Develop., Growth and Differ., 25 (l), 23-37 (1983)
Early Events in Anuran Amphibian
Fertilization
: An Ultrastructural Study
of
Changes Occurring in the Course of
Monospe rmic Fertilization and Artificial
Activation
(anuran/egg cortex/exocytosis/fertilization/microvilli)
M. CHARBONNEAU AND B. PICHERAL
Laboratoire de Cytologie Expdrimentale. L. A . N o 256, C . N . R . S., Universitt! de Rennes, Campus de Beaulieu. 35042 RENNES Cedex, France
In unfertilized frog eggs, the plasma membrane displays an animal vegetal polarity characterized by the presence of short microvilli in the vegetal hemisphere and long microvilli or ridge-like protru- sions in the animal hemisphere. The densities of microvilli are similar in the two hemispheres.
The fertilizing sperm always fuses with the animal hemisphere of the egg and induces a wave of exocytosis of cortical granules from its site of penetration. Similar spreading of the cortical reaction is seen on activation by pricking the egg cortex. The integration of the cortical granule membrane with the plasma membrane is rapidly followed by elongation of microvilli, which is progressively realized all over the egg surface from the site of sperm entry or the site of pricking. At this time, the length and shape of the microvilli in the animal and vegetal hemispheres are similar and their den- sities are the same as in unfertilized eggs.
A “smoothing” wave can be seen on the living egg, 40-60 seconds after pricking, starting around the site of pricking. This wave of microvillar elongation is accompanied by changes in intensity of diffracted light spots observed at the surface of the egg. This pattern might result from rapid and progressive thickening of the cortex that would drive pigment granules into the cytoplasm. The Brownian movement of these granules is thought to be responsible for the observed diffracted light spots.
Electrical stimulus or the ionophore A231 87 induced activation reactions similar to those trig- gered by the sperm or by pricking, except that the cortical reaction began simultaneously in several distinct sites of the cortex.
The early events of fertilization are relatively well understood in echinoderms (1, 2, 3), but not in amphibians, particularly with respect to binding and fusion of gametes. Though there have recently been many ultrastructural studies on anuran fertilization (4), none have actually been on early sperm-egg interactions, except that by PICHERAL and CHARBONNEAU (5). The only reports on the earliest morphological events in urodele fertilization are also those by PICHERAL (6, 7). The overall transformations of the cortex in anurans have been observed mainly by transmission electron microscopy. The present study provides scanning electron micrographs of marked changes of the surface of the egg during fertilization and artificial activation.
The present study showed the configuration of the plasma membrane before and after exocytosis of cortical granules and the series of events occurring in the immediate vicinity of exocytosing cortical granules. These phenomena could not be seen on examination of sections
24 M. CHARBONNEAU AND B. PICHERAL
through the egg cortex by light microscopy.
The exocytotic wave in anurans has so far been studied only in artificially activated eggs here we describe the transformation of the cortex surface of fertilized monospermic eggs during gamete interactions and at different stages of cortical exocytosis within the same egg from the site of sperm entry to the front of the cortical wave. We also used artificial activating stimuli to define the time course of the cortical reaction. Observations on cortical transformations in living eggs made by stereo-light microscopy were completed with observations by scanning electron microscopy (SEM). Eggs of Ranidae are particularly suited for this type of study because they have a relatively large diameter (1.5 to 1.8 mm) allowing clear observations by stereomicroscopy. Moreover, the high pigmentation of the animal hemisphere, especially in Rana temporaria is essential for clear in vivo observation of some phenomena taking place at the egg surface. However, egg jelly layers, which are normally necessary for successful ferti- lization, make it difficult to examine details of the egg surface. The kinetics of some early events affecting the egg surface were consequently studied using coelomic or mechanically dejellied eggs, after artificial activation. These in vivo observations are the necessary complement to SEM and TEM studies, in that they make it possible to deduce the overall sequence of events and to establish reference marks that can be related to ultrastructural observations.
MATERIALS AND METHODS
Sexually mature Runu temporaria were collected either during the hibernation period in Besanqon, France (November), or just at the breeding period (January) in Rennes, France, and were stored at 2 to 8°C untile use. Sexually mature Runu esculentu and Runu ridibundu were collected during the breeding season in Concarneau and Rennes respectively, France, in May-June and kept at 1622°C until use.
Females were induced to ovulate by injection of one female pituitray plus two male pituitaries from the same species. Sometimes ovulation was induced by natural mating and the male was then separated from the female before natural insemination. Mature body cavity eggs (coelomic eggs) were obtained by ligating the anterior oviducts before pituitary injection. Sperm were obtained by mincing the testes with forceps in insemination medium, the suspension was used immediately or stored for at most a few hours at 4°C until use.
The normal medium was a tenfold dilution of OR, (1/10 OR,), described by WALLACE et uI. (8). Its final composition was (mM) as follows: 8.25 NaCl; 0.25 KCI; 0.1 CaCI,; 0.1 MgCl,; 0.1 Na,HPO,; 0.5 Hepes buffer; 0.38 NaOH; pH: 7.2-7.5.
Eggs were activated by pricking with a fine glass or tungsten needle, or by an electrical stimulus (70 pF under 70 V; 1 to 2 seconds) or by application of the calcium ionophore A23187 (Calbiochem) (stock solution: 2 x lo-, M in 100 % ethanol). Egg activation was tested by checking the following criteria (chronologically) : observation of cortical granule exocytosis, change in appearance of the egg surface (5); raising of a vitelline envelope, cortical contraction of the pigmented area, emergence of the second polar body and fragmentation and cytolysis of the egg in the case of an artificial activation, observation of the sperm entry site and formation of the first cleavage furrow in the case of activation by the sperm. The procedures for SEM and TEM were as described previously (5). All experiments were done at 18-20°C.
RESULTS
1. Pattern of the exocytotic wave
1-1.
The size and density of the cortical granules differ in different species; the granules are larger
EARLY EVENTS IN A N U R A N FERTILIZATION 25 and more numerous in Rana temporaria than in Rana esculenta, but are uniform within the same species. In these two species, there is no difference in the size or ultrastructural appearance
of
granules in the animal and vegetal halves (Figure l), whereas in Xenopus there is an animal-vegetal difference (9, 10).
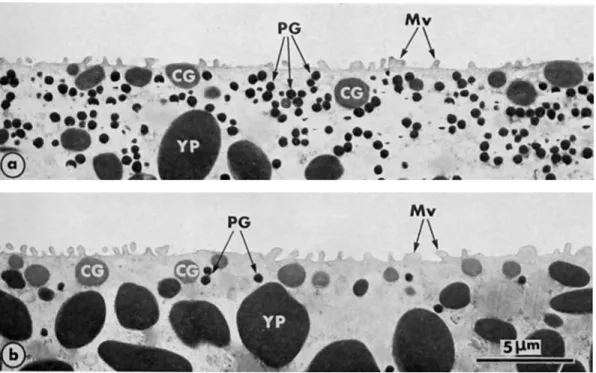
Fig. 1. Cortex and subcortical cytoplasm in the animal hemisphere (a) and the vegetal hemisphere (b) of unferti- lized mature eggs of Rono escuienru. Pigment granules (PG) are much more numerous in the animal hemisphere than in the vegetal one, whereas the gradient of size and density of the yolk platelets (YP) is oriented on the reverse axis. CG: cortical granule; Mv: microvilli. 4500X.
1-2. Sperm entry site and associated changes in the egg surface
In about 500 cases observed by SEM (from batches of 100
%
monospermic eggs), the ferti- lizing sperm was consistently seen to fuse with the animal hemisphere of the egg. It has previously been demonstrated that the exocytosis of cortical granules spreads over the egg surface from the site of sperm entry (5). This phenomenon is also shown in Figure 2, where different areas of the surface of the same egg displaying different steps in the cortical reaction are shown for a monospermic egg of Rana ridibundu. To our knowledge, these are the first pictures of early gamete interactions in monospermic anuran eggs. However, from pictures these it is difficult to establish the relation between sperm binding, gamete fusion and cortical exocytosis, since even if the rate of the cortical reaction is known, the time of gamete interaction at which the cortical reaction is triggered and the rate of the sperm penetration are unknown.1-3. Activation by pricking
Activation by pricking the egg cortex appears to closely mimic the activation by sperm in that the cortical reaction spreads all over the egg surface from the site of pricking (Figure
26 M. CHARBONNEAU AND B. PICHERAL
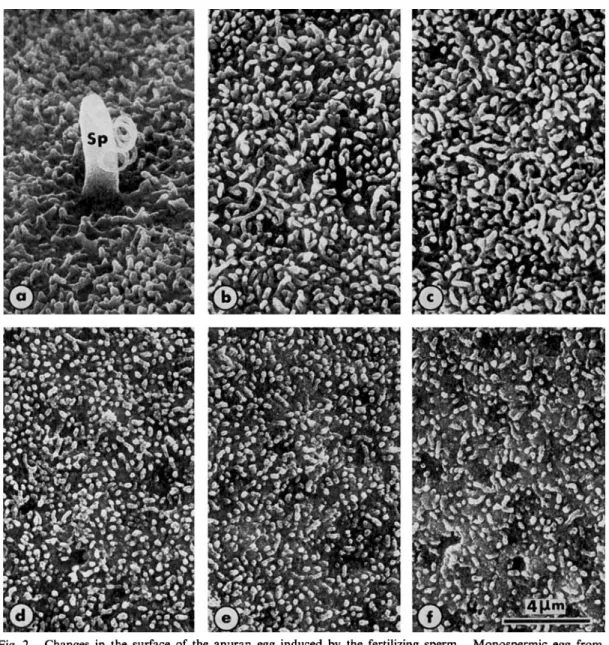
Fig. 2. Monospermic egg from
the sperm entry site (a) to about 100” from it ( f ); a-f are from the same egg of Rana ridibunda 5 min. after insemination. The surface of the egg just reached by the exocytotic wave still displays short microvilli (e, f ).
After cortical breakdown, the microvilli are considerably elongated (a-c).
Fig. 3. a: Front of the exocytotic wave in R a m temporaria propagating from the site of pricking (left) to a region where cortical granules have not yet been extruded 15 seconds after pricking.
b-d: The first visible steps of
exocytosis (in b) consist of the appearance of “craters” with we distinct edges (single black arrow) then, some “bouquets” of microvilli frequently appear (single white arrows in c) among the open granules (single black arrow). In the late steps of the cortical reaction (d), the “craters” progressively disappear with elongation of microvilli (double black arrow).
e, f : In e, the contents of a cortical
granule (CG) are being extruded. After exocytosis (in f ), the microvilli (Mv), integrating the cortical granule membrane, become very long. 14000X.
Changes in the surface of the anuran egg induced by the fertilizing sperm.
Sp : spermatozoon. 5000 x
.
3000 x.
Surfaces of eggs of Rana esculenta fixed 10-15 seconds after electrical stimulus.
5000 X
.
28 M. CHARBONNEAU AND B. PICHERAL
3a). Pricking activation of coelomic or dejellied eggs makes it possible to observe many phenomena that cannot be seen during the course of normal fertilization because of the presence of jelly layers. It also makes it possible to determine the exact the time of activation, and to observe all early events with respect to this time. All these phenomena were observed in the highly pigmented area, because few details can be obtained by observing the vegetal hemisphere. The sequence of these events observed in vivo in Rana temporaria is summarized in Table 1 . The latent period represents the time between the application of the stimulus and the time when cortical exocytosis is first detected by stereomicroscopy ; in the case of prick activation, this period is 6 to 10 seconds. Exocytosis of the cortical granules results in the appearance of “craters”, conferring a rough and granular appearance to the egg surface. In most cases, the propagation is asymmetrical; i.e., the exocytosis rate is not the same in all directions. More- over, the wave seems to slow down considerably just before it reaches the equator. Therefore, we made measurements only to an angle of about 150” (75” from either side of the animal pole) to obtain data during the period when the fast moving part of the wave was estimated to have a constant rate. The part of the cortical wave moving more rapidly traverses the 150“ angle in 30 to 50 seconds. When the vegetal pole was pricked, the cortical wave reached the animal
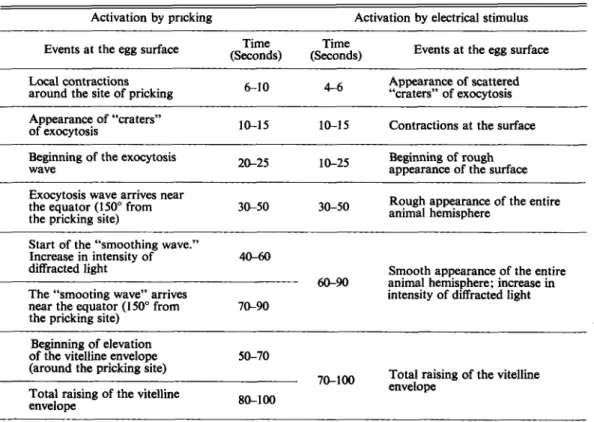
Table 1. Sequence of early events occurring at the egg surface of Rana temporaria after artificial activation. In vivo observations were made with a stereomicroscope. The eggs were pricked at about 75” from either side of the animal pole, with respect the to the egg center, and the exocytosis wave was followed over another 75”, in the opposite direction.
Activation by pricking Activation by electrical stimulus
Events at the egg surface
Time Time
(Seconds) (Seconds) Events at the egg surface
Appearance of scattered “craters” of exocytosis 6 1 0 4-6
Local contractions around the site of pricking Appearance of “craters”
of exocytosis 10-15 10-15 Contractions at the surface Beginning of rough appearance of the surface 20-25
Beginning of the exocytosis wave
Rough appearance of the entire Exocytosis wave arrives near
the equator (150” from 3&50
the pricking site) 30-50 animal hemisphere
Start of the “smoothing wave.”
Increase in intensity of 40-60 diffracted light
The “smooting wave” arrives near the equator (150° from the pricking site)
Smooth appearance of the entire intensity of diffracted light 60-!XI animal hemisphere: increase in 70-90
Beginning of elevation
of the vitelline envelope 50-70 (around the pricking site)
Total raising of the vitelline envelope
70-100 Total raising of the vitelline envelope 80-100
EARLY EVENTS IN ANURAN FERTILIZATION 29
pole after 2 to 3 min and in some cases, 2 to 3 prickings were needed to induce the cortical reaction.
1-4. Activation by an electrical stimulus and by the ionophore A23187
When the egg is activated by electrical stimulus or by the ionophore A23187 (Figure 4),
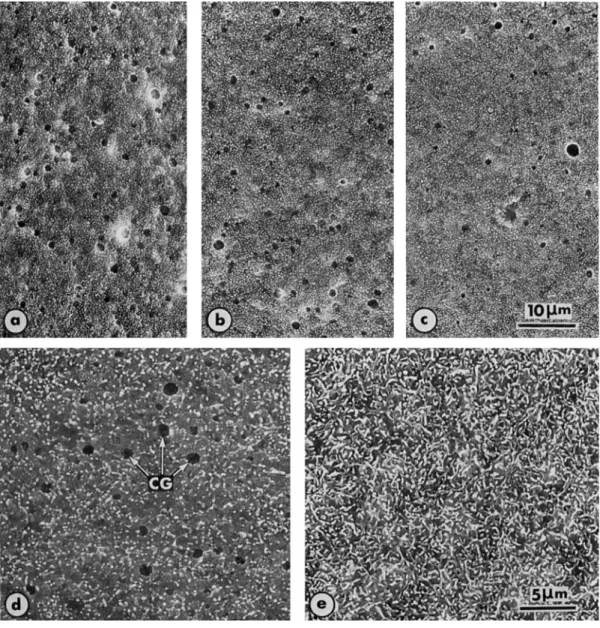
Fig. 4. a, b and c: Egg of Rana esculenta fixed 3 seconds after electrical stimulus, showing areas separated from each other by about 60" (with respect to the egg center), that is about 120" from a to c, b located near the animal pole. The regions where cortical granules simultaneously burst out represent a surface area much more extensive than upon pricking or sperm activation, therefore suggesting that electrical stimulus triggers the cortical reaction in several distinct sites in the egg cortex.
d, e: Eggs of Rana temporaria activated by the calcium ionophore A23187 (final concentration. 2 x lO-'M), at the time of exocytosis of the cortical granules (CG) in d, and after elongation of the microvilli in e. 2600 x
.
30 M. CHARBONNEAU AND B. PICHERAL
cortical granules burst out in distinct sites of the cortex (three or four visible sites), starting the cortical reaction, which then spreads all over the egg surface. This phenomenon is best seen
in vivo. Observations on electrically activated eggs examined by scanning microscopy suggested that the portion of the egg surface on which open granules were seen at a given time was much broader on these treatments than after pricking or fertilization (Figures 4a, b, c).
2. 2-1.
Microvillar elongation accompanying cortical exocytosis
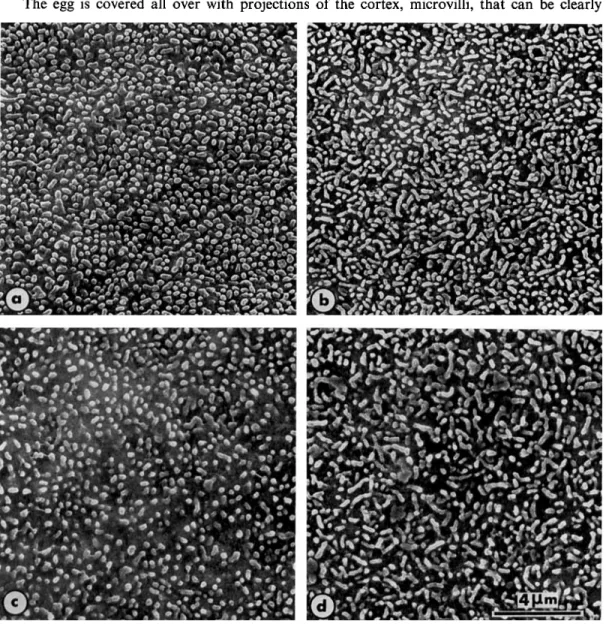
The egg is covered all over with projections of the cortex, microvilli, that can be clearly
Animal vegetal polarity in microvilli in the unfertilized egg
Fig. 5 . Scanning micrographs showing the surface of unfertilized mature eggs. The vegetal hemisphere (a) and animal hemisphere (b) of the same egg of Rana esculenta. The vegetal hemisphere (c) and animal hemisphere (d) in Rana ridibunda. Microvilli are longer in the animal hemisphere than in the vegetal one, another feature of cell polarity in the anuran egg. 5000 x
.
EARLY EVENTS IN ANURAN FERTILIZATION 31 seen in scanning electron micrographs. In mature unfertilized eggs of Rana temporaria, Rana
esculenta and Rana ridibunda, the microvilli are longer in the animal hemisphere than in the vege- tal one (Figure 5). In the animal hemisphere, most of the microvilli appear as ridges, but this appearance could be produced by bending of long finger-shaped microvilli along their axis (Figure 5b and d). In contrast, most of the microvilli in the vegetal hemisphere are short (Figure 5a and c). A very similar pattern has been observed in Rana pipiens (4). In Rana
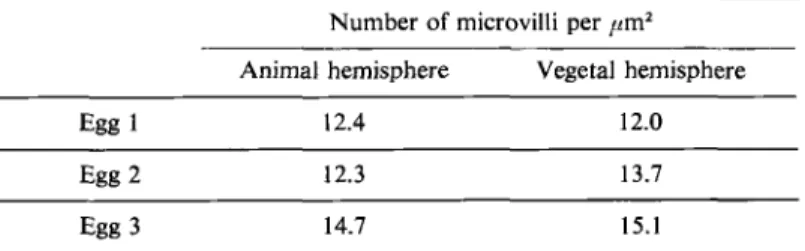
temporaria, Rana esculenta and Rana ridibunda, the density of microvilli is similar in the two hemispheres and very constant from one egg to another (Table 2).
Table 2. Density of microvilli in animal and vegetal hemispheres of unfertilized mature eggs of Runa ridibundu. Measurements were made from scanning micrographs and concern real areas of 20 ,urn*.
Number of microvilli per pmZ Animal hemisphere Vegetal hemisphere
Egg 1 12.4 12.0
Egg 2 12.3 13.7
Egg 3 14.7 15.1
2-2. Changes in length of microvilli after ,fertilization or activation
Just after fertilization it is possible to see all the steps in cortical exocytosis in the same egg (Figure 2). The front of the cortical wave consists of a well marked region with cortical granules
opening outside. These are easily seen between the microvilli and looking like large “craters” (see also Figure 3a). Between this region and the site of sperm entry, as well as just around the sperm, the microvilli become considerably elongated, and the “craters” are no longer visible, the membrane of the cortical granules having fused with the plasma membrane. In some in- termediate steps, remnants of the cortical “craters” can be seen beneath the elongated microvilli, suggesting that the elongation process is very rapid (see also Figure 3b, c, d). In the other direction, beyond the front of the cortical wave, the cortical granules have not yet been extruded and the microvilli are much shorter than in the region reached by the cortical reaction. The complete sequence of the sperm penetration process and of the formation of the fertilization body has been described previously (5).
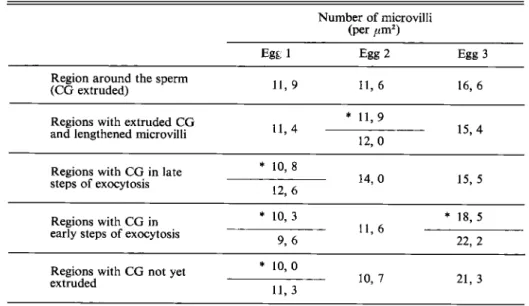
The density of microvilli remains constant just after fertilization in both hemispheres (Table 3), suggesting that increase in area of the membrane surface results only from lengthening
of the microvilli, not from increase in their number. Interestingly, it was no longer possible to recognize differences in length or appearance of microvilli in the two hemispheres (Figure 6 ) ,
although the animal hemisphere eventually shows more ridges than the vegetal hemisphere (Figure 7a and b).
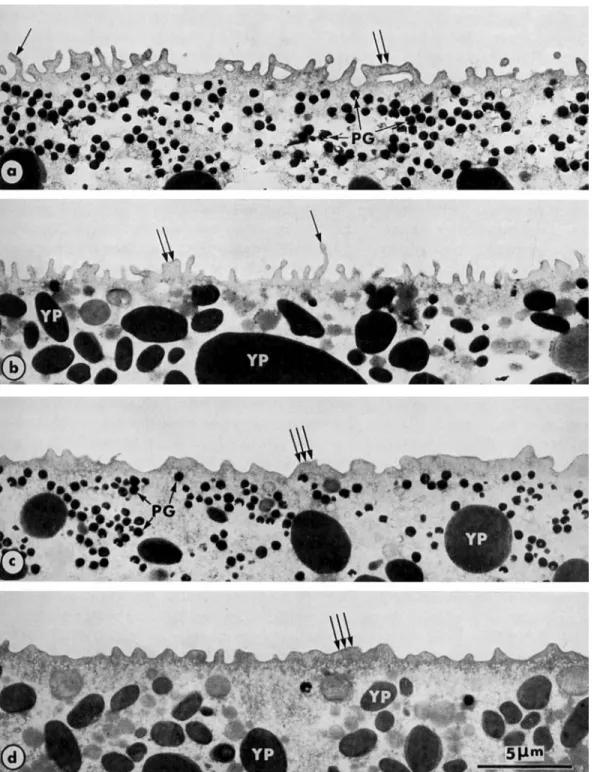
The considerable lengthening of microvilli following the cortical reaction is also very apparent in ultrathin sections through the cortex of fertilized eggs (Figure 3e and f, Figure 7a and b). Thirty min after fertilization, the egg cortex thickens and finger-like microvilli are replaced by short, stubby protrusions (Figure 7c and d).
32 M. CHARBONNEAU AND B. PICHERAL
Table 3. Density of microvilli in several different areas of fertilized eggs of Rana ridibunda 5 to 10 min after insemination. Procedures were as for Table 2.
Number of microvilli (per ,m2)
11, 9 11, 6 16, 6
Region around the sperm (CG extruded)
Regions with extruded CG
and lengthened microvilli 11,4
*
11,9 12, 015, 4
Regions with CG in late steps of exocytosis * 10,8 12, 6 14. 0 15, 5 * 10,3 * 1 8 , 5 11, 6 Regions with CG in
early steps of exocytosis 9, 6
22, 2 * 10,o
1 1 , 3 10, 7 21, 3
Regions with CG not yet extruded
* Two areas of the characterized region were examined on the same egg. CG: cortical granules.
Fig. 6. After the cortical reaction
the microvilli elongate becoming the same size in the vegetal hemisphere (a) and the animal hemisphere (b). (Cf Fig. 5 . ) 5 0 0 0 ~ .
Surface of fertilized eggs of Rana ridibundu 5 min after insemination.
3 . A ‘Smoothing” wave follows the wave of exocytosis
A second wave starts around the pricking site in the animal hemisphere, 40-60 sec after
EARLY EVENTS I N ANURAN FERTILIZATION 33
Fig. 7. Transformation of the cortex in fertilized eggs of Runu esculentu, 10 min (a, b) and 30 min (c, d) after insemination, in the animal hemisphere (a and c) and the vegetal hemisphere (b and d).
The microvilli, which are short in the unfertilized egg (see Figure l), become elongated after extrusion of cortical granules triggered by fertilizing sperm. The lengthening is notable (single arrows). Sometimes, the microvilli look like ridges (double arrows). Thirty min after fertilization, the egg surface is less unfolded and microvilli
34 M. CHARBONNEAU AND B. PICHERAL
of progressive re-smoothing of the surface, contrasting with the previous rough appearance, described above. The rate of this smoothing wave appears to be fairly similar to that of the exocytotic wave, for it reaches the region near the equator (always referring to the 150” angle), 70 to 90 seconds after pricking. In the meantime, elevation of the vitelline envelope begins 50- 70 seconds after activation, also from the pricking site. The vitelline envelope is completely lifted over the entire surface of the egg 80-100 seconds after pricking. We have interpreted the appearance of smoothing of the egg surface as resulting from lengthening of the microvilli, following the cortical reaction. Evidence for this interpretation is suggested by SEM obser- vations (see Figs. 2 and 3d), where the elongation of the villi is seen to follow immediately after cortical exocytosis. The observed smoothing of the egg surface would be due to the disappear- ance of the “craters” left by the open granules, their membrane being covered by, or integrated into, the microvilli. The occurrence of this wave is also accompanied by change in intensity of small spots of diffracted light at the surface of the egg. Since these spots are also observed on eggs from which the cortex has been peeled, we believe that they account for the movements of the pigment granules generated by Brownian movements. Therefore, the changes in the intensity of the diffracted light spots observed at the time when the smoothing wave is in progress might be due to the fact that the pigment granules are driven deeper into the cytoplasm as a result of the thickening of the cortical zone described above (see Figures 3e, f and 7).
4. Attempts to inhibit cortical exocytosis
We tested the effects of several substances known to delay, inhibit or shunt the cortical reaction in sea urchins. However, the following agents or conditions did not produce any similar effect in Rana temporaria: 10 or 100 mM NH,Cl; pH 9.4; 2 % urethane; 0.05
%
nicotine; 10 mM procaine; 10 or 50 mM soybean trypsin inhibitor; 0.2 M phosphate buffer.5 . Activation in Ca++-free medium
In Rana temporaria, mature coelomic eggs immersed for 5-10 min in Ca++-free 1/10 OR, containing EGTA 10 mM become unresponsive to electrical stimulation or pricking. After application of the stimulus, the eggs became partially covered with a whitish material presumably, as a result of precipitation of the contents of cortical granules that were only partially extruded. Subsequent applications of the same kind of stimulus resulted in the appearance of further precipitate in other areas of the egg, and eventually the egg lysed. A nonspecific cytotoxic effect of the EGTA at the concentration used can be ruled out, since mature dejellied eggs of
Xenopus laevis immersed for 10 min in Ca++-free 1/10 OR, containing 10 mM EGTA are normally activated by 5 x M ionophore A23187 (CHARBONNBAU, unpublished data).
DISCUSSION
In unfertilized eggs of Rana temporaria, Rana esculenta and Rana ridibunda, the microvilli are longer in the animal hemisphere than in the vegetal hemisphere, as also reported for
Rana pipiens (4). Just after the cortical reaction, the microvilli of the two hemispheres become similar. In the same eggs the densities of microvilli in the two hemispheres are equal, and the same before and after fertilization, suggesting that increase in surface area of the membrane is achieved only by lengthening of pre-existing microvilli, and not by formation
EARLY EVENTS IN ANURAN FERTILIZATION 35 of microvilli. This situation is quite different from that in Xenopus, where the density of micro- villi is higher in the animal hemisphere than in the vegetal hemisphere of unactivated mature eggs and ionophore A23187 induces an increase in the density of microvilli in the animal hemis- phere and a decrease in that in the vegetal hemisphere (11).
Another feature distinguishing the two hemispheres in anuran eggs is that sperm entry is restricted to the animal hemisphere, and the plasma membrane in the vegetal hemisphere appears not to be able to bind sperm (12). The animal and vegetal cortices also differ in their capacity to respond to activating stimuli (13, 14) and in the distribution of junctions between the plasma membrane and endoplasmic reticulum (GARDINER and GREY, unpublished data).
The exocytotic process of cortical granules in Rana temporaria and Rana esculenta is similar to that described in Rana pipiens (15, 13) and in Xenopus laevis (9, 16, 17). In mature eggs of
Rana temporaria external calcium is required for activation by pricking or electrical stimulus, at least for the cortical reaction, as reported for Xenopus laevis (17). Furthermore, the appli- cation of the calcium ionophore A23187 (18, 19, 20) and calcium iontophoresis (14) indicate that triggering of the cortical reaction, as well as of all the activation reactions of the anuran egg, depends directly on increase in intracellular calcium. This together with the fact that calcium in the external medium is necessary for activation, suggests that increased in intracel- lular calcium is, in part, due to calcium influx, or alternatively that a calcium influx may trigger calcium release from intracellular stores. Increase in intracellular calcium is also an early event during fertilization of egg of the sea urchin (21, 22) and the fish Oryzias (23, 24).
In Rana temporaria, the latent period of the cortical reaction was found to be 4-6 seconds after electrical activation and 6-10 seconds after pricking the animal hemisphere at 18-20°C. These findings agree well with those reported by KAS’YANOV et al. (25) in the same species, that is 8.7 seconds in the animal hemisphere and 12.5 seconds in the vegetal one, after pricking at 15°C. In Rana temporaria, we found that the cortical reaction was propagated to an angle of 150” over the animal hemisphere in 30-50 seconds. This rate of 40 to 65 pm/s is higher than those reported in the literature; i.e., 20-30 pm/s in Rana pipiens (15), 12-24 pm/s in Rana temporaria at 15°C (25); 10 pm/s in Xenopus laevisat 21°C (26) and 26 pm/s in Oryzias latipes (27). How- ever, our value is only for the animal hemisphere of the egg and it has been reported that the exocytosis wave slows down considerably when it reaches the vegetal hemisphere in Rana
pipiens (1 3) and Oryzias latipes (24).
Electrical stimulus and the ionophore A23187, are similar in that they both induce increase in intracellular calcium simultaneously in distinct sites of the cortex. This characteristic action of the ionophore A23187 has already been described with respect to activation of medaka eggs (24). Electrical stimulus may cause transient “breakdown” of the plasma membrane, weakening it in several places and this allowing calcium to enter the egg and trigger activation. These two artificial activators differ from pricking and sperm activation, which trigger the cortical reaction from a precise site, as demonstrated by scanning microphotographs. Activation by pricking may result from leakage of Ca++ into the wound, as suggested for Xenopus (17), but nothing is known about the mechanism by which the sperm acts in inducing activation reactions. After both pricking and activation by sperm, elongation of the microvilli was observed immediately after the exocytotic wave. On in vivo examination of the surface of pricked eggs we observed a wave of re-smoothing of the surface, which in fact corresponded to the pheno- menon of microvilli lengthening; at a given site of the cortex, the smoothing wave follows the
36 M. CHARBONNEAU AND B. PICHERAL
exocytotic wave by 40-60 seconds. So the elongation of the microvilli is not actually so rapid as thought. Alternatively, it is possible that the microvilli become elongated before this second wave is seen to start from the pricking site. In this case, the wave of smoothing might be the result of the beginning of thickening of the egg cortex, which would cause the pigment granules to be displaced away from the plasma membrane, that would affect the pattern of diffracted light observed a t the surface of the egg, and might result from Brownian movements of the pigment granules. The observation that the rates of the two waves (exocytotic and smoothing) are fairly similar and constant, at least in the animal hemisphere suggests that the mechanisms involved in the exocytotic reaction and microvilli elongation are perfectly Synchronized and that the kinetics of these early events are identical in all sites of the egg cortex. Elongation of the microvilli following insemination has also been observed in Runa pipiens (13) and sea urchins (28, 29, 30, 31, 32 33). However, in some sea urchins, various chemical treatments cause lengthening of the microvilli in the absence of a cortical reaction (34, 35). Consequently, in frog and sea urchin eggs, the possibility of new membrane synthesis in addition to integration of cortical granule membranes into the egg plasma membrane cannot yet be excluded.
The present data also provide essential basic information for the electrophysiological studies recently carried out on anurans and urodeles (36). One of our main interests is in mechanisms preventing polyspermy. Our basic model in amphibians consists of a comparison of four situations : physiological polyspermy of urodele eggs, normal monospermy of mature anuran eggs, artificially-induced polyspermy of mature anuran eggs and normal polypermy of immature anuran oocytes. The present data provide information about gamete interactions and the time course of the cortical reaction and its occurrence with respect to sperm penetration. However,
these microscope observations are difficult to correlate chronologially with electrical phenomena. So further studies are required on gamete interactions at a molecular level, both in monospermic and polyspermic anuran eggs to define the occurrence of the so-called fast and slow blocks to polysermy (37, 9, 38, 39) with respect to the actual binding and fusion of the sperm(s) with the egg. One possible way to obtain information on this problem is t o study gamete interactions by measurements of capacitance change and voltage noise.
We are greatly indebted to Dr. R. D. Grey for valuable discussion and for help in preparing this manuscript. We also thank Mr. E. Lelannic for help in scanning electron microscopy and Ms. F. de Sallier-Dupin for typing the manuscript.
REFERENCES 1. EPEL, D., 1977. Scient. Amer. 43, 128-138.
2. SCHUEL H., 1978. Gamete Res., 1, 299-382.
3. SHAPIRO, B. M., SCHACKMANN, R. W. and GABEL, C. A., 1981.
4. ELINSON, R. P., 1980. In: “The Cell Surface: mediator of developmental processes”. S. Subtelny and N. K. Wessells, eds., Academic Press, N. Y., pp. 217-234.
5. PICHERAL, B. and CHARBONNEAU, M., 1982. J. Ultrastruct. Res., 78, (in press). 6. PICHERAL, B., 1977a. J. Ultrastruct. Res., 60, 106-120.
7. PICHERAL, B., 1977b. J. Ultrastruct. Res., 60, 181-202.
8. WALLACE, R. A., JARED, D. W. and SEGA, M. W., 1973. J. Exp. Zool., 184, 321-334. 9. GREY, R. D., WOLF, D. P. and HEDRICK, J. L., 1974. Develop. Biol., 36, 44-61. 10. CAMPANELLA, D. and ANDREUCCETTI, P., 1977. Develop. Biol., 56, 1-10.
EARLY EVENTS IN ANURAN FERTILIZATION 37
11. EZZELL, R. M. and BROTHERS, A. J., 1980. J. Cell Biol., 87, 137a. 12. ELINSON, R. P., 1975. Develop. Biol., 47, 257-268.
13. GOLDENBERG, M. and ELINSON, R. P., 1980. Develop. Growth and Differ., 22, 345-356. 14. CROSS, N. L., 1981. Develop. Biol., 85, 380-385.
15. KEMP, N. E. and ISTOCK, N. L., 1967. J. Cell Biol., 34, 111-122. 16. WOLF, D. P., 1974a. Develop. Biol., 36 62-71.
17. WOLF, D. P., 1974b. Develop. Biol., 40, 102-115.
18. STEINHARDT, R. A., EPEL, D., CARROLL, E. J. and YANAGIMACHI, R., 1974. Nature, 252,4143. 19. BELANGER, A. and SCHUETZ, A. W., 1975.
20. SCHLICHTER, L. C. and ELINSON, R. P., 1981.
21. STEINHARDT, R. A., ZUCKER, R. and SCHATTEN, G., 1977. Develop. Biol., 58, 185-196. 22. ZUCKER, R. S., STEINHARDT, R. A. and WINKLER, M. M., 1978.
23. RIDGWAY, E. B., GILKEY, J. C. and JAFFE, L. F., 1977. Proc. Nat. Acad. Sci. USA, 74,623-627. 24. GILKEY, J. C., JAFFE, L. I., RIDGWAY, E. B. and REYNOLDS, G. T., 1978. J. Cell Biol., 76,448-466. 25. KAS'YANOV, V. L., SVYATOGOR, G. P. and DROZDOV, A. L., 1971. Ontogenez, 2, 507-511. 26. HARA, K. and TYDEMAN, P., 1979. Wilhelm Roux's Archiv., 186, 91-94.
27. YAMAMOTO, T., 1956. Exp. Cell Res., 10,387-393.
28. EDDY, E. M. and SHAPIRO, B. M., 1976. J. Cell Biol., 71, 3548. 29. SCHROEDER, T. E., 1978. Develop. Biol., 64, 342-346.
30. SCHROEDER, T. E., 1979. Develop. Biol., 70, 306-326. 31. KIDD, P. and MAZIA, D., 1980. J. Ultrastruct. Res., 70, 58-65. 32. CHANDLER, D. E. and HEUSER, J., 1979. J. Cell Biol., 83, 91-108. 33. CHANDLER, D. E. and HEUSER, J., 1981. Develop. Biol., 82, 393-400.
34. MAZIA, D., SCHATTEN, G. and STEINHARDT, R. A., 1975. Proc. Nat. Acad. Sci., USA, 72,4469-4477. 35. SPIEGEL, E. and SPIEGEL, M., 1977. Exp. Cell Res., 109, 462-465.
36. CHARBONNEAU, M., MOREAU, M., PICHERAL, B., VILAIN, J. P. and GUERRIER, P., 1983. Develop. Biol. (in press).
37. CROSS, N. L. and ELINSON, R. P., 1980. Develop. Biol., 75, 187-198. 38. GREY, R. D., WORKING, P. F. and HEDRICK, J. L., 1976.
39. GREY, R. D., BASTIANI, M. J., WEBB, D. J. and SCHERTEL, E. R., 1982. Develop. Biol., 89, 475-484. Develop. Biol., 45, 378-381.
Develop. Biol., 83, 3341.
Develop. Biol., 65, 285-295.
Develop. Biol., 54, 52-60.