Iron-Based Layered Double Hydroxide Implants: Potential Drug
Delivery Carriers with Tissue Biointegration Promotion and Blood
Microcirculation Preservation
Mariana P. Figueiredo,
†Vanessa R. R. Cunha,
*
,†Fabrice Leroux,
‡Christine Taviot-Gueho,
‡Marta N. Nakamae,
§Ye R. Kang,
§Rodrigo B. Souza,
∥Ana Maria C. R. P. F. Martins,
⊥Ivan Hong Jun Koh,
§and Vera R. L. Constantino
*
,††Departamento de Química Fundamental, Instituto de Química, Universidade de São Paulo - USP, Av. Prof. Lineu Prestes 748, 05508-000 São Paulo, São Paulo, Brazil
‡Université Clermont Auvergne, CNRS, Institut de Chimie de Clermont-Ferrand, BP 10448, F-63000 Clermont-Ferrand, France §Departamento de Cirurgia, Universidade Federal de São Paulo - UNIFESP, Rua Botucatu 740, CEP 04023-900 São Paulo, São
Paulo, Brazil
∥Departamento de Morfologia e Genética, Universidade Federal de São Paulo −UNIFESP, Rua Botucatu 740, CEP 04023-900, São Paulo, São Paulo, Brazil
⊥Instituto Biológico, Secretaria da Agricultura e Abastecimento, Av. Conselheiro Rodrigues Alves 1252, CEP 04014-002, São Paulo, São Paulo, Brazil
*
S Supporting InformationABSTRACT: This work explores the synthesis,
physicochem-ical characterization, and in vivo biocompatibility of iron-based layered double hydroxides (LDHs) with molar ratio M2+/(Fe3+ + Al3+) equal to 2, Fe3+/Al3+ equal to 1, and chloride anions as charge-compensating ion (abbreviated Mg4FeAl-Cl and Zn4FeAl-Cl) prepared by the coprecipitation
method. The higher structural organization of Zn4FeAl-Cl in comparison to Mg2+analogous material was noticed by X-ray diffraction and scanning electron microscopy images. Bio-compatibility of LDH was evaluated by intramuscular implantation in rats. Tablets of M4FeAl-Cl (M = Mg, Zn) were readily identified macroscopically after 7 and 28 days of
implantation, denoting slow dissolution in the internal medium; adjacent to the tablets, blood flow was preserved without tortuosity or pathological dilatations, according to the Sidestream Dark Field Imaging technique. The histological analysis showed no inflammatory response and the presence of angiogenesis and tissue remodeling with the reconstruction of the extracellular matrix and cells around the tablets, besides the induction of collagen type-I formation. Prussian blue histochemical reaction suggested higher solubility of Mg4FeAl-Cl in the extracellular matrix compared to zinc LDH. Considering the positive biocompatibility results obtained for M4IIFeAl-LDH materials, experiments were conducted to intercalate the anti-inflammatory
naproxen, as a model drug, into the iron-based LDHs (M4IIFeAl-NAP). The release profile of NAP in phosphate buffer showed
90% of the drug delivered after about 80 h. However, divalent metal leaching was verified mainly for Mg-LDH (around 50%) when compared to Zn2+(around 1%). Iron-based LDHs have great potential for medical and technological applications as local
drug delivery biomaterials exhibiting biocompatibility and biointegration properties.
■
INTRODUCTIONLayered double hydroxides containing iron cations in their intralayer composition have received considerable attention in the field of catalysts for electrochemical water splitting,1,2 magnetic materials,3−5 photon Fenton reaction,6 and adsorb-ents7since their physical and chemical properties can be tuned by the interlayer species and also by the content and ratio of metal cations’. Besides, iron is an earth-abundant and endogenous element, which amplifies its sphere of applications and justifies efforts to develop iron-based materials.
The growing interest in the use of layered double hydroxides (LDHs) as vehicles for drug delivery systems is related to their properties such as biocompatibility, biodegradability, wide capacity of storage, and promotion of a modified release of immobilized species.8−13Performance of LDHs in contact with biological systems, accessed through in vitro or in vivo assays,
Received: September 26, 2018
Accepted: December 13, 2018
Published: December 26, 2018
Article http://pubs.acs.org/journal/acsodf
Cite This:ACS Omega 2018, 3, 18263−18274
copying and redistribution of the article or any adaptations for non-commercial purposes.
Downloaded via 118.70.52.165 on June 24, 2021 at 14:49:19 (UTC).
appears to be related to their chemical composition,14 layer charge density,15 surface properties,15 and particle size.16,17 Therefore, synthetic parameters can modulate the drug release regarding the rate, time, and/or release targeted when compared to the pharmaceutical conventional systems.
LDHs are hydrated materials comprising positive electric layers stacked face to face and negatively charged species in the interlayer region, which present the general composition [M(1−x)2+M3+x(OH)2](An−)x/n·zH2O, where M is a metal ion
and An− is an anion of electrical charge n (abbreviation: MR2+M3+-A with R equal to M2+/M3+molar ratio).18
The layers are formed by octahedral units, composed of hydroxyl anions coordinated to divalent and trivalent metal cations, [M(OH)6],
sharing the edges as shown inFigure 1. As mentioned before, positive layers are neutralized by the presence of interlayers of anions and water molecules.
The versatility of LDHs is related to the appreciable number of metal cations that can support the layers (Mg2+, Ca2+, Zn2+,
Mn2+, Ni2+, Co2+, Cu2+, Cr3+, Fe3+, Al3+, etc.) and the wide variety of anions that can be accommodated between them, including organic and inorganic bioactive anions.12,18Design of LDHs constituted by essential metals seeks to decrease or minimize the possible biological harmful effects coming from the use of species containing exogenous elements since life organisms can cope with them. Aluminum is the trivalent cation mostly used in the LDH synthesis for biomedical applications; the substitution of exogenous Al3+ cations by Fe3+ can be interesting. Free iron only exists in trace amounts in the organism. In a general way, iron forms are present in circulating erythrocytes, bone marrow, muscle myoglobin, liver paren-chyma, reticuloendothelial macrophages, and plasma trans-ferrin.19Iron excretion is not regulated, occurring minimally by normal exfoliation from body surfaces, as dermal, intestinal, pulmonary, and urinary tissues, loss in sweat, bile, by gastrointestinal bleeding, or during menstruation.19
Besides iron, Mg and Zn ions can be used as divalent cations in the composition of LDH layers. The Mg2+ cation is mainly present in bone, complexed with phosphate, but high concentrations are also found in the liver and muscles.19 Magnesium is found in plasma in concentrations varying between 0.7 and 1 mmol L−1, and about 20−25% of this concentration is protein bounded. Serum Mg2+ions arefiltered
at the glomerulus; most of them are reabsorbed and the remaining amount is excreted in the urine. Zinc is important in bone formation, male fertility, and wound healing. Free Zn2+is found in a more significant amount compared to iron within biological cells: approximately 10−3mol L−1in some vesicles and 10−11 mol L−1in the cytoplasm.20Zinc is eliminated in fecal excrements and in urine.
The total substitution of Al3+ cations by Fe3+ in the LDH
layers is still challenging. Heredia et al.4synthesized a series of
Mg3M3+-CO3 LDHs, varying the Fe/Al molar ratio from the
Mg3Al-CO3 to the Mg3Fe-CO3material. As the iron amount increased in the layers, a progressive loss of the material’s crystallinity was observed. The higher the structural disorgani-zation presented by a material, the more difficult is its characterization. The synthesis of ternary LDHs containing magnesium, iron, and aluminum (MgFeAl) or zinc, iron, and magnesium (ZnFeAl) can be an alternative to increase the presence of endogenous cations in the drug carrier. The presence of Al3+in the composition is a key to avoid impurities of
iron oxo-hydroxo phases,21 as well as to achieve high organization and crystallinity, which is an important aspect when searching a modified drug release system.22,23
In this work, LDHs with molar ratio M2+/(Fe3++ Al3+) equal
to 2, Fe3+/Al3+ equal to 1 and chloride anions as charge-compensating ions (abbreviated Mg4FeAl-Cl and Zn4FeAl-Cl) were synthesized and characterized by physical chemistry methods. Considering the possibility of application of such matrices as drug delivery systems, the biocompatibilities of iron-based LDHs were evaluated using an in vivo model, as reported previously by our group.13Lack of toxicity through cell-based assays does not eliminate the possibility of harmful effects on tissues (such as nephrotoxicity and hepatotoxicity), or consequences resulting from prolonged exposition (for instance, genotoxicity and oncogenicity), or the induction of an immune response.24
Biocompatibility of LDH was evaluated by intramuscular implantation of M4FeAl-Cl (M = Mg, Zn) tablets in rats after 7 and 28 days through histological assays and the observation of bloodflow adjacent to the tablets by the sidestream dark field (SDF) imaging technique. Muscular or bone implantation is recommended to assess materials biocompatibility for the long term by the International Standards Organization (ISO).25In addition to being an indorsed test for biocompatibility of materials, the intramuscular or bone implantation results can motivate the application of LDHs in implantable systems for therapeutic purposes. Studies have reported the performance of middle ear prosthesis implants, coated with LDH impregnated with the antibiotic ciprofloxacin, in the local infected with Pseudomonas aeruginosa26,27and the implantation of a scaffold comprising LDH, hydroxyapatite, and gelatine (with or without rabbit adipose stem cell seeding) to induce bone regeneration.28 Considering the positive biocompatibility results obtained in this work for M4IIFeAl-LDH materials through muscular
implantation tests, experiments were conducted to intercalate the anti-inflammatory naproxen, as a model drug, into these ion-based LDHs (M4IIFeAl-NAP), aiming to explore local drug
Figure 1.Schematic representation of the LDH structure.
Figure 2.X-ray diffraction patterns of Zn4FeAl-Cl and Mg4FeAl-Cl samples.
delivery biomaterials. Naproxen intercalated into LDH materials of different chemical compositions and obtained by coprecipi-tation, ion-exchange, or reconstitution methods was reviewed by Rives et al.29 The hybrid M4IIFeAl-NAP materials were
investigated by a set of physicochemical techniques, and the release of naproxenate anion was evaluated using tablets in simulated physiological media, as previously reported.30
■
RESULTS AND DISCUSSIONCharacterization of Zn4FeAl-Cl and Mg4FeAl-Cl
Sam-ples. X-ray diffraction patterns of Mg4Al-Cl and Zn4FeAl-Cl
materials are presented inFigure 2. If we assume the 3R1 three-layer polytype most frequently observed in the LDH system, Zn4FeAl-Cl and Mg4FeAl-Cl show basal spacing related to
(003) plane of 7.8 and 8.0 Å and harmonic reflection (006) related to the basal spacing with interplane spaces equal to 3.9
and 4.0 Å, respectively. For the zinc LDH sample, the reflections are well defined, intense, and narrow, suggesting an enhance-ment in the structural organization compared to the magnesium layered material, as observed in other related materials without iron.31X-ray diffraction (XRD) patterns do not show extra peaks that could be related with impurities.
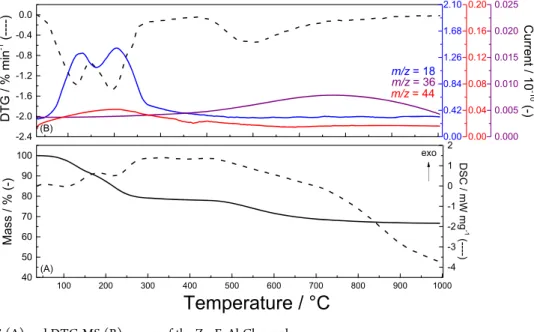
TGA−DSC and DTG-MS curves of Zn4FeAl-Cl and
Mg4FeAl-Cl materials are shown inFigures 3and4, respectively.
All curves were smoothed applying Netzsch Proteus software. DTG curves indicated two main thermal events. Thefirst one, an endothermic process, related to the release of absorbed and intercalated water molecules, occurs from 25 up to 150°C for Zn4FeAl-Cl and up to 250°C for Mg4FeAl-Cl samples. A similar
behavior was reported for free iron Zn2Al-Cl and Mg2Al-Cl
samples.11,27 The second event (an endothermic process) occurs in the temperature range above 150°C for Zn4FeAl-Cl
Figure 3.TGA−DSC (A) and DTG-MS (B) curves of the Zn4FeAl-Cl sample.
and above 250 °C for Mg4FeAl-Cl and is assigned to the dehydroxylation of the matrices. A release of a small quantity of CO2 (m/z = 44) is observed with higher intensity for
magnesium LDH due to the higher pH value used to synthesize the magnesium phase compared to the zinc phase, which intensifies the solubilization of CO2in the reaction medium. For both materials, dehydrochlorination process (release of HCl, m/ z = 36) occurs at temperatures higher than 400°C, as observed for free iron Zn2Al-Cl and Mg2Al-Cl samples.13
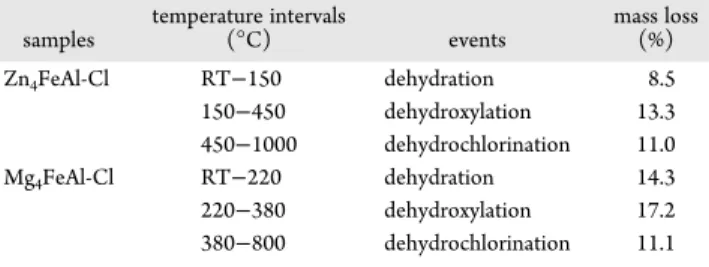
The detailed weight loss events obtained by thermal analysis for both iron-based LDH materials are presented inTable 1.
According to chemical and thermal analyses, it was possible to obtain both ternary materials with a reasonable amount of substitution of aluminum for iron. The proposed chemical formulae of Zn4FeAl-Cl and Mg4FeAl-Cl are shown inTable 2.
To verify if the dehydrochlorination process involving the release of chlorine as HCl removed all the halogen from the decomposed iron-based LDHs, samples calcined at 1000°C for 2 h were submitted to X-rayfluorescence analysis (Figure S1in the Supporting Information). The equipment employed allows identification of atoms above aluminum in terms of Z atomic number (Z = 13). The titanium signals are related to the internal standard used. No chlorine emission lines were observed (2.6 keV), even after high-scale magnification, confirming that the dehydrochlorination process occurring at high temperatures is complete. To identify the phases produced in the endothermal process occurring at about 1000°C (Figures 3and4), X-ray diffraction patterns of MII4FeAl-Cl samples after calcination
were recorded (Figure 5). Two hours of calcination at 1000°C were necessary to promote the phase crystallization. For the magnesium LDH, three phases are observed: magnesium oxide, MgO, and the spinel phases MgFe2O4and MgAl2O4. On the
other hand, the calcined zinc LDH is decomposed to zinc oxide, ZnO, and the spinel phase Zn(FeAl)O4, both with high
crystallinity. InFigure 5, the dashed lines indicate the position of the peaks related to other candidates for calcined products but not observed: ZnFe2O4, ZnAl2O4, and Mg(FeAl)O4.
The presence of both trivalent cations in the Zn-LDH propitiated the formation of a mixed spinel after calcination, instead of the separated spinel phases ZnAl2O4and ZnFe2O4,
usually observed for matrices of zinc/aluminum or magnesium/ aluminum composition, respectively.32Heredia et al.4submitted a series of Mg3M3+-CO3LDHs, including a material similar to
the ternary Mg4FeAl-Cl presented in this work (Fe/Al molar
ratio equal to 1), to air calcination at 550°C for 9 h. The authors observed X-ray diffraction peaks related to the MgO for all samples, and for those containing iron in particular, it was also observed the formation of spinel MgFe2O4was also observed
but not the MgAl2O4 phase, possibly because of the lower
temperature applied.
Taking into account the proposed chemical compositions, the thermal analysis data, and the X-ray diffraction results of the calcined products, the decomposition steps were interpreted as follows (to simplify the calculation, it was assumed that the Fe/ Al molar ratio is equal to 1).
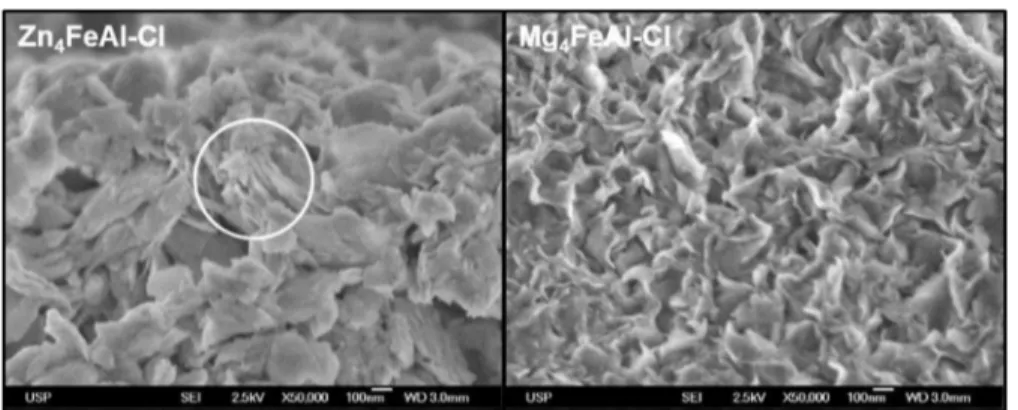
Figure 6 shows the scanning electron microscopy (SEM) micrographs of ternary M4IIFeAl-Cl materials. Among the
samples, it was possible to observe plate-like-shaped particles, characteristic of LDH materials. Zn4FeAl-Cl sample showed
larger particles (circulated spot) than the magnesium LDH, which can be related to the increase in crystallinity observed by Table 1. Thermal Analysis Data of Zn4FeAl-Cl and Mg4
FeAl-Cl Samples samples temperature intervals (°C) events mass loss (%) Zn4FeAl-Cl RT−150 dehydration 8.5 150−450 dehydroxylation 13.3 450−1000 dehydrochlorination 11.0 Mg4FeAl-Cl RT−220 dehydration 14.3 220−380 dehydroxylation 17.2 380−800 dehydrochlorination 11.1
Table 2. Chemical Compositions and Proposed Formulae for the Synthesized Iron-based LDH-Cl Materials
sample proposed formula MII/Ala MII/Fea Fe/Al % H
2Ob
Zn4FeAl-Cl [Zn2.00Fe0.47Al0.49(OH)5.94]Cl·1.6H2O 4.08 (4.11)c 4.26 0.96 8.5
(4.26) (0.96) (8.5)
Mg4FeAl-Cl [Mg2.00Fe0.50Al0.49(OH)5.97]Cl·2.1H2O 4.08 (4.06) 4.00 1.02 14.2
(3.99) (1.03) (14.2)
aMolar ratio where MII= Mg or Zn.bAmount of water (w/w) obtained by TGA experiments.cExperimental values are given in parentheses.
Figure 5.X-ray diffraction patterns of Zn4FeAl-Cl and Mg4FeAl-Cl samples calcined at 1000°C for 2 h. The peaks expected for possible calcination products are indicated by dashed lines (---), and the identified phases are indicated by asterisks (*). The diffraction patterns were consulted in the ICSD database,33and the collection code of each phase is indicated between parentheses.
XRD, as already reported for M2IIAl-CO32−(MII= Mg or Zn)
phases.31The small thickness of the Mg4FeAl-Cl phase provides
flexibility to the particles.
FT-Raman spectra of the iron-based materials are shown in Figure S2 in the Supporting Information. The low-intensity band at 1060 cm−1present only in the Mg4FeAl-Cl spectrum is
assigned to the symmetric stretching of carbonate anion, νsCO32−,
34
which can be related to the adsorption of carbonate anions in the material. For both LDH-Cl samples, the band is observed around 540 cm−1, which is related to the metal-OH translation modes.35Fourier transform infrared (FT-IR) spectra of Zn4FeAl-Cl and Mg4FeAl-Cl (Figure S3 in the Supporting
Information) have a similar profile: a broad band at around 550 cm−1 and a low-intensity band at 1360 cm−1 related to the antisymmetric stretching of the carbonate anion.
In Vivo Biocompatibility Tests of Zn4FeAl-Cl and
Mg4FeAl-Cl Materials. The host’s biointeraction with the
implanted tablets between the muscular layers of the abdominal wall was analyzed macroscopically, along with the aspect of microvessels and bloodflow (Figure 7). The tablets of Zn4 FeAl-Cl and Mg4FeAl-Cl were readily identified macroscopically
during the 7 and 28 day periods, denoting the slow dissolution of the tablets in the internal medium. The blood vessels adjacent to the tablets showed the usual appearance of intramuscular vascular branching under the transillumination; in addition, the observation of microcirculation without tortuosity or patho-logical dilatations and with preserved bloodflow, under SDF monitoring, suggested the absence an inflammatory response for both iron-based LDH tablets. Theflow of red blood cells can be visualized in Videos S1−S4 accessible in the Supporting Information. Next, histological analysis was performed to reconfirm the observed biocompatibility results at the cellular level.
The physiological repair of an injured tissue is done with the reconstruction of the extracellular matrix, cells, and collagens, which usually ends in a period of 28 days in the absence of acute or persistent inflammatory factor. In the presence of non-biocompatible exogenous materials, tissue repair events are delayed or even disrupted or inhibited according to the degree of the material-related-antigenicity factor to the host biology.
In the first 7 days after surgical trauma, the neutrophils are usually the predominant leukocyte within thefirst 5 days and are replaced by macrophages around day 7. These events arise from the need to remove cellular debris damaged by surgical trauma for the implantation of materials, extracellular matrix recon-struction, and repopulation by cells tofill voids. In the presence of antigenic materials in the tissue, the following are observed: the predominance and persistence of neutrophils beyond 7 days,
Figure 6.Scanning electron micrographs of Zn4FeAl-Cl and Mg4FeAl-Cl samples.
Figure 7.Color photographs show the shadows of Zn4FeAl-Cl and
Mg4FeAl-Cl tablets between muscle layers on both sides of the
abdominal wall, anatomically separated by the xiphoidal appendix (implanted tablets’ diameter: 5 mm); gray pictures show the pattern of the microcirculation over the tablets, captured by SDF video microscopy“in vivo”, on 7th (left column) and 28th P.O. days (right column). The colored images were taken from the peritoneal side, following a large U-shaped incision of the abdominal wall and with the
aid of transillumination. SDF image sizes are of 1 mm2/frame
presence of tissue edema, cellular necrosis, empty spaces without cellularfilling, disorganized extracellular matrix, and absence of collagen productiontypical signs of a persistent inflammation that will be present while the antigenic implant remains.
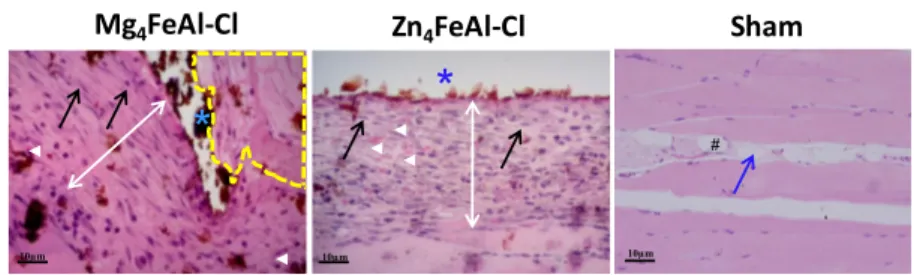
The intramuscular implantation of Mg4FeAl-Cl and Zn4 FeAl-Cl LDHs supported tissue repair in the absence of neutrophils, giant cells, mast cells, and eosinophils, usually present in inflammatory processes (Figures 8 and 9), indicating the biocompatibility of LDHs. However, an increased number of macrophages was seen as compared to a previous study of our research group with Mg4Al2-Cl and Zn4Al2-Cl LDHs (molar
ratio [Mg or Zn]/Al equal to 2) applying the same biocompatibility assay.12The higher presence of macrophages in the absence of other inflammatory cells suggested a possible influence of iron in the macrophage mobilization. According to Xie et al.36and Rodrigues et al.,37macrophages have an affinity for iron ions. According to Ganz and Nemeth,38phagocytosis of iron ions by macrophages can contribute to extracellular iron reduction and decrease their availability to bacteria using iron for their multiplication, thus contributing to the host defense mechanism.
To check the correlation between the presence of high quantity of macrophages and the availability of iron from the LDHs, we evaluated the presence of Fe3+ ions in the tissue containing the LDH tablets on the 28th day of implantation by means of the Prussian blue technique (Figure 10). Interestingly, iron granules were predominantly found inside macrophages, fibroblasts, and fibrocytes. Nevertheless, the extracellular iron was present only for the animals implanted with Mg4FeAl-Cl in
comparison to the Zn4FeAl-Cl tablet. This difference suggests
possible leaching of the ions from the LDH tablets more rapidly and to a greater extent for the Mg4FeAl-Cl sample. As previously
reported,30 LDH composed of magnesium and aluminum is more soluble than the LDH of zinc and aluminum in an aqueous solution of pH 7.2, which may justify the greater defragmenta-tion of the Mg4FeAl-Cl tablet and the subsequent higher amount
of iron ions in the ECM observed post implantation in vivo. Besides, the easier defragmentation of the Mg4FeAl-Cl tablet
may justify also the expressive invagination of ECM into the implanted tablet as shown by histology. In the previous study reported by our research group,12the predominance of collagen type-III disposed in a parallel way around the Zn4Al2-Cl sample
Figure 8.Histological results following Mg4FeAl-Cl LDH and Zn4FeAl-Cl LDH tablet implants between the abdominal wall intermuscular space, showing their interaction with host cells after the 7th postoperative or P.O. day. (*) LDH tablet; (↔ white) new tissue adjacent to LDH; (○yellow) new tissue invagination; (→) fibroblast; (▶ white) neomicrovessels; (→ blue) space between abdominal wall muscle layers; (#) adipose tissue. Hematoxylin−eosin staining.
Figure 9.Histological aspect of the implants showing thefinal tissue reconstruction and the persistence of implanted LDH tablets after 28 days: Mg4FeAl-Cl (left row); Zn4FeAl-Cl (middle row), and Sham control group (right row) showed a normal aspect of tissue. LDH tablet-implanted area
(*); new tissue (↔ white); fibroblast (→ arrow black); macrophage (→ arrow white); tissue invagination (→ dotted arrow); collagen type-I (▶
white); collagen type−III (▶ yellow); muscle perimysium between abdominal wall muscle layers (→ blue); vessels (#). Hematoxylin−eosin
was observed. However, collagen type-I was predominant for the Mg4Al2-Cl sample and assumed a parallel arrangement, besides
presenting invagination process of the repaired tissue to the interior of the tablet region. In the presence of Fe3+ions in LDH compositions, the type-I collagen formation was predominant for both materials, Mg4FeAl-Cl and Zn4FeAl-Cl, suggesting a
modification of the fibroplasia process modulated by the iron’s presence (Figure 9). According to Ruiz et al.,39Fe3+ ion can induce the transcription activation of the Col1α1 gene, which is crucial for the formation of type-I collagen, which may have been supported by the iron-LDHs. Hosaka et al.40have reported that connective tissues can accumulate minerals from the extrac-ellular space. In addition, the work of the group of Deng et al.,41 based on in vitro methods, showed Fe3+ion incorporation from the supernatant into the molecules of Human like collagen (type-I). Thus, the extracellular matrix in contact with
iron-LDHs from implanted samples may have propitiated the insertion of dissolved Fe3+ ions into the collagen molecules and influenced the maturation process favoring the formation of type-I collagen. Interestingly, the new collagen tissue around the Mg4FeAl-Cl tablet presented poor birefringence, unlike previous
observation for the Mg4Al2-Cl material,14 that triggered the production of type-I collagenfibers with strong birefringence. The low birefringence around the iron-LDH samples may be explained by the possible noncompetitive inhibitor activity of iron toward the lysyl hydroxylase, which is a crucial enzyme that promotes stabilization for the triple procollagen helix formation modifying the post-translational fiber conformation, although apparently not inhibiting the binding of the enzyme to the substrate. In the extracellular matrix around the Zn4FeAl-Cl
sample, a very interesting scenario was observed in sites where type-III, type-I, or both types of collagens predominated. These data can be understood as a local effect of Zn2+ or Fe3+ ions separately or the presence of both ions.
These results demonstrate the strong influence of LDH composition on the modulation of collagenfiber type during the tissue repair process. The absence of an inflammatory response allied with the presence of angiogenesis and tissue remodeling around the tablets indicate that iron-based LDHs have great potential for medical and technological applications as drug carriers exhibiting biocompatibility and biointegration proper-ties, besides allowing the induction of collagen type-I formation, mostly.
Characterization of Zn4FeAl-NAP and Mg4FeAl-NAP
Samples and in Vitro Release Tests. It is important to quote that the intercalation of NAP into the LDHs was not achieved by the coprecipitation method, maintaining the same conditions applied to the M4IIFeAl-Cl materials. Moreover, by the
ion-exchange method, an NAP/(Fe3++ Al3+) molar ratio equal to 3 was necessary to obtain a satisfactory percentage of drug intercalation. Furthermore, the thermal postsynthesis treatment was important to improve the crystallinity of the Mg4FeAl-NAP material.
To the best of our knowledge, few papers have reported the intercalation of organic species into iron-containing LDHs, that is, magnesium or zinc and iron cations in the layers: (i) ascorbate by ion-exchange,42 reconstruction,43,44 or coprecipitation methods;45(ii) phenylalanine by ion exchange, reconstruction, or coprecipitation in the presence of urea;45and (iii) tartrate or vinylbenzenesulfonate by ion exchange.46 Regarding the syn-thesis, even though the coprecipitation method is the most used for LDHs containing only aluminum as the trivalent ion, it does not seem to be the most appropriate approach for the isolation of LDHs composed by exclusively iron as the trivalent ion according to the studies reported in the literature. For example, ascorbate was adsorbed in the external surface of the Mg3 Fe-NO3material.45Rives et al.47have reported the intercalation of
drugs (mefenamate, meclofenamate, and naproxenate) into the ternary phase Mg,Fe,Al-LDH by coprecipitation and ion-exchange methods. However, the inorganic carrier was synthesized with small quantities of Fe3+ ions (Al3+/Fe3+
molar ratio equal to 9). Hence, intercalation of organic species by the coprecipitation method into the iron−LDH matrix is challenging even using molar ratio Fe3+/Al3+equal to 1, as tried
in this work.
X-ray patterns of Zn4FeAl-NAP and Mg4FeAl-NAP materials
are presented inFigure 11. With the intercalation of NAP, the displacement of the peak (003) to a low angle (2θ) is observed when compared to the LDH-Cl profiles of the precursors. The Figure 10.Iron histochemistry findings following Mg4Al2-Cl LDH,
Zn4Al2-Cl LDH, Mg4FeAl-Cl LDH, and Zn4FeAl-Cl LDH tablet
implants between abdominal wall intermuscular spaces, after 28th P.O. The white dotted line with the blue arrow indicates the macroscopic appearance of the implanted LDH tablet and the cut line for histological processing. Sham (control group), Mg4Al2-Cl (A), Zn4Al2-Cl (B),
Mg4FeAl-Cl (C), Zn4FeAl-Cl (D), and LDH tablet (*); positive
staining (→ arrow black); positive staining in fibroblast (→ arrow
green); positive staining in extracellular matrix (→ yellow); perimysium between abdominal wall muscle layers (▶). Prussian blue staining.
basal spacing is 22.2 Å for the LDH of zinc and 19.1 Å for the LDH of magnesium. For Zn4FeAl-NAP samples, six (00S) harmonic peaks are observed with the respective interlayer spaces of 11.2, 7.4, 5.6, 4.5, 3.7, and 3.2 Å. On the other hand, for the Mg4FeAl-NAP sample, four (00S) harmonic peaks can be
indexed according to the interplanar spaces of 9.0, 6.3, 4.4, and 3.8 Å.
Works regarding the intercalation of naproxenate ion into non-iron LDH have reported interlayer values quite closely related to those obtained with Mg/Al (21.2−23.5 Å) and Zn/Al (23.9 Å) LDHs.29,48For the Zn4FeAl-NAP material, the quite
high intensity of the (009) peak may actually indicate the presence of a chloride phase for which the (003) peak is expected at the same position. Furthermore, the observation of a double peak for (110)/(113) reflections while a single peak is expected as a consequence of the increase of c parameter after drug intercalation also indicates the presence of the remaining LDH-Cl phase besides the LDH-NAP one. Having in mind these important observations concerning the XRD analysis of LDH, we can see that signs of the presence of an additional LDH phase intercalated with simple anions (OH−, CO32−, Cl−,
NO3−) are also often present in the X-ray patterns of LDH-NAP systems reported in the literature but not always interpreted as such.
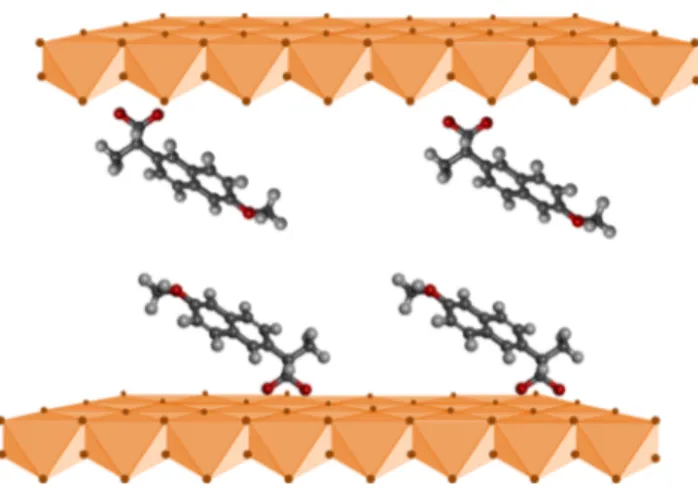
On the basis of computational simulation, Gu et al.48 proposed the NAP orientation between the Mg4Al2-LDH layers as a bilayer arrangement of the anions without overlapping, with the carboxylate groups oriented vertically to the layers (Figure 12). This proposal is consistent with the thickness of the layer (about 4.8 Å) plus the interlayer distance: 17.4 Å for Mg4 FeAl-NAP and 14.3 Å for Zn4FeAl-NA.
The elemental chemical composition of LDH-NAP samples and the amount of water obtained by TGA experiments (Figures S4 and S5in the Supporting Information) are shown inTable 3. The amount of drug in Zn4FeAl-NAP and Mg4FeAl-NAP was
about 29 and 35 wt %, respectively. No formulae were proposed since both samples also contain LDH-Cl phases as evidenced by XRD.
Oral administration is globally the most used solid pharmacological formulation since it is economic and desirable due to its simplicity.49Therefore, the in vitro tests performed in this work aimed to provide the dissolution profiles of free and intercalated naproxenate anions in buffer phosphate (pH = 7.4) to simulate the oral administration, considering that the absorption of the drug is mainly by the intestine.
LDH ternary samples intercalated with NAP were also studied by SEM, and some microphotograhps can be seen inFigure 13. A pronounced loss of crystallinity was observed for Zn4 FeAl-NAP compared to the corresponding chloride phase. Both materials present pronounced aggregated lamellar particles.
FT-Raman spectra of NAP after the intercalation are similar for Zn4FeAl and Mg4FeAl samples (Figure S6in the Supporting Information). The most intense bands are attributed to C−C ring vibrations at 1631 and 1415 and to CH3deformation at 1386 cm−1.50Figure S7in the Supporting Information shows the FT-IR spectra of Zn4FeAl-NAP and Mg4FeAl-NAP. The most important bands are those related to the symmetric and antisymmetric stretching of the carboxylate group COO− at 1266 and 1606 cm−1, respectively, and the C−H in plane bending of the rings at 1233 cm−1. The FT-IR spectra for the NAP-LDHs were very similar to those reported in the literature for Mg4Al2-NAP.29,51
The presence of the vibrational modes related to the rings, as well as their substituents in FT-Raman and FT-IR, indicates the preservation of the NAP drug structure after intercalation between LDH layers.
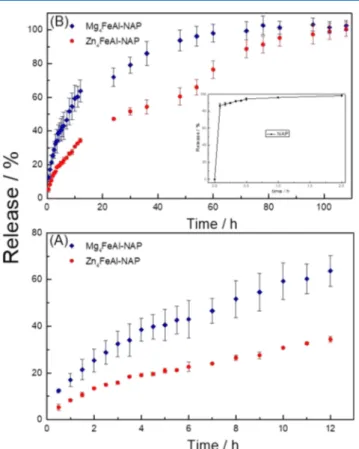
Figure 14shows the release curves obtained for the systems until 12 h (A) and after 108 h (B). The curves present different behaviors over time: at the beginning, the release rate is constant up to 3 h, decreases, and remains constant up to approximately 6 h and increases again up to 12 h. By expanding the assay time (Figure 14B), it is verified that this shape of the curves is maintained. After 30 min, the percentage of the pure NAP released is practically constant and the tablets were visually completely dissolved. The release is sustained for over 100 h for the ZnFeAl-NAP sample. However, for Mg4FeAl-NAP, the percentage released started to become constant, reaching almost 100% (>95%) after 60 h of assay. For all samples, the shapes of the tablets were maintained at the end of the test and the metals were quantified by ICP from the buffer solutions. The dissolution of the Mg4FeAl-Cl tablet observed in the in vivo
tests was also noticed during the in vitro study. The amounts of Al3+ and Fe3+ ions were found below the limit of detection, whereas the amounts of Mg2+and Zn2+ions were 2.92 and Zn
0.15 mg/L, respectively. These concentrations indicate a divalent cation leaching from the tablet of around 50% for magnesium LDH and 1% for zinc LDH after 108 h, which is consistent with the highest solubility product (Kps) value for the magnesium hydroxide.
Figure 11.X-ray patterns of Zn4FeAl-NAP and Mg4FeAl-NAP samples. Figure 12.Schematic representation of the LDH structure containing
The study of the release of NAP from LDHs of magnesium and aluminum composition have already been reported.52−54 Carriazo et al. have tested Mg2Al-NAP samples in the tablet
form, in a buffer solution of pH 7.4 at 37 °C with a stirring rate of 60 rpm, that is, reasonably similar conditions to those applied in this work. A complete NAP release was reached after 45 min, and data were modeled using the Korsmeyer−Peppas model. Hou and Jin51 performed tests with Zn-Al-NAP suspended in a Na2HPO4−NaH2PO4 buffer (pH 6.86), and the system was
maintained under stirring at 37°C. After 130 min, the release was higher than 90%.
■
CONCLUSIONSIron-based LDHs Zn4FeAl and Mg4FeAl, constituted by a high level of the endogenous iron ions, are potential candidates for use in the therapeuticalfield. In vivo tests showed biocompat-ibility and biointegration, without signs of inflammation and toxic effect. In addition, presence of iron induced the predominance of type-I collagen. The ternary LDH phases can intercalate drug species like naproxen in a suitable amount and display a modified release compared to the free drug. Such materials can be interesting to the development of biomaterials of the third generation, allied to drug delivery for local therapies.
■
EXPERIMENTAL SECTIONMagnesium chloride hexahydrate (MgCl2·6H2O), zinc chloride
(ZnCl2), aluminum chloride hexahydrate (AlCl3·6H2O), sodium hydroxide (NaOH), iron(III) chloride hexahydrate (FeCl3·6H2O), silver nitrate (AgNO3), sodium naproxenate (NaC14H13O3), monopotassium phosphate (KH2PO4),
diso-dium hydrogen phosphate heptahydrate (Na2HPO4·7H2O), sodium chloride (NaCl), and potassium chloride (KCl) were purchased from Sigma-Aldrich. Paraformaldehyde solution 4% (Synth), historesin (Technovitz 7100, Kulzer), hematoxylin (C16H14O6, Vetec), chloride acid (HCl, Vetec), eosin (C20H6Br4Na2O5, Vetec), Picrosirius red (C45H26N10Na6O21S6,
Alfa Aesar), and potassium ferrocyanide (K4Fe(CN)6·3H2O, Cromoline) were used in the histological evaluation. All chemical reagents were used without further purification or treatment.
Synthesis of Zn4FeAl-Cl and Mg4FeAl-Cl Samples.
Mg4FeAl-Cl was prepared by the coprecipitation method at
pH 10.5 by adding concomitantly a mixture of an orange-brown 0.1 mol L−1solution of MgCl2·6H2O, FeCl3·6H2O, and AlCl3·
6H2O (molar ratio Mg/[Fe + Al] equal to 2) and a 0.2 mol L−1 NaOH solution into deionized water. During the addition, vigorous stirring was maintained under N2 atmosphere. The resultant material was kept at room temperature for 1 h. The colored solid was washed with deionized water and dried at room temperature under reduced pressure. The Zn4FeAl-Cl
Table 3. Chemical Composition of the Synthesized Iron-based LDH-NAP Materials
sample MII/Ala MII/Fea Fe/Al % C % H
2Ob % NAP (w/w)
Zn4FeAl-NAP 3.97 3.96 1.00 21.46 6.9 29
Mg4FeAl-NAP 4.18 4.00 1.05 25.44 6.4 35
aMolar ratio where MII= Mg or Zn.bWater amount (w/w) obtained by TGA experiments.
Figure 13.Scanning electron micrographs of Zn4FeAl-NAP and Mg4FeAl-NAP.
Figure 14.Release profiles of Zn4FeAl-NAP, Mg4FeAl-NAP, and NAP samples in buffer phosphate (pH = 7.4) at 37 °C, (A) from 0.5 to 108 h and (B) from 0.5 to 12 h.
sample was prepared by the same procedure, replacing magnesium salt by ZnCl2salt and keeping the pH value of the
coprecipitation reaction at 7.5. Calcination of M4IIFeAl-Cl
samples was performed in a carbonite furnace, model RF 1500, for 2 h at 1000°C in an air atmosphere.
Preparation of M4IIFeAl-NAP Samples. As-prepared and
no-dried Mg4FeAl-Cl samples, obtained as described above,
were resuspended in 50 mL of an aqueous solution of 0.13 mol L−1sodium naproxenate (NaNAP), whereas Zn4FeAl-Cl was
resuspended in 0.17 mol L−1NaNAP to obtain about 1 g of MIIFeAl-NAP samples. For both materials, the NAP/(Fe3+ +
Al3+) molar ratio was equal to 3 and the suspensions were kept under N2atmosphere and vigorous stirring for 24 h at 80°C (for the Mg4FeAl-NAP preparation) or room temperature (for
Zn4FeAl-NAP). After the ion-exchange step, the solids were washed with deionized water and then dried at room temperature under reduced pressure.
To check the presence of chloride ions in the LDH-NAP samples, due to incomplete ion-exchange reaction, both samples were reacted with silver ions as follows: approximately 3 mg of LDH-NAP sample wasfirst mixed with 3 mL of 0.1 mol L−1 nitric acid solution. After the solid solubilization, about 1 mg of silver nitrate was added to monitor the formation of silver chloride (AgCl) precipitate.
Materials Characterization. X-ray diffraction (XRD) patterns of powdered LDH samples were recorded on a PANalytical diffractometer, model X́Pert PRO, equipped with an X’Celerator detector, using Cu Kα radiation (1.5418 Å) in a scan range of 1.5−70° (2θ) with a scan step of 0.02°(2θ)/s) at Laboratório de Caracterização Tecnológica (LCT) of Escola Politécnica of Universidade de São Paulo (USP). XRD patterns of powdered LDH calcined samples were recorded on a D8 Discover Bruker diffractometer using Cu Kα radiation (1.5406 Å) in a scan range of 1.5−70°(2θ) with a scan step of 0.05°(2θ)/ 8s) at Laboratório de Cristalografia of Instituto de Física of USP. Mass coupled thermal analyses (TGA-MS) were recorded on Netzsch thermoanalyser model TGA/DSC 490 PC Luxx coupled to an Aëolos 403 C mass spectrometer using a macro alumina crucible (3 mL of capacity) and a heating rate of 10°C/ min under synthetic air flow of 50 mL/min. To verify the chloride ions; presence in the calcined LDH-Cl samples, X-ray fluorescence spectra were obtained in an S2 PICOFOX TXRF spectrometer with Mo K radiation. The samples were prepared suspending approximately 1 mg of the calcined samples in 100 μL of n-propanol (from Synth), 10 μL of a 1 g L−1 titanium
standard solution (from Fluka), and 40μL of poly(vinyl acetate) (PVA). Two drops of the suspension were deposited on a quartz sample holder and dried in an oven. Fourier transform infrared (FT-IR) spectra of powdered samples were recorded in the 4000−400 cm−1range on a Bruker spectrophotometer, modelα, by ATR with acquisition step of 4 cm−1and 512 scans. Fourier transform Raman (Raman) spectra were recorded in an FT-Raman Bruker FRS-100/S spectrometer using 1064 nm exciting radiation (Nd:YAG laser Coherent Compass 1064−500 N), a Ge detector, laser power of 100 mW, acquisition step of 4 cm−1, and 2048 scans. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analyses of metals were performed in duplicate on Spectro Analytical Instrument at the Central Anali ́tica of Instituto de Qui ́mica of USP. Chemical elemental analyses of carbon, hydrogen, and nitrogen were recorded on Perkin Elmer-CHN 2400 equipment at the Central Anali ́tica of the Instituto de Qui ́mica of USP. Images by scanning electron microscopy (SEM) were obtained in an FE-SEM Jeol JSM
7401F (FREG) equipment using uncoated samples deposited on a copper tape.
Intramuscular Implantation Assessment. The in vivo biocompatibility assays were performed following the procedure described by Cunha et al.14The solids were pressed (0.25 ton for 1 min) into small tablets (5 mm in diameter and 2 mm in thickness). The biological response toward implanted LDH tablets was analyzed by microcirculatory and histological findings on 7th (n = 1/group) and 28th (n = 2/group) postoperative (P.O.) days. In the control, the surgical procedure used was similar to other groups, but without the LDH tablet implantation (sham-operated animals), to check only the surgical trauma effect involved in this process (n = 3). All adult female Wistar rats were purchased from UNIFESP animal colony (CEDEME) and kept in adequate environment, fed with proper food pellets and water ad libitum. International guidelines for the care and use of animals were followed, and the experimental protocol was approved by the Local Ethical Committee (CEUA N° 873141013).
Microcirculatory Monitoring by Sidestream Dark Field Imaging (SDF). At postoperative periods, before the tissue sample collection containing LDH tablets, the microcirculatory hemodynamic images of muscular tissues, around and over the tablets, were captured as described by Cunha et al.14
Histological Assessment. The histological assays of the tissues were performed as reported by Cunha et al.14
Prussian Blue Reaction: Histology Stain for Iron Identification. The samples were embedded in resin, and histologic sections of 3μm thickness of each sample were stained by Blue Prussia staining formed in situ. The section was immersed in a solution prepared with equal parts of 0.5% hydrochloric acid and 1% potassium ferrocyanide for 1 h at 56 °C. The slides were washed with deionized water and stained with Harris hematoxylin for counterstaining. The histological analyses were performed under light microscope Carl-Zeiss Axio Scope A1.
In Vitro Naproxenate Release from LDH-NAP Samples. Naproxenate anion release experiments were conducted by the basket method, whereas the samples were compacted (0.25 ton for 1 min), forming tablets approximately 5 mm in diameter and 2 mm in thickness. Three tablets of each sample were prepared using 40 mg of Mg4FeAl-NAP (14 mg of drug) or Zn4FeAl-NAP
(12 mg of drug); for comparison, three tablets of 15 mg of sodium naproxenate (free drug) were also produced. The release assays were performed in a Pharma Test Dissolution Instrument type PTWS 610 at 37°C and 200 rpm. A phosphate buffer of pH 7.4, used as dissolution media, was prepared using KH2PO4(0.2 g L−1), Na2HPO4·7H2O (1.5 g L−1), NaCl (8.0 g L−1), KCl (0.2
g L−1), and 1 L of deionized water. At specified time intervals, 2 mL of solution was collected and replaced with an equivalent amount of dissolution medium. The naproxenate concentration was determined by UV−visible absorption spectrophotometry at a maximum absorption (λmax) equal to 232 nm using a naproxenate calibration curve.
■
ASSOCIATED CONTENT*
S Supporting InformationThe Supporting Information is available free of charge on the ACS Publications websiteat DOI:10.1021/acsomega.8b02532. X-ray fluorescence (XRF) spectra of Mg4FeAl-Cl and Zn4FeAl-Cl samples calcined at 1000 °C for 2 h,
samples, TGA−DSC and DTG-MS curves of Zn4
FeAl-NAP and Mg4FeAl-NAP samples, thermal analysis data
observed for Zn4FeAl-NAP and Mg4FeAl-NAP samples,
FT-Raman and FT-IR spectra of Zn4FeAl-NAP and
Mg4FeAl-NAP samples (PDF)
Videos of the microcirculatory networks of the Zn4
FeAl-Cl and Mg4FeAl-Cl tablets examined after 7 and 28 days captured by SDF video microscopy “in vivo” over the LDH tablet (AVI) (AVI) (AVI) (AVI)
■
AUTHOR INFORMATION Corresponding Authors *E-mail:vrrc@iq.usp.br(V.R.R.C.). *E-mail:vrlconst@iq.usp.br(V.R.L.C.). ORCID Vanessa R. R. Cunha:0000-0002-1328-7851 NotesThe authors declare no competingfinancial interest.
■
ACKNOWLEDGMENTSThe graphical abstract includes images obtained fromhttps:// smart.servier.com/ under Creative Commons attribution unported (CC BY 3.0). The authors are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2011/50318-1, 2014/15900-0, and 2016/13862-9) and Conselho Nacional de Desenvolvimento Cienti ́fico e Tecnológico (CNPq). We are thankful to Roberta Mancini (PhD student) and the Laboratório de Química Supramolecular e Nanotecnologia (LQSN, Instituto de Qui ́mica−USP) for the XRF spectra experiments and to M.Sc. Alfredo Duarte for the SEM images acquisition. Laboratório de Espectroscopia Molecular Hans Stammreich (LEM, Instituto de Qui ́mica− USP) is also acknowledged for the FT-IR and Raman spectra recording. This work is part of the Research Academic Cooperation Agreement PRC-CNRS-FAPESP (PRC-Projets de recherché conjoints 1688 and SPRINT-São Paulo Researchers in International Collaboration 2016/50317-9).
■
REFERENCES(1) Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered Double Hydroxide Nanosheets with Multiple Vacancies Obtained by Dry Exfoliation as Highly Efficient Oxygen Evolution Electrocatalysts. Angew. Chem., Int. Ed. 2017, 56, 5867− 5871.
(2) Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, No. 4477. (3) Heredia, A. C.; Oliva, M. I.; Zandalazini, C. I.; Agú, U. A.; Eimer, G. A.; Casuscelli, S. G.; Herrero, E. R.; Pérez, C. F.; Crivello, M. E. Synthesis, Characterization, and Catalytic Behavior of Mg−Al−Zn−Fe Mixed Oxides from Precursors Layered Double Hydroxide. Ind. Eng. Chem. Res. 2011, 50, 6695−6703.
(4) Heredia, A. C.; Oliva, M. I.; Agú, U. A.; Zandalazini, C. I.; Marchetti, S. G.; Herrero, E. R.; Crivello, M. E. Synthesis, character-ization and magnetic behavior of Mg−Fe−Al mixed oxides based on layered double hydroxide. J. Magn. Magn. Mater. 2013, 342, 38−46.
(5) Agú, U. A.; Oliva, M. I.; Marchetti, S. G.; Heredia, A. C.; Casuscelli, S. G.; Crivello, M. E. Synthesis and characterization of a mixture of CoFe2O4and MgFe2O4from layered double hydroxides: Band gap energy and magnetic responses. J. Magn. Magn. Mater. 2014, 369, 249−259.
(6) Laipan, M.; Zhu, R.; Zhu, J.; He, H. Visible light assisted Fenton-like degradation of Orange II on Ni3Fe/Fe3O4 magnetic catalyst prepared from spent FeNi layered double hydroxide. J. Mol. Catal. A: Chem. 2016, 415, 9−16.
(7) Guo, Y.; Zhu, Z.; Qui, Y.; Zhao, Z. Enhanced adsorption of acid brown 14 dye on calcined Mg/Fe layered double hydroxide with memory effect. Chem. Eng. J. 2013, 219, 69−77.
(8) Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2018, 28, No. 1703868.
(9) Kuthati, Y.; Kankala, R. K.; Lee, C. H. Layered double hydroxide nanoparticles for biomedical applications: Current status and recent prospects. Appl. Clay Sci. 2015, 112−113, 100−116.
(10) Chimene, D.; Alge, D. L.; Gaharwar, A. K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261−7284.
(11) Gu, Z.; Atherton, J. J.; Xu, Z. P. Hierarchical layered double hydroxide nanocomposites: structure, synthesis and applications.
Chem. Commun. 2015, 51, 3024−3036.
(12) Choi, S. J.; Choy, J. H. Layered double hydroxide nanoparticles as target-specific delivery carriers: uptake mechanism and toxicity.
Nanomedicine 2011, 6, 803−814.
(13) Gil, O. M.; Rocha, M. A.; Constantino, V. R. L.; Koh, I. H. J.; de Faria, D. L. A. Modified drug release system based on Sulindac and layered double hydroxide: An in vivo Raman investigation. Vib. Spectrosc. 2016, 87, 60−66.
(14) Cunha, V. R. R.; de Souza, R. B.; Martins, A. M. C. R. P. F.; Koh, I. H. J.; Constantino, V. R. L. Accessing the biocompatibility of layered double hydroxide by intramuscular implantation: Histological and microcirculation evaluation. Sci. Rep. 2016, 6, No. 30547.
(15) Alekseevaa, T.; Prevot, V.; Sancelmec, M.; Forano, C.; Besse-Hogganc, P. Enhancing atrazine biodegradation by Pseudomonas sp. strain ADP adsorption to Layered Double Hydroxide bionanocompo-sites. J. Hazardous Mater. 2011, 191, 126−135.
(16) Xu, Z. P.; Walker, T. L.; Liu, K. L.; Cooper, H. M.; Lu, G. Q. M.; Bartlett, P. F. Layered double hydroxide nanoparticles as cellular delivery vectors of super-coiled plasmid DNA. Int. J. Nanomed. 2007, 2,
163−174.
(17) Chen, M.; Cooper, H. M.; Zhou, J. Z.; Bartlett, P. F.; Xu, Z. P. Reduction in the size of layered double hydroxide nanoparticles enhances the efficiency of siRNA delivery. J. Colloid Interface Sci. 2013, 390, 275−281.
(18) Braterman, P. S.; Xu, Z. P.; Yarberry, F. Handbook of Layered Materials; Auerbach, S. M., Carrado, K. A., Dutta, P. K., Eds.; Wiley: New York, 2004; Chapter 8.
(19) Bhagavan, N. V. Medical Biochemistry, 4th ed.; Academic Press, 2002; Chapters 29 and 37.
(20) Silva, J. J. R. F.; Williams, R. J. P. The Biological Chemistry of the Elements, 2nd ed.; Oxford University Press, 2001; Chapter 11.
(21) Jolivet, J. P.; Chanéac, C.; Tronc, E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem. Commun. 2004, 5, 481−487.
(22) Rojas, R.; Jimenez-Kairuz, A. F.; Manzo, R. H.; Giacomelli, C. E. Release kinetics from LDH-drug hybrids: Effect of layers stacking and drug solubility and polarity. Colloid Surf. A. 2014, 463, 37−43.
(23) Gu, Z.; Li, L.; et al. Influence of Hydrothermal Treatment on Physicochemical Properties and Drug Release of Anti-Inflammatory Drugs of Intercalated Layered Double Hydroxide Nanoparticles. Pharmaceutics 2014, 6, 235−248.
(24) Parboosing, R.; Mzobe, G.; Chonco, L.; Moodley, I. Cell-based Assays for Assessing Toxicity: A Basic Guide. Med. Chem. 2017, 13, 13− 21.
(25) Use of International Standard ISO -110993, Bioloical Evaluation of Medical Devices Part 1: Evaluation and Testing, Draft Guidance for
Industry and Food and Drug Administration Staff. Food and Drug
Administration, 2013. www.fda.gov/downloads/medicaldevices/
deviceregulationandguidance/guidancedocuments/ucm348890.pdf
(accessed Sept 24, 2018).
(26) Hesse, D.; Badar, M.; Bleich, A.; Smoczek, A.; Glage, S.; Kieke, M.; Behrens, P.; Müller, P. P.; Esser, K. H.; Stieve, M.; Prenzler, N. K. Layered double hydroxides as efficient drug delivery system of
ciprofloxacin in the middle ear: an animal study in rabbits. J. Mater. Sci.: Mater. Med. 2013, 24, 129−136.
(27) Badar, M.; Rahim, M. I.; Kieke, M.; Ebel, T.; Rohd, M.; Hauser, H.; Behrens, P.; Mueller, P. P. Controlled drug release from antibiotic-loaded layered double hydroxide coatings on porous titanium implants in a mouse model. J. Biomed. Mater. Res., Part A 2015, 103, 2141−2149. (28) Fayyazbakhsh, F.; Hashjin, M. S.; Keshtkar, A.; Shokrgozar, M. A.; Dehghan, M. M.; Larijani, B. Novel layered double hydroxides-hydroxyapatite/gelatin bone tissue engineering scaffolds: Fabrication, characterization, and in vivo study. Mater. Sci. Eng., C 2017, 76, 701− 714.
(29) Rives, V.; del Arco, M.; Martín, C. Layered double hydroxides as drug carriers and for controlled release of non-steroidal antiinflamma-tory drugs (NSAIDs): a review. J. Controlled Release 2013, 169, 28−39. (30) Rocha, M. A.; Petersen, P. A. D.; Teixeira−Neto, E.; Petrilli, H.
M.; Leroux, F.; Taviot−Gueho, C.; Constantino, V. R. L. Layered
double hydroxide and sulindac coiled and scrolled nanoassemblies for storage and drug release. RSC Adv. 2016, 6, 16419−16436.
(31) Troutier-Thuilliez, A.; Taviot-Guého, C.; Cellier, J.; Hintze-Bruening, H.; Leroux, F. Layered particle-based polymer composites for coatings: Part I. Evaluation of layered double hydroxides. Prog. Org. Coat. 2009, 64, 182−192.
(32) Babakhani, S.; Talib, Z. A.; Hussein, M. Z.; Ahmed, A. A. A. Optical and Thermal Properties of Zn/Al-Layered Double Hydroxide Nanocomposite Intercalated with Sodium Dodecyl Sulfate. J. Spectrosc. 2014, 2014, No. 467064.
(33)https://icsd.fiz-karlsruhe.de/search/basic.xhtml.
(34) Faria, D. L. A.; Constantino, V. R. L.; Baldwin, K. J.; Batchelder, D. N.; Pinnavaia, T. J.; Chibwe, M. Raman microspectroscopy of phthalocyanine intercalates: tetrasulphonated cobalt and nickel phthalocyanines in layered double hydroxide. J. Raman Spectrosc. 1998, 29, 103−108.
(35) Kim, T. H.; Heo, I.; Paek, S. M.; Park, C. B.; Choi, A. J.; Lee, S. H.; Choy, J. H.; Oh, J. M. Layered metal hydroxides containing calcium and their structural analysis. Bull. Korean Chem. Soc. 2012, 33, 1845− 1850.
(36) Xie, W.; Lorenz, S.; Dolder, S.; Hofstetter, W. Extracellular Iron is a Modulator of the Differentiation of Osteoclast Lineage Cells. Calcif. Tissue Int. 2016, 98, 275−283.
(37) Rodrigues, D.; Freitas, M.; Costa, V. M.; Lopez-Quintela, M. A.; Rivas, J.; Freitas, P.; Carvalho, F.; Fernandes, E.; Silva, P. Quantitative Histochemistry for Macrophage Biodistribution on Mice Liver and Spleen After the Administration of a Pharmacological-Relevant Dose of Polyacrylic Acid-Coated Iron Oxide Nanoparticles. Nanotoxicology 2017, 11, 256−266.
(38) Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500−510.
(39) Ruiz, I. G.; Torre, P.; Diaz, T.; Esteban, E.; Morillas, J. D.; Yagüe, T. M.; Herruzo, J. A. S. Sp Family of Transcription Factors Is Involved in Iron-Induced Collagen alpha1(I) Gene Expression. DNA Cell Biol. 2000, 19, 167−178.
(40) Hosaka, Y.; Yamaguchi, M.; Takehana, K. Functional ability of tendinocytes to take up Fe substances in an inflamed tendon. Arch. Histol. Cytol. 2005, 68, 161−169.
(41) Deng, J.; Chen, F.; Fan, D.; Zhu, C.; Ma, X.; Xue, W. Formation and characterization of iron-binding phosphorylated human-like collagen as a potential iron supplement. Mater. Sci. Eng., C 2013, 33,
4361−4368.
(42) Gasser, M. S. Inorganic layered double hydroxides as ascorbic acid (vitamin C) delivery systemIntercalation and their controlled release properties. Colloids Surf., B 2009, 73, 103−109.
(43) Gao, X.; Lei, L.; O’Hare, D.; Xie, J.; Gao, P.; Chang, T.
Intercalation and controlled release properties of vitamin C intercalated layered double hydroxide. J. Solid State Chem. 2013, 203, 174−180.
(44) Aisawa, S.; Higashiyama, N.; Takahashi, S.; Hirahara, H.; Ikematsu, D.; Kondo, H.; Nakayama, H.; Narita, E. Intercalation behavior of L-ascorbic acid into layered double hydroxides. Appl. Clay Sci. 2007, 35, 146−154.
(45) Seftel, E. M.; Cool, P.; Lutic, D. Mg−Al and Zn−Fe layered double hydroxides used for organic species storage and controlled release. Mater. Sci. Eng., C 2013, 33, 5071−5078.
(46) Meng, W.; Li, F.; Evans, D. G.; Duan, X. Preparation and intercalation chemistry of magnesium-iron(III) layered double hydroxides containing exchangeable interlayer chloride and nitrate ions. Mater. Res. Bull. 2004, 39, 1185−1193.
(47) Del Arco, M.; Fernández, A.; Martín, C.; Rives, V. Release studies of different NSAIDs encapsulated in Mg,Al,Fe-hydrotalcites. Appl. Clay Sci. 2009, 42, 538−544.
(48) Gu, Z.; Wu, A.; Li, L.; Xu, Z. P. Influence of Hydrothermal Treatment on Physicochemical Properties and Drug Release of Anti-Inflammatory Drugs of Intercalated Layered Double Hydroxide Nanoparticles. Pharmaceutics 2014, 6, 235−248.
(49) Dokoumetzidis, A.; Macheras, P. A century of dissolution research: From Noyes and Whitney to the Biopharmaceutics Classification System. Int. J. Pharm. 2006, 321, 1−11.
(50) Cunha, V. R. R.; Guilherme, V. A.; Paula, E.; Araujo, D. R.; Silva, R. O.; Medeiros, J. V. R.; Leite, J. R. S. A.; Petersen, P. A. D.; Foldvari, M.; Petrilli, H. M.; Constantino, V. R. L. Delivery system for mefenamic acid based on the nanocarrier layered double hydroxide: physicochem-ical characterization and evaluation of anti-inflammatory and antinociceptive potential. Mater. Sci. Eng., C 2016, 58, 629−638.
(51) Hou, W. G.; Jin, Z. L. Synthesis and characterization of Naproxen intercalated Zn−Al layered double hydroxides. Colloid Polym. Sci. 2007, 285, 1449−1454.
(52) Carriazo, D.; Del Arco, M.; Martín, C.; Ramos, C.; Rives, V. Influence of the inorganic matrix nature on the sustained release of naproxen. Microporous Mesoporous Mater. 2010, 130, 229−238.
(53) Rojas, R.; Kairuz, A. F. J.; Manzo, R. H.; Giacomelli, C. E. Release kinetics from LDH-drug hybrids: Effect of layers stacking and drug solubility and polarity. Colloids Surf., A 2014, 463, 37−43.
(54) Rojas, R.; Linck, Y. G.; Cuffini, S. L.; Monti, G. A.; Giacomelli, C. E. Structural and physicochemical aspects of drug release from layered double hydroxides and layered hydroxide salts. Appl. Clay Sci. 2015, 109-110, 119−126.
■
NOTE ADDED AFTER ISSUE PUBLICATIONThis paper published on 12/26/2018 with an important graphic omission. The revised version was reposted on 12/28/2018.