HAL Id: hal-02810176
https://hal.umontpellier.fr/hal-02810176
Submitted on 6 Jun 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Antioxidant and Anti-inflammatory Potential of Shiitake
Culinary-Medicinal Mushroom, Lentinus edodes
(Agaricomycetes), Sporophores from Various Culture
Conditions
Ibrahima Diallo, Frédéric Boudard, Sylvie Morel, Manon Vitou, Caroline
Guzman, Nathalie Saint, Alain Michel, Sylvie Rapior, Lonsény Traoré, Patrick
Poucheret, et al.
To cite this version:
Ibrahima Diallo, Frédéric Boudard, Sylvie Morel, Manon Vitou, Caroline Guzman, et al.. Antiox-idant and Anti-inflammatory Potential of Shiitake Culinary-Medicinal Mushroom, Lentinus edodes (Agaricomycetes), Sporophores from Various Culture Conditions. International Journal of Medici-nal Mushrooms, Begell House, 2020, 22 (6), pp.535-546. �10.1615/IntJMedMushrooms.2020034864�. �hal-02810176�
Short Title: Biological Activities of Cultivated Lentinus edodes
Antioxidant and Anti-inflammatory Potential of Shiitake Culinary-Medicinal Mushroom,
Lentinus edodes (Agaricomycetes) Sporophores from Various Culture Conditions
Ibrahima Diallo,a,b,c Fréderic Boudard,d Sylvie Morel,b Manon Vitou,b Caroline Guzman,d
Nathalie Saint,e Alain Michel,a Sylvie Rapior,b Lonsény Traoré,c Patrick Poucheret,a,† &
Françoise Fonsb,*,†
a
Laboratoire de Pharmacologie et Physiopathologie Expérimentale, UMR Qualisud, CIRAD –
Université de Montpellier, Montpellier, France; bLaboratoire de Botanique, Phytochimie et
Mycologie, CEFE, CNRS – Université de Montpellier – Université Paul-Valéry Montpellier –
EPHE – IRD, Montpellier, France; cLaboratoire de Technologie Alimentaire du Département de
Génie Chimique de l’Université Gamal Abdel Nasser de Conakry, BP : 1147, Conakry, Guinée;
d
Laboratoire d’Immunologie, UMR Qualisud, CIRAD – Université de Montpellier, Montpellier,
France; ePHYMEDEXP, Université de Montpellier, CNRS, INSERM, Montpellier, France
*
Address all correspondance to: Françoise Fons, Laboratoire de Botanique, Phytochimie et
Mycologie, CEFE, CNRS – Université de Montpellier — Université Paul-Valéry Montpellier —
EPHE — IRD, Montpellier, France; Tel.: 33 (0) 4 11 75 96 60, E-mail:
francoise.fons@umontpellier.fr
†These authors contributed equally to this work.
ABSTRACT: Lentinus edodes (=Lentinula edodes) is an edible mushroom grown and marketed
for centuries due to its nutritional and medicinal properties. L. edodes has multiple
pharmacological activities as antioxidant and anti-inflammatory. Few studies were performed
taking into account the influence of culture conditions to optimize the biological properties of L.
anti-inflammatory activity of L. edodes fruit bodies cultivated by three mushroom producers in the
French Occitanie region using the same strain in various growing conditions (organic and
non-organic). Sequential extraction was performed on freeze dried fungal materials. All extracts have
a quantifiable but moderate antioxidant activity using the DPPH and ORAC tests. The
anti-inflammatory activity of the ethanol and aqueous extracts was evaluated on a model of
inflammatory macrophages. The ethanol extracts inhibit NO production in a dose-dependent
manner when the cells are pre-treated for 4 h with a 24 h stimulation time.
KEY WORDS: Lentinus edodes, anti-inflammatory, antioxidant, DPPH, food health benefit,
J774.A1 macrophages, medicinal mushrooms, nitric oxide, ORAC, pharmacology
ABBREVIATIONS: APPH, 2,2’-azo-bis(2-methypropionamide); CA, chlorogenic acid; DMSO, dimethyl sulfoxide; DPPH, 1,1-diphenyl-2-picrylhydrazyl; IFN, Interferon gamma; LPS, lipopolysaccharide; NO, nitric oxide; ORAC, oxygen radical absorbance capacity; ROS,
reactive oxygen species; RPMI, rosewell park memorial institute; TE, Trolox equivalent; TNFα, tumor necrosis factor alpha.
I. INTRODUCTION
Shiitake culinary-medicinal mushroom, Lentinus edodes (Berk.) Singer (=Lentinula edodes,
Marasmiaceae, Agaricomycetes) is among the most edible cultivated mushrooms in the world.1-3
Its production is intensive in Asia, United States of America and in Europe.4,5 L. edodes can be
grown using a wide range of conditions and substrates. L. edodes is appreciated for its fragrant
taste and nutritional properties, and as medicinal mushroom.6-8
L. edodes is a source of bioactive agents as ergosterol,9 ergothioneine,10 phenolic
compounds and polysaccharides,11,12 responsible for therapeutic activities (anti-inflammatory,
inhibit or slow down the production of free radicals and to protect lipids against peroxidation due
to its content in antioxidant molecules.16-20 Others data reported that L. edodes has an inhibitory
effect on NO production from LPS/IFNγ activated macrophages explaining its anti-inflammatory potential.21
The aim of our work is to compare the influence of L. edodes growing conditions using
the same strain (cultivated by organic and non-organic French mushroom professionals) on its
antioxidant and anti-inflammatory properties.
II. MATERIAL AND METHODS A. Chemicals
Trolox (98%), NaH2PO4, 2-amino-ethyl diphenyl borinate and acetic acid are from Fluka
Chemicals. DPPH and AAPH radicals, chlorogenic acid (95%), cyclohexane (99.8%),
acetonitrile, and dimethyl sulfoxide (DMSO; 99.9%) were purchased from Sigma-Aldrich.
Fluorescein is from Panreac. Ethanol (96%) and methanol (99.9%) are obtained from VWR.
Chloroform (99%) and Na2HPO4 (99%) are from Honeywell Research Chemicals.
RPMI medium 1640 GlutaMAX®, penicillin-streptomycin, murine recombinant
Interferon , Hank’s Balanced Salt Solution (HBSS) and fetal bovine serum were obtained from
Gibco. LPS E. coli 055: B5 and sodium nitroprusside were purchased from Sigma Aldrich. MTS
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
was purchased from Promega France. PMS (phenazinemethosulfate) was purchased from ICN
Biomedical.
B. Mushroom Material
Mycelia-3782 strain of L. edodes was cultivated by three producers located in Occitanie (France)
commercial suppliers (producers A and C). The L. edodes sporophores from producer A
(Fontiès-d’Aude) grow on a mixture of wood chips and straw (non-organic conditions; substrate
of Eurosubstrat©) with temperatures ranging from 10 to 20°C and 80% hygrometry. The
mushrooms generated by producer B (Saint-André-de-Lancize) are cultivated in the Cevennes
National Park on organic sawdust of chestnut, wheat bran and rye (organic conditions) with
temperatures ranging from 18 to 21°C and 60-70% hygrometry. The mushrooms from producer
C (Saint-Bonnet-de-Salendrinque) grow on a mixture of wood chips, oak sawdust and straw
(non-organic conditions, substrate of “Lentin de la buche” SA©) and temperatures vary from 15
to 17°C and 100% hygrometry.
C. Sample Preparation
Five kg of L. edodes from each producer were cleaned, sliced, gauged, and carefully packaged in
plastic bags and snap frozen. Then they were lyophilized in a RP2V lyophilizer.
D. Extract Production Process
A sequential process based on solvents of increasing polarity is used as previously described.22
50 g of each lyophilized mushroom sample are crushed with a Thermomix Vorwerk crusher.
Then 5 g of crushed mushroom are placed in 50 ml cyclohexane, sonicated for 90 min at 30°C
and then filtered using a Büchner device. The cyclohexanic filtrate is stored for subsequent
evaporation procedure: extract 1. Retentate is then submitted successively to extractions with
chloroform (50 mL, extract 2), ethanol (50 mL; extract 3) and water (50 mL; extract 4) under the
same conditions.22 The sequential extraction is carried out in triplicate. After extractions,
solvents were evaporated to dryness. To fully dry aqueous extracts, a lyophilization is performed.
All dried extracts were stored in the darkness at 4°C.
𝑅 =Mass of dried extract in g ∗ 100Mass of dried mushroom in g The values were expressed as a percentage.
Total yield was calculated as (Sum of masses of dried extracts/Mass of dried mushroom
in g) × 100, and was expressed as a percentage.
E. Antioxidant Activity
1. DPPH Test
Antioxidant activity is assessed using the DPPH as previously described.22 Extracts are
solubilized in DMSO (4 mg/mL) and then diluted in absolute ethanol to reach a concentration
range of 0.2, 0.5 and 1 mg/mL. Ethanol is used as blank. A standard curve with Trolox is
performed (12.5, 25, 50 and 75 µM). Rosmarinus officinalis ethanol extract (0.2 mg/mL) and
chlorogenic acid (0.01 mg/mL) are used as positive controls. On a 96 wells plate, 100 µL of
either a positive controls or mushroom extracts are deposited in each well. The assay is run in
triplicate at each tested concentration. Then 75 µL of absolute ethanol and 25 µL of
extemporaneously prepared DPPH (0.4 mg/mL) solution are introduced in each well. Plate is
incubated for 30 min at room temperature and protected from light. Absorbance reading is
performed at 550 nm with a microplate reader (MDS Inc., Toronto, Canada). Results are
expressed as mean plus or minus standard deviation of three independent experiments and are
expressed as Trolox equivalents (TE µmoles per gram of dry extract). Results are also expressed
as inhibition percentage (% inhibition) and was calculated as follows:
%𝑖𝑖ℎ𝑖𝑖𝑖𝑖𝑖𝑖𝑖 =𝐷𝐷 𝑖𝑏𝑏𝑖𝑏 − 𝐷𝐷 𝑒𝑒𝑖𝑒𝑏𝑒𝑖𝐷𝐷 𝑖𝑏𝑏𝑖𝑏 ∗ 100 2. ORAC Test
DMSO to a concentration of 1 mg/mL. They are then diluted to 25 µg/mL using a phosphate
buffer solution (75 mM) at pH 7.4. On a 96 wells microplate, are deposited Trolox standard
curve solutions (20 µL at 6.25, 12.5, 25, 50 and 75 µM), or chlorogenic acid (0.01 mg/mL), or
rosemary ethanol extract (12.5 µg/mL) as positive control, or shiitake extracts from all producers
at a concentration of 25 µg/mL. Then 100 µL of phosphate buffer and 100 µL of
extemporaneously prepared fluorescein solution (0.1 µM in phosphate buffer) are added.
Microplate is incubated at 37°C for 10 min under stirring. Reaction is initiated with 50 µL of
AAPH extemporaneously prepared. Fluorescence is recorded at an excitation wavelength of 485
nm and an emission wavelength of 535 nm, for 70 min using a microplate reader Tristar LB 941.
Final ORAC values are calculated using a regression equation between Trolox concentration and
the area under the curve of fluorescein decreasing. Data are expressed as µmoles of Trolox
equivalents per gram of dry extract. Results avec expressed as mean plus or minus standard
deviation (n=3).
F. Anti-inflammatory Assay
1. Macrophage Culture and Cell Treatment
The J774.A1 macrophage cell line (ATCC, TIB67) was obtained from LGC Standards. Cells
were cultured in RPMI medium 1640 GlutaMAX® supplemented with streptomycin (100
µg/mL) and penicillin (100 Units/mL) and 10% heat inactivated fetal bovine serum (complete
RPMI medium), and incubated at 37°C in a humidified incubator containing 5% CO2. For NO
production, the cells (5 x 105 cells/well) were seeded onto a 24-well culture plate in complete
RPMI medium and pretreated with different concentrations of ethanol or aqueous extracts (50 to
6.25 µg/mL) for 4 h. After, cells were activated with LPS (100 ng/mL) and Interferon γ (10 ng/mL) and incubated at 37°C for another 18 h.
2. Cell Viability by MTS Assay
In order to test the cytotoxicity, 105 cells/well was seeded in a 96-well culture plate in complete
RPMI medium and incubated at 37°C with different concentrations of ethanol or aqueous
extracts (50 to 6.25 µg/mL) for 20 h. After incubation, 20 µL/well of tetrazolium salt, MTS,
mixed with an electron coupling reagent, PMS in HBSS, was added. The plate was incubated for
another 4 h and the absorbance at 490 nm was measured in a microplate reader (Molecular
Devices) as previously described.23
3. Nitrite Determination
Ethanol and aqueous extracts was used for nitrite determination. The presence of nitrite, a stable
oxidized product of nitric oxide, was determined in cell culture media as previously described.24
Briefly, 100 µL of supernatant was combined with 100 µL of Griess reagent in a 96-well plate,
incubated 10 min at room temperature. Nitrite concentration was determined by measuring the
absorbance at 550 nm and by using a standard curve of NaNO2 (1-100 µM).
G. Statistical Analyses
Values are presented as mean ± standard error of the mean (SEM). Statistical analysis of the data
was carried out using Prism® software by two-way ANOVA followed by Bonferroni
post-test. P values <0.05 were considered to be significant.
III. RESULTS
A. Extraction and Extraction Yield
A sequential extraction was used to get the broader spectrum of bioactive molecules from the
shiitake materials. Total yield of the fourth extractions were 21.88, 28.58 and 24.73% for
producers A, B and C, respectively. For all producers, the highest extraction yields are obtained
followed by the ethanol extracts (extracts 3). No significant differences of extraction yields were
found between the three producers for extracts 1, 2 and 3; the aqueous extract yield for organic
producer B was significantly higher than that of the other two producers (Fig. 1).
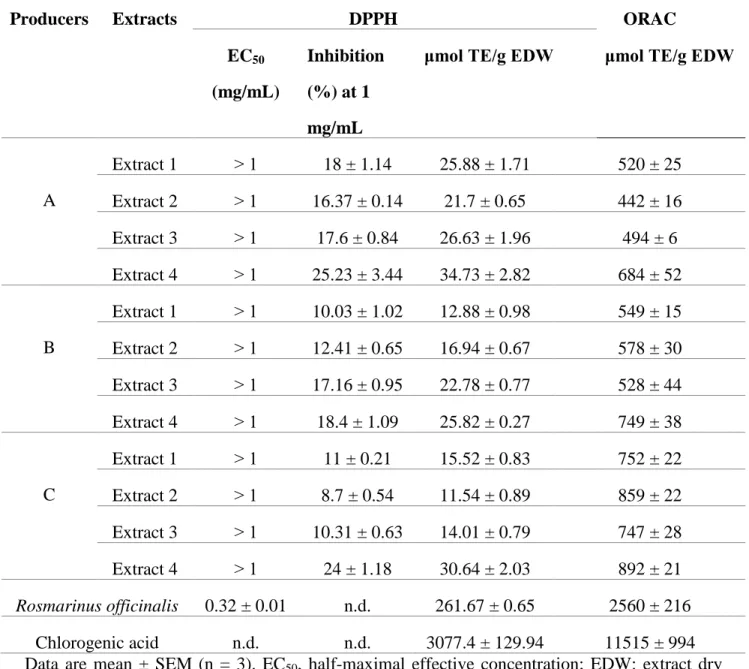
B. Antioxidant Activities
The DPPH and ORAC tests results on our extracts (Table 1) were compared with two positive
controls in terms of antioxidant activity: an ethanolic extract of R. officinalis that has been
considered as a food antioxidant additive (E392) and chlorogenic acid (CA), a caffeic acid
derivative.22 Rosemary extract was used as a reference of natural crude extract and CA as a
natural purified standard.
1. DPPH Antioxidant Activity
Results from the DPPH test (Table 1, Fig. 2) are expressed as percentage of inhibition and as
Trolox equivalents (TE). Trolox is used as positive control. Shiitake extracts demonstrate an
antioxidant effect ranging from 11.54 to 34.73 μmol TE/g of extract. These values are, in average, 22 times lower than rosemary extract antioxidant potential (261.67 μmol TE/g of extract). If all mushroom extracts (1, 2, 3, 4) demonstrate moderate DPPH antioxidant activity,
aqueous extracts appear the most antioxidant whatever the producer when compared with the
organic extracts: 34.73 μmol TE/g for producer A, 25.82 μmol TE/g for producer B, 30.64 μmol TE/g for producer C. The average value is 8.6 times lower than rosemary extract antioxidant
effect and 100 times lower than CA antioxidant effect (3077.4 μmol TE/g of standard) (Fig. 2). In addition, cyclohexane, chloroform and ethanol extracts from producer A have statistically the
highest DPPH antioxidant ability when compared to producer C. Cyclohexane and aqueous
extracts from producer A have statistically the highest DPPH antioxidant ability when compared
have the lowest DPPH antioxidant capacities with 12.88 and 11.54 μmol TE/g, respectively. It should be noted that when comparing producers, the highest DPPH antioxidant properties are
recorded with non-organic producer A, whatever the type of extract. However, there is no
significant difference between producer A and B regarding their ethanol extracts with values of
26.63 and 22.78 μmol TE/g, respectively. About organic producer B, DPPH antioxidant potential of extracts increases with extracts polarity whereas no correlation can be done between polarity
of the extracts and DPPH values for non-organic producers A and C.
2. Oxygen Radical Absorbance Capacity (ORAC) Antioxidant Activity
Results are expressed in µmol of Trolox Equivalents (TE) per gram of dry matter. Similarly, to
DPPH, results from ORAC indicate that aqueous extracts have the highest ORAC antioxidant
activity for all producers (684, 749 and 892 μmol TE/g for producers A, B and C, respectively) (Table 1). These values are followed by those of the cyclohexane extract for producer A and
chloroform extracts for producers B and C. There is no significant difference between ORAC
values of cyclohexane, chloroform and ethanol extracts from producers A (non-organic) and B
(organic) as shown (Fig. 3). All extracts from non-organic producer C have significantly higher
ORAC values when compared with two other producers. The highest average value of ORAC
antioxidant activity for shitake extracts were three times lower than rosemary activity (2560 μmol TE/g EDW) and 15 times lower than CA (11515 μmol TE/g EDW), respectively.
C. Anti-inflammatory Activity
Apolar extracts were not investigated for their anti-inflammatory properties due to poor
solubility at 10 mg/mL in DMSO.
1. L. edodes Aqueous and Ethanol Extracts Effects on Cell Viability
concentration range from 50 to 6.25 µg/mL. L. edodes extracts assay on cell viability, performed
on macrophage cell line J774.A1 using the MTS/PMS method, demonstrate the absence of
cytotoxic effect on cells. Indeed, none of the concentrations tested, ranging from 50 to 6.25
µg/mL during 4 h incubation, impaired cell culture when compared to control cells (data not
shown). In consequence, these extracts concentrations are tested on NO production by activated
macrophages in vitro.
2. L. edodes Aqueous and Ethanol Extracts Effects on Nitric Oxide (NO) Production
NO is an unstable mediator of inflammation. Upon production by cells it is readily converted in
nitrites that can be dosed by Griess colorimetric method. Anti-inflammatory effects of ethanol
extracts are assessed on stimulated macrophage J774.A1. After cells stimulation by LPS/IFNγ, nitrite production, is quantified in the cell culture supernatant. Extracts concentrations range
from 50 to 6.25 µg/mL. We record that LPS/IFNγ strongly stimulates NO production by macrophages as expected.
Ethanol extracts from the three producers significantly inhibit NO production in a
concentration dependent manner (Fig. 4). At 50 µg/mL, inhibition reaches 29.66, 31.30 and
27.56% for producers A, B and C, respectively, on cells pre-treated by extracts for 4 h with
stimulation time of 24 h. It should be noted that NO production is also concentration dependently
decreased after the 48 h stimulation condition but in this case the effect is globally more
important at the highest concentration (41.6, 42.6 and 39% for producers A, B and C,
respectively). When comparing producers, anti-inflammatory potential appears equivalent
showing no statistical differences. Results presented in Fig. 4 shown that cell pretreatment with
shiitake aqueous extract induced a low inhibition of nitrite production and this effect was
14.23, 15.60 and 20.93% for producers A, B and C, respectively at 50 µg/mL. About 48 h
stimulation, results indicate 17.1, 15.22 and 18.71% for producers A, B and C at higher
concentration, respectively. When comparing producers or stimulation time (24 h and 48 h), we
found no statistically differences (data not shown).
The NO inhibition activities of aqueous and ethanol extracts of L. edodes were compared.
They were the highest for the ethanol extracts whatever the producer or concentration used; a
significant difference was only observed between producers A and B (p<0.05) at the
concentration of 50 µg/mL (data not shown).
IV. DISCUSSION
In order to extract bioactive molecules from various cultivated L. edodes strain (Mycelia-3782)
conditions, a sequential extraction was performed using cyclohexane, chloroform, ethanol and
water. Aqueous and ethanol extracts generated the highest extraction yields. Differences of total
extraction efficiency for each solvent and producer may be explained by growing conditions
influence, stage of harvest as well as mushroom size.25 Methanol, ethanol, water and
hydro-ethanolic mixes are the most commonly used solvents, for the extraction of bioactive molecules,
since they allow to reach high yields.26 Few studies reported chloroform and cyclohexane to
extract secondary metabolites on L. edodes mushroom.26,27 Extraction yields for water extracts
from producers A, B and C (13.45, 19.52 and 15.36%, respectively) were similar to data from
Cheung et al.,16 (16.2%) but lower than those reported by Da Silva and Jorge (30%).28 Our
results for chloroform extracts ranged from 0.68 to 1.15% and from 6.49 to 7.49% for ethanol
extracts. More recently, Ferrari et al.,29 using a non-sequential extraction, recorded extraction
yields of 11, 13.6, 14, 27.2, 32.7 and 33.8% with ethanol, methanol, acetone, ethanol-water,
literature may be explained by differences in extraction methods, mushroom strain and growth
conditions when compared with literature.
In our study, we used two complementary methods, i.e., DPPH as the mixed mode assay
(hydrogen atom transfer and electron transfer reaction-based assay) and ORAC as a hydrogen
atom transfer reaction-based assay to evaluate antioxidant properties of shiitake.22 DPPH assay
results indicate that all shiitake extracts demonstrate a moderate but quantifiable antioxidant
activity. With an extract concentration of 1 mg/mL, the inhibition levels of the ethanolic L.
edodes extracts (17.6, 17.16 and 10.31% from producers A, B and C, respectively; Table 1) were
similar to those reported by Da Silva and Jorge.28 Regarding cyclohexane extracts, our results
suggest a lower inhibitory ability for mushroom material from producers A, B and C (18, 10 and
11%, respectively) when compared to literature.27 A large variety of shiitake extracts were tested
for their antioxidant potential using DPPH assay; nonetheless most studies were performed on
aqueous, ethanolic and hydro-ethanolic extracts.7,16,20 Regarding antioxidant efficacy against
DPPH radicals, hydro- ethanolic extract demonstrated the highest potential.29
Few investigations were performed on L. edodes with the ORAC assay. Our results
ranged from 442 to 892 µmol TE/g of extract (Table 1). Previous studies recorded various
ORAC values expressed with different units.30,31,32 DPPH and ORAC are quantitative assays
allowing to compare radical scavenging capacities between extracts as well as producers. Our
results, and their comparison to data from the literature, clearly indicate that shiitake biological
activity as antioxidant capacity may vary significantly as a function of mushroom strain, growth
conditions, extraction process and experimental conditions of biological activities evaluation.
Plant extracts (as rosemary extract, the E392) or purified natural compounds (as chlorogenic
and related compounds can mitigate oxidative and inflammatory stresses and show several health
promoting properties.33 That’s why there were used as positive controls in our investigations for
oxidant and inflammatory potential of L. edodes.
In addition to antioxidant effects, shiitake ethanol extracts demonstrate an inhibitory
activity on NO production from LPS/IFNγ activated macrophages J774.A1. This result indicates that shiitake extracts have an anti-inflammatory potential in vitro. Ethanol extracts from the three
producers, at a concentration of 50 µg/mL, inhibit NO production in concentration dependent
manner (41.6, 42.6 and 39% inhibition for producers A, B and C, respectively) on 4 h pre-treated
cells with a stimulation time of 48 h. Phenolic compounds and ergothioneine extracted by
ethanolic or hydro-ethanolic solvent, might be responsible for this effect as previously
suggested.19,21 These results are in agreement with those reported by authors34,35 studying the
effect of an ethanolicshiitake extract on NO inhibition.
Several mechanisms of action for inhibition of NO production are described in the
literature such as NF-κB, a transcription factor composed of a protein complex, located in cytoplasm and associated with the inhibitor IκB.36,37 In response to various stimuli such as pro-inflammatory cytokines (IL-1β), LPS, or other forms of stress, IκB inhibitor is phosphorylated by the IKK kinase and then degraded.36,38 This degradation promotes the activation of NF-κB and its release to join the nucleus where it attaches to inflammatory genes and activates the
transcription of specific genes.39,40 This will lead to synthesis of cytokines and inflammatory
mediators including TNF-α and NO.38 NF-κB transcription factor plays an important role in immune response and then inflammation. Consequently, its deregulated activation contributes to
the pathogenic processes of various inflammatory diseases.40,41 Therefore, inhibition of this
In our work, the inhibition would be associated with a decreased expression of iNOS (induced
nitric oxide synthase) responsible for NO production.42 A cytotoxic impact of the shiitake
extracts on cells was ruled out by the cell viability test that produced negative results.
Inflammation reaction being a complex process involving many mediators such as cytokines, in
addition to NO, further molecular investigations are required to fully understand the
pharmacology of antioxidant andanti-inflammatory properties of L. edodes extracts.
V. CONCLUSIONS
Our study focused on the antioxidant and anti-inflammatory potential of L. edodes fruit bodies
cultivated by non-organic and organic mushroom professionals using the same strain. The L.
edodes extracts from the three French mushroom producers obtained by a sequential extraction
showed moderate antioxidant activity with greater free radical inhibition in aqueous extracts.
Regarding the anti-inflammatory property of the polar extracts tested, all ethanolic extracts
strongly inhibit NO production in a concentration-dependent manner thus suggesting L. edodes
potential interest in the treatment against certain types of non-communicable diseases. On the
other hand, the inhibition observed for aqueous extracts remains low whatever organic and
non-organic producers. Concerning the shiitake growing conditions used, our study shows some
significant differences in the antioxidant and anti-inflammatory activities among the L. edodes
extracts from the three mushroom producers. Other growing conditions will need to be tested
both organic and non-organic to improve differences. In addition, further investigations should
be carried out to identify the main bioactive components of cultivated L. edodes extracts
responsible for the antioxidant capacity and anti-inflammatory activity, and especially to study
their mechanism of action.
Thanks are extended to R. Loubet, C. Veenstra and V. Lehnebach for supplying the fresh L.
edodes mushrooms and reporting cultivation methods. This study was financially supported by
the French Embassy in Guinea (Campus France).
REFERENCES
1. Wasser SP. Medicinal mushroom science: history, current status, future trends, and unsolved
problems. Int J Med Mushrooms. 2010;12(1):1–16.
2. Gaitan-Hernandez R, Zavaleta MAB, Aquino-Bolaños EN. Productivity, physicochemical
changes, and antioxidant activity of shiitake culinary-medicinal mushroom Lentinus edodes
(Agaricomycetes) cultivated on lignocellulosic residues. Int J Med Mushrooms.
2017;19(11):1041—52.
3. Kupcova K, Stefanova I, Plavcova Z, Hosek J, Hrouzek P, Kubec R. Antimicrobial,
cytotoxic, anti-inflammatory, and antioxidant activity of culinary processed Shiitake
medicinal mushroom (Lentinus edodes, Agaricomycetes) and its major sulfur sensory-active
compound-lenthionine. Int J Med Mushrooms. 2018;20(2):165—75.
4. Bernaś E, Jaworska G, Kmiecik W. Storage and processing of edible mushrooms. Acta Sci Pol Technol Aliment. 2006;5(2):5—23.
5. Chakravarty B. Trends in mushroom cultivation and breeding. AJAE. 2011;2(4):102—9.
6. Chang ST, Wasser SP. Current and future research trends in agricultural and biomedical
applications of medicinal mushrooms and mushroom products. Int J Med Mushrooms.
2018;20(12):1121—33.
7. Mata G, Valdez K, Mendoza R, Trigos A. HS/GC-MS analyzed chemical composition of the
aroma of fruiting bodies of two species of genus Lentinus (higher Basidiomycetes). Int J Med
8. Wasser SP, Nevo E, Sokolov D, Reshetnikov S, Timor-Tismenetsky M. Dietary supplements
from medicinal mushrooms: diversity of types and variety of regulations. Int J Med
Mushrooms. 2000;2(1):1–19.
9. Mattila P, Salo-Väänänen P, Könkö K, Aro H, Jalava T. Basic composition and amino acid
contents of mushrooms cultivated in Finland. J Agric Food Chem. 2002;50(22):6419—22.
10. Dubost NJ, Ou B, Beelman RB. Quantification of polyphenols and ergothioneine in
cultivated mushrooms and correlation to total antioxidant capacity. Food Chem.
2007;105(2):727—35.
11. Reis FS, Martins A, Barros L, Ferreira IC. Antioxidant properties and phenolic profile of the
most widely appreciated cultivated mushrooms: A comparative study between in vivo and in
vitro samples. Food Chem Toxicol. 2012;50(5):1201—7.
12. Chang ST, Wasser SP. The role of culinary-medicinal mushrooms on human welfare with a
pyramid model for human health. Int J Med Mushrooms. 2012;14(2):95–134.
13. Poucheret P, Fons F, Rapior S. Biological and pharmacological activity of higher fungi:
20-year retrospective analysis. Cryptogamie, Mycol. 2006;27(4):311—33.
14. Lo H-Ch, Wasser SP. Medicinal mushrooms for glycemic control in diabetes mellitus:
history, currenr status, future perspectives, and unsolved problems (review). Int J Med
Mushrooms. 2011; 13(5): 401-26.
15. De Silva DD, Rapior S, Sudarman E, Stadler M, Xu J, Alias AS, Hyde KD. Bioactive
metabolites from macrofungi: ethnopharmacology, biological activities and chemistry.
Fungal Divers. 2013;62:1—40.
16. Cheung LM, Cheung PCK, Ooi VEC. Antioxidant activity and total phenolics of edible
17. Mujic I, Zekovic Z, Lepojevic Ž, Vidovic S, Živkovic J. Antioxidant properties of selected
edible mushroom species. J Cent Eur Agric. 2010;11(4):387—92.
18. Shao S, Hernandez M, Kramer JKG, Rinker DL, Tsao R. Ergosterol profiles, fatty Acid
composition, and antioxidant activities of button mushrooms as affected by tissue part and
developmental stage. J Agric Food Chem. 2010;58(22):11616–25.
19. Benson KF, Ager DM, Landes B, Aruoma OI, Jensen GS. Improvement of joint range of
motion (ROM) and reduction of chronic pain after consumption of an
ergothioneine-containing nutritional supplement. Prev Med. 2012;54:S83—S89.
20. Choi EJ, Park ZY, Kim EK. Chemical composition and inhibitory effect of Lentinula edodes
ethanolic extract on experimentally induced atopic dermatitis in vitro and in vivo. Molecules.
2016;21(8):993-98.
21. Gunawardena D, Bennett L, Shanmugam K, King K, Williams R, Zabaras D, Head R, Ooi
L, Gyengesi G, Münch G. Anti-inflammatory effects of five commercially available
mushroom species determined in lipopolysaccharide and interferon-γ activated murine macrophages. Food Chem. 2014;148:92—96.
22. Morel S, Arnould S, Vitou M, Boudard F, Guzman C, Poucheret P, Fons F, Rapior S.
Antiproliferative and antioxidant activities of wild Boletales mushrooms from France. Int J
Med Mushrooms. 2018;20(1):13—29.
23. Almaksour Z, Boudard F, Villareal M, Grosmaire L, Guzman C, Isoda H, Larroque M,
Margout D. Anti-inflammatory and anti-oxidant activities of different extra virgin olive oil
varieties extracts. AJMAP. 2017;3(2):163—83.
24. Boudard F, Vallot N, Cabaner C, Bastide M. Chemiluminescence and nitrite determination of
25. Vitrac V, Reignier A, Henry-Vitrac C, Minvielle N, Mérillon JM, Savoie JM. Changes in
antioxidant activities and compounds during cultivation of Shiitake (Lentinula edodes).
Proceedings of the 7th International Conference on Mushroom Biology and Mushroom
Products (ICMBMP7); 2011 Oct 4—7; Arcachon, France. Paris: INRA, 2011.
26. Kalyoncu F, Oskay M, Kayalar H. Antioxidant activity of the mycelium of 21 wild
mushroom species. Mycology. 2010;1(3):195—199.
27. Rahman MA, Abdullah N, Aminudin N. Lentinula edodes (Shiitake mushroom): An
assessment of in vitro anti-atherosclerotic bio-functionality. Saudi J Biol Sci.
2018;25(8):1515—23.
28. Da Silva AC, Jorge N. Antioxidant properties of Lentinus edodes and Agaricus blazei
extracts. J Food Qual. 2011;34:386—94.
29. Ferrari GP, Soares AA, Bazanella GCD, Bracht A, De Souza CGM, Boer CG, Peralta RM.
Antioxidant properties of the most common edible mushrooms consumed in Brazil. In book:
Mushrooms: Types, Properties and Nutrition. New York: Nova Science Publishers Inc.,
2012, p. 285—97.
30. Kettawan A, Chanlekha K, Kongkachuichai R, Charoensiri R. Effects of cooking on
antioxidant activities and polyphenol content of edible mushrooms commonly consumed in
Thailand. Pak J Nutr. 2011;10(11):1094—1103.
31. Ito T, Kato M, Tsuchida H, Harada E, Niwa T, Osawa T. Ergothioneine as an
anti-oxidative/anti-inflammatory component in several edible mushrooms. Food Sci Technol Res.
2011;17 (2):103—10.
32. United States International Trade Commission (USITC). Mushrooms industry & trade
33. Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic acid: recent
advances on its dual role as a food additive and a nutraceutical against metabolic syndrome.
Molecules. 2017;22(3):358-63.
34. Chien RC, Lin LM, Chang YH, Lin YC, Wu PH, Asatiani MD, Wasser SG, Krakhmalnyi M,
Agbarya A, Wasser SP, Mau JL. Anti-inflammation properties of fruiting bodies and
submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int J
Med Mushrooms. 2016;18(11):999—1009.
35. Xu X, Yasuda M, Nakamura-Tsuruta S, Mizuno M, Ashida H. β-Glucan from Lentinus edodes inhibits nitric oxide and tumor necrosis factor-α production and phosphorylation of mitogen-activated protein kinases in lipopolysaccharide-stimulated murine RAW 264.7
macrophages. J Biol Chem. 2012;287(2):871—78.
36. Baud V, Jacque E. Voie alternative d’activation de NF-kB et cancer. Amis ou ennemis? Méd
Sci. 2008;24(12):1083—88.
37. Suryavanshi SV, Kulkarni YA. NF-κβ: A potential target in the management of vascular complications of diabetes. Front Pharmacol. 2017;8:798-803.
38. Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2001;25(6):280—88.
39. Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7—11.
40. Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metabolism. 2011;13(1):11—22.
41. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023.
42. Pacheco-Sánchez M, Boutin Y, Angers P, Gosselin A, Tweddell RJ. Inhibitory effect of
CDP, a polysaccharide extracted from the mushroom Collybia dryophila, on nitric oxide
synthase expression and nitric oxide production in macrophages. Eur J Pharmacol.
2007;555(1):61—66.
TABLE 1: Antioxidant Capacity of Lentinus edodes Extracts from Organic and Non-Organic
French Producers
Producers Extracts DPPH ORAC
EC50 (mg/mL)
Inhibition (%) at 1 mg/mL
μmol TE/g EDW μmol TE/g EDW
A Extract 1 ˃ 1 18 ± 1.14 25.88 ± 1.71 520 ± 25 Extract 2 ˃ 1 16.37 ± 0.14 21.7 ± 0.65 442 ± 16 Extract 3 ˃ 1 17.6 ± 0.84 26.63 ± 1.96 494 ± 6 Extract 4 ˃ 1 25.23 ± 3.44 34.73 ± 2.82 684 ± 52 B Extract 1 ˃ 1 10.03 ± 1.02 12.88 ± 0.98 549 ± 15 Extract 2 ˃ 1 12.41 ± 0.65 16.94 ± 0.67 578 ± 30 Extract 3 ˃ 1 17.16 ± 0.95 22.78 ± 0.77 528 ± 44 Extract 4 ˃ 1 18.4 ± 1.09 25.82 ± 0.27 749 ± 38 C Extract 1 ˃ 1 11 ± 0.21 15.52 ± 0.83 752 ± 22 Extract 2 ˃ 1 8.7 ± 0.54 11.54 ± 0.89 859 ± 22 Extract 3 ˃ 1 10.31 ± 0.63 14.01 ± 0.79 747 ± 28 Extract 4 ˃ 1 24 ± 1.18 30.64 ± 2.03 892 ± 21 Rosmarinus officinalis 0.32 ± 0.01 n.d. 261.67 ± 0.65 2560 ± 216 Chlorogenic acid n.d. n.d. 3077.4 ± 129.94 11515 ± 994 Data are mean ± SEM (n = 3). EC50, half-maximal effective concentration; EDW: extract dry
FIG. 1: Extraction yields for producers A, B and C. Values are presented as mean ± SEM (n=3).
Extract 1 = cyclohexane extracts; Extract 2 = chloroform extracts; Extract 3 = ethanol extracts;
Extract 4 = aqueous extracts. Extraction yields (R) were calculated according to this formula:
(Mass of dried extract in g /Mass of dried mushroom in g) × 100, and was expressed as a
percentage; ***p˂0.001 for producer A versus producer B; $$$ p˂0.001 for producer B versus producer C.
FIG. 2: DPPH values for producers A, B and C. Values are presented as mean ± SEM (n=3).
Extract 1 = cyclohexane extracts; Extract 2 = chloroform extracts; Extract 3 = ethanol extracts;
Extract 4 = aqueous extracts. ***p˂0.001 for producer A versus producer B; $$$p˂0.001 for
producer A versus producer C; #p˂0.05, ###p˂0.001 for producer B versus producer C.
FIG. 3: ORAC values for producers A, B and C. Values are presented as mean ± SEM (n=3).
Extract 1 = cyclohexane extracts; Extract 2 = chloroform extracts; Extract 3 = ethanol extracts;
Extract 4 = aqueous extracts. **p˂0.01, ***p˂0.001 for producer A versus producer C; $p˂0.05,
$$p˂0.01, $$$p˂0.001; for producer B versus producer C.
FIG. 4: The bar graphs summarize the NO inhibition for producers A, B and C after 4 h
pretreatment with 24 h of stimulation, (a) by ethanolic extracts or (b) by aqueous extracts. Values
are presented as mean ± SEM (n=3). ***p˂0.001, inhibition resulting from 50 µg/mL of extract vs. no inhibition; $p˂0.05, $$p˂0.01, $$$p˂0.001, inhibition resulting from 25 µg/mL of extract vs. no inhibition; ££p˂0.01, inhibition resulting from 12.5 µg/mL of extract vs. no inhibition;
α
2