Magnesium
Insertion in Vanadium Oxides:
A Structural
Study
By
Petr NóvákPaul ScherrerInstitute, ElectrochemistrySection,CH-5232Villigen PSI,Switzerland
Valéry
Shklover and ReinhardNesper
Institute of
Crystallography
andPetrography,and LaboratoryforInorganic
Chemistry,-Zentrum,CH-8092Zürich,Switzerland
Dedicatedto
Professor
Dr.Wolf
Vielstichontheoccasionof
his 70thbirthday
(Received July7, 1993;acceptedAugust 30, 1993)
Magnesium battery
/
Vanadium oxides/
Mg2
+ ion insertion/
X-ray diffraction / Electrochemistry
X-ray powderdiffraction patterns ofV205-related electrode materials
[V205,
NaV308 and Mg(V308)2] as well asofpoly(tetrafluoroethylene)-bonded composite
electrodes,containing chemically andelectrochemicallyinserted
Mg2
+,
wereanalysed
before andafter
Mg2+
insertion. Transmission electronmicroscopyandbothwavelengthand energydispersiveelectronprobe microanalysiswereappliedfor thedetermination of the
compo-sition andstructureofdifferenttypesofmicroparticlesfoundinthe electrodemass.
Röntgen-Pulverdiffraktogramme
einiger V205-verwandtenMg2
+-einlagernden
Elektro-denmaterialien[V205,NaV308und Mg(V308)2]sowie derPolytetrafluoroethylen-halti-genVerbundelektroden wurdenvorund nachderchemischen sowie elektrochemischen
Einlagerungvon
Mg2+
ausgewertet. Transmissions-ElektronenmikroskopiesowieWel-lenlängen- und energieaufgelöste
Elektronen-Mikroprobenanalyse
wurden für dieBe-stimmungvon Zusammensetzungund Struktur derinder Elektrodenmassegefundenen
verschiedenen Mikroteilchen
eingesetzt.
1.
Introduction
High
energydensity rechargeable
iontransferbatteriesarecurrently
underdevelopment
in manylaboratories.They
consistofanalkali metalnegative
electrode,
anaprotic (nonaqueous) electrolyte,
and apositive
insertionValéry Nesper
generated
at one of the electrodes is at the same time consumed at theopposite
electrode.Thus,
incontrasttocustomary
batteries there isnoneed forcomparatively large electrolyte
volumes.Due to its natural
abundance,
lowequivalent
weight,
and lowprice,
metallicmagnesium might
beanalternativetolithiumorsodium inafuture ion transferbattery [1 —4].
Unfortunately,
themagnesium
electrochemistry
at ambienttemperatures
is far frombeing
wellunderstood,
andonly
fewcompounds containing
insertedmagnesium
ions have been mentioned in the literature[1-14].
We
regard
V205
and other related vanadium oxides aspromising
electroactive materials for thepositive
insertion electrode ofasecondary
Mg-battery [3, 4].
Mg2+
ions can be inserted in the oxidechemically
orelectrochemically.
An overall scheme for the electrochemical reaction can be written asV205
+Mg2+
+2xe-«MgxV205
.Electrochemicaltestshave shown that the insertion reactionsare
fairly
reversible. The amountofMg2+
inserted inV205
depends
on the nature oftheelectrolyte,
on the ratio between the amounts ofH20
andMg2+
in the solution aswell as on the absolute amountofH20
in theelectrolyte
[3].
Thehighest
coulombiccapacities ',
about200Ah/kg,
werereached onV205
(and V6013)
in acetonitrile solutioncontaining
1 MMg(C104)2
+1 M
H20
[3, 15].
TheamountofMg2
+insertedin
V205
decreases with thedecreasing
amount ofH20
in the solution andapproaches
about 20Ah/
kg
infairly dry electrolytes [3].
In order to facilitate the
Mg2+
insertion in theinterlayer
space of theoxide,
weattempted
to increase the distance between theV205
layers
by
introducing
metal ions in theV205 lattice,
forming,
thus,
vanadium bronzes.Indeed,
several vanadium bronzes such asNaV308
andMg(V308)2
showpromising
coulombiccapacities
ofup to 110Ah/kg
inrigorously dry electrolytes [4].
Obviously,
variations in thecrystal
structureof the insertion materials influence theirability
to accommodateMg2+
or other metal ions. Thepresent
workattempts
to determine the structures of theMg2
+-inserted
V205
aswellasofthe vanadium bronzes(which
arebothpoorly
crystalline).
Efforts have been made to correlate the results of the
crystallographic
structuralanalysis
of the vanadium bronzes with their electrochemicalbehaviour,
with the aim ofgaining
information necessary for the futuredesign
andoptimisation
of thesynthesis
of bronzes withhigher
coulombiccapacity.
1 Coulombiccapacityisdefined
aschargestored in1kgof theoxide andcorresponds with theamountof
Mg2+
insertedinthe oxide.Table 1. SchemeofpreparationofMg-insertedvanadium oxides*. v2o5 powder (I)" Synthesisof bronzes[4] (H20)y (III)" Driedat: 50°C 100°C NaV308. (H20)y (IX)"
Mg(V308)2|
(H20)y (IV) 200°C Nav3ug. ,(H20)y (X)" Nav3u8. (H20)y (XI)" Electrochemical cycling^aMgxV308
(H20)y (XVI)"^aMgxV308
(H20)y+TC| (XVII)" MgxV205 ÍV)" at100°C Mg(V308)2, (H20)y (XII)· Chemical Mg2+-insertion STOPioxidised^aMgxV308.
(H20)y+TC| (XVIII)" Mg1+X. (v3o8)2. (H20)y (XIX)" Mg¿+-insertion Chemically I Electrochemically STOPreduced! cycles 20.5w:yc!es MgxV205 +TC (XIII)"· MgxV205 +TC (XIV)"(*)The chemical formulas of thesamplesmay indicate eitherpreliminary compositionorthe compositionof the mainphaseof thesample (*")Samplestudiedby X-raypowderdiffraction
2.
Experimental
2.1. Materials
AsreceivedV205powder(I, Aldrich,99.6+ %)andV205 single
crystals2
(11)wereused asstartingmaterials fortheexperiments (Table 1).Thebronzes,NaV308andMg(V3Os)2were prepared asin [4]: anaqueous solution ofNaOH, or an aqueous suspension of
MgO, was stirred witha stoichiometricamount ofV205 (I)at ~50°C overnight. The
orange-yellow colourofV205 turnedgradually tored-brown under slowprecipitation of thehydratedbronze,
NaV308(H20),,
(III)orMg(V308)2(H20)),
(IV). The latterwas filtered, washed with H20, and vacuum-dried at room temperature for several days. Afteramildgrinding,thehydrated
bronzewasfurtherdried underdynamic
vacuumat50, 100, or200°C overnight (IX-XII, Table1). Note,the formula
(H20),,
denotesanunknown,variableamountof bound water,
including
y = 0. 2 The V205single crystals,
agift
of Dr. K. Kato of the National Institute for Research in Inorganic Materials, Tsukuba,Japan, wereprepared byY. Uchida andE. Bannai fromreagentgradeV205 of 99.99% purity usingthefloating-zone
techniqueinanimagefurnace
[16].
Detailed results ofinvestigationsofMg2
+-insertedsingle crystals
Valéry Nesper
-1.5 -1.0 -0.5 0.0 0.5
Potential/ Vvs.
Ag/Ag*
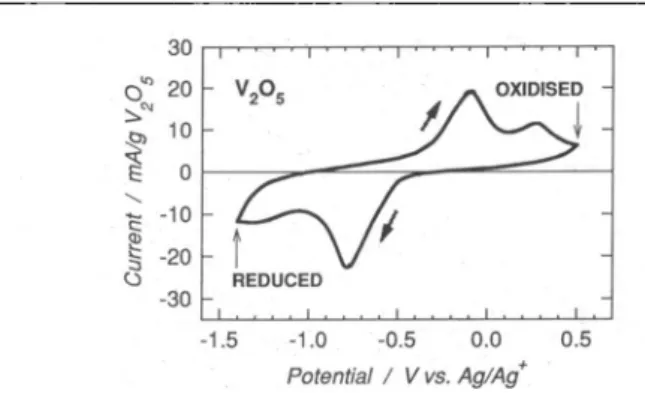
Fig. 1. Cyclic voltammogram (first cycleat0.02 mV/s) in the 1 M Mg(C104)2 + 1 M
H20/AN electrolyteofaV205electrodecontaining50wt.% teflonised carbon(VI).
2.2.
Magnesium
insertionThecompoundsV205(I, II),NaV308(IX-XI)andMg(V308)2 (XII)werescreened for
their ability to insert (excess)
Mg2+
via chemical and electrochemical tests. Chemical insertion experiments involved placingeither about 0.5g ofa powdery material or aV205single crystal (II)incontactwithexcess1 Mdibutylmagnesiumsolutioninheptane
(Aldrich)underanAr
atmosphere,
andallowing
themto reactunder occasionalstirring
atroomtemperaturefortwomonths. The materialswerethen rinsed withheptaneseveral
times, dried,and examined for structuralchangesviaX-raydiffraction.
The electrochemical insertion of
Mg2+
in V205, NaV308 and Mg(V308)2 wasinvestigated using cyclic voltammetry at very slow potential sweeprates. To give the workingelectrodes sufficient electronicconductivityand mechanicalstability,eachofthe electroactive materialswasdry-mixedwith anequalpartof TC(TeflonisedCarbon: 25
wt.% PTFE + 75 wt.%acetylene black),and the mixture was
spread
by pressingon a currentcollector. To avoid oxygen and moisturecontamination,allsucceeding manipu-lations and measurementsdescribed belowwereperformed
eitherin an Ar-filled gloveboxinwhichtheH20and02levelswerenotpermittedtoexceed 5 ppm,orinhermetically
sealedcells assembledintheglove box.
Electrochemicalexperimentswereperformedintwokinds ofelectrolytes: (i)atroom
temperature in acetonitrile (AN) solutions containing 1 M Mg(C104)2 and various
amounts(0.02M
—
2.5M) ofH20,and (ii) at80°C in room temperature molten salts.
The salt melt contained 3 wt.% MgCl2, 56 wt.% A1C13 and 41 wt.% l-ethyl-3-methylimidazoliumchloride(EMIC).Theexperimentaldetails have beendescribed else-where [3, 4], Conventionalglasscells with a
free-hanging working
electrode, aswell ascells withworkingandcounterelectrodespressed togetherwithaspringwereemployed.
(With the lattercells, better electrochemical
performance
athighersweep rates, due tobetter electrical contact of the electroactive particles to the currentcollector, was ob-served.) The
working
electrodes hadgeometrical
areas of 1.3 —1.5cm2and contained10—20 mg of electroactive material. For the sake ofcomparison, the currents were
normalised bythemassof the electroactive material.A
magnesium
counterelectrode andan
Ag/Ag+
reference electrode were used in AN based solutions. In the salt melt,metallic aluminium servedasthe counterelectrode
against
which thepotentialswerealsomeasured.
Electrochemicallytreated
samples
for X-raypowder
diffractionmeasurementswerepreparedasfollows: The
working
electrodewasvoltammetrically cycled, starting
withareduction sweepfrom theopencircuitpotential. Then,itwasstabilised in the oxidised orreduced stateatthe
appropriate
potential(Fig.
1)overnight, removed from thecell,washed indrydeoxygenatedacetonitrileovernight,and dried inthegloveboxatmosphere
for severaldays.The electrodemassscrapedfrom thecurrentcollectorwasloadedin a
glasscapillarywhichwassealedafterwards.
Electrochemical magnesium insertion experimentswere performed also with V205 single crystals. Thecrystals were contacted by pressing them against a glassy carbon
currentcollector,
using
thespring-loadedelectrochemicalcelldescribed above. Theelec-trolytewas 1 MMg(C104)2 + 0.9MH20inAN. Inatypical experiment,thepotential
was sweepedat0.5µ /s fromits opencircuit valueto —1.4 V(vs. Ag/Ag+
). Then,the single crystal wasreduced at
—
1.4V for 5weeks. During thisprocedure, the orange-yellow colour of the V205 turned dark. The reduced crystal was washed with dry
deoxygenated
AN,dried in thegloveboxatmosphere,andanalysedafterwards.2.3. Structural
investigations
X-ray powderdiffractionpatternsofsamplesI,III, V,VIandIX—XIX,sealed underan
inert atmosphere in glass capillaries, were recorded using a STOE automatic powder diffractometer(CuKaradiation,Gemonochromator,small linearpositionsensitive detec-tor, data collection inDebyemode, 150stepswithin the20 interval of5
—
80°,1000sec
perstep). Thepatternsofthe sampleswere indexed with the TREOR and LATCON programs.
Asingle crystalfour-circle Nicoletdiffractometeras wellas alight microscopewere
usedtostudytheelectrochemicallytreatedV205sampleVIII.
Transmission electronmicroscopy (TEM)studies of thesamplesXIIIand XIVwere
performed on a Philips SM 30 ST transmission electron microscope equipped with a
detector forenergydispersive X-rayspectrometry (EDX) anda STEM attachment,as
wellaswithaPhilips500scanning microscopewithanEDXattachment.
Wavelength dispersiveelectron
probe microanalysis
( )[17]wasapplied
forthedetermination of theMgcontentinV, XII,XVI andXIXusingaCameca SX50equipped
with fivespectrometers(accelerating voltage20kV,beamcurrent20µ ).
Solidstate NMRspectraof the
sample
IIIwere recorded witha BruckerAMX400spectrometer equipped with a Wide-Bore-Magnet (B0 = 9.4 Tesla) and a high-speed
double-bearing MAS-probe.
3.
Results anddiscussion
3.1.
Hydrated
sodium vanadiumbronzesIII, IX,
X and XITo avoid a
possible
reaction of metallicmagnesium
with water,fairly
dry electrolytes
arepreferred
in aMg battery.
Because ourpreliminary
electrochemicalexperiments
have shown that the coulombiccapacity
ofpure
V205
significantly
decreases with thedecreasing
amount ofwaterinthe
electrolyte
[3],
vanadium bronzes of theLiV308
type
weresynthesised
and tested for
Mg2+
insertion. Incontrast to theV205
case, the bronzesallowanelectrochemical insertion of
Mg2+
fromarigorously dry
environ-ment. As
reported
in[4],
coulombiccapacities
ofup to 110Ah/kg
werereached for the best
samples
ofNaV308.
It follows from the
comparison
ofcoulombiccapacities
ofthe bronzesValéry Nesper
40 r
Fig.2.Cyclic voltammograms(thirdcyclesat0.05mV/s)in theMgCl2/AlCl3/EMICsalt melt of
NaV308(H20)v
dried at 50° C (IX) and 200° C (XI). The electrode contained 50 wt.% teflonised carbon.I_
(a)I
20.0 40.0 60.0 2 ,degr.
Fig.
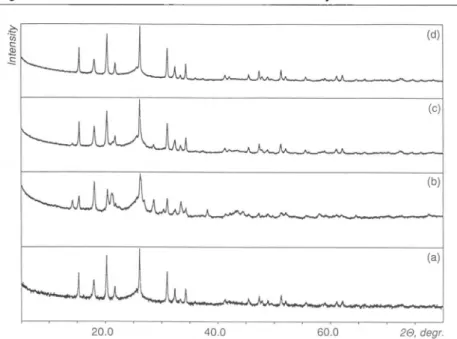
3.X-ray powderdiffraction patternsof(a)thebronzeNaV308(H20),
(III),and of theproductsof itsdrying
at(b)50° C(IX), (c) 100° C(X)and(d)200° C(XI).
electrochemically
much less active than the bronzes dried at <100°C.(There
is nosignificant
variance between the coulombiccapacities
of the50and 100°C
samples.)
This effect isillustrated inFig.
2.X-ray
diffractionpatterns
of the(poorly
crystalline)
bronzeNaVjOgtHzO),,
(III)
and theproducts
IX,
Xand XI ofdrying
of IIIat50,
100 and 200° C are similar. Thepatterns
have four common basicpeaks
(found
at 2 =11.31°,
25.84°,
28.31° and 50.73° forTable 2.Positions(20,degree) and intensities(/)ofthepeaksontheX-raydiffraction
patternsof
NaV308(H20),,
(III)and theproductsofdryingofIIIat50°C (IX),100°C(X),and200° C (XI). Ill IX X XI 20 / 20 / 20 / 20 / hkl* 7.30 13 8.13 40 8.05 25 8.03 35 100 8.90 52 8.24 20 8.23 35 11.42 44 11.31 100 11.61 100 11.78 100 11.72 40 001 12.55 88 14.64 10 200 22.88 36 23.32 16 25.84 79 25.87 85 25.82 51 25.73 82 110 27.30 13 28.31 76 28.34 67 28.30 58 28.28 87 28.52 74 111 28.88 53 30.12 20 30.15 32 -211 34.86 14 -103 38.90 19 38.68 27 -410 39.85 28 -303 40.04 25 40.29 27 40.60 26 50.73 50 50.70 98 50.66 47 50.50 100 204 60.12 30 66.34 27 -323 66.47 10 305 *
Reflections indexed with the unit cell of barnesite(a = 12.17
Â,
b = 3.602Â,
c7.78
Â,
ß = 95°2', V= 342.0Â3[18]).Table
2).
The shift tolarger
2 values in the seriesIII -> IX -> X -* XIand the
splitting (samples
X andXI)
of the firstpeak
(at
2 = 11.31° inIII),
and theapproximate
conservation of thepositions
of the three other basicpeaks
areworthy
of attention. Thesingle crystal
datapublished
forNaV308(H20)15
[18]
(existing
in nature as the mineralbarnesite)
were used for theindexing
of thepatterns
of III and IX —XI. Minorchanges
of thetwo unit cellparameters
bandc,and alarge
successive decrease of theparameter
aaccompanied
withasuccessive decreaseofthe unit cellvolume with theincreasing drying
temperature
(transition
III -» IX -» X ->XI)
wereobserved.
(Note
thatalatticeconstantvariationwithinonephase
was observed alsoforthe-phase
ofLixV205
bronzes[19].)
One may
suggest
alayered
character of thecrystal
structure of theNóvák,Valéry Nesper
lai
20.0 40.0 60.0 2 ,degr.
Fig.4.X-ray powderdiffractionpatternsof(a)thebronze
NaV308(H20)y
driedat100° C (X), and the products of(b) chemical (XVI) and (c, d) electrochemical (XVII, c and XVIII,d)Mg2+
insertion inX.andadecrease of the
interlayer separation
withwaterremoval. Thesplitting
ofthefirst basicpeak
ofX andXI mayindicateacoexistence oftwophases
with different watercontentinthesamples
Xand XI(Xa
andXb,
XIa andXlb).
Thus,
thedrying
processprobably proceeds
in distinctsteps,as, e.g.,reported
for the removal ofexcess water from thesolidgel
V205
·y
H20
[20].
A similar
abrupt change
of the unit cellparameters,
occurring during
awater removal in the course of
heating
as observed for III and IX—XI,
wasreported
earlier for the transition ofhydrated
sodium metavanadate:NaV03(H20)2
->(transition
phase)
-»/?-NaV03
[21].
The closeness of theunit cell sizes of the studied
hydrated
sodium vanadium bronzes and thecited metavanadates is
remarkable,
but a51V
solid state NMRstudy
ofthe
sample
INclearly
indicated an octahedralcoordination,
and not thetetrahedral coordination of vanadium atomscharacteristic for metavana-dates
(óiso
= -533±
10 ppm, <5 = 657 ± 10 ppm,^
=2
= -290±
10 ppm,
3
= —947 ± 10 ppm; fortheinterpretation
ofthe solid stateNMR dataon vanadium
compounds
see[22]).
Combining
the electrochemical and structuralresults,
it seems to be reasonabletosuggest
that thehydrated
bronze,
NaV308(H20)i.5,
iselec-trochemically
the most active constituent of the obtained Na-bronzes.Table 3. Positions(2<9, degree)and intensities (/)of thepeakson theX-raydiffraction
patterns ofthe products of chemical (XVI)and electrochemical (XVII, XVIII)
Mg2
+insertion in thehydratedsodium vanadium bronze(X).
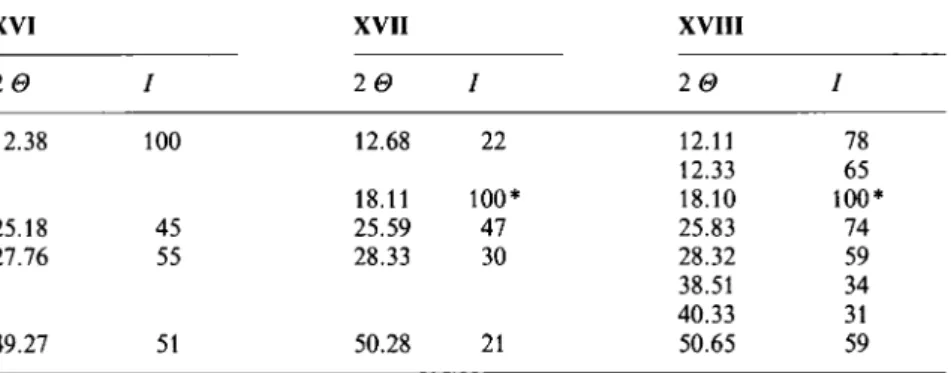
XVI XVII XVIII
2 I 2 I 20 I 12.38 100 12.68 22 12.11 78 12.33 65 18.11 100* 18.10 100* 25.18 45 25.59 47 25.83 74 27.76 55 28.33 30 28.32 59 38.51 34 40.33 31 49.27 51 50.28 21 50.65 59 * Peak
100of triclinicPTFE[23] (fromteflonisedcarbon).
100°C,
and a new structure is formed which is able to accommodatesignificantly
lessMg2
+than the bronze
NaV^^ri-jO)^.
Infraredspectro-scopic
investigations [4]
have shown thatwaterisexpulsed,
anddehydrated
NaV308
is formedduring
thedrying
above 100° C.3.2. Productsof chemical
(XVI)
and electrochemical(XVII,
XVIII)
insertion ofMg2
* inthepartially dehydrated
sodiumvanadiumbronzeXThe
patterns
of thepoorly
crystalline products
XVI—XVIII ofMg2
+insertion in X
(Fig.
4,
Table3)
can also be describedusing
the four basicpeaks,
asthepatterns
ofIIIand thedrying products
of III discussedabove,
andcanbe indexed with the unit cell of barnesite.A
layered crystal
structurecan be
proposed
for thesamples
XVI —XVIII. The size of the unit cell(Xa:
344A3,
Xb: 293Â3)
decreasesduring
both the chemical(XVI:
242Â3)
and the electrochemicalMg2+
insertion(XVII:
227A3
and XVIII: 262Â3).
Theelectrochemically
reducedproduct
XVII has the smallest value of the unit cell volume. Note that recent electrochemicalexperiments [4]
have shown thatasignificant
amount ofMg2+
ions inserted inNaV308(H20)j,
during
the first voltammetriccycle
isirreversibly
bonded in thecrystal
lattice of the bronze.
Therefore,
theelectrochemically cycled,
oxidisedsample
XVIII containedtrapped
Mg2
+ ions.The contractionof the unitcell volumeof
NaV308(H20)y
during
Mg2
+insertion isnot
surprising;
asimilar effectwasalready
observed for cation-intercalatedV205(H20)i.6 xerogels [24].
Theélectrostrictionphenomenon
can
explain
this behaviour: theintensity
oftheelectrical fieldsurrounding
Valéry Nesper (d) (c) \******
LJxJ
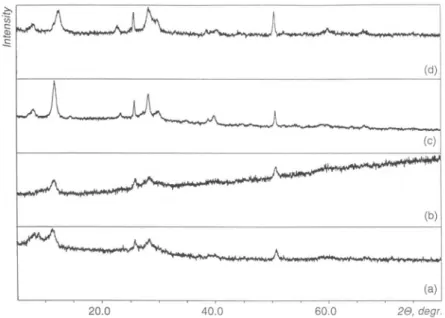
(b) (a) 20.0 40.0 60.0 2 ,degr.Fig.5.X-raypowderdiffractionpatternsof(a) V205(I), (b) Mg^^Os (V),(c)the bronze
Mg(V308)2(H20)^
driedat100°C(XII),and(d)theproduct Mg!+*( 308)2( 20),
(XIX)of chemical
Mg2+
insertionin XII.The
Mg
and Nacontents,foundby
for differentmicroparticles
in theproduct
XVI of chemicalMg2+
insertion inX,
varied in narrowintervalsof 11.7-12.2 at.% for
Mg,
and of 5.4-5.8 at.% for Na.3.3. ProductsV and XIX ofchemical
Mg2
+ insertioninV2Os (I)
andin the
Mg
bronzeIV,
XIIThe
X-ray
diffractograms
of thesamples
I, V,
XIIand XIXarecompared
inFig.
5;
thepositions
of the diffractionpeaks
aregiven
in Table 4. Thepatterns of the
crystalline sample
I,
and of theproduct
V of the chemicalMg2+
insertion in I are very similar andrepresent
V205.
TheV205
unit cellparameters
calculated forIandV,
aswellasfor thosepeaks
ofXIIandXIX,
whichcan be indexed with theV205
unitcell,
arecompared
in Table 5 with the latticeparameters
ofelectrochemically prepared Mg0 2V205 [10],
and of
MgV205
synthesised
at900°C[25].
In contrast toMg0 2V2O5,
the distribution ofpeak
intensities in V is very similarto those observed inI,
andcould indicateanessentially
smalleramountof insertedMg2+
in Vascompared
toMg0.2V2O5. Indeed,
theinvestigation
showed acon-tent of 1.0 —1.2 at.% of
Mg
insample
V.(This
is in agreement with the conclusionsuggested
in[3]
thatwatermoleculesareessential for theMg2
+ insertion reaction ofV205.
Note that adry
environment is used for theTable 4.Positions(20,degree)andintensities(/)ofthepeaksontheX-raydiffraction
patterns ofthe products V and XIX ofchemical
Mg2+
insertion in V205 (I)and Mgbronze(XII).
I V XII XIX
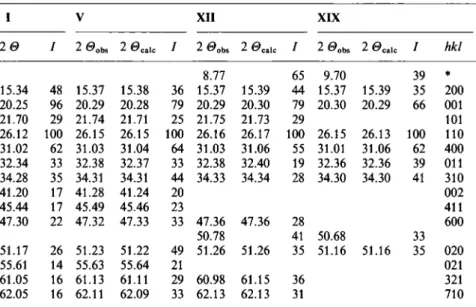
20 I 20obs 20calc / 2<9obs 2 6»calc / 20obs 2 0calc / hkl
8.77 65 9.70 39 * 15.34 48 15.37 15.38 36 15.37 15.39 44 15.37 15.39 35 200 20.25 96 20.29 20.28 79 20.29 20.30 79 20.30 20.29 66 001 21.70 29 21.74 21.71 25 21.75 21.73 29 101 26.12 100 26.15 26.15 100 26.16 26.17 100 26.15 26.13 100 110 31.02 62 31.03 31.04 64 31.03 31.06 55 31.01 31.06 62 400 32.34 33 32.38 32.37 33 32.38 32.40 19 32.36 32.36 39 011 34.28 35 34.31 34.31 44 34.33 34.34 28 34.30 34.30 41 310 41.20 17 41.28 41.24 20 002 45.44 17 45.49 45.46 23 411 47.30 22 47.32 47.33 33 47.36 47.36 28 600 50.78 41 50.68 33 51.17 26 51.23 51.22 49 51.26 51.26 35 51.16 51.16 35 020 55.61 14 55.63 55.64 21 021 61.05 16 61.13 61.11 29 60.98 61.15 36 321 62.05 16 62.11 62.09 33 62.13 62.13 31 710 * This reflection is
contrarytotheothers,verybroadand, thus,
assigned
toanother phase.Table 5.Unit cellparametersofV205 (I),V205-relatedcomponentsofV,XIIandXIX,
and vanadiumpentoxidebronzesMg0 2V2O5 [10]andMgV205 [25],
I V XII XIX Mg0.2V2O5 MgV205
a(k)
11.5204(9) 11.513(2) 11.508(1) 11.508(8) 11.42 11.019b(k)
3.5674(5) 3.5640(6) 3.5617(3) 3.5679(6) 3.552 3.696c(Â)
4.3780(4) 4.3745(8) 4.370(2) 4.372(2) 4.468 9.965K(Â3)
179.93 179.50 179.12 179.50 181.2 405.8In
sample
XII,
thestudy
showed thepresenceofmicroparticles
with
distinctly
differentMg2+
content, viz.yellow microcrystals
with 0.14at.% of
Mg,
and brownmicrocrystals
with 1.3—
1.5 at.% of
Mg.
Themicroparticles
oftheproduct
XIX of thesubsequent
chemical insertion ofMg
in XII contain 3.8—
4.0 at.% of
Mg.
Note that severalphases
related toV205,
and acoexistence of differentphases having
the sameLicontent(multiphase
domains)
werereported
forLixV205
bronzes[19].
X-ray
powder
diffractionpatterns
of thepoorly crystalline samples
XII and XIXessentially
differ from thepatterns
ofI and V. Somepeaks
ontheNovak,Valéry Nesper
Fig.6.CrystalstructureofV205 [26].
in
comparison
withIandV,
but therearealsosome"new"peaks: (i) Strong
and diffuse
peaks
at 2 = 8.77°(XII)
and 9.70°(XIX)
whichcannot beindexed with the unit cell of IorV
(the
first reflection100,
which isactually
absent in the measuredpattern
ofI,
has 2 =7.67°);
and(ii)
newsharp
peaks
at 2 = 50.78°(XII)
and 50.68°(XIX)
which areonly slightly
shifted from the
V205
020peak
at 2 = 51.23° in thepattern
of V.Moreover,
there isa hintofasmall contraction of theaseparation
of theV205
crystalline
component
in the series I -* V -» XII -» XIX. Aslight
shortening
of the aseparation
and aslight
increase of the cseparation
(corresponding
to theinterlayer separation
in the structure ofV205
[26],
Fig. 6)
is characteristic for atopotactic
insertion inV205,
see, e.g., thestructural
study
ofelectrochemically
formedMg0.2V2O5
bronze[10].
Besides the
phase
with theV205
structure, we believe that in the newphase(s)
of thesamples
XII andXIX,
agood
ordering
existsonly
in onedirection,
b'. Thelength
of this axis isslightly
increased incomparison
with the bseparation
in I and V. The value of b' is 3.59Â
inXII,
and 3.60A
in XIX(calculated
underanassumption
that the newsharp peak
inpatterns
of XII and XIX is a 020peak).
The newphase(s)
of XII and XIX aredisordered in two other directions
(i.e.
in the a'c'planes).
Note that an earlierX-ray powder
diffractionstudy
ofelectroformedLixV205
bronzesshoweda conservation of the b
separation
of theV205
structurewith an increased Li contentparallel
to morepronounced
changes
ofthe a and cseparations
[27].
3.4. Products XIII —XV of electrochemical
cycling
oftheV2Os
electrode VIA
cyclic
voltammogram
of theV205/TC
electrode VI in anacetonitrile-based
electrolyte
is shown inFig.
1. TheX-ray
diffractionpatterns
ofthe
electrochemically
cycled
electrodes XIII —XV arecompared
with thepattern
ofa newelectrode VI inFig.
7and in Table 6. Thepatterns
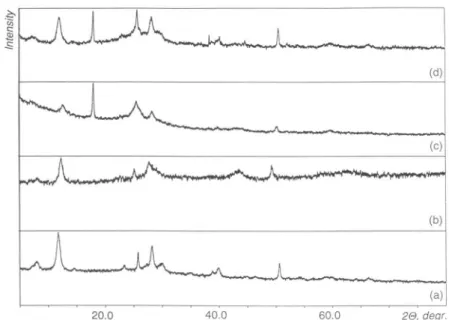
of the20.0 40.0 60.0 2 ,degr.
Fig.7.X-ray powderdiffractionpatternsof(a)theelectrodemassV2Os/TC (VI)andof theproducts of electrochemical
Mg2+
insertion: (b)XIII(reduced electrode,after2l/2
cycles), (c)XIV (reducedelectrode, after20l/2
cycles), and (d) XV (oxidised electrode,after20cycles).
represent
asuperposition
of thepattern
ofV205
(or
components
with the unit cell closetoV205,
Table7),
and the mixture ofcrystalline
PTFE and carbon black. The electrochemical reduction of theelectrode VI leads tothe appearance of some "new"
peaks
in thepatterns
of XIII and XIV(besides
ofthepeaks
oftheV205
and TCcomponents).
It can be
speculated
that the appearance of theV205
features in the reduced electrode means that a fraction of theV205
is not accessible for theelectrochemical reduction. Thereasonmight
beapoorelectricalcontact ofV205
particles
tothecurrentcollector.Thishypothesis
is consistent with the fact that in thespring-loaded
cell,
where theworking
electrode ismechanically pressed
against
thecurrentcollector,
significantly
higher
cou-lombiccapacities (up
to230Ah/kg V205)
werereached incomparison
withthe
free-hanging
electrodes(about
170Ah/kg V205).
But
essentially
differentdistributionof thepeak
intensitiesof theV205
component
insample
XIII,
incontrast to thedistributionobserved for theV205-containing
new electrodeVI,
might
indicate the chemicalcompo-sition
MgxV205
of thiscomponent.
The presence ofMg2+
in theV205
microcrystals
found in the reducedelectrode XIIIwas confirmedby
EDXanalysis.
There is nopronounced change
of the unitcellparameters
of theValéry Nesper
Table 6. Positions(2 0,degree)and intensities( )ofthepeaks ontheX-raydiffraction
patterns ofthe productsXIII —XV ofelectrochemicalcycling of the V205-containing electrodemassVI.
VI XIII* XIV* XV*
2 0 / 20obs 2 0calc / 2 0obs 20calc / 20obs 20calc / hkl
14.25 22 14.23 5 15.33 53 15.42 15.37 38 15.45 15.40 52 15.46 15.41 54 200 18.02 79** 18.17 79** 18.16 43** 18.17 31** 20.26 91 20.39 20.36 63 20.35 20.34 75 20.38 20.35 82 001 21.10 56 21.35 41 21.36 8 21.70 28 21.81 21.79 23 21.79 21.77 20 21.81 21.78 25 101 22.03 13 22.49 12 25.79 16 25.87 25.61 34 25.68 25.61 21 25.94 25.62 16 201 26.12 100 26.22 26.20 100 26.21 26.18 100 26.24 26.23 100 110 27.01 34 28.40 20 28.65 34 28.67 11 30.36 13 31.02 65 31.06 31.02 48 31.12 31.04 60 31.12 31.05 62 400 32.39 29 32.44 32.47 18 32.43 32.42 26 32.48 32.48 26 011 33.48 33.41 37 33.44 33.37 16 111 34.27 31 34.39 34.34 22 34.38 34.35 33 34.40 34.40 32 310 38.18 23 41.33 11 41.44 41.41 11 41.33 41.35 12 41.36 41.37 12 002 43.54 17 43.56 8 43.76 12 44.44 17 44.43 5 45.42 13 45.46 45.52 10 45.50 45.54 13 45.57 45.59 13 411 47.40 20 47.29 12 47.42 22 47.44 20 600 47.93 11 47.95 47.97 11 47.91 47.94 10 302 48.98 10 49.00 48.99 15 012 51.27 22 51.35 51.34 18 51.28 51.27 22 51.31 51.39 22 002 52.09 13 52.14 52.00 19 52.10 52.09 13 601 58.05 18 59.10 11 412 61.11 12 61.19 61.18 11 61.21 61.27 11 321 62.17 14 62.19 62.20 13 62.20 62.26 13 710
* See the discussion ofnotindexed reflectionsinthetext. ** Peak ofPTFE(fromteflonisedcarbon).
indicates that
V205
can beregarded
as a three-dimensional frameworkhost,
rather thanatwo-dimensional one(as already suggested
in studies ofTable 7. Unit cellparametersofV205-relatedcomponentsof the electrodemassVI and
theproductsXIII —XVof its electrochemicalcycling.
VI XIII XIV XV
a(Â)
11.533(6) 11.520(7) 11.495(3) 11.489(3)è
(A)
3.562(1) 3.556(2) 3.561(1) 3.553(1)c(Â)
4.376(4) 4.357(2) 4.363(2) 4.3609(9)K(Â3)
179.93 178.50 178.62 178.02The unitcell
parameters
ofchemically
(V)
andelectrochemically
(XIII)
formedMgxV205
arevery close(cf.
Tables 5 and7).
Thus,
theproducts
ofMg2+
insertion inV205
might
be similar in bothcases.The new
peaks appearing
in theX-ray
diffractogram
of theV205/TC
electrode reduced at —1.4 V(vs.
Ag/Ag+)
after2l¡2
voltammetriccycles
(XIII)
can beseparated
into two groups:(i)
fivepeaks
presentalso in thesample
XIV reducedafter20i/2
voltammetriccycles,
and(ii) peaks
present
only
in XIII(Table 6).
Thesetwogroupsofpeaks
havetobelong
todifferentphases (e.g.
XHIa andXHIb),
because,
e.g., thestrongest
newpeak
in thepattern
of XIII(at
2 =21.10°)
is absent in thepattern
ofXIV.Thus,
thereduced electrode XIII may consist of five
phases:
V205
(or MgxV205),
carbonblack, PTFE,
XHIa(which
exists also inXIV),
and XHIb.The amount of the
phase
XHIb in the electrodeobviously
decreasesduring
cycling
as evidencedby
the absence ofcorresponding
peaks
in thediffractogram
of the electrode XIV reduced after2OV2
cycles.
Thisbehav-iour is consistent with the known fact that
MgxV205
exists in twophases
—
a stable orthorhombic
phase
(0
< <0.11)
andametastablephase
[6].
Note the XHIa and XHIbphases
are absent in theoxidised,
cycled
electrodeXV,
sothey
areproducts
of reversibleV205
reduction. Theindexing
of the XHIbphase
isambiguous.
One of the solutions is an orthorhombic unit cell[a
=17.69(1)
A,
b =13.003(4)
A,
c =3.360(2)
Â,
V = 773
A3]
with oneperiod equal
to that observed inMgV205
and theunit cell volume four time
larger
as ofMgV205.
Also the unit cell of thephase
XHIacannotbeelucidated,
because theindexing
of thecorresponding
linesgives
several solutions ofcomparable
reliability.
Thus,
the electro-chemical reduction of VIseemstolead toboth(i)
Mg2+
insertion inV205
with the conservation of theV205
structure, and(ii)
formation ofnewMg,V-containig phase(s)
(XHIa
or XHIa +XHIb).
The TEM
study
of XIII confirmed the presence ofmicrocrystals
withthe unit cell closeto
V205
inelectrochemically
reducedsamples.
Therewereobtained,
besides the commonV205 diffraction,
two kinds ofdiffractionpatterns.
Theinterplanar
distances from the firstone aredx
= 9.7Â
andd2
= 25.0Â.
Thepatterns
ofthe second kind indicate asuperstructure
Fig.8. Diffractionpatternofamicrocrystalof the reduced electrodeXIII.
beam
direction;
recording
of diffractionpatterns
suitable for the determi-nation of theinterplanar
distance for the "third direction"wasthereforenotpossible.
Wedidnotsucceed inperforming
areliableanalysis confirming
thepresenceof
Mg
within thegrains exhibiting
thesuperstructure.
The electrode XIV consists of four
phases:
one with the unit cell ofV205,
carbonblack, PTFE,
and a newphase structurally
relatedtoV205.
The
change
of thestructureof theproducts
of electrochemicalcycling
with the number ofcycles
(note
the observed differencebetween the electrodesXIII and
XIV)
was discussed also in[19]
forelectrochemically
insertedLixV205.
3.5. Electrochemical
Mg2+
insertionintheV2Os
single crystal
IIThe
V205
single crystals (II)
lost thesingle crystallinity
after theelectro-chemical
Mg2
+insertion.An
optical micrograph
ofthecrystal
VIIIshowedbroad lines and shadows of the dark
MgxV205
within theorange-yellow
V205
crystal.
Obviously,
the insertion process is very nonuniform. TheEDX
analysis
did not indicate the presence ofMg.
Thus,
the amount ofMg2
+ inserted in thesingle
crystals
wasrather low. Thechemical insertionof
Mg2+
in theV205
single crystal
resulted in noapparent
colourchange.
4.
Conclusions
The chemical and the electrochemical
Mg2+
insertion inV205
seems toV205
inPTFE-bonded electrodes ismorecomplex
and rather nonuniform.Atleasttwonew
phases (both possibly
Mg^V205
phases)
areformedduring
the reversible reduction ofV205
inwetacetonitrilecontaining Mg(C104)2.
The presence ofone ofthe newphases
in the electrode decreasesduring
electrochemicalcycling.
Upon
reoxidation,
theoriginal
V205
structureof the
MgxV205 phases
appears to be restored. In thehydrated
sodium vanadiumbronzes,
NaV308(H20)y,
twolayered phases
withdifferentwater contentcoexist. Thephase
NaV308(H20)i.5
iselectrochemically
themostactive one,which allows
Mg2+
insertion fromrigorously
dry electrolytes.
Dehydration occurring
above 100°C causes a decrease of theinterlayer
separations
aswell as a decrease in the coulombiccapacity
of thebronze,
sothepreparation
anddrying
of the bronzes below 100°C is recommended.Acknowledgements
We thank Dr. O.
Haas, PSI,
formanystimulating
discussions. Wearealso indebted to Dr. K. Kato of the National Institute ofInorganic
Materials,
Tsukuba,
Japan,
for the donation of theV205
single
crystals,
R.Wessicken,
,
for STEM measurements, H.Meyer
zuAltenschildesche, ,
for NMR measurements, F.Ried, ,
forstudy,
and W.Scheifele,
PSI,
for technical assistance. This work wassupported by
the Swiss "Bundesamt fürEnergiewirtschaft"
(Grant
No.EF-PROCC(91)018).
References
1. T. D.Gregory,R.J. Hoffman andR.C.Winterton,J.Electrochem.Soc. 137(1990) 775.
2. R. J. Hoffman,R. C.Winterton andT. D.Gregory,USPat.4,894,302(1990).
3. P. NovakandJ.Desilvestro,J. Electrochem. Soc. 140(1993)140. 4. P.Novak,W.Scheifele and O. Haas,Molten SaltForum 1
-2(1993/94)389. 5. J.Galyand M.Pouchard,Bull.Soc. Chim. France(1967)261.
6. J. Galy, M. Pouchard, A. Casalot and P. Hagenmuller, Bull. Soc. Fr. Mineral. Cristallogr. 90(1967)544.
7. R. Schöllhorn and H.Meyer, Mat. Res. Bull. 9(1974)1237. 8. R. Schöllhorn and W.Schmucker, .Naturforsch.30b(1975)975. 9. A. Lerf andR.Schöllhorn,Inorg.Chem. 16(1977)2950.
10. J. P. Pereira-Ramos,R. Messina and J. Perichon,J. Electroanal. Chem. 218(1987) 241.
11. P.G.Bruce,F.Krok,J.Nowinski,V. C. Gibson and K.Tavakkoli,J. Mater. Chem. 1(1991)705.
12. P.
Lightfoot,
F. Krok,J. L. Nowinski and P. G. Bruce, J. Mater. Chem.2 (1992) 139.13. P. G. Bruce, F. Krok, P.
Lightfoot,
J. L. Nowinski andV. C. Gibson, Solid State Ionics53-56(1992)351.14. R. E.Dueber,J. M.Fleetwood andP.G.Dickens,Solid State Ionics50(1992)329. 15. F.Joho,P.Novák,O. Haas and R.Nesper,Chimia 47(1993)288.
Nesper
16. K.Rato, personalcommunication,June 1992.
17. R.Gutmann,J.
Hulliger
andE.Reusser,J.Cryst.Growth 126(1993)578. 18. A. D.Weeks, D. R.Ross and R. F.Marvin,Am. Mineral.48(1963) 1187.19. J. M.Cocciantelli,J. P.Doumerc,M.Pouchard.M.Brousselyand J.Labat,J. Power Sources 34(1991) 103.
20. J. A. Jacobson: "Intercalation ReactionsofLayered Compounds", in Solid Stale
Chemistry. Compounds (A. K.Cheetham andP. Day, Eds.),p. 217.Clarendon Press,
Oxford,(1992).
21. K.Kato and E.Takayama,ActaCrystallogr. B40(1984) 102.
22. O. P. Lapina.V. M.Mastikhin,A. A.Shubin,V. N.Krasilnikov and K.I.Zamaraev. Prog.Nucl. Magn. Reson.Spectrosc.24(1992)457.
23. H.G.Killian,Kolloid-Z. 185(1962)13.
24. N.Baffier,L.Znaidi and J.-C.Badot,J.Chem. Soc.
Faraday
Trans. 86(1990)2623. 25. J. C. Bouloux,I. Milosevic andJ.Galy,J.Solid State Chem. 16(1976)393. 26. H. G. Bachmann, F. R.Ahmedand W. H. Barnes,Z.Kristallogr. 115(1961) 110. 27. S. Hub,A.Tranchantand R.Messina, Electrochim.Acta 33(1988)997.28. D. W. Murphy,P. A. Christian,F. J. DiSalvoand J.V.Waszczak, Inorg.Chem.18
![Table 1. Scheme of preparation of Mg-inserted vanadium oxides*. v2o5 powder (I)" Synthesis of bronzes [4] (H20)y (III)" Dried at: 50°C 100°C NaV308](https://thumb-eu.123doks.com/thumbv2/123doknet/14925916.663941/3.627.71.548.109.529/table-scheme-preparation-inserted-vanadium-oxides-synthesis-bronzes.webp)

![Fig. 6. Crystal structure of V205 [26].](https://thumb-eu.123doks.com/thumbv2/123doknet/14925916.663941/12.627.92.574.78.293/fig-crystal-structure-of-v.webp)