EGF- and CPA-induced mitogenic stimuli are differentially
down-regulated by TGF-
β1 in cultured rat hepatocytes
Reto Fasciati
1and Peter Maier
2be the crucial step in clonal selection and promotion of
preneoplastic cells towards cancer. More likely, endogenous
Institute of Toxicology, Swiss Federal Institute of Technology and
and exogenous mitogens affect different cell populations
University of Zu¨rich, CH-8603 Schwerzenbach, Switzerland
within a tissue, populations which differ in their response to
1Present address: Amplimmun AG, Via Gravulaina, Ch-7523 Madulain,
negative growth stimuli or in their vulnerability to acquiring
Switzerland
mutations. In rodents, the liver was found to be particularly
2To whom correspondence should be addressed
sensitive towards non-genotoxic carcinogens (5). Therefore,
Down-regulation of the mitogenic activity of the rodent liver
we addressed the question whether cultured rat hepatocytes,
carcinogen cyproterone acetate (CPA) and of epidermal
stimulated by an exogenous or an endogenous mitogen, respond
growth factor (EGF) were compared in cultured rat
differentially to negative growth control initiated by
trans-hepatocytes. Both hepatomitogens produce an increase in
forming growth factor
β
1 (TGF-
β
1*). This was carried out
the expression of proliferating cell nuclear antigen (PCNA)
with two rodent liver mitogens, the carcinogenic and synthetic
and in [
3H]thymidine incorporation in a dose-dependent
steroid cyproterone acetate (CPA) and the endogenous peptide
manner. In combination, the two mitogens induced an
epidermal growth factor (EGF).
additive mitogenic response. Concomitant exposure to the
CPA is found in oral anti-acnegenic contraceptives and is
growth inhibitory cytokine transforming growth factor
β1
used to treat prostate carcinoma, male hypersexualism and
(TGF-
β1) resulted in a differential dose-dependent down-
signs of androgenization in women. In a rat liver foci bioassay,
regulation of PCNA-expressing cells. The correspond-
CPA induced altered foci in female rats (6) but not in male
ing down-regulation of CPA-induced PCNA expression
rats (7). At lower doses CPA increased benign liver proliferation
required a 3- to 5-fold higher TGF-
β1 concentration than
(8) and at higher levels hepatic tumours in rats and mice, both
for EGF-induced expression. In contrast, CPA-exposed
males and females (9). The mitogenic activity of CPA in rat
hepatocytes become vulnerable to and EGF-exposed cells
liver was demonstrated in vivo (10) and in vitro (11), whereas
protected against the apoptosis-inducing activity of TGF-
in human hepatocytes the mitotic activity was found only in
β1 (.0.1 ng/ml). Under culture conditions that mimicked
the presence of growth factors (12). The synthetic steroid
a pericentral-equivalent microenvironment (low oxygen
exerted no mutagenic activity in tests with bacteria and
tension, low glucagon concentration), PCNA expression
mammalian cells (13,14), but recent findings demonstrated an
was 3-fold lower and CPA-specific resistance was no longer
induction of DNA repair synthesis (15,16). Reports about its
detectable. It is concluded that EGF and CPA induce their
clastogenic activity in hepatocytes were inconclusive (17).
growth stimuli preferentially in the periportal area of the
Highly persistent DNA adducts of the metabolite 3
α
-hydroxy-liver but in different hepatocyte sub-populations, which
cyproterone acetate were found (18,19), particularly in female
differ in their down-regulation of premitotic events by
rat liver (20). Together, the data imply that CPA is a complete
TGF-
β1. At low TGF-β1 concentrations, EGF-stimulated
carcinogen in female rodent liver at high doses, whereas in
cells shift back into a resting cell cycle phase, whereas
male liver it is predominantly the mitogenic stimulus that
CPA-treated hepatocytes are eliminated by apoptosis at
contributes to tumourigenesis.
higher TGF-
β1 concentrations.
Negative growth control in hepatocytes can be initiated
by low levels of TGF-
β
1 (21). This cytokine is a potent
inhibitor of cell growth in parenchymal liver cells as well as
Introduction
in rat and human hepatocytes (22,23). In addition, TGF-
β
1 is
Cell proliferation is an important promoting factor in tumour
one of the most effective endogenous growth inhibitors after
induction and progression in rodents (1,2). Chemically induced
partial hepatectomy (24). It is expressed in most liver cells,
cell cycle progression can be stimulated directly by interactions
including hepatocytes (25), and acts via paracrine and autocrine
with growth stimulating factors (3) or indirectly by regenerative
mechanisms.
growth as a consequence of chemically induced cell necrosis
In order to obtain in vitro information which adequately
(4).
represents the situation in liver, cultured hepatocytes should
Endogenously controlled high rates of cell proliferation are
be growth arrested and should preserve or re-acquire
tissue-well tolerated in many tissues, whereas exogenous chemicals
equivalent metabolic competence. This was achieved by the
that induce a mitogenic stimuli often act as non-genotoxic
use of a tissue-specific extracellular matrix (26,27), high cell
carcinogens. This suggests that mitogenesis per se cannot
densities and a serum-free culture medium supplemented with
low insulin concentrations and a protease inhibitor (28). Under
*Abbreviations: TGF-β1, transforming growth factor-β1; CPA, cyproteronethese conditions, within 2 days in culture hepatocytes express
acetate; EGF, epidermal growth factor; PCNA, proliferating cell nuclear
their liver-specific metabolic competence (29), they respond
antigen; CMF, crude membrane fraction; PBS, phosphate-buffered saline;to tissue-equivalent oxygen tensions (29), to glucoregulatory
DMSO, dimethylsulphoxide; BSA, bovine serum albumin; TdR, thymidine;hormones (29) and to the apoptotic stimuli of cytokines (30).
LDH, lactate dehydrogenase; PC cultures, pericentral-equivalent cultures; PP
scraped off in 5% trichloracetic acid and transferred to glass fibre filters in a
mitogenic stimuli of EGF and CPA are differentially
down-cell harvester. Non-precipitable DNA was removed by washing with distilled
regulated by TGF-
β
1. Three possible differences were
water. The3H radioactivity on each filter was determined in a scintillation
addressed: (i) a different dose response towards TGF-
β
1;
counter (Irga-Save scintillation cocktail; Beckmann LS-6000LL).(ii) a difference in down-regulation by apoptosis; (iii) the
Measurement of lactate dehydrogenase (LDH) release and total protein contentsensitivity of this down-regulation to factors characteristic of
LDH release was analysed spectrophotometrically (COBAS Fara
auto-different regions of the liver lobules, namely oxygen tension
analyser) using a commercially available kit (Boehringer-Mannheim,and ratio of the glucoregulatory hormones (insulin/glucagon).
Mannheim, Germany). Protein content was determined by the method ofLowry et al. (34) using BSA as standard.
Apoptotic events
Materials and methods
Apoptotic cells (condensed and fragmented nuclei) were identified 48 h after
Chemicals and biologicals the beginning of treatment in Hoechst dye 339342 stained hepatocytes as
Chemicals and biochemicals were of reagent grade and purchased from reported earlier (30). Cells with condensed nuclei were rarely seen. A Sigma (St Louis, MO). Cell culture products were purchased from Gibco fragmented nucleus was defined as (i) one or more brightly fluorescent (Paisley, UK) and from Seromed (Berlin, Germany). Porcine collagen type I (condensed) chromatin pieces within an area equivalent to a nucleus surrounded was from Pentapharm (Basel, Switzerland). Human recombinant TGF-β1 was by cytoplasmic material (apoptotic bodies) or (ii) a cluster of chromatin pieces a generous gift of Dr K.Frei (Institute for Clinical Immunology, University within a cell without a nucleus.
Hospital of the University of Zurich, Switzerland). Human recombinant EGF
Data analysis
was purchased from Becton Dickinson (Bedford, MA).
The mean value and the standard error of the mean (SEM) were calculated Antibodies were purchased from the following sources: monoclonal mouse
for each parameter measured. Significance of differences between means was anti-proliferating cell nuclear antigen (PCNA) antibody (PC-10) from DAKO
calculated using Student’s t-test for paired or unpaired samples as appropriate. (Glostrup, Denmark); Cy3™-conjugated AffiniPure F(ab’)2 fragment goat
The level of significance chosen was P, 0.05. The description of the dose– anti-mouse IgG 1 IgM (H1L) from Milan Analytica AG (La Roche,
response curves and calculation of the IC5 and IC50values were performed Switzerland).
according to the model described by Bruinink (35).
Isolation, cultivation and treatment of hepatocytes
Hepatocytes were isolated from male Sprague–Dawley rats (random bred,
Results
240–270 g body wt, ZUR/SIV; Institute of Laboratory Animals, Universityof Zurich, Switzerland) by two-step in situ collagenase perfusion as described
Constitutive PCNA expression and DNA synthesis in untreated
previously (31). The isolated hepatocytes were washed and concentrated bycells
three centrifugation steps (2 min at 20 g and 4°C) in serum-free William’s Emedium supplemented with 10 mM HEPES, 2 mM L-glutamine, 10 nM
Three hours after seeding, ~40 PCNA-positive nuclei/mm
2insulin, 1µg/ml aprotinin, 100 IU/ml penicillin/streptomycin and cultured in
were detectable. This corresponds to ~2% of the originally
the same medium without HEPES but supplemented instead with 100 nMseeded hepatocytes. This spontaneous PCNA expression
dexamethasone, 10 nM glucagon and 30 nM SeCl2. Hepatocytes were seeded
decreased over culture time down to
,0.1% after 96 h. In
on Costar 24-well plates (2 cm2/well) at a density of ~1.53105 cells/cm2
under 5% CO2/air. The influence of oxygen tension and glucoregulatory
contrast, [
3H]TdR incorporation was not markedly changed
hormone ratio were investigated in gas-permeable Teflon membrane dishesduring the 4 day culture period; on day 2 a slight but
(Petriperm, 20 cm2, 23105 cells/cm2; Heraeus AG, Zurich, Switzerland)insignificant increase was observed. In cultures under low,
cultured under 13 or 4% O2. All culture dishes were precoated with a mixture
periportal-equivalent oxygen tension (4% O
2) and low glucagon
of liver crude membrane fraction (CMF) and porcine collagen type I [6µg
rat liver CMF and 60 ng COL in 71µl/cm2phosphate-buffered saline (PBS),
concentrations (1 nM), this down-regulation of PCNA within
overnight precoating at 4°C] as described earlier (27,28,31).
48 h was effective in a similar way.
In all the experiments the medium was changed 4 and 20 h after seeding andCPA and EGF increase PCNA expression and DNA synthesis
then daily. After 2 days in culture, hepatocytes were exposed to the mitogensand/or to TGF-β1 for 48 h. Stock solutions of CPA were freshly prepared in
CPA, up to a concentration of 6.25
µ
M, dose-dependently
dimethylsulphoxide (DMSO) and diluted with culture medium (final DMSOincreases the number of PCNA-expressing nuclei within 48 h.
concentration,0.1%). Human recombinant TGF-β1 (106U/mg; Genzyme,Above 12
µ
M CPA, the response plateaued; 12
µ
M CPA
Cambridge, MA) and the human recombinant EGF were freshly prepared in
increased the control value 3.5-fold and 25
µ
M CPA only
0.1% bovine serum albumin (Sigma; initial fractionation by cold alcohol
precipitation) in PBS and diluted up to 103-fold with culture medium.
4-fold. EGF at 1 ng/ml increased PCNA expression almost
PCNA analysis and measurement of Hoechst dye fluorescence
2.5-fold; at 10 ng/ml the increase was 7-fold (Figure 1A).
Hepatocytes were immunostained for PCNA, an auxiliary protein of DNA
[
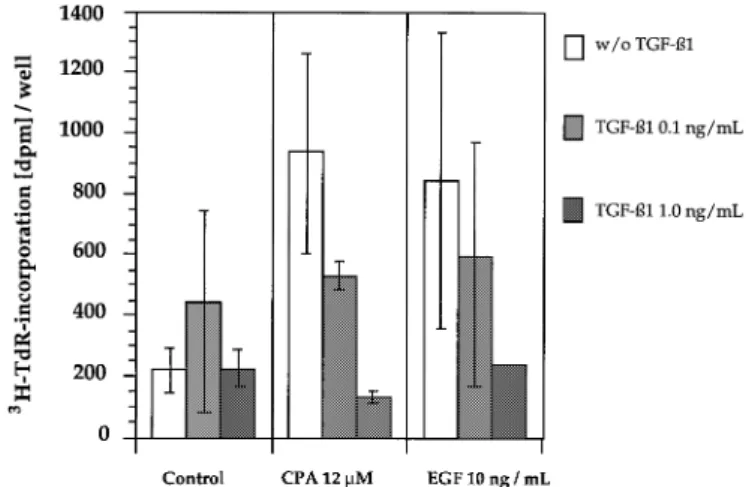
3H]TdR incorporation was induced dose-dependently up to
polymerase δ, by a method modified from Kurki et al. (32). Briefly, the
a concentration of 12
µ
M CPA. A doubling of this dose led
hepatocytes were fixed and permeabilized as follows: 15 min at 4°C in 1%to a decrease in DNA synthesis. EGF, given in concentrations
formalin in PBS containing 20µg/mlα-lysophosphatidylcholine, then 15 minof 10 ng/ml, increased [
3H]TdR incorporation 3- to 4-fold
in ice-cold methanol and subsequently 15 min in 0.1% NP-40, both at 4°C.
(Figure 3).
Non-specific binding sites were blocked in 1% BSA in PBS (30 min, room
temperature). Hepatocytes were then exposed to the anti-PCNA mAb (PC-10,
TGF-
β
1 down-regulates PCNA-expressing hepatocytes
1:100, 1% BSA in PBS) or to mouse ascites fluid (1:100). PCNA-expressingEGF-induced PCNA expression was down-regulated by
con-cells were stained with the second antibody (Cy3-conjugated, 1:250) and
hepatocyte nuclei with Hoechst 339342 (Calbiochem; 2 µg/ml in PBS).
centrations of 0.04 ng/ml TGF-
β
1 and higher, whereas
CPA-PCNA-positive nuclei were identified by fluorescence microscopy (Ex/Eminduced stimulation was unaffected up to 0.1 ng/ml TGF-
β
1
340/460 nm) and the total DNA content by area measurements per well or(Figure 1A). Between 0.1 and 1 ng/ml, TGF-
β
1 inhibited
dish (Cytofluor™ 2350 multiplate scanner, Millipore, Bedford, MA; Ex/Em
both CPA- and EGF-mediated mitogenic activity in a
dose-340/460 nm).
dependent manner. The IC
5and IC
50values of TGF-
β
1 were
Measurement of DNA synthesis
0.012 and 0.124 ng/ml for EGF- and 0.065 and 0.305 ng/ml for
[3H]Thymidine (TdR) incorporation of the hepatocytes was determined
CPA-stimulated hepatocytes respectively. Among hepatocytes
according to Vintermyr et al. (33) with minor modifications. After the 15 h
pulse (1µCi/well or plate [3H]TdR, 33–48 h after addition of the mitogens)
exposed concomitantly to EGF and CPA, the mitogenic
the cells were fixed in ethanol (30 min) and DNA content determinedresponse was additive or even slightly synergistic, whereas the
(propidium iodide, Cytofluor™ 235, Ex/Em530/620 nm). Subsequently, thesensitivity toward TGF-
β
1 was intermediate between the IC
5
monolayers were rinsed (ice-cold PBS) and fixed (5% aqueous trichloracetic
and IC
50values of TGF-
β
1 for the two mitogens alone
acid, ice-cold 70% ethanol, 10 min each). RNA and bulk protein were
Fig. 3. Inhibition of EGF- (10 ng/ml) and CPA (12µM)-induced [3H]TdR
Fig. 1. Dose-dependent down-regulation of EGF- (10 ng/ml), CPA- (12µM)
incorporation (15 h pulse) by low and high levels of TGF-β1. CPA or EGF (A) and EGF1CPA-induced PCNA expression (B) by TGF-β1 (mean of
were administred together with TGF-β1 from days 2–4 in culture. Mean of three independent experiments6 SD). CPA or EGF were administred
three independent experiments6 SD. together with TGF-β1 from days 3–4 in culture. PCNA-positive nuclei were
counted with a microscope as described in Materials and methods.
Fig. 4. Dose-dependent induction of apoptosis (fragmented nuclei) by
TGF-β1 and stimulation or inhibition by concomitant exposure to CPA or EGF respectively (A). (B) Simultaneous exposure to CPA and EGF. Measurements were performed after 4 days in culture. Values represent the means6 SD of three independent experiments.
Fig. 2. Down-regulation of PCNA expression after exposure to low and
high concentrations of the hepatomitogens (mean of three independent experiments6 SD) by low (A) and high (B) levels of TGF-β1. Values
4), as measured by fluorescence microscopy of Hoechst dye
represent relative inhibition when compared with cultures without TGF-β1.
33
9342 stained nuclei (30). After exposure to 1 ng/ml TGF-
β
1,
the CPA-exposed hepatocytes showed a 3-fold higher incidence
When exposed to 0.1 ng/ml TGF-
β
1, the relative inhibition
of fragmented nuclei (Figure 4A) and the EGF-exposed cells a
of PCNA nuclei was the same in control cells as in those
50% lower incidence (Figure 4B) than cells treated with
TGF-treated with 1 or 10 ng/ml EGF, namely ~40% (Figure 2A).
β
1 alone. CPA and EGF alone had no effect on the spontaneous
In contrast, at this low TGF-
β
1 dose 3
µ
M and 12
µ
M
CPA-level of apoptotic cells. In cells exposed concomitantly to EGF
treated cells showed a further stimulation of PCNA nuclei
and CPA the higher sensitivity to TGF-
β
1-induced apoptotic
(20 and 10% respectively). At a higher dose of TGF-
β
1
processes was abolished (Figure 4B).
(0.5 ng/ml) all cells showed inhibition of PCNA expression,
LDH release after 48 h exposure to TGF-
β
1, in combination
but much more pronounced in EGF-treated, in EGF
1CPA-with aberrant nuclei, reflects the late phase of apoptotic cells
treated or in control cells (60–80% inhibition) than in
CPA-(30). Extracellular LDH activity was not significantly increased
exposed cells (35%) (Figure 2B).
after exposure to the mitogens (Figure 5). With increasing
TGF-[
3H]TdR incorporation was not differentially affected: both
β
1 concentration, in parallel with the incidence of fragmented
CPA- and EGF-induced [
3H]TdR incorporation was reduced
nuclei, extracellular LDH activity increased; it was higher in the
by one third at 0.1 ng/ml and returned to control levels at
CPA- (Figure 5A) and lower in the EGF-exposed hepatocytes
1 ng/ml TGF-
β
1. In untreated hepatocytes, after exposure to
(Figure 5B) than in the corresponding cultures without mitogen
0.1 ng/ml TGF-
β
1 [
3H]TdR incorporation was doubled, but
treatment.
the difference was not significant (Figure 3).
Differential inhibitory activity of TGF-β1 on PCNA expression
Differential susceptibility of CPA- and EGF-exposed cells
is abolished in a pericentral equivalent microenvironment
towards TGF-
β
1-induced apoptosis
The experimental conditions used in the experiments described
Exposure of hepatocytes to TGF-
β
1 concentrations
.0.1 ng/ml
approxi-of CPA-stimulated hepatocytes to down-regulation by low levels
of TGF-
β
1.
Discussion
Investigations on growth stimuli or impaired negative growth
control in cell cultures might be greatly affected by the
proliferative capacity and the metabolic state of the cells. In
the present study, at the beginning of the exposure to the
mitogens the hepatocytes expressed liver-specific functions
(28,29) and a low spontaneous mitogenic activity comparable
with that found in the intact organ.
Negative growth control and liver-specific metabolism
Fig. 5. Dose-dependent induction of LDH release by TGF-β1 and
stimulation (A) or inhibition (B) by concomitant exposure to the
It was shown that during liver perfusion and the following
hepatomitogens. Values represent the means6 SD of three independenttissue dissociation, growth stimuli similar to those observed
experiments.
after partial hepatectomy are induced in hepatocytes (37). This
entry into the cell cycle after liver perfusion might be the
reason for the initially high levels of PCNA expression in our
hepatocytes. However, only a small proportion of these
PCNA-positive cells enter S phase, as indicated by the only slightly
increased DNA synthesis rate within 48 h after seeding. Thus,
enzymic dissociation shifts hepatocytes from G
0into middle
to late G
1, but not into S phase. Accordingly, the majority of
PCNA-expressing hepatocytes in our cultures represent cells
arrested in mid to late G
1phase (38).
The efficient spontaneous down-regulation of this
‘com-pensatory’ growth stimulus within 2 days in our cultures is
most likely the consequence of the high cell density
(down-regulation of immediate early genes; 39,40) and the culture
conditions chosen. Expression of liver-specific metabolism
seems to be linked to active negative growth control. This is
concluded from the fact that the loss of P450 mRNAs after
hepatocyte isolation parallels an increase in proliferative
activ-ity (37) and an increase in xenobiotic metabolism after 2–7
days in culture (41) parallels the decrease in PCNA expression.
Mitogenic effects of EGF and CPA
The present experiments demonstrate that EGF and CPA
shift quiescent hepatocytes with well-preserved liver-specific
functions (29,41) into G
1and some into S phase. No serum,
and in the case of CPA no co-administration of EGF, was
required, in contrast to a previous report (42), in which PCNA
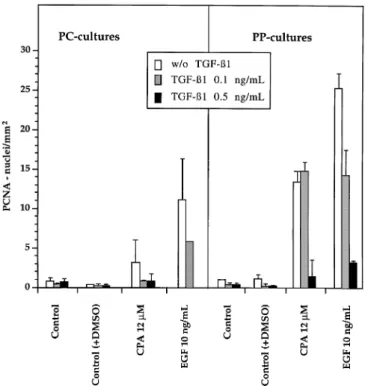
Fig. 6. Induction of PCNA expression in PC cultures (1 nM glucagon,expression was induced by EGF but not by CPA. A
well-incubation atmosphere 4% O2) and down-regulation by TGF-β1. The
functioning liver metabolism might be of specific importance
reference culture (10 nM glucagon, incubation atmosphere 13% O2)
corresponds to 24-well cultures. Simultaneous exposure to CPA, EGF and
for CPA because this chemical affects microsomal
mono-TGF-β1 from days 3–4 in culture. Values represent the means6 SD of
oxygenase activity (43) and, prior to DNA binding, requires
three independent experiments.metabolic activation (20), most likely via a hydroxysteroid
sulfotransferase (44). Whether metabolic activation is also
required for the mitogenic activity of CPA remains to be
mate periportal conditions (36). Pericentral-equivalent cultures
(PC cultures) can be mimicked by culturing cells at low glucagon
elucidated.
Effective CPA concentrations in our in vitro
investiga-concentrations (1 nM) and low oxygen tension. This can be
achieved by the use of Teflon membrane culture dishes kept at
tions are in the range of those found in the serum of rats
treated with the synthetic hormone (45). It is noteworthy that
4% O
2(28). As a corresponding reference, hepatocytes were
cultured in parallel in Teflon membrane culture dishes under
PCNA expression plateaus at CPA concentrations which induce
maximal DNA synthesis and remains at higher concentrations
13% O
2in the same medium as used in the 24-well dishes (PP
cultures). The oxygen tension is then comparable to that under air
where DNA synthesis is inhibited. Clearly, only a limited
number of hepatocytes are able to respond to the mitogenic
in conventional culture dishes. Accordingly, differential
down-regulation was again detectable (Figure 6). However, two find-
stimulus of CPA. The additive, partially synergistic response
after combined treatment with CPA and EGF (Figure 1B)
ings differed in PC cultures (Figure 6): (i) EGF and CPA were
both much less effective (EGF 1/2 and CPA 1/4 of ‘periportal’
indicates that CPA stimulates additional cells that are not
responding to EGF. A similar additive response was reported
values); (ii) CPA-stimulated hepatocytes lost their resistance to
0.1 ng/ml TGF-
β
1. The metabolic status of the hepatocytes,
for the induction of DNA synthesis (12). The two mitogens
therefore act via different pathways and as a consequence
which is differentially affected by the two culture conditions
The differential mitoinhibitory activity of TGF-
β
1
of TGF-
β
1 and by that they become resistant to
apoptosis-inducing levels of TGF-
β
1. In contrast, CPA-stimulated
hepato-TGF-
β
1 is a key factor in pre-replicative negative growth
cytes cannot re-enter the resting cell cycle phase initiated by
control. After partial hepatectomy, TGF-
β
1 mRNA is increased
low levels of TGF-
β
1, but become a target for higher
TGF-in non-parenchymal liver cells before the major wave of
β
1 levels and escape via apoptosis. In favour of the second
DNA synthesis has been initiated (46). Accordingly, the
interpretation are the additive/synergistic induction of
PCNA-mitoinhibitory activity of TGF-
β
1 acts in cells at or prior to
expressing cells (Figure1B), the fraction of cells responding
the G
1/S cell cycle phase transition (47).
to 0.1 ng/ml TGF-
β
1 after combined exposure to CPA and
Normal hepatocytes release TGF-
β
by an autocrine
EGF (Figure 2A) and, as discussed in the following section,
mechanism in its latent form (25) and are unable to activate
the influence of an altered metabolism as induced under PC
TGF-
β
complexes in vitro (24). This and the dose–response
culture conditions.
relationship seen in our study confirms that the added activated
Regiospecific response
recombinant TGF-
β
1 antagonizes endogenous and exogeneous
growth signals in rat hepatocytes. The TGF-
β
1 concentrations
Theoretically, liver lobules can be divided into a proliferative/
used were physiologically relevant. For the corresponding
mitogenic (periportal) and a functional/apoptotic (pericentral)
down-regulation of CPA-exposed cells a 3- (IC
50) to 5-fold
compartment (50–52). Hepatocyte cell populations within liver
(IC
5) higher TGF-
β
1 concentration is required when compared
lobule regions might differ in their degree of differentiation
with EGF-stimulated cells (Figure 1). This differential down-
and their metabolic competence. Reduction of glucagon
con-regulation can be observed at low or high concentrations of
centration and of oxygen tension shift cellular metabolism
the two mitogens (Figure 2A). This demonstrates that the
towards that described for the pericentral region (28). The
resistance is related to the type of mitogen and not to the
reduced activity of the two mitogens and the loss of resistance
intensity of the mitogenic response. The absence of a differen-
to low levels of TGF-
β
1 in PC cultures (Figure 6) correspond
tial response in cells that enter DNA synthesis (Figure 3)
well with the reported increased sensitivity to TGF-
β
1 under
suggests that down-regulation of PCNA expression occurs
these culture conditions (30). It also corresponds well with
prior to the G
1/S phase transition.
in vivo studies in which the mitotic activity of EGF (53) and
of CPA (8,54) was mainly found in the periportal area,
Down-regulation of CPA-stimulated hepatocytes via an
including down-regulation by apoptosis after withdrawal of
apoptotic pathway
CPA (54). The present in vitro findings support data from an
It is well established that high levels of TGF-
β
1 induce
animal study in which apoptosis induced by CPA withdrawal
apoptosis in vivo (48) and in vitro (30,49). This was also
did not occur in DNA synthesizing cells (54).
found in our study at 48 h culture time at concentrations above
The loss of the differential response in PC cultures can be
0.1 ng/ml TGF-
β
1. The differential dose-dependent increase
attributed either to low glucagon concentrations or low oxygen
in fragmented nuclei (Figure 4A) and LDH release (Figure 5A
tension. The interaction of glucagon with mitogenic processes
and B) indicate that CPA-treated hepatocytes are sensitized to
is controversial and seems to depend on the exposure levels
and EGF-treated ones protected against the apoptotic activity
and cell density chosen. High levels of glucagon (10 nM), as
of TGF-
β
1. The differential down-regulation can also be seen
used in our 24-well or PP cultures, might even be mitoinhibitory
at higher TGF-
β
1 levels (Figure 4B). In EGF-exposed cells
(55). No interaction was detectable between TGF-
β
1 and
the IC
50(0.124 ng/ml) of TGF-
β
1 for PCNA down-
glucagon in rat hepatocyte cultures (47). However, glucagon
regulation induced no apoptosis, whereas the IC
50for CPA
concentrations chosen affect basal metabolism in the
hepato-(0.305 ng/ml) significantly increased the number of
frag-cytes (28) and low oxygen tension, as reported earlier (56,57),
mented nuclei (Figure 4A). This fits well with an in vitro study
modulates chemically induced growth stimuli.
in which CPA increased the apoptotic activity of TGF-
β
1 in
In conclusion, the experiments demonstrate differences
cultured rat hepatocytes (42). The protective effect of EGF is
between the down-regulation of an endogenous (EGF) and an
stronger than the apoptotic stimulus of CPA, as indicated by
exogenous (CPA) mitogenic stimulus by TGF-
β
1. The exposure
the additive response after simultaneous exposure to CPA
levels of the growth inhibitory cytokine and the metabolic
and EGF (Figure 4B). This indicates that the EGF-mediated
state of the cultured hepatocytes are crucial. It seems that
protection against apoptosis precedes sensitization and is also
endogenous and exogenous growth stimuli affect different
effective in CPA-stimulated hepatocytes.
hepatocyte
populations.
CPA-stimulated
cells
are
less
Taken together, the results suggest that EGF-stimulated
susceptible to down-regulation by low levels of TGF-
β
1
PCNA-expressing hepatocytes are down-regulated by a shift
than are EGF-exposed cells. Down-regulation in CPA-exposed
from a prereplicative into a resting cell cycle phase, whereas
hepatocytes, in contrast to EGF-exposed hepatocytes, occurs
CPA-stimulated cells are more resistant to this pathway and
preferentially via apoptosis inducible at higher TGF-
β
1 levels.
are eliminated by apoptosis. Two different modes of action
Therefore, at low (e.g. tissue equivalent) TGF-
β
1 levels,
CPA-are possible. Firstly, EGF-exposed hepatocytes CPA-are arrested in
stimulated hepatocytes have less chance of re-entering the
mid to late G
1phase and thus are sensitive to the mitoinhibitory
resting cell cycle phase compared with EGF-stimulated cells
activity of low levels of TGF-
β
1, whereas CPA-exposed
and have a higher risk of acquiring and fixing a spontaneous
hepatocytes proceed further into the cell cycle (e.g. late G
1premutagenic lesion into a mutation which could contribute to
phase), at which point they lose their responsiveness to low
tumour formation.
levels of TGF-
β
1 but become more vulnerable to the
apoptosis-inducing signal at higher concentrations. Alternatively, EGF
Acknowledgements
and CPA induce PCNA expression in different, as yet
unidenti-This research project was funded by Interpharma, Basle, Switzerland. We
fied or uncharacterized, hepatocyte sub-populations. EGF-
thank Prof. Dr K.Frei of the Section of Clinical Immunology, Universitystimulated cells are able to switch from PCNA expression into
Hospital of Zu¨rich, Switzerland and Prof. Dr D.R.Dietrich, University ofKonstanz, Germany, for helpful suggestions and discussions.
23. Petersen,B., Yee,C.J., Bowen,W., Zarnegar,R. and Michalopoulos,G.K.
References
(1994) Distinct morphological and mitoinhibitory effects induced by TGF-1. Pitot,H.C. and Sirica,A.E. (1980) The stages of initiation and promotion β1, HGF and EGF on mouse, rat and human hepatocytes. Cell Biol.
in hepatocarcinogenesis. Biochim. Biophys. Acta, 605, 191–205. Toxicol., 10, 219–230.
2. Schulte-Hermann,R., Ohde,G., Schuppler,J. and Trosiener-Timmerman,I. 24. Jakowlew,S.B., Mead,J.E., Danielpour,D., Wu,J., Roberts,A.B. and (1981) Enhanced proliferation of putative preneoplastic cells in rat liver Fausto,N. (1991) Transforming growth factor-β isoforms in rat liver following treatment with the tumor promoters phenobarbital, hexachloro- regeneration: messenger RNA expression and activation of latent TGFβ. cyclohexane, steroid compounds and nafenopin. Cancer Res., 41, 2556– Cell Regulat., 2, 535–548.
2561. 25. Bissell,D.M., Wang,S.S., Jarnagin,W.R. and Roll,F.J. (1995) Cell-specific 3. Cunningham,M.L., Foley,J., Maronpot,R.R. and Matthews,H.B. (1991) expression of transforming growth factor βin rate liver. Evidence for Correlation of hepatocellular proliferation with hepatocarcinogenicity autocrine regulation of hepatocyte proliferation. J. Clin. Invest., 96, induced by the mutagenic noncarcinogen:carcinogen pair 2,6- and 2,4- 447–455.
diaminotoluene. Toxicol. Appl. Pharmacol., 107, 562–567. 26. Saad,B., Schawalder,H.P. and Maier,P. (1993) Crude liver membrane 4. Butterworth,B.E., Popp,J.A., Conolly,R.B. and Goldsworthy,T.L. (1992) fractions maintain liver specific functions in long term, serum free rat
Chemically induced cell proliferation in carcinogenesis. IARC Sci. Publ., hepatocyte cultures. In Vitro Cell Dev. Biol., 29A, 32–40.
116, 279–305. 27. Saad,B., Scholl,F.A., Thomas,H., Schawalder,H-.P., Streit,V., Waechter,F.
5. Ashby,J. and Tennant,R.W. (1985) Chemical structure, Salmonella and Maier,P. (1993) Crude liver membrane fractions and extracellular mutagenicity and carcinogenicity as indicators of genotoxic carcinogenesis matrix components as substrate regulate differentially the preservation and among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat. Res., inducibility of cytochrome P-450 isoenzymes in cultured rat hepatocytes.
204, 17–115. Eur. J. Biochem., 213, 805–814.
6. Deml,E., Schwarz,L.R. and Oesterle,D. (1993) Initiation of enzyme altered
28. Ohno,K. and Maier,P. (1994) Cultured rat hepatocytes adapt their cellular foci by the synthetic steroid cyproterone acetate in rat liver foci bioassay.
glycolytic activity and adenylate energy status to tissue oxygen tension:
Carcinogenesis, 14, 1229–1231.
influences of extracellular matrix components, insulin and glucagon. J. 7. Schuppler,J., Damme,J. and Schulte-Herman,R. (1983) Assay of some
Cell. Physiol., 160, 358–366.
endogenous and synthetic sex steroids for tumor-initiating activity in rat
29. Maier,P., Saad,B. and Schawalder,H.P. (1994) Periportal- and centrilobular-liver using the Solt–Farber system. Carcinogenesis, 4, 239–241.
equivalent oxygen tension affect liver specific functions and xenobiotic 8. Schulte-Hermann,R., Hoffman,V., Parzefall,W., Kallenbach,M., Gerhardt,
metabolism in long-term rat hepatocyte cultures. Toxicol. In Vitro, 8, A. and Schuppler,J. (1980) Adaptive response of rat liver to the gestagen
423–435. and anti-androgen cyproterone acetate and other inducers. II. Induction of
30. Ohno,K., Amman,P., Fasciati,R. and Maier,P. (1995) Transforming growth growth. Chem.-Biol. Interactions, 31, 287–300.
factor β1 preferentially induces apoptotic cell death in rat hepatocytes 9. Schuppler,J. and Gu¨nzel,P. (1979) Liver tumors and steroid hormones in
cultured under pericentral-equivalent conditions. Toxicol. Appl.
rats and mice. Arch. Toxicol., 2, 181–195.
Pharmacol., 132, 277–236.
10. Schulte-Hermann,R., Ochs,H., Bursch,W. and Parzefall,W. (1988)
31. Maier,P., Saad,B. and Ohno,K. (1995) New approaches for the preservation Quantitative structure–activity studies on effects of sixteen different
of metabolic zonation in rat hepatocyte cultures. In Goldberg,A.M. and steroids on growth and monooxygenase of rat liver. Cancer Res., 48,
van Zutphen,L.F.M. (eds), The World Congress on Alternatives and Animal 2462–2468.
Use in the Life Sciences: Education, Research, Testing. Alternative Methods
11. Parzefall,W., Monschau,P. and Schulte-Hermann,R. (1989) Induction by
in Toxicology and the Life Sciences. Mary Ann Liebert, New York, Vol.
cyproterone acetate of DNA synthesis and mitosis in primary cultures of
11, pp. 213–219. adult rat hepatocytes in serum free medium. Arch. Toxicol., 63, 456–461.
12. Parzefall,W., Erber,E., Sedivy,R. and Schulte-Hermann,R. (1991) Testing 32. Kurki,P., Ogata,K. and Tan,E.M. (1988) Monoclonal antibodies to for induction of DNA synthesis in human hepatocyte primary cultures by proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating rat liver tumor promoters. Cancer Res., 51, 1143–1147. cells by immunofluorescence microscopy and flow cytometry. J. Immunol. 13. Lang,R. and Redmann,U. (1979) Non-mutagenicity of some sex hormones Methods, 109, 49–59.
in the Ames’s Salmonella microsome mutagenicity test. Mutat. Res., 67, 33. Vintermyr,O.K., Boe,R., Bruland,T., Houge,G. and Døskeland,S.O. (1993) 361–365. Elevated cAMP gives short-term inhibition and long-term stimulation of 14. Lang,R. and Reimann,R. (1993) Studies for a genotoxic potential of some hepatocyte DNA replication: roles of the cAMP-dependent protein kinase
endogenous and exogeneous sex steroids. I. Communication: examination subunits. J. Cell. Physiol., 156, 160–170.
for the induction of gene mutations using Ames Salmonella/microsome 34. Lowry,O.H., Rosebrough,N.J., Farr,A.L. and Randall,R.J. (1951) Protein test and HGPRT test in V 79 cells. Environ. Mol. Mutagen., 21, 272–304. measurement with the Folin phenol reagent. J. Biol. Chem., 193, 265–275. 15. Neumann,I., Thierau,D., Andrae,U., Greim,H. and Schwarz,L.R. (1992) 35. Bruinink,A. (1992) Serum-free monolayer cultures of fetal chick brain Cyproterone acetate induces DNA damage in cultured rat hepatocytes and retina: immunoassays of developmental markers, mathematical data and preferentially stimulates DNA synthesis inγ-glutamyltranspeptidase- analysis, and establishment of optimal culture conditions. In Zbinden,G. positive cells. Carcinogenesis, 13, 373–378. (ed.), The Brain in Bits and Pieces. MTC, Zollikon, Switzerland, pp. 40–46. 16. Martelli,A., Mattioli,F., Fazio,S., Andrae,U. and Brambilla,G. (1995) DNA 36. Holzer,C. and Maier,P. (1987) Maintenance of periportal and pericentral repair synthesis and DNA fragmentation in primary cultures of human oxygen tensions in primary rat hepatocyte: influence on cellular DNA and and rat hepatocytes exposed to cyproterone actetate. Carcinogenesis, 16, protein content monitored by flow cytometry. J. Cell. Physiol., 133,
1265–1269. 297–304.
17. Kasper,P., Tegethoff,K. and Mueller,L. (1995) In vitro mutagenicity studies
37. Padgham,C.R., Boyle,C.C., Wang,X.J., Raleigh,S.M., Wright,M.C. and on cyproterone acetate using female rat hepatocytes for metabolic activation
Paine,A.J. (1993) Alteration of transcription factor mRNAs during the and as indicator cells. Carcinogenesis, 16, 2309–2314.
isolation and culture of rat hepatocytes suggests the activation of a 18. Kerdar,R.S., Baumann,A., Brudny-Klo¨ppel,M., Biere,H., Blode,H. and
proliferative mode underlies their de-differentiation. Biochem. Biophys. Kuhnz,W. (1995) Identification of 3α-hydroxycyproterone acetate as a
Res. Commun., 197, 599–605.
metabolite of cyproterone acetate in the bile of female rats and the potential
38. Dietrich,D.R. (1993) Toxicological and patholocgical applications of of this and other already known or putative metabolites to form DNA
proliferating cell nuclear antigen (PCNA), a novel endogenous marker for adducts in vitro. Carcinogenesis, 16, 1835–1841.
cell proliferation. Crit. Rev. Toxicol., 23, 77–109. 19. Werner,S., Topinka,J., Wolff,T. and Schwarz,L.R. (1995) Accumulation
39. Kumatori,A., Nakamura,T. and Ichihara,A. (1991) Cell density dependent and persistence of DNA adducts of the synthetic steroid cyproterone
expression of the c-myc gene in primary cultured rat hepatocytes. Biochem. acetate in rat liver. Carcinogenesis, 16, 2369–2372.
Biophys. Res. Commun., 178, 480–485.
20. Topinka,J., Andrae,U., Schwarz,L.R. and Wolff,T. (1993) Cyproterone
40. Shimbara,N., Takashina,M., Sato,C., Iizuka,M., Kobayashi,S., Tanaka,K. acetate generates DNA adducts in rat liver and in primary rat hepatocyte
and Icjihara,A. (1992) c-myc expression is down-regulated by cell–cell cultures. Carcinogenesis, 14, 423–427.
and cell–extracellular matrix contacts in normal hepatocytes, but not in 21. Fausto,N. (1991) Multifunctional roles for transforming growth factorβ1.
hepatoma cells. Biochem. Biophys. Res. Commun., 184, 825–831.
Lab. Invest., 65, 497–498.
41. Maier,P., Schawalder,H.P., Milosevic,N., Bouis,P., Thomas,H. and Ohno,K. 22. Nakamura,T., Tomita,Y., Hirai,R., Yamaoka,K., Kaji,K. and Ichihara,A.
(1997) Insulin and glucagon downregulate specifically the phenobarbital (1985) Inhibitory effect of transforming growth factorsβin DNA synthesis
inducible CYP2B1/2 in cultured rat liver cells. Biochem. Pharmacol., of rat hepatocytes in primary culture, Biochem. Biophys. Res. Commun.,
42. Oberhammer,F.A. and Qin Hong-Min (1995) Effect of three tumour promoters on the stability of hepatocyte cultures and apoptosis after transforming growth factor-β1. Carcinogenesis, 16, 1363–1371. 43. Schulte-Hermann,R. and Parzefall,W. (1980) Adaptive response of rat liver
to the gestagen and anti-androgen cyproterone acetate and other inducers. I. Induction of drug metabolizing enzymes. Chem.-Biol. Interactions., 31, 279–286.
44 Schwarz,L.R., Werner,S., Topinka,J. Andrae,U., Neumann,I. and Wolff,T. (1995) The liver as origin and target of reactive intermediates exemplified by the progesterone derivative, cyproterone acetate. In Snyder,R. et al. (eds), Biological Reactive Intermediates V. Plenum Press, New York, NY, 243–251.
45. Bursch,W., Du¨sterberg,B., Schulte-Hermann,R. (1986) Growth, regression and cell death in rat liver as related to tissue levels of the hepatomitogen cyproterone acetate. Arch. Toxicol., 59, 221–227.
46. Braun,L., Mead,L.E., Panzica,M., Mikumo,R., Bell,G.I. and Fausto,N. (1988) Transforming growth factor β mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc.
Natl Acad. Sci. USA, 85, 1539–1543.
47. Thoresen,G.H., Refsnes,M. and Christoffersen,T. (1992) Inhibition of hepatocyte DNA synthesis by transforming growth factorβ1 and cyclic AMP: effect immediately before the G1/S border. Cancer Res., 52, 3598–3603.
48. Bursch,W., Oberhammer,F., Jirtle,R.L., Askari,M., Sedivy,R., Grasl-Kraupp,B., Purchio,A.F. and Schulte-Hermann,R. (1993) Transforming growth factor-β1 as a signal for induction of cell death by apoptosis. Br.
J. Cancer, 67, 531–536.
49. Oberhammer,F.A., Pavelka,M., Sharma,S., Tiefenbacher,R., Purchio,A.F., Bursch,W. and Schulte-Hermann,R. (1992) Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor
β1. Proc. Natl Acad. Sci. USA, 89, 5408–5412.
50. Gebhardt,R. (1992) Metabolic zonation of the liver: regulation and implication for liver function. Pharmacol. Ther., 53, 275–354.
51. Reid,L.M., Fiorino,A.S., Sigal,S.H. and Holst,P.A. (1992) Extracellular matrix gradients in the space of disse: relevance to liver biology.
Hepatology, 15, 1198–1203.
52. Arber,A., Zajicek,G. and Ariel,I. (1988) The streaming liver II. Hepatocyte life history. Liver, 8, 80–87.
53. Gebhardt,R. and Jonitza,D. (1991) Different proliferative response of periportal and perivenous hepatocytes to EGF. Biochem. Biophys. Res.
Commun., 181, 1201–1207.
54. Roberts,R.A., Soames,A.R., Gill,J.H., James,N.H. and Wheeldon,E.B. (1995) Non-genotoxic hepatocarcinogens stimulate DNA synthesis and their withdrawal induces apoptosis, but in different hepatocyte populations.
Carcinogenesis, 16,1693–1698.
55. Parzefall,W., Erber,E., Kainzbauer,E. and Schulte-Hermann,R. (1996) Effects of insulin, glucagon, and triiodothyronine on DNA synthesis in rat hepatocyte primary cultures induced by liver tumor promoters and EGF. Toxicol. In Vitro, 10, 183–193.
56. Maier,P. and Schawalder,H.P. (1993) Physiological oxygen tension modulates the chemically induced mitogenic reponse of cultured rat hepatocytes. J. Cell. Physiol., 156, 119–129.
57. Duivenvoorden,W.M.C. and Maier,P. (1994) Nongenotoxic carcinogens shift cultured rat hepatocytes into G1 cell cycle phase: influence of tissue oxygen tension on cells with different ploidy. Eur. J. Cell Biol., 64, 368–375.
Received on March 29, 1996; revised on October 16, 1996; accepted on January 14, 1997