Controlled Release Microneedle Technologies for the Enhanced
Immunogenicity of Subunit Vaccines
By
ANDREW CHARLES ZMOLEK Bachelor of Science, Chemical Engineering
University of Pittsburgh, 2013
Submitted to the Department of Chemical Engineering in partial fulfillment of the requirements for the degree of
DOCTOR OF PHILOSOPHY IN CHEMICAL ENGINEERING at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
M A SSSACH INS TITU TE OF TECHNOLOGY
NOV
29 2017
LIBRARIES
ARCHIVES
September 2017
( Massachusetts Institute of Technology 2017 All Rights Reserved
Signature of Author:
Certified by:
Certified by:
Signature redacted
Department of Ch mical EngineeringJuly 6 th 2016
Signature redacted
Paula T. Hammond David H. Koch Professor of Chemical Engineering Thesis Supervisor
______________Signature redacted
Darrell J. Irvine Professor of Materials
Accepted by:
Science and Engineering and Biological Engineering Thesis Supervisor
Signature redacted
Dani Ycihtein Herman P. Meissner (1929) Professor of Chemical Engineering, Graduate Officer Committeee for Graduate Students
MIT Libraries
77 Massachusetts Avenue
Cambridge, MA 02139 http://Iibraries.mit.edu/ask
DISCLAIMER NOTICE
Due to the condition of the original material, there are unavoidable flaws in this reproduction. We have made every effort possible to provide you with the best copy available.
Thank you.
The images contained in this document are of the best quality available.
Controlled Release Microneedle Technologies for the Enhanced
Immunogenicity of Subunit Vaccines
By
ANDREW CHARLES ZMOLEK
Submitted to the Department of Chemical Engineering on July 6th 2017 in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Chemical Engineering
Abstract
The poor efficacy of subunit protein vaccines, which typically consist of a protein antigen and a molecular adjuvant, has recently been improved by completing multiple injections of the vaccine with an exponential dosing profile over time. The hypothesis is that as viruses replicate in a host organism, they shed exponentially increasing quantities of pathogen associated molecular patterns (PAMPs) and viral protein, and replicating this pattern during vaccination allows scientists to better mimic the immune response elicited by an actual infection. Instead of completing multiple injections, a promising alternative is to complete a one-time application of a microneedle device to the skin that controllably releases the vaccine to mimic this exponential pattern to manipulate the immune system into providing strong humoral and cellular mediated protection. A number of different novel microneedle constructs have been created in this thesis towards this end. Membrane microneedles consist of tips with a polymeric core that contains vaccine that's encapsulated within a crosslinked layer-by-layer film. The three component film acts as a tunable diffusional barrier to vaccine release. Poly(7-propargyl 1-glutamate) (PPLG) polymer grafted with maleimides was used to make microneedles that function based on the same concept except that the tip's surfaces are chemically crosslinked with poly(ethylene glycol) dithiol to enable controlled release. Importantly, degradable ester bonds are incorporated into the network to allow for tunability. Finally, poly(vinyl alcohol) microneedles were vapor-phase crosslinked to form reversible acetal bonds, and these constructs were also characterized and shown to allow for controlled release. Several other constructs were also generated. The work presented herein involves the microneedle design, fabrication, and characterization of these constructs with various experimental set-ups and techniques.
Thesis Supervisors: Paula T. Hammond
Title: David H. Koch Professor and Department Head of Chemical Engineering
Thesis Supervisors: Darrell J. Irvine
Acknowledgements
This dissertation would not have been possible without the help of so many people. I constantly think about how others have helped me through this difficult process at the various stages (i.e. admission to grad school, moving and settling in Boston, the first semester process, research in grad school, etc). I would specifically like to acknowledge my gratefulness to the following people. I would first like to thank my advisors, Professor Paula Hammond and Professor Darrell Irvine, for all of their help and support over the years. I have realized that it is a significant investment of time and energy to welcome a new graduate student to a laboratory environment, and I'm very glad that they chose me for this project. It has especially been inspiring to watch Professor Paula Hammond make her transition to being the Department Head of Chemical Engineering since she provided each of her lab members with tremendous support and mentorship even despite all of her other commitments. Professor Darrell Irvine, who is also the Director of the Program in Polymers and Soft Matter Program, dedicated a significant amount of time to mentoring me over the years, and I am grateful for the terrific and insightful feedback that he provided in 1-on-1, subgroup, and group meetings. Overall, I'm very thankful for the opportunity to work for two advisors since it gave me an unparalleled experience with exposure to research in two distinct fields (i.e. polymer sciences and immunoengineering).
I would also like to thank my committee members for their terrific feedback and support over the years. Professor Jianzhu Chen inspired me to think like a biologist, and he always provided an alternative point of view on my projects. Professor Robert Langer is a world-renowned expert on controlled drug delivery systems, and I am lucky that he made the time to attend my committee meetings and meet with me in 1-on-I meetings. Finally, Professor Bradley Olsen has provided invaluable input with regards to the polymer science aspects of my projects starting from the first thesis proposal meeting.
I am also extremely grateful for lab members from both the Hammond & Irvine laboratories and also for other MIT employees & researchers who provided technical support. I was lucky enough to be able to work in two laboratories for my PhD as I was able to learn about science and research from two fundamentally distinct bodies of knowledge. From the Hammond Lab, I would like to sincerely thank Anasuya Mandal and Archana Boopathy for microneedle expertise, Mohi Quadir to polymer synthesis expertise, and Wade Wang for synthesizing the PPLG for the PPLG microneedle project. I would also like to thank Connie Wu, Sheryl Wang, Elizabeth Galoyan, Xiuyun Hou, Chibueze Amanchukwu, Lawrence Mensah, Santiago Correa Echavarria, Jouha Min, Kevin Shopsowitz, Ki Young Choi, Li Gu, Samin Akbari, Bryan Hsu, Jason Deng, Nasim Hyder, Nisarg Shah, Po-Yen Chen, & Stephen Morton. From the Irvine Lab, I would like to thank Michael Zhang for discussions and support related to microneedles, Talar Tokatlian for teaching me how to do ELISAs, Sudha Kumari for assistance with confocal microscopy, Peter Demuth for initial discussions related to microneedle technologies, and Mariane Bandeira Melo for help with my gene delivery project and general assistance in the lab. I would also like to sincerely thank Mark Miller, Valerie Corapi, Kelly Moynihan, Naveen Mehta, Sabrina Yang, Jake Martin, Kavya Rakhra, Nitasha Bennett, Yiran Zheng, Chyan-Ying Ke, Melissa Hanson, Prabhani Atukorale, Adrienne Li, Yuan Zhang, Li Tang, and Greg Szeto. I would especially like to both of the MIT undergraduate students who worked on these projects with me. Sarah Stern worked on membrane microneedles and PPLG microneedles, is currently finished with her sophomore year of college, and is now working in the Langer Lab. Jae Hyun Kim worked on membrane microneedles, is
currently finishing with her junior year of college, and she plans to attend Law School. You were both a source of inspiration, and I appreciate all of your hard work in the lab to help with completing the necessary experiments that are shown here in this thesis.
The acknowledgements section for my parents and family members has been the most difficult to write since I am closest to them and they have made the most significant investment in me over the years. First and foremost, without the love and support from my parents, I would most certainly not even have been accepted into graduate school in the first place. Also, this accomplishment and other milestones would not have meant much to me without them with which to share it. They've been extremely supportive of me in my endeavors, and I am very grateful for them. I also don't entirely understand why they've been willing to make the sacrifices that they've made in their own lives to help me succeed. For instance, when I was applying to college, I didn't receive any major scholarships. To help me pay for college, my Mom, Barbara Zmolek, started working full-time again and has since been promoted to the Director of Teaching and Learning at the College where she works and is also a Professor. My Dad, Randy Zmolek, has always been someone who has been very talented in figuring out how things work, and we would frequently work together on small projects, such as repairing bikes, putting together our trampoline, and completing the various stages of gardening. I think this really taught me how to think critically about problems, and set me up to be able to complete graduate school. He is also a one of the driving forces behind me pursuing a career as an engineer since he is an electrical engineer and recently received a promotion to a managerial engineering role! I love you both.
Both of my parents are also from large families with my Mom & Dad being one of 5 and 9 siblings, respectively. I think one of the great advantages of coming from a big family is the insight that comes from sharing the human experience together. Together, we've celebrated, been discouraged, agreed, disagreed, planned, traveled, and reflected. All of these actions happen over time, and it's very enjoyable to see how they strengthen the family bonds over the years. In particular, I would like to thank Sarah Zmolek, my sister, for her friendship - we have lots of fun memories! Overall, there are too many people for me to thank in this section and too many specific instances to remember for it all to be mentioned here. I hope you all understand, and if you ever read this thesis, I hope that you accept my apology.
I would like to sincerely thank all of my Chemical Engineering classmates - I really enjoyed the camaraderie, encouragement, insightful comments, different perspectives and opinions, and of course, the get-togethers (i.e. game nights, BBQs, Super Bowl parties, athletic events, etc). I would particularly like to thank Naveed Bakh, John Barton, Steven Brown, Jae Cho, Kameron Conforti, Nabeel Dahod, Leia Dwyer, Jeff Kowalski, Brinda Monian, Daniel Parker, Brian Seifried, Zsigmond Varga, and Min Hao Wong. Garrett Dowdy, also a member of the 2013 incoming ChemE class, was also a terrific roommate for the past two years, and it was fun to hear about all of your adventures. I also enjoyed several vacations with Apoorv Gupta and Lynna Chen, who work(ed) in ChemE labs, during our time in grad school.
Speaking of housing accommodations, I lived in Ashdown House, which is one of MIT's graduate dormitories, for the duration of my time at MIT. I served the community as an officer for two years as an events committee member and later an outings and athletics officer. Notably, I enjoyed working with Christopher Foy and Ammar El Seed to plan the events and coordinate with the residents. I would also like to sincerely thank Richard Zhang and Karim Raafat Gadelrab for being terrific roommates. Other residents, such as Louis Chen, Kevin Sabo, and Yamini Krishnan
continually made Ashdown an exciting place to live. Finally, I enjoyed the facilities at Ashdown as swinging in the hammocks, running on the treadmills, and visiting the Hulsizer for house events was a great way to relax. I also sometimes think of the basketball court at MIT as my home away from home, and I would sincerely like to thank the community there for the rare mix of fierce competition and nurturing companionship. I would specifically like to thank Pranam Chatterjee, Edward Chen, Joel Paulson, David, Darius & Robert Hill, Ravi Netravali, Kevin Kauffman, Octavio Sandoval, Jude Safo, David Bierman, Mike McEldrew, Joseph Brady, Justin Nelson, Neil Dalvie, Yalcin Cayir, John Wigneswaran, Aaron Rose, Hannah Lippe, Fulton Wang, Emilio Nanni, Mark Guttag, Daniel Lerner, and McLain Leonard.
I would also like to thank the National Science Foundation for awarding me a NSF Graduate Research Fellowship, which covered my stipend costs and a portion of my tuition fees for the three years that I was on the fellowship. In accordance with their request for the acknowledgements section, I would like to include the following text. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1122374. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. I would also like to thank the Singapore-Alliance for Research and Technology for providing funds to both fund our research efforts and also to serve as terrific collaborators. Abdul Rahim Mohamed Sharif and Shaillender Mutukumarsaswamy from Professor Jianzhu Chen's group were my primary contacts from the Singapore, and I value our Skype conversations and general discussions regarding the microneedle technologies.
I would like to thank several friends in particular from the University of Pittsburgh where I completed my undergraduate work -Ryan Stolakis was an incredibly supportive roommate and apartmentmate for all four years, and congratulations on almost finishing med school! All of your MCAT studying, long volunteer hours at UPMC, and fruit fly research paid off. I would also like to thank Lisa Volpatti, who also works as a ChemE grad student in the Koch Institute at MIT, and Chad Ringel, for their friendship and for being teammates with me on the various ChemE problem sets at PITT. I've also made several trips to New York City over these past few years in graduate school to meet back up with Danny Butler, Dr. Atif Mustafa, and Samantha Larsen from college. I would also like to thank my friends from high school who provided support during my time in grad school. Kieran McGhee and I overlapped in regards to our time in Boston since she attended Simmons College to complete dual Masters degrees in History and Archival Sciences, and it was always fun to meet to talk and reminisce about high school. I've also gotten the chance to meet up with a number of friends since we've graduated, and there are too many people to mention everyone here. Briefly, I am lucky to be friends with Nick Clingan, Matt Vaupel, and Ben Cable. My most sincere apologies if I forgot anyone.
Table of Contents
CHAPTER 1 INTRODUCTION AND BACKGROUND... 13
1.1 Controlling the spread of HIV and dengue is a difficult engineering problem... 13
1.2 Vaccine-induced immunity would greatly reduce HIV and dengue-related mortality... 13
1.3 Microneedles for vaccine delivery are of great interest to academia and industry... 15
1.4 Microneedles target the skin... 16
1.5 Controlled drug delivery techniques offer us tools to build better vaccines... 18
1.6 Eliciting strong immune responses with the combination of polyelectrolyte multilayers 20 an d m icroneedles... 1.7 A im s and scope of thesis... 22
CHAPTER 2 INTRADERMAL IMMUNIZATIONS WITH EXPONENTIAL KINETICS MOTIVATE THE DEVELOPMENT OF NOVEL CONTROLLED RELEASE MICRONEEDLE DEVICES...25 2 .1 In tro d u ction ... 2 5 2 .2 M eth o d s... 2 6 2 .2 .1 M aterials... 2 6 2.2.2 Im m unizations... 27 2.2 .3 E L ISA assays... 27
2.2.4 Flow cytom etry... 28
2.3 R esults and D iscussion... 29
2.3.1 Low dose immunization... 29
2.3.2 High dose immunization... 30
2.4 C onclu sion s... 33
CHAPTER 3 MEMBRANE MICRONEEDLES FOR TRANSCUTANEOUS IMMUNIZATIONS...34
3.1 Introduction ... 34
3 .2 M eth o d s... 3 5 3 .2 .1 M aterials... 3 5 3.2.2 Layer-by-layer film and membrane microneedle fabrication... 35
3.2.2.1 Layer-by-layer assembly conditions... 35
3.2.2.2 Polymer labeling for LbL film quality control... 36
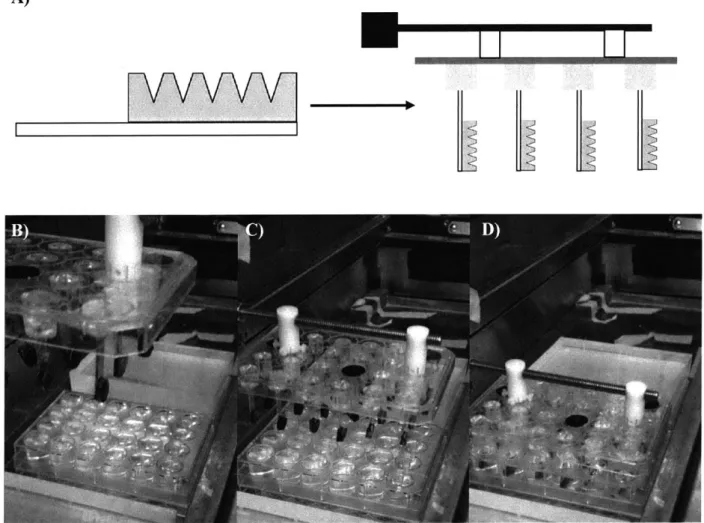
3.2.2.3 Custom apparatus design and implementation... 36
3.2.2.4 Membrane microneedle fabrication... 36
3.2.4 A TR -FTIR analysis... 37
3.2.5 Confocal and scanning electron microscopy... 37
3.2.6 Im ageJ Processing... 38
3.2.7 'H NMR quantification of polyelectrolyte presence in the films... 38
3.2.8 In vitro agarose gel release assay device... 38
3.3 R esults and D iscussion... 39
3.3.1 Overview of membrane microneedles... 39
3.3.2 High throughput membrane microneedle fabrication enabled by new 41 tech n o lo gy ... 3.3.3 Layer-by-layer film optimization... 43
3.3.4 Imaging fully-designed membrane microneedle constructs... 45
3.3.5 Crosslinking the polyelectrolytes in the LbL membrane... 48
3.3.6 Tunability of membrane and processing condition analysis... 50
3.3.7 In vitro analysis of performance... 53
3.4 C onclusion s... 55
CHAPTER 4 CHEMICALLY CROSSLINKED PPLG MICRONEEDLES FOR SUSTAINED VACCINE RELEASE...57
4 .1 Introduction ... 57
4 .2 M eth o d s... 5 8 4 .2 .1 M aterials... 58
4.2.2 PPLG synthesis and maleimide grafting... 58
4.2.3 Solubility studies... ... 59
4.2.4 Covalent crosslinking reactions... 59
4.2.5 'H NMR spectroscopy to quantify the extent of coupling... 60
4.2.6 Microneedle fabrication... 60
4.2.7 In vitro studies to quantify adjuvant signaling... 60
4.2.8 In vitro release profiles... 61
4.2.9 PPLG microneedle immunizations... 62
4.2.10 Histological examination of PPLG microneedle treated skin... 62
4.3 R esults and D iscussion... 62
4.3.1 Overview and fabrication of PPLG microneedles... 62
4.3.2 PPLG MN crosslinking solvent selection and optimization... 63
4.3.3 Polymer backing material identified... 65
4.3.4 In vitro analysis of adjuvant activity and microneedle device performance... 66
4.3.5 In vivo work optimization... 69
CHAPTER 5 CHEMICALLY CROSSLINKED PVA MICRONEEDLES FOR
SUSTAINED VACCINE RELEASE... 73
5.1 In tro du ction ... 7 3 5.2 M eth o d s... 74
5 .2 .1 M aterials... 74
5.2.2 PVA microneedle fabrication... 74
5.2.3 PVA microneedle immunization studies... 74
5.2.4 Vapor-phase crosslinking reactions... 75
5.2.5 Release profiles from PVA microneedles... 75
5.3 R esults and D iscussion... 76
5.3.1 Overview and fabrication of PVA microneedles... 76
5.3.2 PVA microneedle immunization studies... 77
5.3.3 Glutaraldehyde based vapor-phase crosslinking reaction validity... 79
5.4 C on clu sion s... 82
CHAPTER 6 LAYER-BY-LAYER MICROPARTICLES IN MICRONEEDLES FOR TRANSCUTANEOUS GENE DELIVERY...84
6.1 Introdu ction ... 84
6 .2 M eth od s... .... 84
6 .2 .1 M aterials... 84
6.2.2 PLGA microparticle synthesis... 85
6.2.3 Layer-by-layer microparticle coating... 85
6.2.4 Layer-by-layer microparticle in microneedle synthesis... 85
6.2.5 Microneedle array ex vivo application and characterization of treated 86 m u rin e sk in ... 6.3 R esults and D iscussion... 86
6.3.1 Overview and fabrication of layer-by-layer microparticles in microneedles... 86
6.3.2 Tunable PLGA microparticle synthesis to target or avoid macrophage 87 p h ago cyto sis... 6.3.3 Layer-by-layer microparticle optimization... 88
6.3.4 Microneedle array ex vivo application and characterization of treated murine 90 sk in ... 6 .4 C on clu sion s... 92
CHAPTER 8 SUPPLEMENTARY FIGURES ... 95
8.3 Membrane Microneedles for Transcutaneous Immunizations... 95
8.4 Chemically Crosslinked PPLG Microneedles for Sustained Vaccine Release... 96
CHAPTER 9 APPENDICES...97
9.1 PNMP Polymer Reproducibility Studies for Microneedle Vaccinations... 97
9.2 Layer-by-Layer Coated Alginate Particles for Sustained Vaccine Release... 101
List of Figures
Figure 1-1: Microneedle patches for transdermal vaccine delivery.
Figure 1-2: Immune cell networks and locations within the layers of the skin. Figure 1-3: Layer-by-layer film growth mechanism is illustrated.
Figure 2-1. Experimental set-up and the low dose exponential injection pattern is shown. Figure 2-2. Antibody production and avidity is higher for the exponential injection mice. Figure 2-3. Experimental set-up and the high dose exponential injection patterns are shown. Figure 2-4. Antibody production is comparable for the vaccinated mice.
Figure 2-5. Antigen-specific T cells are most abundant with the 2 week exponential dosing regime.
Figure 2-6. Significant differences are not observed in memory T cell populations across the experimental groups.
Figure 3-1. Mechanism of action for membrane microneedles.
Figure 3-2. Membrane microneedle synthesis and workflow is shown. Figure 3-3. Custom apparatus design and implementation.
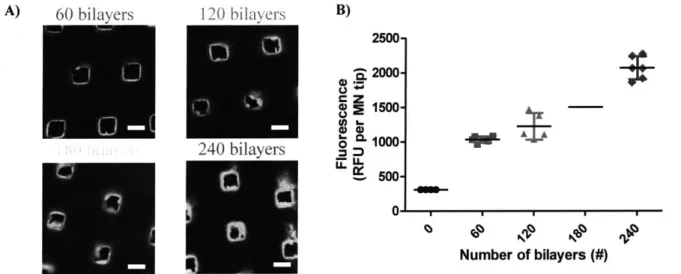
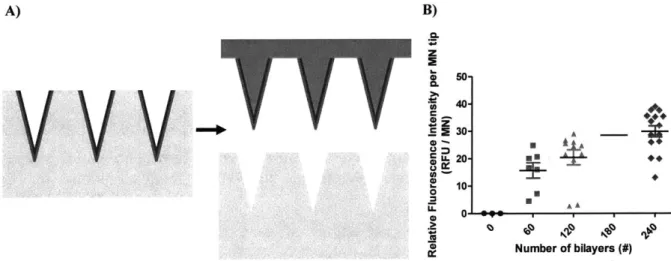
Figure 3-4. Membrane thickness is dependent on the number of assembled bilayers.
Figure 3-5. Membranes are transferred onto polymeric microneedles and the film thickness remains dependent on the number of assembled bilayers.
Figure 3-6. Membrane microneedle tips have micron-scale surface roughness. Figure 3-7. Three dimensional reconstruction of membrane microneedles.
Figure 3-8. Dual imaging of vaccine cargo and exterior membrane on the microneedles. Figure 3-9. Chemical and thermal crosslinking of the LbL films are successful and does not ablate the fluorescence from the conjugated fluorophore.
Figure 3-10. Quantification of polymer presence in ternary LbL films.
Figure 3-11. Membrane microneedle synthesis is a highly tunable and robust process.
Figure 3-12. Two hundred and forty bilayers in the membrane microneedle's film are needed to retain the membrane properties of these constructs in skin mimicking gels.
Figure 3-13. Membrane microneedle's release profile is shown.
Figure 4-1: Introduction to novel crosslinkable PPLG polymeric MN arrays. Figure 4-2: Successful PPLG MN crosslinking solvent selection.
Figure 4-3: Successful PPLG MN backing material selection. Figure 4-4: Successful PPLG MN fabrication.
Figure 4-5: PPLG MNs do not effectively generate antibody and T cell responses while traditional PAA MNs do.
Figure 4-6: PPLG MNs do not effectively pierce mouse skin while traditional PAA MNs do. Figure 5-1: Crosslinked poly(vinyl alcohol), (PVA), MNs can prolong the release of subunit vaccines.
Figure 5-2: PVA microneedles successful deliver a model subunit vaccine intradermally to prime a strong humoral immune response.
Figure 5-3: ATIR-FTIR identifies PVA's unique chemical signatures. Figure 5-4: Vapor-phase crosslinking forms intermolecular acetal bonds.
Figure 5-5: Controlled vaccine release properties from crosslinked PVA microneedles are tunable and robust.
Figure 5-6: Excess PVA material is removed from the patch and replaced with water-soluble PAA polymer.
Figure 6-1: Layer-by-layer microparticle in microneedle array overview and synthesis. Figure 6-2: Particle synthesis conditions elucidated to provide particle templates above and below the size cut-off for macrophage phagocytosis.
Figure 6-3: Surface charge reversal during particle-based layer-by-layer for a range of polycations and double-stranded DNA.
Figure 6-4: Total double-stranded DNA loading in layer-by-layer particle coating experiments. Figure 6-5: Particle containing microneedle arrays deliver payload into the dermis.
Figure 6-6: In vivo particle retention and luciferase expression after intradermal delivery via injections or LbL particles in microneedles.
Supplementary Figure S3-1: Relevant 1H NMR for membrane microneedle quantification studies.
Supplementary Figure S4-1: Maleimide - thiol coupling proceeds spontaneously in an aqueous solvent.
Supplementary Figure S9-1: Previously published polymer multilayer tattooing for enhanced DNA vaccination schematic, overview, and early replication results.
Supplementary Figure S9-2: 'H NMR comparisons between the polymer used in the reproducibility studies and the original paper.
Supplementary Figure S9-3: PNMP thickness after UV exposure and aqueous solution treatments at different pH values.
Supplementary Figure S9-4: PNMP allows for partial release of a fluorescent tagged LbL film after UV exposure to cleave the nitrobenzyl group and activate the polymer.
Supplementary Figure S9-5: Overview of the alginate microparticle for vaccine delivery project. Supplementary Figure S9-6: Alginate particle fabrication optimization.
Supplementary Figure S9-7: Alginate particle vaccine loading optimization.
Supplementary Figure S9-8: Fluorescent (DiD) labeled particles were detected in the gastrointestinal tracts of mice that had been administered these particles by oral gavage.
1. Introduction and Background
1.1 Controlling the spread of HIV and dengue is a difficult engineering problem
Infectious diseases have been estimated to cause over 13 million deaths per year. To curb these fatalities and loss of associated economic productivity, it has been estimated that 120 billion dollars are spent on treating infectious diseases in the United States annually2. Additionally, the U.S. spent $15 billion dollars on HIV programs in 2014 alone3. HIV, which is a retrovirus that likely originated in chimpanzee populations in Cameroon4, has claimed 39 million lives to date and 2.1 million individuals were infected in 2013 alone5. Dengue fever virus (DENV), which is a flavivirus, claims 24,000 lives per year6, and 30 million cases occur each year7. Importantly, HIV and dengue are transmitted via sexual activity and mosquito bites, respectively, and these diseases are particularly present in developing countries where medical interventions are limited.
Viral spread can be mitigated by inhibiting viral replication in infected individuals, developing programs that decrease transmission rates by either educating the public or by eliminating organisms that carry the virus, or by vaccinating at risk individuals. Anti-retroviral drugs (ARV) have gained widespread use to decrease HIV virus titers, and the first ARV was a synthetic peptide that blocked HIV - CD4' T cell fusion and viral entry8. Although future generations of anti-retrovirals have been engineered to be more effective, ARV's are prohibitively expensive with only 75,000 of the millions of HIV' individuals in Africa receiving them9. ARV's are also not curative, and antiretroviral drug resistance can develop' . DENV treatment prospects are even worse since there are no current therapies. During the 1960's, a program in Central America greatly reduced the spread of dengue fever by eliminating Aedes aegypti mosquitos, but these programs have since been discontinued. Taken together, all of this evidence motivates work on developing vaccines that can potentially elicit potent immune responses against these two infectious diseases.
1.2 Vaccine-induced immunity would greatly reduce HIV and Dengue-related mortality Vaccines are used to educate the adaptive arm of the immune system to be cognizant of the invasion of pathogens. These entities introduce 'non-self and 'danger' components into the organism, and vaccines are also designed to introduce these two components. Toll-like receptors (TLRs) and pattern recognition receptors (PRRs) of the innate immune system can detect these
conserved danger signals and trigger the release of inflammatory cytokines. A series of selection processes occur to generate a repertoire of T and B cells that can recognize the foreign proteins from the invading species. The clonally expanded effector T cells and the produced antibodies can then be used to recognize intracellular and extracellular pathogens, respectively. If the pathogen ever enters the vaccinated host, the host's already primed immune system will be ready to kill the invading pathogens by a variety of mechanisms". Vaccines can take the form of attenuated whole virus vaccines or subunit vaccines. The former are typically strongly immunogenic, but there are a number of safety risks - Baba et al discovered that macaques vaccinated with live attenuated, multiply deleted SIV developed AIDs and similar prototypes for humans might be equally dangerous". Subunit vaccines are safer but are not as potent as viral vector vaccines. They involve two parts: (i) viral proteins or DNA / RNA that encodes for a viral protein and (ii) an adjuvant. The enormous design space that can be explored when designing subunit vaccines is also advantageous because components can be targeted to lymph nodes'3, the vaccine can be
incorporated into particulate forms to mimic the virus'4, and a variety of potent adjuvants can be
used. For example, Moon et al developed a subunit vaccine strategy that involved incorporating ovalbumin and MPLA into interbilayer-crosslinked liposomes that remained intact until they entered endolysosomal compartments15. They showed that these vaccines were more effective than traditional liposomal vaccines, and this work and the results of others suggest that subunit vaccine immunogenicity can still be greatly increased.
The development of HIV vaccines have hit a number of roadblocks since the first clinical trial in 198716. The seminal studies in this field have used macaque models since the field lacks a suitable small animal model that adequately represents the human immunology that's needed to study HIV. Initial studies showed that neutralizing antibody directed against HIV- 1 blocked viral plaque production in in vitro cultures and also resulted in pig-tailed macaques having substantially fewer SHIV DNA copies in the blood after vaccination". In two separate papers, Shiver et al and Barouch et al used Ad5 viral vectors that expressed the SIV gag protein and cytokine augmented DNA vaccination, respectively, to suppress viremia and maintain stable CD4* T cell levels'"9.
However, Barouch et al later discovered that viral particles could evade the adaptive immune system by mutating several amino acids. Additionally, in comparison to other viruses, such as the
flu virus, numerous studies have documented that HIV can mutate extremely rapidly due its error-prone reverse transcription-in fact, it's been estimated that the HIV-1 reverse transcriptase is about 1 million times more error-prone than eukaryotic DNA polymerase 20. Baba et al found that adult macaques don't develop SIV after infection with an attenuated viral strain but showed that CD4' T cell levels in neonatal macaques decrease rapidly after injection of the same virus21. Several studies have reported similar findings and noted that this makes it extremely unethical for attenuated whole virus to be administered to humans12 22. More recently, it's been shown that broadly neutralizing antibodies and inducer combinations can decrease the size of the latent HIV-1 reservoirs23, antibody immunotherapy has been shown to suppress viraemia in macaques2, and recent results suggest that computational, epitope-focused protein design may be able to induce potent neutralizing antibodies against HIV25.Although these results are extremely promising, there
is not currently an approved HIV vaccine.
It's been difficult to develop a successful dengue (DENV) vaccine formulation because there are four different serotypes of the virus (DENV-I through DENV-4). Thus, it has been determined that a successful DENV vaccine must ideally protect against all four serotypes, and this requires eliciting a balanced antibody response against all of the serotypes7. It has also been shown that a secondary infection with a DENV serotype can allow the virus to complex with non-neutralizing antibodies and get phagocytized by mononuclear cells, which is the primary site where more viral particles are produced26. Next, Konishi et al used four different DNA vaccines to elicit an immune response against all four serotypes in a mouse model27. Recently, Sanofi Pasteur funded a proof-of-concept trial to assess the efficacy of a recombinant, live, attenuated tetravalent dengue vaccine, and they found that their candidate vaccine allowed for mild protection against three of the four serotypes.
1.3 Microneedles for vaccine delivery are of great interest to academia and industry for several key reasons
Microneedles are constructs that are between 300 and 900 um in height, and they can either be made of metal or degradable materials with ~100 individual tips (Figure 1-1). The first patent that outlined the use of microneedles to deliver molecules into the skin was issued in 197628. Since then, vaccines have been incorporated onto or within microneedles as either dried powders29 or
solutions3 0, and these materials are subsequently delivered into the dermis. Academic labs have
been particularly interested in microneedles to deliver vaccines, and early work involved metal MNs31. The vaccines delivered by these MNs demonstrated complete protection as a result of high
hemagglutination inhibition titers and CD4 and CD8+ T cell responses. Overall, MNs have been
used to deliver subunit and attenuated viral vaccines for HIV33, influenza32, hepatitis C34,
diphtheria toxin35, anthrax36, malaria37, and tetanus37. More recent MN designs have incorporated biodegradable and biocompatible polymers, such as polyvinylpyrrolidone (PVP), and one paper showed that MN vs. intramuscular immunizations resulted in 1,000-fold more efficient viral clearance from the lungs38. In addition to microneedles being used to deliver vaccines in the academic setting, they've also been used extensively by companies.
A) B)
4
Figure 1-1: Microneedle patches for transdermal vaccine delivery. (a) Microneedle patch picture. Scale bar: 0.5 cm. (b) Scanning electron microscopy image of polymeric microneedle tips.
Three companies are actively developing MN technologies to deliver influenza vaccines. The FDA has approved an intradermally injected vaccine that's produced by Fluzone. Their technology doesn't utilize an array of MNs to inject the solutions but instead uses a single MN. NanoPass has developed micropyramidal silicon MNs, and BD has published several papers that outline strategies to deliver vaccine injections intradermally with a MN3 9,40. Furthermore, Wick et al from
3M is developing microstructured transdermal systems for vaccine applications41. There are a
number of reasons that academia is interested in studying this technology, and industry envisions that a considerable profit can be made from this technology in the near future.
1.4 Microneedles target a site that contains high levels of immune cells and this technology has advantages over hypodermic needle injections
The skin is an ideal site for vaccine delivery. Its structure and the prolific presence of immune cells in the skin makes MINs an ideal modality to deliver vaccine components. As the first line of defense against pathogens, the skin is filled with sentinel immune cells, such as Langerhans cells, macrophages, and dermal dendritic cells (DCs) (Figure 1-2)". Macrophages and DCs, which are antigen presenting cells (APC), can take up peptides from the vaccine into endosomes and present them on MHC class II molecules. APCs with internalized foreign peptide and adjuvant can migrate to the lymph nodes via the lymphatics where they can help to initiate an immune response. There are also Toll-like receptors (TLRs) on keratinocytes and other resident cells which can detect foreign components. For example, ssRNA, CpG DNA, and lipopolysaccharide (LPS) can be recognized by TLR7, TLR9, and TLR4, respectively. Furthermore, Kenney et al discovered that 1 unit of intramuscular influenza vaccine and 1/5th of a unit of intradermal vaccine elicited comparable immunogenicity42. This dose sparing effect could be particularly useful for vaccine components that can only be manufactured on a small scale. Also, since the skin is also easily accessible and injections into the skin via microneedles were found to be significantly less painful than an injection with a hypodermic needle43, there also might be fewer patient compliance issues with MNs.
-Corneocytes Stratum
corneum .
CD8+ T cell Langerhans cell Epidermis
*J
CD4+ T cell Dermal DC Macrophage
Figure 1-2: Immune cell networks and locations within the layers of the skin. Immune cell locations in the skin are shown.
Microneedles are also ideal for a number of other non-immunological reasons. MINs can potentially be self-applied in the developing world whereas traditional injections can only be done by a trained employee. Donnelly et al found that MN arrays could be reproducibly applied by human volunteers who were given adequate instruction44. This is particularly interesting upon
considering the fact that in underdeveloped countries in Africa, such as South Africa, some medical clinics are not yet functional due to scarce or non-existent allocated funds. The direction that social determinants will lead this situation are unclear45. Also, preliminary MN storage data suggests that MNs can also potentially be easily shipped to third world countries lyophilized, and they would be able to maintain their bioactivity for prolonged periods of time before administration33. Lastly, a smaller amount of shipping space might be required to ship MNs when compared to shipping space for hypodermic needles and vaccine solutions. Furthermore, degradable needles could entirely eliminate sharps waste46.
Microneedles should also be of particular interest in light of the shortcomings of the current vaccine administration routes. There have been a number of documented problems with the quality of the cold chain between the production facilities and eventual vaccine distribution. It's been noted that it's prohibitively expensive or impossible in some regions of the world to maintain the samples in the 2-8 'C optimal range and to prevent inadvertent sample freezing4 7 48. The issue of uncapped needles or needles that have been used multiple times has also been a recurring problem
in underdeveloped countries, and one study even found that over 50% of injections in the developing world are unsafe49. Additionally, while controlled drug delivery technology is applicable to vaccines that can be injected intramuscularly or intravenously, it is particularly useful with microneedles since the needles act as a platform where these systems can be tuned for maximal immunity.
1.5 Controlled drug delivery techniques offer us tools to build better subunit vaccines
Significant enhancement of vaccine efficacy might be achieved by coupling microneedles with controlled drug delivery techniques and polymer science technology. Controlled drug delivery,
specifically, is about manufacturing synthetic structures or particles that interact with or regulate a biological system to achieve a desired outcome. Biological systems are compartmentalized on many different levels (i.e. organs/tissues, cells, subcellular level) and processes occur in a time-dependent fashion. For example, the process of T cell activation and B cell somatic hypermutation and class-switching to generate high affinity antibodies of the appropriate isotype can take several days after the antigen is first detected, and this process occurs in several tissues and involves many different cell types and proteins". With controlled drug delivery, an application for a material with certain properties is typically identified, and then a polymer is designed to serve that specific function. Controlled drug delivery vehicles are assembled from these polymers and the cargo, and the vehicle is engineered to disassemble in a time-dependent fashion. For example, pellets made of ethylene-vinyl acetate copolymers were originally used to release antigen for over six months50. In the field of immunobioengineering, Irvine et al developed antigen-containing nanoparticles made with PLGA-PEG-PLGA that were engineered to be taken up by dendritic cells5 1.
Drug delivery materials and specific polymers have been designed for specific applications and have a wide range of properties. Lynn el al synthesized a high-throughput library of cationic, poly(j-amino esters), (PBAE) that complexed DNA to form polyplexes. Several candidate polymers were identified that generated high levels of transfection after administering polyplexes of luciferase DNA and PBAE to COS-7 cellss2-4. With regards to material design in general, Liu
et al designed diacyl lipid tails and attached them to CpG DNA or PEG-peptide conjugates, and these macromolecules trafficked to lymph nodes where they enabled robust immune responses13.
Polymers have also been designed to undergo surface erosion where the outer layers degrade before hydrolysis can occur on the interior5 5, and this potentially has applications to protecting
very labile macromolecules.
Polyelectrolyte multilayers (PEMs) are a subset of the controlled drug delivery field and have seen great interest in recent years 6-59. The PEM synthesis process involves dipping a substrate into aqueous solutions of complementary charges with interspersed wash steps to remove weakly bound polyelectrolyte (Figure 1-3a). The resulting PEM films have nanometer-scale layers of polymer that is electrostatically bound to each other (Figure 1-3b). Although it's also been used for many non-biological applications, the incorporation of biocompatible components into these
multi-layered structures have been used for various immunomodulatory and biomedical applications60,61. These films that are assembled layer-by-layer (LbL) can incorporate a wide
variety of components without exposing them to harsh processing conditions. Also, only aqueous solvents are used during the self-assembly process, and the film!s have been shown to protect encapsulated components from degradation62,63 .Data in the literature also suggests that these films can be targeted to tumors56 59, and the films can be constructed to disassembly on the timeline that
is most appropriate for the biological application of interest63 64.
A) B) Substrate Polycation Wash Polyanion Polycation Polyanion
Polycation Polyanion Polycation
Solution Solution
Polyanion
Substrate
Wash
Figure 1-3: Layer-by-layer film growth mechanism is illustrated. (a) Protocol used to grow LbL films is shown. (b) Resulting LbL film is shown on a substrate.
1.6 Eliciting strong immune responses with polyelectrolyte multilayers on microneedles Layer-by-layer (LbL) is a technique used to assemble films of polymers with complementary interacting groups in order to form tightly intertangled structures. Theories regarding many aspects of LbL films including counterion concentrations, spray or solution assembly methods (kinetic vs thermodynamic), and polymer combinations have been extensively studied6 5
,66. Although electrostatic interactions are typically used as the driving force for these films to self-assemble, hydrogen bonding58 and hydrophobic interactions 67 have also been studied in the context of LbL films. Importantly, it's been shown that these films can grow at linear or exponential rates with the addition of each bilayer and that interdiffusion between the film's layers occurs as the film grows68. This technology has been used to coat medical devices, bandages, particles, and biological
entities 69-71, and the films have been constructed to contain polymeric particles, polyelectrolytes, liposomes, viruses, and inorganic materials72 7 .
Layer-by-layer films that contain DNA or RNA that encode an antigen's sequence need to be delivered to the nucleus or cytoplasm, respectively, for the antigen needed to initiate the immune response to be produced. The quantity of antigen produced from these subunit vaccines has been correlated to the vaccine's immunogenicity76. From a mechanistic standpoint, a cell must engulf the cargo by endocytosis, the cargo must escape the endosome, and it must enter the cytoplasm. If the delivered cargo is mRNA, it can then be translated to protein. If DNA has been delivered, it must additionally traffic to and traverse the nuclear membrane for it to be transcribed and translated. The engulfed cargo can either be a section of the LbL film that has delaminated from a coated surface or it can take the form of a coated micro or nano-scale particle. Jewell et al has studied how plasmid containing LbL films have been able to transfect cells in vitro and has suggested that the polycation in these films may help with film internalization and expression of plasmid77. In general, cationic polymers that have secondary or tertiary amines may be the most efficient at escaping endosomes8. Specifically, Ghosn et al found that chitosan, which contains primary amines, can be functionalized with secondary and tertiary amines to achieve a 100-fold increase in plasmid DNA transfection efficiency 8. Although high transfection efficiency is crucial to the immunogenicity of plasmid DNA subunit vaccines, it is also important for transfection to occur over the desired timeframe.
By incorporating boundaries to diffusion within the assembled films, it is possible to tune the release kinetics and dissociation of the films from minutes to months63,64. As a baseline release
profile, Min et al showed that gentamicin containing films destabilize over the course of a week and that the active agent retained antibacterial properties. They next extended the duration of release by adding laponite clay barrier layers and achieved sustained release for over three weeks64. Hong et al showed that by incorporating graphene multilayer 'gates' into his films, he could get sequential release of ovalbumin for up to 100 days63. While the sequential release of agents is particularly useful to engineers who aim to modulate the immune system to capitalize on how temporal events occur between immune cells, it may also be possible to further control these LbL systems by increasing their specificity for certain cell types. For example, Mintern et al targeted
LbL 800 nm particles to dendritic cells by functionalizing the nanoparticles with antibodies that target CD1lc and CD20579.
Previous work on microneedle vaccines and transdermal delivery with layer-by-layer films from the Hammond and Irvine labs have explored both subunit vaccine and viral vector delivery. First, Su et al used a transcutaneous approach to deliver ovalbumin and immunostimulatory CpG DNA into murine ear skin, and showed that 14% of Langerhans cells in the skin engulfed ovalbumin (OVA) from the films. Demuth et al then followed up this OVA-oriented research by publishing three more papers where OVA was used as the antigen. Different components were included in the MNs in these papers, including lipid nanocapsules7 5, microparticles (MPs) in a dissolving MN
matrix80, and silk tips that allowed for programmable release of antigen 1. Demuth et al also published two systems where a subunit vaccine incorporating plasmid DNA showed substantial amounts of transfection. First, polymer nanoparticles embedded in PEM coatings of varying thickness were applied to mouse skin for different durations of time and the in vivo transfection was then tracked in a murine ear skin mode174. Next, a system that employed a release layer that detached the film from the MN surface was used, and it was found that NIN delivery elicited 140-fold higher gene expression in non-human primate skin relative to intradermal polyplex injection and that led to strong CD8 T cell and IgG responses". In Demuth et al's final paper, Ad5 viral vectors encoding SIV-Env or SIV-Gag were dried into sucrose matrices on microneedles, applied to macaques, and robust T cell and antibody responses were observed via ELISPOT assays and ELISAs, respectively, for sixteen weeks82
1.7 Aims and Scope of Thesis
When I started my PhD, I decided that I needed to have a project that would be deemed a "scientific home-run" if it were to be successful. Since the Irvine lab had recently published a PNAS paper on exponential dosing profiles to prime strong immune responses, there was tremendous momentum for designing microneedles that would produce this profile. This is in contrast to the studies in this paper where multiple subcutaneous injections were performed so that the summation of the individual injections would approximate an exponential profile. Thus, I began a selection process to narrow down the list of options with regards to which projects would have the greatest probability of making microneedles that could release a subunit vaccine at an exponential rate.
To choose these projects, several assumptions were first made about what types of mechanisms would need to be in play to be able to attain an exponential release profile of a vast array of subunit vaccines. We first assumed that the microneedles would need to function correctly regardless of the antigenic protein and molecular adjuvant. This allowed us to rule out systems that would complex the vaccine solely based on electrostatics or other molecular interactions. Further, we assumed that the antigenic protein, which could vary in molecular weight by one to two orders of magnitude, could easily be incorporated into the bulk of microneedle tips, but that it would require extra experiments and optimization to incorporate these with polymeric, metallic, or liposomal particles within microneedles. This would also defeat the purpose of using microneedles since engineered particles would be the novelty instead of the microneedles, which were chosen to be the focus of this project for the aforementioned reasons. Thus, particle-based systems were eliminated from the list of options. Molecular adjuvants, such as the vast array of toll-like receptor agonists, can vary from single or double stranded RNA, DNA, small molecules, lipids, and even flagellum. We also wanted to be able to incorporate any potential collaborator's 'adjuvants of choice' into the system, so we exclusively looked further into microneedles that could be cast from an aqueous solution of polymer and vaccine directly into the microneedle tips. Next, we decided to compartmentalize the vaccine from the treatments as much as possible and began thinking about
exterior membranes that could act as a diffusional barrier to vaccine release.
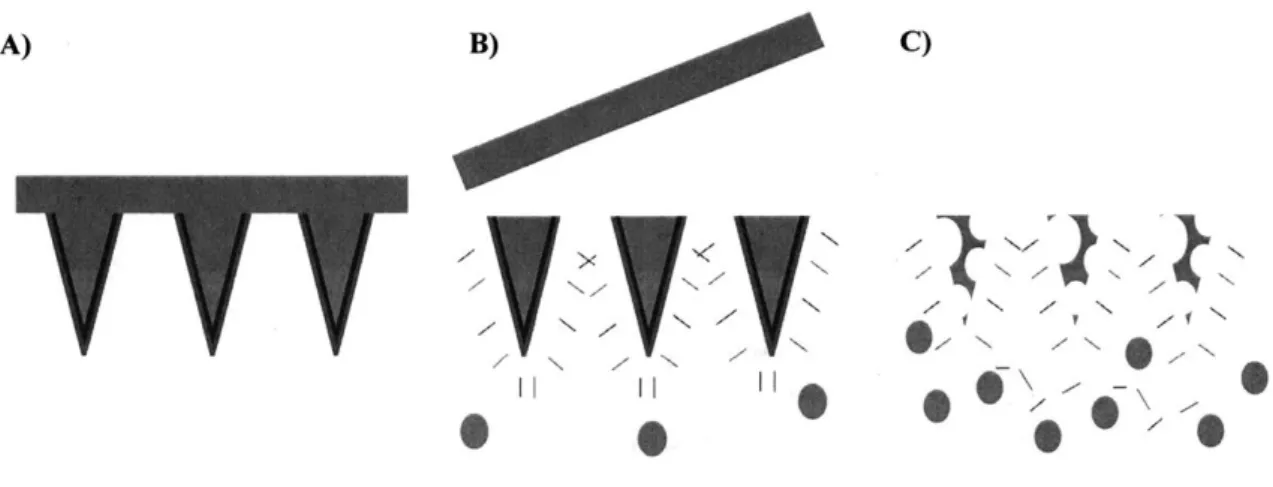
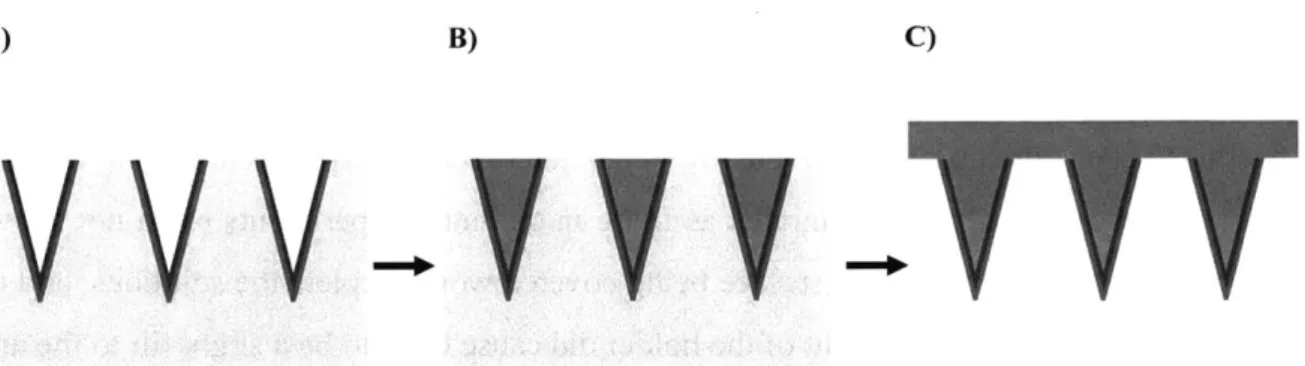
In order to make systems that could theoretically achieve exponential release, however, we assumed that the membrane would need to fit into one of the following categories. Herein, we define exponential release as a profile with progressively more vaccine being discharged from the device over time instead of a strict definition of a linear plot of instantaneous vaccine remission versus time. An alternative definition is that the slope of the cumulative egress of vaccine versus time should become larger with time as opposed to conventional drug delivery systems where this slope will approach zero. Category I: a membrane composed of multiple polymers that would selectively release one or more of the components and progressively lose its membrane due to increased membrane porosity. Category II: a degradable membrane that would initially be a tight, interconnected mesh of polymers of defined thickness but that would contain degradable units spread uniformly throughput the mesh that would hydrolyze over time to allow for increased chain
mobility and diffusion of the vaccine outwards from the construct. Category III: a membrane with truly reversible crosslinks that could be pushed towards an equilibrium at later time points that would exhibit a very low extent of crosslinking.
Membrane microneedles (category I), PPLG microneedles (category II), and PVA microneedles (category III), respectively, then became the three specific aims of my thesis, and the different development strategies for each project increased the probability that one of the projects would yield exponential profiles. While much of this work is too broad in scope to be completed in any one thesis, we hope that a future graduate student will be able to bring this work to completion since the fundamentals and framework are now in place.
2. Intradermal Immunizations with Exponential Kinetics Motivate the
Development of Novel Controlled Release Microneedle Devices
2.1 Introduction
Subunit vaccines are composed of an antigen and an adjuvant, and are frequently given in soluble form8 3. The antigen is a protein that is part of the virus, and a successful immunization will elicit both antibodies that can specifically bind to this antigen and T cells that can recognize a fragment of the antigen that is presented on an antigen presenting cell. These two processes, which are both hallmarks of an adaptive immune response, would allow the individual's immune system to combat the virus if the individual is infected. The adjuvants, which are typically toll-like receptor (TLR) agonists and are a necessary component, activate immune cells by both initiating signaling cascades to produce inflammatory cytokines and to initiate other relevant processes 84,85. To date, however, these subunit vaccines are typically not as effective as live attenuated vaccines3 3. To
increase the immunogenicity of this class of vaccines, alternative dosing patterns have been investigated extensively86. The exponential kinetic pattern has been studied since it mimics the manner that pathogen replication produces increasing amounts of antigenic protein and TLR agonists87.
Recent immunizations studying the exponential dosing regimen employed subcutaneous injections over the course of several days to mimic this kinetic pattern. While all groups received the same total quantity of vaccine, exponential dosing groups received the majority of the vaccine during the final injections. A study of a peptide vaccine for T cell responses showed that the exponential pattern elicited vaccine responses with the highest percentage of CD8* T cells producing IFN-y, the highest value for T cell proliferation, and performed the best with regards to resisting viral challenge and promoting survival87. Recent work from the Irvine and Anderson labs has studied
these exponential dosing patterns for antibody responses88. They have shown that this pattern elicits the highest antibody titers to the stripped-core gp 120, which is a model HIV immunogen, as well as promoting antigen retention in the lymph nodes. The antigen retention is currently attributed to antibody-antigen immune complex formation in these nodes, and they are also studying the role of T follicular helper cells. Computational studies from the Chakraborty group at MIT have confirmed these results in silico.
It remained unclear, however, if this exponential kinetic pattern would also show promise if the injections were done intradermally instead of subcutaneously. Soluble vaccine injected intradermally will be captured by both Langerhans cells and dermal dendritic cells, which can then migrate to lymph nodes to activate the adaptive immune response 9. Subcutaneous vaccine administration mainly elicits monocyte-derived dendritic cells to take up, present antigen, and migrate through the lymphatics90. The inherent differences in the relevant dendritic cell populations for these two types of injections is indeed one of the most relevant issues that needs to be considered when studying exponential dosing regimens.
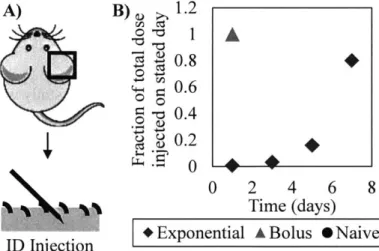
In this chapter, ovalbumin (OVA), a model antigen derived from chicken egg whites, and poly(I:C), a double-stranded RNA adjuvant that signals via TLR3, were injected intradermally either in a bolus or in an exponential pattern. We found that the humoral immune response was dependent on the subunit vaccine dosage and that higher antibody titers and avidity could be observed with an exponential dosing pattern. Since previous work suggested that the exponential pattern delayed dendritic cell recruitment and thus had an impact on the T cell response87, we
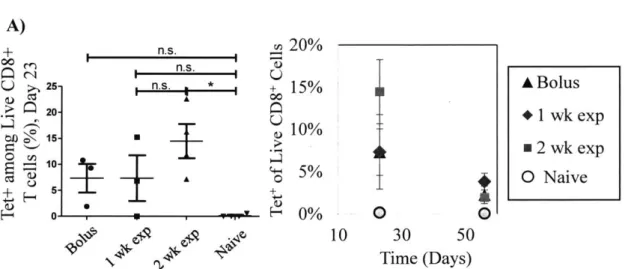
tested the hypothesis that the exponential kinetic pattern would elicit a more antigen-specific T cells. We found that there were significantly more CD8* T cells that were specific for the H-2 Kb OVA (SIINFEKL) tetramer at day 23 if the exponential pattern of injections was completed over the course of 2 weeks relative to the nafve control. Finally, central memory and effector memory T cell responses were studied at day 64 in this experiment and shown to be comparable across all groups. These results suggest that immunizations completed intradermally with exponential kinetic patterns can enhance humoral immunity and the clonal expansion of antigen-specific T cells.
2.2 Methods 2.2.1 Materials
Purified ovalbumin (OVA) was purchased from Worthington Biochemical (Lakewood, NJ), poly(I:C) HMW was purchased from Invivogen, and imiquimod was purchased from Sigma Aldrich. MAXIsorp NUNC plates, purified anti-mouse CD16/CD32 antibody, and anti-mouse PECy7-anti-CD62L antibody were purchased from eBioscience. The anti-OVA IgG-HRP was
purchased from BioRad. TMB (3,3',5,5'-tetramethylbenzidine), ACK lysis buffer, and DAPI were purchased from ThermoFisher Scientific. APC rat anti-mouse CD8 and FITC-UCD44 antibodies were purchased from BD Pharmingen. H-2 Kb OVA (SIINFEKL) tetramer was purchased from MBL International. Concentrated skim milk was purchased from Lab Scientific, Inc. H2SO4 and
micro hematocrit tubes with heparin coatings were purchased from VWR. Urea, fetal bovine serum (FBS), and bovine serum albumin (BSA) were purchased from Sigma-Aldrich. PBS-Tween tablets were purchased from EMD Millipore. Dasatinib was purchased from Selleckchem.
2.2.2 Immunizations
Animal studies were approved by the MIT IUCAC and animals were cared for in the USDA-inspected MIT Animal Facility under federal, state, local, and NIH guidelines for animal care. Groups of 4 or 5 C57B1/6 mice were immunized with intradermal injections in the same ear so that vaccine drainage occurred to the same lymph node. For the low dose immunizations, the mice were only subjected to a prime with a total of 1 pLg OVA and 0.5 [ig imiquimod. For the high dose immunizations for each the prime and the boost, 10 pg OVA and 1 pg poly(I:C) were administered. To show an example calculation for the exponential kinetics groups in the high dose experiment, both the prime and the boost included masses of OVA and poly(I:C) given over time as follows, respectively: 1st injection (0.072 pg, 1.2 ng), 2 nd injection (0.32 ptg, 32 ng), 3 rd injection (1.6 pig,
160 ng), and 4th injection (8.008 ig, 806.8 ng). At pre-determined time points, mice were bleed
retro-orbitally with capillary tubes (VWR). The blood was collected into EDTA treated vials (Greiner Bio-One, Monroe, NC) or serum test tubes (Sarstedt, Ntimbrecht, Germany) for processing for flow cytometry and ELISAs, respectively.
2.2.3 ELISA Assays
OVA titers were determined by enzyme-linked immunosorbent assays (ELISA), defined as the dilution of sera at which the OD reading was 0.5. Briefly, 96 well microtiter MAXIsorp plates (eBioscience) were coated at room temperature overnight with OVA at 100 pig/mL in PBS solution using 100 pL/well on a rocking platform. After discarding the OVA solution and allowing the plates to dry for 1 hour, 200 iL/well of blocking buffer (5% skim milk concentrate by weight, 1% FBS, and 0.2% Tween-20 by volume in PBS) was added, and the plates were incubated for 2 h at room temperature with agitation. The plates were then washed once with the ELISA wash buffer
that were prepared with the PBS-Tween tablets (EMD Millipore). Sera was diluted in dilution buffer (1% FBS by weight, 0.2% Tween-20 by volume in PBS) at the desired range of dilutions, and 100 pL was added per well. Plates were incubated for 1 h at room temperature on the rocking platform and then washed four times with wash buffer. Secondary antibody conjugated with horseradish peroxidase (anti-mouse IgG-HRP, Biorad) was diluted 1:5000 in dilution buffer and then added to each sample at 100 ptL/well, incubated for 1 h at room temperature, the solutions was aspirated out of the wells, and the plates were then washed 4x. Plates were then developed by addition of 100 pL/well of 1-step Ultra TMB-ELISA Substrate Solution (ThermoScientific). After a 20 minute incubation step and a 50 ptL addition of 2 N H2SO4 solution as a stop reagent, plates
were read for absorbance at both 450 nm and 540 nm (reference wavelength) with a Tecan microplate reader (Tecan, Switzerland). The difference between the measurement and reference wavelength absorbances were found, and an interpolation algorithm was used to approximate the dilution factor that corresponds to an absorbance reading of 0.5. The log I o values of this value was then reported as the titer91. For avidity indices, duplicate serum dilutions were prepared for each sample, and for one set of dilutions, wells were incubated for 10 min with 6 M urea prior to detection with the anti-mouse secondary antibody. The avidity index is reported as the ratio of the serum dilution that corresponds to the titer of the urea-treated sample to those of the non-urea-treated sample92
2.2.4 Flow cytometry
The collected blood was subjected to ACK lysis buffer to lyse the red blood cells. The cells were washed and pipetted into NUNC v-bottom plates (ThermoScientific) where their Fc receptors were blocked with 250x diluted anti-mouse CD16/CD32 antibody (eBioscience) in FACS buffer (0.5% BSA by weight in PBS). For the tetramer specific experiments, anti-CD8 antibody (50x dilution), SIINFEKL tetramer (50x dilution), and desatinib (1:1000 dilution) were then incubated in FACS buffer with the cells for 10 minutes at room temperature while protected from light. For the memory T cell experiments, 50x dilutions were made of anti-CD8 antibody and tetramer while 250x dilutions were made of anti-CD44 and anti-CD62L antibodies and a 1 000x dilution was made of dasatinib. After staining the cells, the cells were resuspended in FACS buffer containing a 1:1000 dilution of DAPI and transferred to polystyrene 12 mm x 75 mm test tubes (VWR). Frequencies of antigen specific, central memory, and effector memory T cells were then