings of $.50 for
members, $1.00 for non-members (off the
regu-lar price for preprints).
TECHNICAL PAPERS (PREPRINTS) COUPON BOOK
CONTAINS 10 COUPONS
ORDER No. CBOOOI
$40 (ASME MEMBERS) I $80 (NON-MEMBERS)
PUBLICATIONS COUPON BOOK
CONTAINS 10 COUPONS ($10 EACH)
ORDER No. CB0002
$100 (MEMBER
&NON-MEMBER)
TELEPHONE
800-THE-ASME
(800-843-2763)
USA & CANADA
95 800-843-2763
MEXICO
201-882-1167
OUTSIDE No. AMERICA