-Cu
Precipitations
at
the
Surface
of
Crystalline
Cu
—Zr
Alloys
after
Annealing
in
Hydrogen
*Xin-Nan Yu, Laboratorium
fur Festkorperphysik,
ETH Zurich, CH-8093 Zurich andL.Schlapbach,
Institut dePhysique,
Universite deFribourg.
CH-1700Fribourg
Crystalline
Cu-Zralloys
of variouscomposition
were annealed (200-400C) in
ultrahigh
vacuum and inhydrogen,
and their surfacecomposition
wasanalyzed
by
X-ray
photoelectron
spectroscopy.Contrary
to themostly
observed oxygen induced surface segregation of Zr we found strong Cu
segregation
afterannealing
Cu-richsamples
inhydrogen
atmosphere.
Scanning
electronmicrographs
show surface Cuprecipitates,
apparently
formed under the effect of the heat ofchemisorption
ofhydrogen
on Cu. IntroductionAmorphous
and alsocrystalline alloys
of Cu with thed-transition metalsZr,
Niand Pd have been studied
quite
intensively
forthe last fewyears among otherreasons because of their
special
dynamical
and diffusionalproperties
in the bulk and at the surface which results ininteresting
catalytic
andhydrogen
sorption phenomena
[1-15].
Studies of theelectronicstructure of
amorphous
CuxZr-|-x
alloys
reveal that in the middle of theglass forming
range(20
-75 at%
Cu),
i.e. around 50 at%Cu,
the Fermi level
Ef
is situated in the Zr 4d band and there is notendency
forordering.
At Cuconcentrations > 80 at%CuEf
shiftstotheedge
of the Zr 4dband so that the
density
ofstatesN(Ef)
gets
drastically
reduced to s-bandvalues,
inagreement
with asign
reversal of the Hall coefficient[1]
andincreased
tendency
for local order. These drasticchanges
of electronicproperties
are interrelated to total energychanges
and structural instabilitiesand relaxation effects
[2].
Activationenergies
for metal atom diffusion inamorphous
Cu
-Zr
alloys
are rathersmall,
by
far smaller than forrecrystallization,
implying
thatrecrystallization
is limitedby
nucleation rather than surface diffusion[3].
Theenthalpy
variation uponcrystallization
amountsto5 kJ/mol
[4].
As many other intermetallic
compounds
alsoalloys
of Cu with d-transitionmetals show surface
segregation
effects. The ratherhigh mobility
of the metalsurface more
easily
than in otheralloys.
Indeed,
the solid solutionsystem
CuxNh-x
is the best studied surfacesegregation alloy [6-8].
On the cleansurface
segregation
of Cu is observed forcompositions
0 < x < 0.75 and Nisegregation
is observed at the Cu-rich end of thecompositional
range.Electronic
theory
based on atight binding
Hartreeapproximation
iscapable
ofexplaining
this crossoverby preserving charge
neurality [6]
in acomparable
model
by Mukeherjee
et al.[7],
in which crossover is absent. Cu enrichment was also observed in thetopmost
surfacelayers
ofsingle crystalline
Cu3f\li97
and
Cu24Ni76
afterannealing
at700 to 800 K and aftersputtering [8].
Oxygen
chemisorption
leads to the "normal" surfacesegregation by
selectiveoxydation
of the more reactivecomponent
(Zr).
Activated bimetallic Zr-Nicatalysts
thus consist of active Ni metal clustersdispersed
on Zroxidesupport,
both of which are
supported
on theunderlying
bulk intermetallicalloy [9].
Attemperatures
around400°
surface enrichment ofNi intheform of NiO and Ni is found afterannealing
ofZrNi3
inO2
andH2,
resp.[9].
Preferential oxidationof Zr and Zr enrichment at the surface was also observed upon oxygen
exposure of
amorphous
Cu -Zralloys
of variouscompositions [10].
Whereas surface
segregation
effects inducedby
oxygen are known to bestrong
andimportant
e.g. for bimetalliccatalyst,
activation ofhydrogen
storage
materials andgetters
[9,11-13],
surface effects inducedby
theadsorption
orabsorption
ofhydrogen
areusually
rather weak or absent[12,13,14].
In thecaseof Cu-d transition metal
alloys,
however,
the results onhydrogen
induced surfacephenomena
are rather controversial[14]. Hydrogen adsorption
reduces the Cu
segregation
of Cu-Nialloys
ascompared
tocleansurfaces,
inagreement
withtheory [15]. Strong
surface enrichment of Cu and Pd wasfound upon
hydrogen
exposure ofamorphous
Cu-Zr andPd-Zr,
resp., around200C,
but on the Pd and Cu rich sideonly [14,16].
Itwasargued recently
thatthis unusual Cu
segregation
is characteristic ofamorphous alloys
and does not exist oncrystalline alloys.
In view of theimportance
of thesesegregation
effects and their
potential application,e..g.
in thepreparation
of bimetalliccatalyst,
we have studiedhydrogen adsorption phenomena
oncrystalline
Cu-Zr
alloys.
Photoelectron
spectroscopy
was used toinvestigate
the surfacecomposition
after various treatments of thealloys
in anultrahigh
vacuumsystem.
The formation of Cuprecipitates
was observed afterannealing
of Cu richsamples
in
hydrogen.
Scanning
electronmicrographs
show that theprecipitates
reachurn diameter.
Experimental
Cu forms with Zr many intermetallic
compounds.
Thecongruently melting
compounds
areCuZr2
(MoSi2
type
structure[17])
andCu3Zr
orCu5-|Zr-|4
(Ag5-|Gd-|4
type
structure[17]) according
to older[18]
and newer[19] phase
Cu3ZrOn.04. Cu5lZr-|4
andCuZr2
by
RF levitationmelting
in a watercooled Cu crucible at 5.
10"7
mbar the constituants Cu(99.99%, Johnson-Matthey),
Zr
(99,9%,
Johnson-Matthey)
and in the case ofCu3ZrOn.o4
some CuO(99.9%, Merck).
GuinierX-ray
patterns
ofCuZr2
andCu5-|Zr-|4
agree withthose
reported
in the literature[17],
thepatterns
ofCu3Zr
andCu3ZrOo.o4
show additional linesprobably
due to the presence of Zr richercompounds.
The lines of
Zr02
and ofCuareabsent.Heat treatment and surface
analysis
of thesamples
wereperformed
on a VG Escalab 5spectrometer
at basepressurel
.10~1u
mbarusing MgKa
radiationof 1254eV
(Au
4f at 84.0eV,
1.2 eVFWHM).
Thespectrometer
isequipped
with ahigh
pressurecell,
fracturestage,
heating
andsample cleaning
(diamond
file,
Ar+bombardement)
facilities. Thus allsample
treatment wasperformed
inside theultrahigh
vacuum(UHV)
spectrometer
avoiding
thetransfer of the
sample
atair betweentreatment andanalysis.
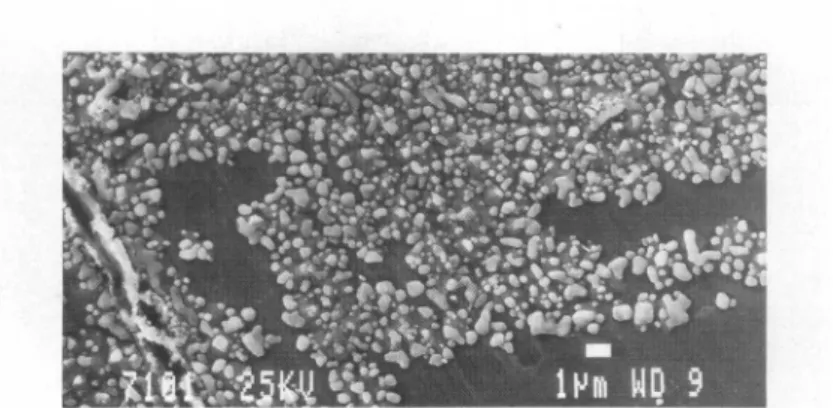
Micrographs
ofsomesamples
weretakenby
ascanning
electronmicroscope.
Results and Discussion
Typical precipitates
observed after 10 to15n
annealing
at200C and 1 barH2
of
previously
UHV-cleanedsamples
ofCu3Zr,
Cu3ZrOrj.04
andCu5-|Zr-|4
areshown in
Fig.
1. Thealloy
surface is coveredto ahigh degree
withprecipitates
of 0.1 to 1 mm diameter.
Analysis
of the characteristicX-rays
by
EDAX andXPS data revealthat the
precipitates
are ofpure Cu. Thosesamples
are Cucoloured,
contrary
to thegrey-metallic
colour of the fracturedsamples.
Fig.1: SEM micrographotaCu3Zr alloy(UHVfractured, 10hoursannealedat200Cat1 bar
H2) showingthespherically shapedsurface Cuprecipitates.
It is
questionable
whetherhydrogen chemisorption
isresponsible
for thisCu
segregation
and it isinteresting
to learn whathappened
to Zr.Surface
Typical
Zr3dphotoelectron spectra
and Cu : Zr ratios are shown inFig.2.
The more bulk orsubsurface(B)
and the moretop
surface(S)
sensitive ratios asevaluated from
Cu3p
andCu2p
core levelpeaks, respectively,
are indicated as well as the colour. Airexposed samples
showheavily
oxidized Zr and a ratherstrong
Cu enrichment at the surface(Cu
: Zr(S)
= 6.22:1).
The Zr3dbinding
energy(BE)
of 182.5 eV is smaller than that ofZrC>2
pointing
to a suboxide.UHV clean surfaces areZr rich.
Annealing
in UHV(200°
for10n)
causessome oxidation of Zr due to oxygen diffusion from bulk to surface and due to thereaction with residual gas. A mixture of Zr suboxide and Zr metal is found. Furthermore
annealing brings along
astrong
Zr enrichment in the nearsurfaceregion
and relativetothe latteroneaCuenrichment ontop
of thesurface.No Cu
precipitates
and no Cu colour were found on the airexposed,
UHVcleaned and UHV annealed
samples, respectively.
I i i i i—i—i—i-1 XPS _* I_^Zrmetal' Zro>ide y UHV I .Oil B 200°C 1.8:1 S 10HOURS _l_I_I_I_I I l_ 188 184 180 176 eV — BINDING ENERGY
Fig
2: Photoelectronspectraof the Zr3dcorelevels ofcrystalline CU51
Zri4aftervarious sampletreatments. The Cu:Zr atomic ratios S and Bwereevaluatedusing
thelargely
topsurface sensitiveCu2p peak
(S)
and thelargely
bulksensitiveCu3p
peak(B),
respectively.
Annealing
inhydrogen (1
barH2, 200C,
10n)
causesfull oxidation ofall surface and nearsurface Zr into
Zr02
aand does notsignificantly
affect the Cu : Zrratio,
although
thesample changes completely
to Cu colour in asurface
layer
of several tenth of a millimeter thickness. On somesamples
asplitting
of the Cu core levelpeaks
was observedapparently
due to theformation ofsome
electrically
isolated Cuprecipitates
whichget
charged
uponphotoelectron
emission.Apparently
annealing
in UHV leads to thenormal, fast,
oxygen induced surfacesegregation
of Zr with the formation ofZr02
at thesurface,
whereasannealing
inhydrogen
ends up in asurfacedisproportionation
of Cu-Zralloys
intoZr02
and Cuprecipitates.
In order toanalyse
the influence of time andtemperature,
hydrogen
and oxygenpartial
pressure as well as of thealloy
composition
on the formation and size of the Cuprecipitates
annealing
ofvarious
samples
attemperatures
upto400C at0..1 to 30 barH2
duringg
0.5to50n
was studied. Detailed results will bepublished
elsewhere;
they
can besummarized as follows:
-nosurface Cu
precipitates
areformed ontheZrrichcompound CuZr2.
-minordifferenceswere
only
observed betweenCu5-|Zr-|4
and
Cu3Zr.
-size and number of
particles
per unit surface areadepend
on theannealing
parameters time, temperature,
andpressure.-the unannealed air
exposed
samples
show a ratherhigh
oxygen contentdown to 100 nmdepth.
-samples
with additional oxygen(Cu3ZrOo.04)
show similar Cuprecipitates.
Based on the above resultswe
suggest
thefollowing
mechanism:The Cu rich
alloys
have a ratherhigh
oxygen content. The oxygen is dissolvedin the metal matrix or
present
in aternary
suboxide. Residual gas andhydrogen
gasare furthersources of oxygen. Elevatedtemperature
favours theformation of the
thermodynamically
more stableZr02
by
adisproportionation
reaction of the
type
Cu-Zr-Ox
+O2
Zr02
+ Cu + Cu-ZrUpon annealing
in UHV Zr isselectively
oxidized andstrongly
enrichedontop
of the
surface,
i.e. Cu is coveredby Zr02-
The presence ofhydrogen
blocksthe Zr enrichment and the free surface Cu forms
precipitates, possibly
favouredby
the heat ofhydrogen
chemisorption
on Cu. Similardisproportionation
of FeTi with the formation of surface Feprecipitates
was observed earlier[20].
We
gratefully acknowledge P.Wagli,
ETHZ,
fortaking
the SEMmicrographs,
S.BOchler,
M. Erbudak and F. Vanini for discussions and the Swiss NationalEnergy
Research Foundation(NEFF)
for financialsupport.
1. D.NguyenMann, D.Pavuna,F.Cyrot-Lackmann, D.Mayou,and A.Pasturel,
Phys.Rev. B33,5920 (1986)
2. AlfredoL.YeyatiandM.Weissmann,Phys. Rev.. B35,2714(1987) 3. E.C.Stelterand D.Lazarus,Phys.Rev. B36,9545(1987)
4. J.Chevrier,SolidState Commun.65, 1461 (1988)
5. W.Wagner,R.Poerschke andH.Wollenberger, Europhys. Lett, 2,379(1986) 6. Yi-Chen
Cheng,
Phys.Rev. B34, 7400(1986)7. S.Mukherjee, J.L.Moran-Lopez,V.Kumar,andK.H.Bennemann, Phys.
Rev.B25, 730(1982)
8. Th.Berghaus,Ch.Lunau,H.NeddermeyerandV.Rogge,Surface Science
182, 133 (1987)
9. R.B.Wright, M.R.Hankins, M.S.Owensand D.LCocke.J.Vac.Sci.Technol.A5,593 (1987)
10. P.Sen, D.D.Sarma, R.C.Budhani,
K.L.Chopra,
andON.R.Rao, J.Phys.F:Met.Phys.
14, 565(1984)11. L.Schlapbach,A.Seiler, F.Stucki, H.C.Siegmann,J.Less-Common Met.73,
145 (1980)
12. L.Schlapbach,NATO ASI B136,397(1986)
13. L.Schlapbach, in Hydrogen In Intermetalllc Compounds II, ed.
L.Schlapbach, Springer
TAP 64. chapt.2,inpress14. F.Vanini, St.Buchler, X.N.Yu, M.Erbudak,L.Schlapbach,andA.Baiker,
Surface Science189, 1117(1987)
15. S.ModakandB.C.Khanra,Phys.Rev.B,34,5909(1986)
16. P.Oelhafen,R.Lapka,U.Gubler,J.Krieg, A.DasGupta,H.J.Guntherodt,
T.Mizoguchi, C.Hague, J.Kubler and S.R.Nagel, Rapidly Quenched Metals IV,eds. T.Masumoto andK.Suzuki(JapanInstitute ofMetals,
Sendai),1259 (1982)
17. P.Villars andL.D.Calvert, Pearson's Handbook ofCrystallographicDate for IntermetallicPhases,Am.Soc.forMetals,1985
18. T.B.Massalski,ed.,Binary AlloyPhaseDiagramsVolI,American Soc. forMetals, Ohio,1986
19. E.Keller, Y.Khan, U.Gorres,Z.Metallkde.77,43(1986)
20. G.Kirch, R.Hampelmann,E.Schwab, H.Zuechner,Z.Physik.ChemieNF, 126,109 (1981)