ORIGINAL ARTICLE
Clinical impact of NK-cell reconstitution after reduced
intensity conditioned unrelated cord blood transplantation in
patients with acute myeloid leukemia: analysis of a prospective
phase II multicenter trial on behalf of the Société Française de
Greffe de Moelle Osseuse et Thérapie Cellulaire and Eurocord
S Nguyen1,2, A Achour2, L Souchet1, S Vigouroux3, P Chevallier4, S Furst5, A Sirvent6, J-O Bay7, G Socié8, P Ceballos9, A Huynh10, J Cornillon11, S Francois12, F Legrand13, I Yakoub-Agha14, G Michel15, N Maillard16, G Margueritte17, S Maury18, M Uzunov1,C-E Bulabois19, M Michallet20, L Clement21, C Dauriac22, K Bilger23, J Lejeune24, V Béziat2, V Rocha25, B Rio26, S Chevret24and V Vieillard2
Unrelated cord blood transplantation (UCBT) after a reduced intensity conditioning regimen (RIC) has extended the use of UCB in elderly patients and those with co-morbidities without an HLA-identical donor, although post-transplant relapse remains a concern in high-risk acute myeloid leukemia (AML) patients. HLA incompatibilities between donor and recipient might enhance the alloreactivity of natural killer (NK) cells after allogeneic hematopoietic stem-cell transplantation (HSCT). We studied the reconstitution of NK cells and KIR-L mismatch in 54 patients who underwent a RIC-UCBT for AML in CR in a prospective phase II clinical trial. After RIC-UCBT, NK cells displayed phenotypic features of both activation and immaturity. Restoration of their polyfunctional capacities depended on the timing of their acquisition of phenotypic markers of maturity. The incidence of treatment-related mortality (TRM) was correlated with low CD16 expression (P = 0.043) and high HLA-DR expression (P = 0.0008), whereas overall survival was associated with increased frequency of NK-cell degranulation (P = 0.001). These features reflect a general impairment of the NK licensing process in HLA-mismatched HSCT and may aid the development of future strategies for selecting optimal UCB units and enhancing immune recovery.
Bone Marrow Transplantation (2017)52, 1428–1435; doi:10.1038/bmt.2017.122; published online 26 June 2017
INTRODUCTION
The varying probabilities offinding an HLA-identical donor, which depend on the recipient’s ethnic origin, have made it necessary to develop alternative donor sources. While recent advances in the prevention of GVHD have greatly improved the outcome of haploidentical hematopoietic stem-cell transplantation (HSCT) patients, there is still no consensus about whether alternative donor sources produce the best outcomes.1 Myeloablative conditioning regimens (MAC)-UCBT provide excellent results in children and young adults with acute leukemia or myelodysplasic syndrome,2 but are too toxic for elderly patients. RIC regimens combining 200cGy total body irradiation (TBI), cyclophosphamide, and fludarabine (the Minneapolis regimen) have been used to minimize the treatment-related mortality (TRM) rates.3–5
Consistent with previous studies, the French MINICORD Trial reported a low TRM rate but a high rate of relapse in adult AML patients who received a RIC-UCBT in CR.6In transplantations with HLA mismatches between donor and recipients, donor NK cells may play an important role in mediating a GvL effect without GvHD, especially in RIC HSCT, where success depends principally on donor cell alloreactivity. NK cells are thefirst lymphoid cells to reconstitute after HSCT (before T cells), reaching normal values within one month after transplant.7,8 The quality of their reconstitution and education is of particular concern, especially in HLA mismatch situations.
NK cells are a unique component of the innate immune system, play a critical role against malignancies, and can recognize various targets without specific sensitization.9 They differ in their
1
AP-HP, Hôpital Pitié Salpêtrière (AP-HP), Service d’Hématologie Clinique, Paris, France;2
Sorbonne Universités, UPMC Univ Paris 06, INSERM U1135, CNRS ERL8255, Centre
d’Immunologie et des Maladies Infectieuses (CIMI-Paris), Paris, France;3
CHU de Bordeaux Hôpital du Haut-Lévèque, Service d'hématologie clinique et de thérapie cellulaire,
Pessac, France; 4
CHU de Nantes, Hematology Department, Nantes, France;5
Institut Paoli Calmettes, Service de greffe de moelle, Marseille, France; 6
Centre Hospitalier
Universitaire de Montpellier, Montpellier, France;7CHU Estaing Service d’Hématologie Clinique, Clermont-Ferrand, France;8AP-HP, Hôpital Saint-Louis, Service d’Hématologie et
de Transplantation, Paris, France;9
Hôpital Lapeyronie, Montpellier, France;10
CHU de Toulouse, Hématologie Clinique, Toulouse, France;11
Institut de Cancérologie de la Loire,
Service d’Hématologie, Saint-Priest-en-Jarez, France;12
CHU de Angers, Angers, France;13CHU de Nice, Service d’Hématologie, Nice, France;14CHU de Lille, Service d’Hématologie
Lille, France;15
Department of Pediatric Hematology and Oncology, Timone Enfants Hospital and Research Unit EA 3279 Aix-Marseille University, Marseille, France;16
CHU de
Poitiers, Poitiers, France;17CHU Montpellier Pédiatrie, Montpellier, France;18Hôpital Henri Mondor, Service d'Hématologie, Créteil, France;19CHU Grenoble, Grenoble, France;
20
Hôpital Edouard Herriot, Lyon, France;21
University Hospital de Bordeaux, Bordeaux, France;22
CHU de Rennes, Rennes, France;23
CHRU de Strasbourg, Strasbourg, France;
24
Department de Bioinformatique et Statistique Médicale, Hôpital Saint-Louis, Paris, France;25
Eurocord Office, Hôpital Saint-Louis, Paris, France and26
Hôpital Saint-Antoine, Hématologie Clinique et Thérapie Cellulaire, Paris, France. Correspondence: Professor S Nguyen, Clinical Hematological Department, Pitié-Salpêtrière Hospital, 75651 Paris, France. E-mail: stephanie.nguyen-quoc@aphp.fr
or Dr V Vieillard, Centre d’Immunologie et des Maladies Infectieuses (CIMI-Paris), 75013 Paris, France. E-mail: vincent.vieillard@upmc.fr
Received 3 January 2017; revised 11 May 2017; accepted 12 May 2017; published online 26 June 2017 www.nature.com/bmt
proliferative potential, homing characteristics, functional capabil-ities, and responses to a wide range of cytokines. The complexity of the NK-cell compartment is attributable to the vast network of inhibitory and activating receptors expressed on their cell surface. Their major activating receptors are NKG2D, NKG2C, and natural cytotoxicity receptors that recognize stress molecules on the surface of target cells. NK-cell target recognition, as well as cell education, depends on the interaction between their inhibitory receptors and their self-MHC class-I ligands.10Two major families of inhibitory NK receptors recognize MHC class-I molecules: the lectin-like CD94/NKG2A heterodimer, which binds to the non-classical class-Ib HLA-E molecule, and the killer cell immunoglobulin-like receptors (KIRs). Three major inhibitory KIRs have been characterized and named: KIR2DL1, KIR2DL2/KIR2DL3, and KIR3DL1; respectively, they recognize HLA-C alleles with asparagine 80 (group C1), HLA-C alleles with lysine 80 (group C2) and HLA-B alleles with Bw4 motifs at positions 77–83.11–13 Activating KIRs are undoubtedly also important for NK-cell education and functions; their role and ligands have not yet been fully elucidated, although they appear to affect the clinical outcome of patients undergoing HSCT for myeloid diseases.14
Biological studies of NK cells and especially of their reconstitu-tion after HSCT are essential for improving our understanding of the factors that influence NK-cell alloreactivity and its counterpart, education for tolerance. Studies have shown major discrepancies in the impact of alloreactive NK cells on relapse and survival, as well as on adverse outcomes related to infection and GVHD.15 After T-cell-depleted haplo-mismatched HSCT, these cells exhibit an immature phenotype as well as defective ex vivo cytotoxicity against primary mismatched AML blasts.7In contrast, NK cells are quickly reconstituted after UCBT, which is now used as an alternative to allogeneic HSCT for patients without an HLA-matched donor. We and others have shown that after UCBT, NK cells appear very rapidly and become functional against AML blasts despite persistent overexpression of CD94/NKG2A, a marker of immaturity.6,7,16–18
The heterogeneity of underlying diseases, conditioning, types of transplants, and GVHD prophylaxis contributes to the difficulty in reaching a conclusion about the clinical impact of NK-cell alloreactivity, especially in retrospective studies. To improve our understanding of the role of NK cells after UCBT, we studied the quality of NK-cell reconstitution, the impact of KIR–ligand incompatibilities, and their possible involvement in the clinical events observed in a prospective phase II clinical trial of adult AML patients who, in CR, underwent RIC-UCBT; this multicenter trial was conducted by the Société Française de Greffe de Moelle Osseuse et Thérapie Cellulaire and Eurocord.19
PATIENTS AND METHODS Patients
To evaluate RIC-UCBT in adults with AML, this prospective phase II multicenter trial enrolled patients in 23 French hospitals from October 2007 to September 2009, specifically 79 patients in CR of de novo or secondary AML. The primary objective was to show a reduction in non-relapse mortality (NRM) from 40% (based on registry data) to 20%. The conditioning regimen consisted of cyclophosphamide (50 mg/kg)+ fludar-abine (200 mg/m2)+TBI (2Gy), CsA +MMF as GVHD prophylaxis, and GCSF from day +1. Biological samples for the NK-cell study were available for 54 of the 79 patients. Their characteristics are summarized in Table 1. These patients experienced 35 events (relapse or TRM). Median event-free survival (EFS) was 13.2 months (95% confidence interval (CI) 7.1–27.6) and median overall survival 18.3 months (95% CI 12.1-not available; Supplementary Figure 1). The study design, homogeneous conditioning regimen, inclusion/exclusion criteria, and chimerism analysis have previously been described.19For controls, 10 cord blood (CB) samples were provided by the obstetrics department of Pitié-Salpêtrière hospital (Paris, France), and blood samples from 20 healthy adult donors (Ctl) by the Etablissement Français du Sang (EFS). The institutional review board of
Hôtel-Dieu, Assistance Publique-Hôpitaux de Paris, France, approved this study.
Flow cytometry analysis
Peripheral blood samples were collected at different time points during thefirst year post-UCBT for this prospective longitudinal study. CD3−CD56+ NK cells were analyzed by flow cytometry within the CD45+ gate (anti-CD45, #J33 from Beckman Coulter, Villepinte, France) after prepara-tion with an appropriate cocktail of monoclonal antibodies (mAbs), as previously described:20CD3 (#UCHT1), CD56 (#N901) (mAbs), CD16 (#3G8), CD159a/NKG2A (#Z199), CD85J (ILT2; #HP-F1), anti-HLA-DR (#Immu-357) mAbs from Beckman Coulter; anti-CD62L (#DREG-56), and antiCD161 (#DX12) from BD Pharmingen (Le Pont de Claix, France); anti-KIR2DL1 (#143211) and anti-KIR3DL1 (#177407) mAbs from R&D systems (Lille, France), and anti-KIR2DL2/KIR2DL3 (#DX27; Miltenyi Biotech, Paris, France) mAb. At least 20 000 CD45+cells were acquired on a Gallios flow cytometer (Beckman Coulter) and then analyzed with Flow Jo version 9 (TreeStar, Ashland, OR, USA).
Polyfunctional assay
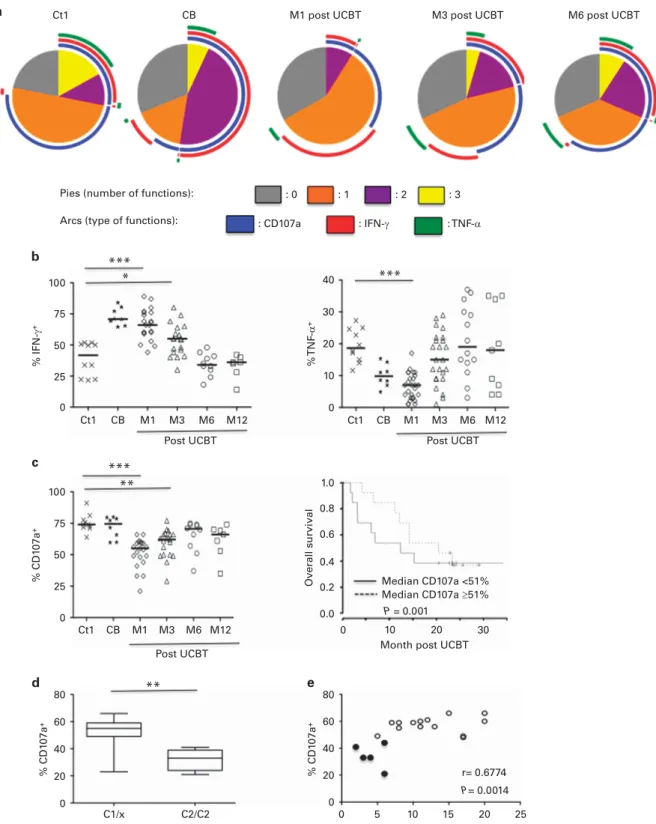
NK cells were incubated with standard HLA class-I negative K562 target cells (ATCC CCL243), at an effector:target (E:T) cell ratio of 1:1, in the presence of anti-CD107a mAb (#H4A3; Becton Dickinson), to measure degranulation. Cells were thereafter incubated for 5 h in the presence of Golgi Stop and Golgi Plug solutions (BD Pharmingen) and then stained with cell-surface markers (anti-CD3 and anti-CD56 mAbs). Cells werefixed, permeabilized with a cytofix/cytoperm kit BD Pharmingen and then intracellularly stained with anti-IFN-γ (#B27; BD Pharmingen) and anti-TNF-α (#Mab11; eBiosciences, Villebon-sur-Yvette, France) mAbs, as described.20,21 Data were analyzed with Flow Jo version 9 (TreeStar, Ashland, OR, USA), with the‘Boolean gate’ algorithm. Pestle software was used to remove the background, and pi charts, generated with Spice software (NIAI freeware, http://exon.niaid.nih.gov/spice/),22 present the frequency of NK cells positive for 0, 1, 2 or 3 responses. Arcs depict the frequency of cells positive for CD107a, IFN-γ or TNF-α.
KIR and HLA genotyping
DNA samples were extracted from patients’ whole blood and from cord blood with the QIAamp DNA blood mini kit (Qiagen, Courtaboeuf, France). KIR genotyping was performed by PCR with the KIR typing kit (Miltenyi Biotec), according to the manufacturer’s instructions. HLA class-I alleles were hybridized with the LABType SSO kit (One Lambda, InGen Technopolis, Chilly Mazarin). HLA sequences were read with a LABScan 200 (Luminex Technology, Thermofisher, Villebon-sur-Yvette, France) and computer-assisted HLA Fusion software.
Statistical analysis
Continuous variables are reported as median values and interquartile ranges, and categorical variables as percentages. The distribution of overall survival and event-free survival were calculated with Kaplan–Meier curves,
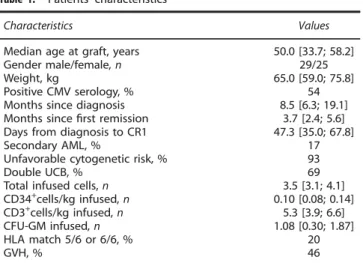
Table 1. Patients’ characteristics
Characteristics Values
Median age at graft, years 50.0 [33.7; 58.2]
Gender male/female, n 29/25
Weight, kg 65.0 [59.0; 75.8]
Positive CMV serology, % 54
Months since diagnosis 8.5 [6.3; 19.1]
Months sincefirst remission 3.7 [2.4; 5.6]
Days from diagnosis to CR1 47.3 [35.0; 67.8]
Secondary AML, % 17
Unfavorable cytogenetic risk, % 93
Double UCB, % 69
Total infused cells, n 3.5 [3.1; 4.1]
CD34+cells/kg infused, n 0.10 [0.08; 0.14] CD3+cells/kg infused, n 5.3 [3.9; 6.6] CFU-GM infused, n 1.08 [0.30; 1.87] HLA match 5/6 or 6/6, % 20 GVH, % 46 1429
according to the median value of the biological markers. The cumulative incidences of relapse and TRM were estimated with the method of Fine and Grey. Univariate analyses used a cause-specific Cox proportional hazards model to test the influence of these markers. Variable selection was based on a stepwise procedure at the .05 level. Statistical analyses were performed with open-source R software, version 3.0.2 (25 September 2013).
Phenotypic and functional NK-cell analyses were performed with Prism 5 software (GraphPad Software). Intergroup comparisons were assessed with the non-parametric Kruskal-Wallis test, with Dunn’s post-analysis test to determine which comparison of results from independent groups of subjects is significant (defined by a P-valueo0.05 with a two-tailed test-. *Po0.05, **Po0.01, and ***Po0.001.
RESULTS
Low CD16 frequency and a high level of cell activation were both associated with high TRM after UCBT
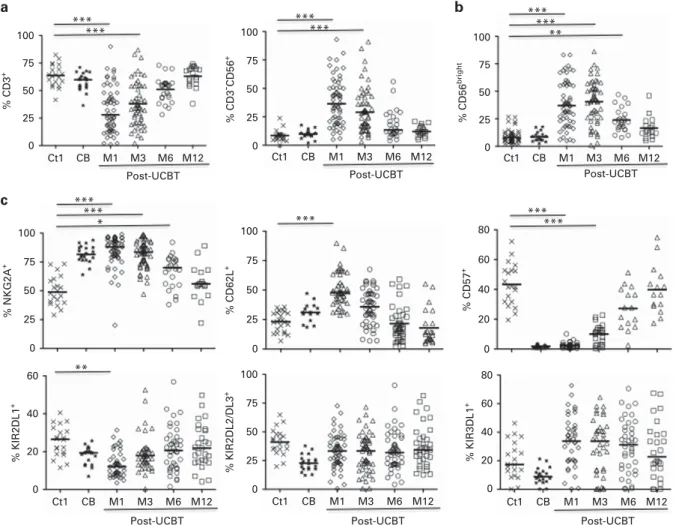
The distribution of CD3+ T lymphocytes remained significantly lower during thefirst 6 months after UCBT (at M6) than in adult and CB controls; at the same time, NK-cell recovery was prompt both in frequency (Figure 1a) and in absolute count with 203 ± 226/mm3 at M1 and 249 ± 202/mm3 at M3, compared with healthy donors (171 ± 74/mm3). These results are consistent with those from previous studies.20,23
NK-cell function is controlled by an array of inhibitory and activating signals that are processed by cell-surface receptors.11,12
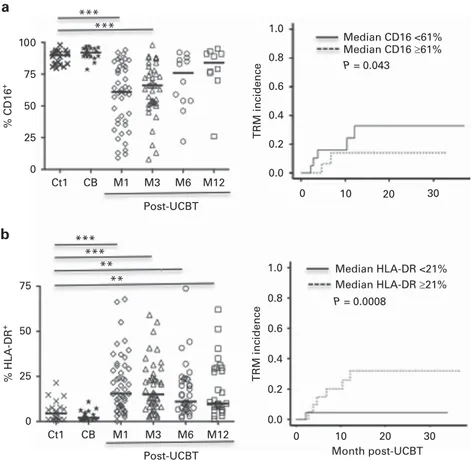
At 3 months post UCBT, phenotypic analyses revealed significantly higher levels of CD56bright, NKG2A, and CD62L than in adult and CB samples from healthy donors (Figures 1b and c), associated with lower levels of CRAAC, CD16, CD8, and CD57 (Figure 1c and Supplementary Figure 2). In accordance with previous studies,15 these data suggest that at the end of thefirst month post UCBT, NK cells have a transient immature phenotype. More importantly, TRM incidence was associated with a low frequency of CD16 on NK cells (HR = 0.97; 95% CI 0.94–1.0, P = 0.043) (Figure 2a). In contrast, NK cells post UCBT were indistinguishable from those of adult healthy donors for natural cytotoxicity receptors (NKp30, NKp44, and NKp46), NKG2D, NKG2C, and ILT-2 (data not shown). KIR2DL2/DL3 and KIR3DL1 were restored promptly after UCBT and were expressed at normal levels throughout the study. On the other hand, at M1, the level of KIR2DL1, which has the strongest inhibitory signal of all KIR family members and is present in 90–95% of healthy individuals worldwide,24,25 was significantly lower than that of controls, although it returned to normal range by 6 months post UCBT (Figure 1c).
To search for differences in NK-cell activation, we tested the expression of HLA-DR markers. Figure 2b shows a high level of HLA-DR after UCBT, compared to healthy controls, in accordance with previous studies. More importantly, HLA-DR was very significantly associated with TRM incidence (HR = 1.08; 95% CI 1.03–1.12, P = 0.0008; Figure 2b). Although almost all patients had full donor chimerism at M2, mixed chimerism was observed 100 75 50 25 0 Ct1 CB M1 M3 M6 M12 Post-UCBT Ct1 CB M1 M3 M6 M12 Post-UCBT 80 60 40 20 0 % KIR3DL1 + % KIR2DL2/DL3 + 100 75 50 25 0 Ct1 CB M1 M3 M6 M12 Post-UCBT % CD3 + *** *** Ct1 CB M1 M3 M6 M12 Post-UCBT % CD3 - CD56 + 100 75 50 25 0 *** *** % CD57 + 80 60 40 20 0 *** *** 100 75 50 25 0 % CD62L + *** 100 75 50 25 0 % NKG2A + *** *** * Ct1 CB M1 M3 M6 M12 Post-UCBT % KIR2DL1 + 60 40 20 0 ** Ct1 CB M1 M3 M6 M12 Post-UCBT % CD56 bright 100 75 50 25 0 *** *** ** a b c
Figure 1. Phenotypic characteristics of NK cells after UCBT. (a) Hematopoietic reconstitution after UCBT. Frequency of CD3+
T cells and CD3−CD56+NK cells gated on peripheral blood lymphocytes. (b) FACS profile of CD56brightsubpopulation gated on CD3−CD56+NK cells. (c) Pattern of receptor expression on CD3-CD56dimNK cells after UCBT. Peripheral blood cells were collected from healthy adult donors
(Ctl, Cross), umbilical cord blood (CB, Star), and from the patients (open symbols) at 1 (M1), 3 (M3), 6 (M6) and 12 (M12) months post-UCBT. Horizontal bars represent the median. P values are for the comparison of samples from adult controls and post-UCBT patients.
during thefirst three months in a few patients, as we reported earlier.19 This mixed chimerism was, however, associated with either high or low frequencies of NK cells expressing CD16 or HLA-DR, and was not associated with TRM (data not shown).
Differential contributions of donor and recipient HLA-KIR to the post-UCBT clinical outcome
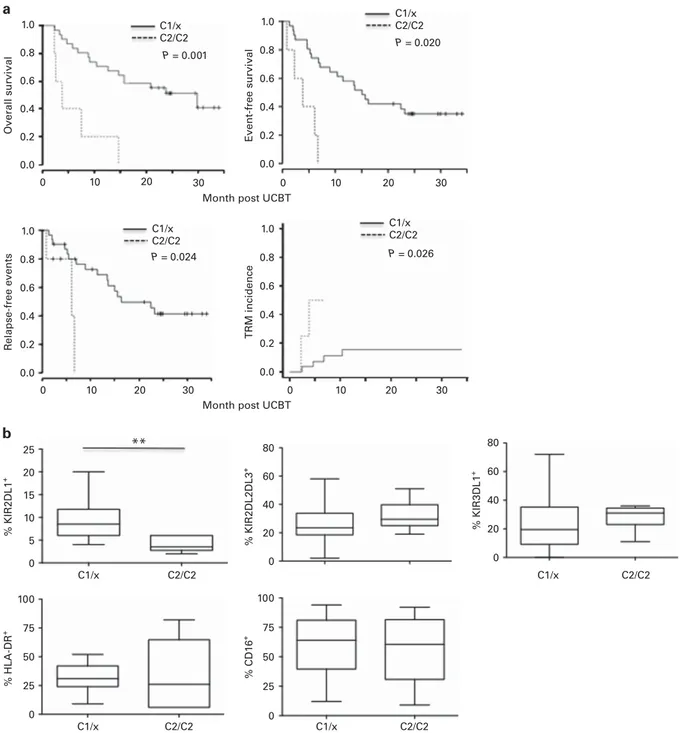
We next analyzed the KIR–ligand mismatch. Despite the small number of patients, the proportion of HLA-C2 homozygous recipients (that is, with HLA-C2/C2) was consistent with the expected rate (about 15%) in Caucasian population. The median EFS for these C2-C2 individuals (n = 5) was 3.8 months vs 15.1 for C1/C1 and C1/C2 recipients (C1/x) (HR = 6.19, 95% CI 1.99–19.19, P = 0.002) (Supplementary Figure 3), and median overall survival 3.8 vs 29.9 months, respectively (HR = 6.12, 95% CI 2.07–18.11, P = 0.001) (Figure 3a). Furthermore, C2/C2 recipients had a higher risk of relapse-free events (HR = 5.04, 95% CI 1.23–20.56, P = 0.024) and a higher TRM incidence (HR = 9.44, 95% CI 1.31–67.88, P = 0.026) than C1/x patients (Figure 3a). Moreover, KIR2DL1, which specifically binds to HLA-C2, was significantly lower at M1 on NK cells from C2/ C2, compared with C1/x, recipients (P = 0.0019) (Figure 2b). In contrast, KIR2DL2/DL3 and KIR3DL1, which are specific for HLA-C1 and HLA-Bw4, respectively, were expressed at similar levels in C2/C2 and C1/x patients (Figure 3b), as were HLA-DR and CD16, which are also associated with TRM risk (Figure 2).
Further exploring the influence of donor KIR haplotypes, we observed that all the C2/C2 individuals were KIR haplotype A/A, compared with 7 of the 15 (46.7%) C1/x recipients tested (Fisher’s
exact test: P = 0.05; Supplementary Table 1). Of note, all C2/C2 patients were genetically positive for KIRD2L1 and negative for KIR2DS1, both binding specifically to HLA-C2 ligands (Supplementary Table 1).
Functional correlation between NK-cell degranulation and overall survival after UCBT
To determine the significance of these genotypic and phenotypic features, we performed polyfunctional assays to determine the ability of NK cells to simultaneously degranulate and produce cytokines (IFN-γ and/or TNF-α) after UCBT, in the presence of K562 target cells. Consistent with the transient immature phenotype of the patients’ NK cells, their polyfunctional activity during thefirst 6 months post UCBT was lower than that of NK cells from the adult and CB control samples (Figure 4a). This difference was associated mainly with the higher production of intracellular IFN-γ by the patients’ NK cells, associated with lower TNF-α production (Figure 4b) and degranula-tion (CD107a surface expression; Figure 4c).
Although the levels of intracellular IFN-γ and TNF-α were similar in C2/C2 and C1/x samples (data not shown), it is important to note that low CD107a expression was significantly associated with worse overall survival (HR = 0.95, 95% CI 0.99–1.0, P = 0.001) (Figure 4c), especially in recipients with the HLA-C2/C2 genotype (P = 0.0079; Figure 4d). Notably, we observed a positive correlation between CD107a expression and frequency of KIR2DL1 surface expression (Figure 4e; Spearman test r = 0.6774, P = 0.0014), with values lowest for HLA-C2 homozygous patients.
*** *** *** *** ** ** 100 75 50 25 0 % CD16 + Ct1 CB M1 M3 M6 M12 Ct1 CB M1 M3 M6 M12 Post-UCBT Post-UCBT 1.0 0.8 0.6 0.4 0.2 0.0 0 10 20 30 Median CD16 <61% Median CD16 ≥61% P = 0.043 P = 0.0008 TRM incidence TRM incidence Median HLA-DR <21% Median HLA-DR ≥21% 75 50 25 0 % HLA-DR + 0 10 20 30 1.0 0.8 0.6 0.4 0.2 0.0 Month post-UCBT a b
Figure 2. Correlation between expression of CD16 or HLA-DR on NK cells and TRM incidence in AML patients after UCBT. Expression of CD16 (a) and HLA-DR (b) were determined by flow cytometry on CD56dimNK cells (left panels). Peripheral blood cells were collected from healthy
adult donors (Ctl, Cross), umbilical cord blood (CB, Star), and from the patients (open symbols) at 1 (M1), 3 (M3), 6 (M6) and 12 (M12) months post UCBT. Horizontal bars represent the median. P values are for the comparison between samples from adult controls and post-UCBT patients. Kaplan–Meier curves (right panels) for TRM incidence were determined at M1. Patients were classified in 2 groups according to the median frequency of HLA-DR (31%), and CD16 (61%), respectively.
DISCUSSION
Although the identification of a large array of NK-cell markers has made it possible to analyze in detail the different stages of cell maturation and to distinguish the cell subsets endowed with different functional capabilities, the respective roles of these markers in clinical outcome after HSCT remain elusive. Some major retrospective studies of large numbers of patients have analyzed the impact of KIR genotype and/or KIR–ligand HLA-C on patient outcome.14,26,27 Very few studies, on the other hand, have conducted prospective longitudinal biological studies combining phenotypic and functional analyses. To reduce the number of confounding factors, our analysis was restricted to AML patients, transplanted with unrelated cord blood according to a standardized clinical protocol with low-dose TBI (2Gy), cyclophosphamide, and
fludarabine in a prospective study. This RIC-UCBT protocol was used to obtain prompt and complete neutrophil recovery and to reduce mortality related to transplantation.19 Our data indicate that after RIC-UCBT, NK cells displayed major phenotypic features associated with transient immaturity, including a high level of CD56bright, persistent overexpression of CD94/NKG2A, and intracellular IFN-γ production, associated with transient down-modulation of three independent markers of advanced differentiation: CD16, CD8, and CD57. These results are consistent with a profound reduction in the polyfunctional capacities of NK cells and produce a positive correlation between CD107a expression and overall survival after RIC-UCBT, compatible with the association between high NK cytotoxic activity and reduced risk of cancer, previously reported in a large 11-year general population follow-up study.28
1.0 Overall survi val Event-free survi val R elapse-free events % KIR2DL1 + % HLA-DR + % CD1 6 + % KIR2DL2DL3 + % KIR3DL1 + TRM incidence 0.8 0.6 0.4 0.2 0.0 1.0 0.8 0.6 0.4 0.2 0.0 1.0 0.8 0.6 0.4 0.2 0.0 1.0 0.8 0.6 0.4 0.2 0.0 0 10 20 30 0 10 20 30 0 10 20 30 0 10 20 30 25 20 15 10 5 0 100 75 50 25 0 100 75 50 25 0 80 60 40 20 0 80 60 40 20 0 P = 0.024 P = 0.026 P = 0.020 P = 0.001 C1/x C2/C2 C1/x C2/C2 C1/x C2/C2 C1/x C2/C2 C1/x C2/C2 C1/x C2/C2 C1/x C2/C2 C1/x C2/C2
Month post UCBT Month post UCBT
a
b
**
Figure 3. Interaction between recipient HLA-C2-C2 status, clinical outcomes, and NK-cell features. (a) Kaplan–Meier curves (right panels) for overall survival (OS), event-free survival (EFS), relapse-free events, and TRM incidence in HLA-C2/C2-positive recipients (bold lines), compared to C1-C1 or C1–C2 (C1-x) patients (dotted lines). (b) Frequency of phenotypic (KIR-L, CD16, HLA-DR) NK-cell markers in HLA-C2/C2 and C1/x recipients.
100 % IFN-γ + % TNF -α + Overall survi val 50 25 0 Ct1 CB M1 M3 M6 M12 Ct1 CB M1 M3 M6 M12 0 10 20 30 Ct1
Pies (number of functions): : 0 : 1
: CD107a : IFN-γ : TNF-α
: 2 : 3
Arcs (type of functions): CB Ct1 CB M1 M3 Median CD107a <51% Median CD107a ≥51% P = 0.001 M6 M12 75 100 *** * *** *** ** ** % CD1 07a + 50 25 0 75 80 % CD1 07a + 40 20 0 C1/x C2/C2 0 5 10 15 20 25 r= 0.6774 P= 0.0014 60 80 % CD1 07a + % KIR2DL1+ 40 20 0 60 40 20 10 0 1.0 0.8 0.6 0.4 0.2 0.0 30 a b c d e Post UCBT
Post UCBT Month post UCBT
M6 post UCBT M3 post UCBT
M1 post UCBT
Post UCBT
Figure 4. Functional characteristics of NK cells post-UCBT. (a) Polyfunctionality of CD3−CD56+
NK cells (able to perform degranulation and produce IFN-γ and/or TNF-α) from patients (open symbols) at 1 (M1, N = 21), 3 (M3, N = 19), 6 (M6, N = 10) and 12 (M12, N = 7) months post UCBT, compared with healthy adult donors (Ctl, N= 10) and umbilical cord blood (CB, N = 8). Cell-surface expression of CD107a and intracellular production of IFN-γ and TNF-α were assessed in the absence of target cells (data not shown) or in the presence of K562. The values were analyzed with a Boolean gate algorithm (Flow Jo; TreeStar, Ashland, OR, USA). Pie and arc charts were generated with SPICE software (National Institute of Allergy and Infectious Diseases freeware). Pies represent the frequency of NK cells positive for 0, 1, 2, or 3 responses (to CD107a, IFN-γ, and TNF-α). Arcs depict the functions for which the cells are functional or polyfunctional. (b) Intracellular production of IFN-γ or TNF-α among CD3−CD56+NK cells. (c) Level of CD107a on CD56dimNK cells (left panels) and Kaplan–Meier curves (right panels) for overall survival (OS) at M1. Patients were classified in 2 groups according to the median frequency of CD107a (51%). Samples provided from healthy adult donors (Ctl, Cross), umbilical cord blood (CB, Star), and from patients (open symbols) at 1 (M1), 3 (M3), 6 (M6) and 12 (M12) months UCBT. Horizontal bars represent the median. P values are for the comparison of samples from adult controls and post-UCBT patients. (d) Frequency of CD107a+NK cells in HLA-C2/C2 and C1/x recipients. (e) Correlation between CD107a and KIR2DL1 expression. Closed and Open circles for HLA-C2/C2 and HLA-C1/x positive patients, respectively.
We observed that TRM incidence is associated with low levels of CD16 and high levels of HLA-DR expression in NK cells. Activated CD56brightCD16‒NK cells are generally considered the precursors of CD56dimCD16+NK cells.29,30We and others have shown that CD56brightCD16+ NK cells may be an intermediate stage of this differentiation, transiently increased after hematopoietic transplantation.31,32CD16 is a major functional NK-cell element, responsible for antibody-dependent cellular cytotoxicity (ADCC). Post-transplant treatment with anti-CD20 rituximab has produced favorable outcomes in patients with non-Hodgkin lymphoma.33,34 It might therefore be important to determine the impact of ADCC on the clinical outcome of AML patients under RIC-UCBT.
Because KIR and HLA class-I genes segregate on different chromosomes, a tolerance mechanism is required to prevent the development of autoreactive NK cells. Only NK cells expressing inhibitory receptors for self-HLA class-I molecules acquire full functional competence, while potentially autoreactive NK cells remain in a hyporesponsive state, a process referred to as education or licensing. Here, we show that adverse clinical outcomes (TRM, relapse, and overall survival) are observed mainly in HLA-C2/C2 patients, as previously reported.35 Although the small number of C2-C2 patients in our study prevents any definitive conclusions, their phenotypic and functional analyses suggest novel pathways that merit more study. Specifically, we observed that NK cells from HLA-C2/C2 patients expressed KIR2DL1 poorly at their cell surface and had less cytotoxic capacity. These findings raise questions about what role the KIR2DL1-HLA-C2 interaction may play in NK-cell education. Delayed expression of KIR2DL1 is consistent with the sequential expression by NK cells of inhibitory KIRs, as previously reported by us and others:20,36–38 it begins with the C1-specific inhibitory KIR2DL2/DL3, at earlier time points and at a higher frequency than the C2-specific KIR2DL1. Interestingly, a previous study reported an impact of KIR2DL1 polymorphism on outcome post HSCT. Patients who had an allele conferring a weak function of KIR2DL1 had a significant worse survival compared to patients whose KIR2DL1 allele conferred a strong function of the inhibitory KIR,39 suggesting a role of inhibitory KIR2DL1 receptor’s functionality on clinical outcome. Alternatively, other reports suggested a negative effect of activating (instead of inhibitory) KIR2DS1 receptor and its C2 ligand on outcome post transplant. In a large genetic study, Venstrom et al.14 reported worse outcomes in post-allogeneic HSCT for C2C2 (compared with C1/x) patients and a (donor or recipient) activating KIR2DS1 gene. It has been hypothesized that the activating KIR2DS1 receptor, which also recognizes HLA-C2, mediates a tolerogenic effect in C2/C2 recipients, thus impairing the NK-cell-mediated GvL effect.14,25,26,37,40In our study, all C2-C2 patients were KIR haplotype AA, and thus were negative for KIR2DS1 gene. The number of samples is not sufficient to conclude on the signification of the lack of KIR2DS1 in HLA- C2/C2. However, this is in accordance with the worldwide strong negative correlations between the presence of activating KIR genes and their corresponding HLA ligand groups across populations.41,42 A better understanding of how inhibitory and activating KIR–HLA interactions dictate specific NK-cell functions could therefore provide important information that might substantially improve selection of the best donor-recipient combinations. Importantly, our results show that the deleterious effect of HLA-C2/C2 recipients after UCBT was predicted not only by the gene’s presence, as previously described, but especially by its impact on NK-cell functionality in the recipient, as shown by the direct correlation between KIR2DL1 expression and NK-cell degranula-tion (CD107a expression), in turn correlated with worse clinical outcome. This functional impairment appears to be independent of the intracellular level of lytic markers (that is, perforin and granzyme-B), given that we have previously reported that these levels are similar in patients and healthy controls.20 Moreover, TRM incidence was also linked to a KIR–ligand-independent
mechanism, by its association with immaturity (low CD16 expression) and high cell activation (HLA-DR expression) in NK cells. Altogether, these date show that HLA-dependent and -independent NK-cell features may play complementary roles in clinical outcome after RIC/UCBT and that their further exploration should help in the development of future strategies to select optimal UCB units and enhance immune recovery.
CONFLICT OF INTEREST The authors declare no conflict of interest. ACKNOWLEDGEMENTS
We thank all transplantation centers participating in this study, the Société Française de Greffe de Moelle Osseuse et Thérapie Cellulaire (SFGM-TC), and the Eurocord office. We also thank the personnel from the Etablissement Français du Sang (EFS) for the healthy adult blood samples and the staff at the gynecology and obstetrics department of Pitié-Salpêtrière Hospital (Paris, France) for the cord blood samples. This work was funded by the Institut National du Cancer (AOM06206), the Ministère des Affaires Sociales et de la Santé (DGOS, AOR12106), the Agence de la Biomédecine, the association Laurette Fugain, and the association Capucine.
REFERENCES
1 Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia
2015;29: 1891–1900.
2 Michel G, Galambrun C, Sirvent A, Pochon C, Bruno B, Jubert C et al. Single- vs double-unit cord blood transplantation for children and young adults with acute
leukemia or myelodysplastic syndrome. Blood 2016;127: 3450–3457.
3 Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood 2013; 122: 491–498.
4 Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K et al. Cord-blood
transplantation in patients with minimal residual disease. N Engl J Med 2016;375:
944–953.
5 Liu H, Stock W, Bishop MR. Expanded indications for allogeneic stem cell transplantation in patients with myeloid malignancies. Curr Opin Hematol 2013; 20: 115–122.
6 Brunstein CG, Wagner JE, Weisdorf DJ, Cooley S, Noreen H, Barker JN et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood
transplant depends on transplantation conditioning intensity. Blood 2009;113:
5628–5634.
7 Nguyen S, Dhedin N, Vernant JP, Kuentz M, Al Jijakli A, Rouas-Freiss N et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood
2005;105: 4135–4142.
8 Mancusi A, Ruggeri L, Velardi A. Haploidentical hematopoietic transplantation for
the cure of leukemia: from its biology to clinical translation. Blood 2016;128:
2616–2623.
9 Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer
immunotherapy. Nat Immunol 2016;17: 1025–1036.
10 Saunders PM, Vivian JP, O'Connor GM, Sullivan LC, Pymm P, Rossjohn J et al. A bird's eye view of NK cell receptor interactions with their MHC class I ligands.
Immunol Rev 2015;267: 148–166.
11 Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8⁺ T cells. Nat Rev Immunol 2011; 11: 645–657.
12 Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL et al. Innate or
adaptive immunity? The example of natural killer cells. Science 2011;331: 44–49.
13 Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors
of natural killer cells in cancer and infection. Trends Immunol 2013;34: 182–191.
14 Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1.
N Engl J Med 2012;367: 805–816.
15 Nguyen S, Béziat V, Roos-Weil D, Vieillard V. Role of natural killer cells in
hematopoietic stem cell transplantation: myth or reality? J Innate Immun 2011;3:
383–394.
16 Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socié G et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after
umbilical cord blood transplantation for acute leukemia. Leukemia 2009;23:
492–500. 1434
17 Foley B, Felices M, Cichocki F, Cooley S, Verneris MR, Miller JS. The biology of NK cells and their receptors affects clinical outcomes after hematopoietic cell
transplantation (HCT). Immunol Rev 2014;258: 45–63.
18 Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Marsh SG et al. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia.
J Immunol 2014;192: 4592–4600.
19 Rio B, Chevret S, Vigouroux S, Chevallier P, Fürst S, Sirvent A et al. Decreased nonrelapse mortality after unrelated cord blood transplantation for acute myeloid
leukemia using reduced-intensity conditioning: a prospective phase II
multicenter trial. Biol Blood Marrow Transplant 2015;21: 445–453.
20 Beziat V, Nguyen S, Lapusan S, Hervier B, Dhedin N, Bories D et al. Fully functional
NK cells after unrelated cord blood transplantation. Leukemia 2009;23: 721–728.
21 Béziat V, Hervier B, Achour A, Boutolleau D, Marfain-Koka A, Vieillard V. Human NKG2A overrides NKG2C effector functions to prevent autoreactivity of NK cells.
Blood 2011;117: 4394–4396.
22 Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of
post-cytometric complex multivariate datasets. Cytometry A 2011;79: 167–174.
23 Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect
Dis 2011;13: 456–465.
24 Parham P. Immunology. NK cells lose their inhibition. Science 2004;305: 786–787.
25 Hou L, Chen M, Steiner NK, Belle I, Turino C, Ng J et al. Seventeen novel alleles add
to the already extensive KIR3DL3 diversity. Tissue Antigens 2007;70: 449–454.
26 Babor F, Manser AR, Fischer JC, Scherenschlich N, Enczmann J, Chazara O et al. KIR ligand C2 is associated with increased susceptibility to childhood ALL and confers
an elevated risk for late relapse. Blood 2014;124: 2248–2251.
27 Miller JS, Blazar BR. Control of acute myeloid leukemia relapse--dance between
KIRs and HLA. N Engl J Med 2012;367: 866–868.
28 Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study
of a general population. Lancet 2000;356: 1795–1799.
29 Béziat V, Descours B, Parizot C, Debré P, Vieillard V. NK cell terminal differentia-tion: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS ONE
2010;5: e11966.
30 Moretta L. Dissecting CD56dim human NK cells. Blood 2010;116: 3689–3691.
31 Dulphy N, Haas P, Busson M, Belhadj S, Peffault de Latour R, Robin M et al. An unusual CD56(bright) CD16(low) NK cell subset dominates the early
posttransplant period following HLA-matched hematopoietic stem cell
transplantation. J Immunol 2008;181: 2227–2237.
32 Béziat V, Duffy D, Quoc SN, Le Garff-Tavernier M, Decocq J, Combadière B et al. CD56brightCD16+ NK cells: a functional intermediate stage of NK cell
differentiation. J Immunol 2011;186: 6753–6761.
33 Shimoni A, Hardan I, Avigdor A, Yeshurun M, Raanani P, Ben-Bassat I et al. Rituximab reduces relapse risk after allogeneic and autologous stem cell transplantation in patients with high-risk aggressive non-Hodgkin's lymphoma.
Br J Haematol 2003;122: 457–464.
34 Pfeiffer M, Stanojevic S, Feuchtinger T, Greil J, Handgretinger R, Barbin K et al. Rituximab mediates in vitro antileukemic activity in pediatric patients after
allogeneic transplantation. Bone Marrow Transplant 2005;36: 91–97.
35 Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human
disease. Semin Immunol 2008;20: 343–352.
36 Nguyen S, Béziat V, Norol F, Uzunov M, Trebeden-Negre H, Azar N et al. Infusion of allogeneic natural killer cells in a patient with acute myeloid leukemia in relapse
after haploidentical hematopoietic stem cell transplantation. Transfusion 2011;51:
1769–1778.
37 Fischer JC, Ottinger H, Ferencik S, Sribar M, Punzel M, Beelen DW et al. Relevance of C1 and C2 epitopes for hemopoietic stem cell transplantation: role for
sequential acquisition of HLA-C-specific inhibitory killer Ig-like receptor. J Immunol
2007;178: 3918–3923.
38 Nguyen S, Kuentz M, Vernant JP, Dhedin N, Bories D, Debré P et al. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical
hematopoietic stem-cell transplantation. Leukemia 2008;22: 344–352.
39 Bari R, Rujkijyanont P, Sullivan E, Kang G, Turner V, Gan K et al. Effect of donor KIR2DL1 allelic polymorphism on the outcome of pediatric
allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2013; 31:
3782–3790.
40 Giebel S, Locatelli F, Wojnar J, Velardi A, Mina T, Giorgiani G et al. Homozygosity for human leucocyte antigen-C ligands of KIR2DL1 is associated with increased risk of relapse after human leucocyte antigen-C-matched unrelated
donor haematopoietic stem cell transplantation. Br J Haematol 2005; 131:
483–486.
41 Single RM, Martin MP, Gao X, Meyer D, Yeager M, Kidd JR et al. Global diversity
and evidence for coevolution of KIR and HLA. Nat Genet 2007;39: 1114–1119.
42 Nakimuli A, Chazara O, Farrell L, Hiby SE, Tukwasibwe S, Knee O et al. Killer cell immunoglobulin-like receptor (KIR) genes and their HLA-C ligands in a Ugandan
population. Immunogenetics 2013;65: 765–775.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)