Publisher’s version / Version de l'éditeur:
Chemical Industry, 58, June 6, pp. 259-270, 2004-06-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Unusual kinetic behaviour in cathodic H2 evolution from KF.2HF melts
at mild-steel and alloy electrodes
Conway, B. E.; Qian, S. Y.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=4d6d03ac-830c-4308-9b59-d72dde7378f5
https://publications-cnrc.canada.ca/fra/voir/objet/?id=4d6d03ac-830c-4308-9b59-d72dde7378f5
http://www.nrc-cnrc.gc.ca/irc
U nusua l k ine t ic be ha viour in c a t hodic H 2 e volut ion from K F.2 H F
m e lt s a t m ild-st e e l a nd a lloy e le c t rode s
N R C C - 4 7 3 2 4
C o n w a y , B . E . ; Q i a n , S . Y .
J u n e 2 0 0 4
A version of this document is published in / Une version de ce document se trouve dans:
Chemical Industry, 58, (6), June, pp. 259-270, June 01, 2004
The material in this document is covered by the provisions of the Copyright Act, by Canadian laws, policies, regulations and international agreements. Such provisions serve to identify the information source and, in specific instances, to prohibit reproduction of materials without written permission. For more information visit http://laws.justice.gc.ca/en/showtdm/cs/C-42
Les renseignements dans ce document sont protégés par la Loi sur le droit d'auteur, par les lois, les politiques et les règlements du Canada et des accords internationaux. Ces dispositions permettent d'identifier la source de l'information et, dans certains cas, d'interdire la copie de documents sans permission écrite. Pour obtenir de plus amples renseignements : http://lois.justice.gc.ca/fr/showtdm/cs/C-42
UNUSUAL KINETIC BEHAVIOUR IN CATHODIC
H2 EVOLUTION FROM KF.2HF MELTS AT
MILD-STEEL AND ALLOY ELECTRODES
Apart from its interest asa"moderprocess for kinetic studies of gas-evolving electrode reactions, the cathodic hydrogen evolution reaction (her) is involved
in severalprocesses that are of importance in applied electrochemistry, e.g., asthe cathodic co-reactions in the commercial production of 012 and F2: in
electrolyses for production of hydrogen, In electrochemical hydrogenation
and, indirectly, as the partiai process in corrosion of base metals in the
absence of oxygen.
The present paper reports several aspects of the unusual behaviour of the her thatis involved as the co-reaction to electroiytic production of F2 from KF/HF melts using carbon anodes and Fe or mild-steel cathodes. Anomalous anodic
polarization behaviour that arises in electroiytlc generation of F2 from KF/HF
melts at carbon electrodesis well known. Itis characterized by vel)' high Tafel
slopes associated with "CP' film formation and by sluggish F2 bubble
disengagement from the electrode. Interest in such effects has, however,
tended to overshadow rathersimilar, so-called "hyperpolarization" effects that
a/so arise in the cathodic reaction, but for different reasons. The origins and
nature of such effects are described and characterizedI)) the present paper,
and comparisons are made between behaviour of H2 evolution from KF.2HF
and that from the aquo-system analogue, KOH.2H20. Animportant aspectis
the vel)' different intelfaciai behaviour in KF/HFor KF.2HF melts from that in the
corresponding aquo-system.
Effects of As-species in HF feeds is investigated in terms of electrocatalysis
and poisoning effects on the her.
(1 )
coupled interfacial effects which are the SUbject of the present paper which will bring together several aspects of such anomalous behaviour, partly in review form, with comparisons with conventional her behaviour from the H20 medium.
Formally speaking, It is to be noted that the reactions of F2, C12, Br2 formation follow the same kinds of two or three-step mechanisms, involving a bound monatomic intermediate, as does the her. Hence, from the electrode-kinetic point of view, similar mechanistic pathways can be formulated and well known diagnostic tests for their applicability can be employed. However, owing to unusual interfacial behaviours that arise in KF/HF melts both at the anode and the cathode of F2
+
H2 generating cells, electrode-kinetic analysis of both these processes become complicated by unusual interfacial effects.
The commercial and laboratory preparation of elemental F2 is usually conducted by electrolysis of KF.HF or KF.2HF melts, using C anodes and Fe or steel cathodes. The overall electrolytic reaction, e.g. for KF.2HF, is the 2-electron process
with K+F remaining In the melt. The temperature re-quired for the above reaction is determined by the ratio of HF to KF in the melt composition. For low temperature operation at ca.
eooc,
the bisolvate, KF,2HF, is preferred. The H2 is evolved at an iron or mild-steel electrode while F2 is generated usually at a carbon anode. In the KF.2HF melt both processes exhibit unusual electrode-kinetics, but for different reasons.B.E. CONWAY' S.Y. QIAN2
'Chemistry Department, University of Ottawa, Ottawa, Canada 21nsutute for Research In Construction, National Research Council, Ottawa, Canada REVIEW PAPER
531.3:66.092.094.25.097:620.193
The cathodic hydrogen evolution reaction has become a model electrochemical reaction for the development of kinetics of mUlti-stage electrolytic reactions and the fundamental principles thereof. It was also the process in which concepts of electrocatalysls were first based [1,2] and is thus also of major historical and contemporary interest in electrochemistry. Cathodic hydrogen evolution has also become of preeminent interest in several processes Involved In applied electrochemistry, e.g. as the co-cathodic reaction to the anodic processes of F2 and C12 production, as the primary reaction in electrolytic generation of pure hydrogen and of deuterium, in persulfate production at Pt and indirectly in electrocatalytic hydrogenation reactions (3J and in formation of metal hydrides.
Historically, most experimental work has been conducted in aqueous solutions but "medium-effects" in the kinetics of the her were studied In some detail by Bockris [4,5] in a variety of organic solvents in relation to proton solvation (6].
An important medium-effect of a somewhat different kind arises with regard to the commercial process for anodic F2 production from anhydrous KF. HF or KF.2HF melts where the co-cathodlc reaction is discharge and H2 evolution from the HF component of the melt, rather than from H20. These latter processes exhibit some qUite unusual electrode-kinetic and
Author address:
a.E.
Conway, Chemistry Department. University of Ottawa. 10 Marie Curle Street, Ottawa, ON. K1 N 6N5,Canada E-mail: wpell@science.uottawa.ca.B.E. CONWAY, et al.: UNUSUAL KINETIC BEHAVIOUR IN... Chern.Ind.58 (8) 259·270 (2004)
Note that the KF.HF system, in HF as solvent, is the analogue of the KOH system in water as regards "acid/base" behaviour, so protons are discharged from HF as the proton-source in the process HF
+
Fe+
e---> FeHad.+
c --> 1/2 H2t
at, e.g. an iron electrode, analogous to H2 evolution from alkaline water solution where H20 is the proton source. Process (1) is thus anaiogous to the electrolysis of aqueous KOH where proton discharge from H20 provides the evolved H2, leaving the OH- ion. The cathodic half-cell reaction in KF-HF melts is hence, by analogy,セN 5.0
"
セ > 4.0,
セ
。N
セ
'.0
••
(2) In studies of anodic F2 generation, much work has been conducted on the electrode-kinetic behaviour of the anode reaction.2,
oMGセbM[BBB⦅QヲMM⦅G[[XMMェ⦅sBBBMMZ⦅セTMMェ⦅G[MMMZ⦅Z[RGMZ⦅G[iMMAo
Log (1/Acm03)
Figure1,High anodic Tafel slope behavIour for F2 generation at a
non-polished Ahone-Poulenccflrbon anode.
It is not possible to conduct anodic F2 evolution at regular metallic electrodes owing to strong corrosion, spalling or passivation. Hence reaction 3 has to be carried out at special kinds of C, e.g. prepared from pitch (Rhone-Poulenc material) as the anode material. Then little corrosion of the anode takes place but some fluorocarbon gases are produced in small amounts in
side-reactions,
Historically, the first successful preparation of F2 was by Moissan (1880). Pt/lr alloy eiectrodes were first used but later it Was found that Cu anodes could be employed at which F2 is formed at a CUF2 film (not completely non-conducting) on the Cu. However, for commercial, large-scale electrolyses, Cu is not a
practical anode material.
Unlike the chemically analogous anodic C12 evolution reaction [7,8], which behaves
electrode-kinetically in conventional ways at various
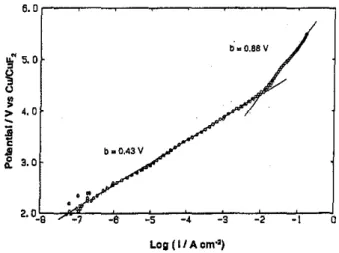
metal anode surfaces, eXhibiting Tafel slopes (at ca. 298 K) of 2.3 x 2RT/F, 2.3 x RT/2F or 2.3 x 2RT/3F [1,2J, anodic F2 evolution at the required C anode material usually exhibits unusually hi9h Tafel slopes (sometimes approaching limiting currents) having values 3 or 4 times 2.3 x 2RT/F or even greater, up to ca. 8 times 2.3 x 2RT/F! This behaviour has been referred to as the "anode effect" in earlier literature (cf.ref.11) and has been studied in several earlier papers by the present and other authors [9-12J. It is exemplified in Fig. 1 for anodic F2 evolution from KF.2HF melt on a Rhone Poulenc carbon electrode.
Interestingly, anomalously high Tafel slopes are also observed in anodic O2 or H2 evolution at the valve metals, Zr. Ta, [13, 14J and also at anodized Au or Au/Pd alloys [15J. Such effects are now clearly recognized as due to development of barrier - layer oxide films, which impede electron transfer due to non-chmic, high-field, resistance effects. Onset of the "anode effecr' is usually associated with the carbon surface taking on an
, , - - - ,
Figure 2. Electrode rotation effects on anodic Tafel polarization behaviour in F2 evolution fromKF.2HFmelt at a carbon electrode
(temperature 85°C): 0 (zero ,p.m.); x (3600 r.p.m.);' (8100
r.p.m.). (Increasing directions of Jog I are the lower curves).
-.
-
.
-.
Log(IIA」ュセI.
,
.,
3 7apparent mirror-like reflectivity, with F2 evolution in the form of "slimey" flat bubbles being discharged sluggishly into the melt at the top of the electrode. Their behaviour is quite different from that of normal, "soda-water' type bubbles that characterize, e.g. CI2 evolution at Pt.
Evidently a thin gas film covers the anode surface. Also,
interfacial capacitance measurements on carbon
electrodes thet are exhibiting the anode--€ffeet, indicate an unusually low capacity on the order of 2-3 flF cm-2 rather than a typical anode polarization capacitance for c at e.g. Hg having a value of ca. 25-30 flF cm-2. This suggests that the low value is that of the dielectric capacitance of a poorly conducting "CP' film [16]. Such a conclusion has been supported by surface-chemical analysis.
Experiments already pUblished [12J show that the Tafel polarization behaviour at unpolished earbon-cone electrodes can be significantly modified by electrode rotation (Fig. 2) from 0, through 3600 to 8100 r.p.m. The polarization is significantly diminished by ca. 1.1 V over
....
.
,
セ
"
セ 5 >,
..
'" 4l
(3) 2c--> F2+
2e 260B.E. CONWAY, etal.: UNUSUAL KINETIC BEHAVIOUR IN...
most of the voltage range but the Tafel-slope itself is little changed. This suggests that the electrode rotation helps to displace the evolved F2 from tha surface but does not affect the underlying "CP' anode film. Note that, above ca. 10-3 A cm-2, there is hysteresis between the positive-golng and the subsequent descending polarization values, dependent on electrode rotation rate (Fig. 2).
Much work has been carried out on the F:M!volution reaction at C because of its commercial significance and In order to establish the origin of the high Tafel slopes, and has formed the basis of our earlier works in this fieid [12,16,17]. Two principal, but not mutually exclusive, factors have been recognized as leading to the anomalously high Tafel slopes, as follows: a) anodic formation of semi-insulating, thin barrier-iayer films of "CF" species at the C anode surface and b) formation of quasi-2-dimensional lyophobic F2 - gas films at the C acting possibly also as a barrier to electron transfe, (In fact, at some C-anode materials, such gas films lead to a mirror-like appearance of the C surface as soon as F2 is generated). This behaviour also leads to difficulty of F2 - bubble nucleation and displacement from the C surface.
Study of the above effects, which is not its subject of the present paper, led us to examine if analogous effects arose in the behaviour of the conjugate cathode reaction of H2 evolution (Eq. 2), normally conducted at Fe electrodes. This was found to be the case and Is thus the subject of the work presented here. Studies of the
cathode reaction in F2 cells have, historically, been
eclipsed by works addressed to the "anode effect" which was perceived to be at more significance with respect to the problem of efficiency and power consumption in operation of the commercial process. However, partially successful procedures for diminution of anode polarization of C [16,17J have led us to focus on origins of cathodic polarization (the her from HF) of Fe cathodes Which, though not so serious as that observed anodically at C, also presents some unusual aspects not encountered in studies of H2 evolution at metals from
aqueous media.
EXPERIMENTAL
i) Mild-steel and electropiated Ni-Mo-Cd composite cathode materiais
A mild-steel, rotating cone electrode, having 45° geometry, was fabricated and mounted in a cylindrical Teflon sheath. The upper end of it was a threaded mandrel that could be screwed into a Pine Instrument
Co. rotator. Use of a rotating-eone electrode
arrangement was found to be necessary in electrolytic experiments in moiten KF.2HF at B5°C, in order to maintain homogeneity of the melt up to the eiectrode sUrface during electroiysis and to facilite disengagement
Chem. Ind. 58(5)259-270 (2004)
of H2 bubbles. A similar carbon-cone electrode was used in the F2-Qeneration experiments (Figs. 1 and 2).
Comparative measurements were made on electroplated Ni-Mo-Cd composite electrodes haVing good catalytic properties (ct. refs. 1B, 19) for cathodic evolution of H2. The composition of such electrodes was Ni (BO at.%), Mo (19 at.%) , Cd (1 at.%).
The counter-electrode in the cell was a small block of the Rhone-Poulenc carbon used commercially, supplied to us by Cameco Corp., Port Hope, ON.
The reference eiectrode was FejFeF2 made by anodizing Fe in the fluoride melt, or sometimes CU/CUF2, both of which give stable potentials in the fluoride melt.
Ii)Electrolyte meit
The KF.2HF melt was prepared by slow stoichiometric addition of HF from a cylinder to potassium bifluoride KHF2 in a cooled vessel until the 2HF-solvate was formed as determined graVimetrically. The HF (Matheson Co.) was 99.9% pure and the KHF2 (Johnson Matthey) was of >99% purity.
Experiments
were
conducted in a specially made Teflon cell maintained in an air thermostat operated, in most experiments, at BOoC,iii) Instrumentation
Steady-state polarization and potential-relaxation measurements were made using an Hokutu Denko HA-301 potentiostat. Data were transmitted to, and recorded and processed by means of an on-line HP mini-computer. In some experiments, time-dependent potential data were recorded on a Nicolet 310 digital
oscilloscope. Some experiments were conducted
potentiostatically using an Hokutu Denko instrument with a programmabla function generator in the usual
way.
RESULTS AND DISCUSSION
i) Cathodic hyperpolarization and its comparison with
the "anode effect".
In order to place the observed anomalous cathodic polarization effects (referred to here as
"hyperpolarizationl
?
in the context of the "anode effect'*,
we first refer to Fig. 1, already shown above, for the steady-state, anodic, Tafel polarization results for F2 generation on a typical Rhone Poulenc carbon electrode, that had been polished under methanol. Tafel behaviour is well defined over a range of current-densities from ca. 10-7 A cm-2 to 10-2 A cm-2, with a Tafel slope of 0.43V Beyond 10-2 A cm-2, Tafel behaviour is still maintained but with a slope almost doubled, viz. O.BBV The anodic Tafel polarization behaviour is found [12,17,19] to be dapendent on pretreatment conditions applied to the carbon anode material, e.g. fine polishing of the electrode under methanol. Polishing tends to generate better defined
B.E. CONWAY, at al.: UNUSUAL KINETiC BEHAVIOUR IN... Cham. Ind.58(S)259-270 (2004) -[
...
NMMMMセMMMMセMMMセMMMMML!i
!
•
-,
-2 -3 Nセ..
..
-.
,
'E
S0 -,2..
-,1•••
·Ls- , 9 , - - - ,
-.
,
Log (I / A cm4)Figure3. Effects of electrode rotation rate on Tafel relations for the her at the mild-steel electrode in KF.2HF melt (BOoC):++ +0 r.p.m.; ... [;00 r.p.m.;
xxx
1600 r.p.m.; 0002500 r.p.m. and ***3600 r.p.m. (Increasing rotation rates progressively increase logI
values, including the limiting currents). N -,7
セ
-.6 "" セ > -.4-Application of rotation of the electrode enabled the "shut-off" effect in Fig. 3 to be understood from results of the experiments to be described below.
It is clear, from comparisons of the forms of the polarization curves of Figs.1 and 2 with those of Fig, 3, that the hyperpolarization effect in the cathodic process at mild-steel is qualitatively different from the
"anode-effect" at carbon.
Evidently, upon rotation of the mild-steel cone electrode, the hyperpolarization behaviour is relieved to extents related to the electrode rotation rate. Under galvanostatic polarization at a stationary cathode, depending on its applied current-density, continuous, constant current passes for long periods of time, e.g. 28 h, as exemplified in Fig, 4, However, the potential eventually jumps In a delta-function way to an hyperpolarization value of ca. 8V(!) remaining constant at that value, onwards in time for a long period,
-0.9
セN
-s .•
セ >1;
-4.0I
.. -, .• If ! '
-Tlme/ hourFigure 4. Sudden potential jump in cathodic polarizationatthe mild-stael electrode after 27h polarization duetoconsumption of
HF, with changeofm.p.ofthe electrolyte(seeFig. 7).
Tafel relationships, as in Fig, 1, The sudden change in
the direction shown, would conventionally correspond to
a change of mechanism of the rate-<1etermining step of
the her from one step to another in a series, consecutive
scheme of reaction steps, (et. ref,20), However, here the sudden change could arise from a discontinuous change in the non-ohmic resistance of a barrier-layer film (et. refs. 13, 14),
The anomalously high Tafel slopes, ca, 4 to 5 times 2.3 x 2RT/F (transfer coefficient
a
= 0,5), found for 02 evolution at anodized Au/Pd alloy electrodes [15) or for H2 evolution at Zr02" covered Zr electrodes [7,8J, arise because a substantial fraction, y, of the overall metal/solution potential differenceM,
falls across the barrier-layer film and the potential difference across the dOUble-layer, determining the electrode-kinetic rate of reactant discharge, is then ca. (1-')j 6$, Assuming the actual charge-transfer coefficient remains ata
(=0.5) over the dOUble-layer, the overall transfer coefficient becomes a(1-')j. Hence, high-valued Tafel slopes canarise as 1-')'becomes smaller, Le. as larger fractions of
the overall
M
fall across the thin, barrier-layer across which an high field arises,In order that high Tafel slopes be maintained (Fig, 1) over appreciable ranges of potential, the film must
behave as a norr-ohmic resistance across which
electron-transfer is a fjeld-assisted activated process, as
treated in refs. 13, 14 and 15, For this condition, the transfer coefficient for the field-assisted passage of electrons across the film combines multiplicatively with that for electron-transfer in the proton-<1ischarge process across the double-layer, leading to unusually small values of the overall transfer coefficient, hence to high Tafel slopes, as also for the "anode effect' but for a chemically different film,
Ii)Cathodic hyperpolarization behaviour at mild-steel electrodes in KF.2HF
During our extensive studies on the anode effect, it came to our attention that, in the cell room of F:Mleneration plants, anomalous cathode polarization (referred to here as "hyperpolarization") also arises under some conditions, StUdy of such effects led to a series of results now to be presented and collated in the following sections of this paper.
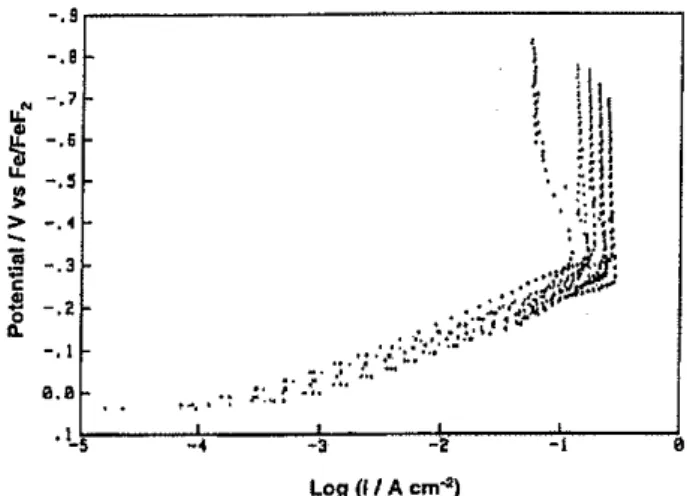
Figure 3 shows a series of cathodic polarization curves for H2 evolution, at five rotation rates, from the fluoride melt at the mild-steel cone electrode, From 10-4 to 10-1A cm-2, a more or less normal value of the Tafel slope (105± 5mV, cf. ref. 20) arises but at ca. 10-<l·9 A cm-2 the electrode process is suddenly shut down, seemingly like In passivation, The transition to the observable limiting currents in Fig. 3 is too sharp to be accounted for by normal diffusion-control limitation, nor can passivation, in the normal sense with anodic reactions involving oxide-film formation, be the reason
forthe observed behaviouc
BE CONWAY, at.1 : UNUSUAL KINETIC BEHAVIOUR IN", Cham, Ind, 58 (6) 269·270 (2004)
°
0.' Mole fraction of HFa.
tI
I
00 f--.I
I-/
°
I/
Oセ
セ
I
セiセ
"
,
セ セ セN-
"
°
•
•
セェ
H
'.0,
,
,
.-
900rpm,
,
\
1600rpm Nセ,
,I-250()rpm,
-2.BFigure 5. Progressive diminution of cathodic hyperpolan"zation fit the mild-steel electrodebyincrease of electrode rotation rate.
1"'"
セ -G.l!l >-:s:
MセN・J
-Ul,e,,'
-Ie 9 1& 28 38 ole 59 60 7Q 88 S8 10l!lTime/sec.
The hyperpolarization potentials, Vhp, for cathodic H2 evolution, thus established, can, however, be brought down substantially by rotating the electrode, as shown in Fig. 5, A rate of 900 r.p,m. has little or no effect but increasing the rate to 1600 r,p,m, cuts down the hyperpolarization by 50% While rotation at 2500 r.p.m. almost eliminates it (Fig, 5),
Figure 6 shows the effects of successively decreasing the rotation rates, followed by increasing them: clearly, the hyperpolarization effect is decreased by increasing rotation rate and the effect is "reversible"
with respect to changes of increase, relative to decrease, of the rotation rate.
These resuits suggest that the cathodic hyperpolarization effects arise not in the way they do at the carbon anode (this is, of course, hardly possibie) but on account of the state of the KF.2HF melt at the steel
electrode interface. Various considerations led us
ultimately to conclude that the cathodic hyperpolarization is not associated with the interfacial kinetics of the her itself at mild-steel but rather due to
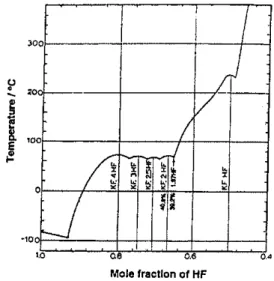
Figure 7.Phase diagrBm for KF.HF melts showing sudden rise of melting point atacritical composition, KF.1.87 HF.
local solidification of the melt in a thin, but still marginally conducting, film of KF.HF arising from local electrolytic removal of HF from the interphase, reSUlting in a rapid increase of melting point.
inspection of the phase diagram for the KF/HF system, shown in Fig. 7, provides an explanation for the above effects, Thus, in an unstirred soiution, the local composition of the KF.2HF melt can move to the right in the phase diagram from the 40,8% HF to a critical composition of KF.1.B7 HF (see Fig.7), beyond which (with further electrolytic removal of HF) a rapid rise of melting point to 240°C for KF.1 HF can take place, resulting in solidification of the electrode at the
meltlsteel interface, Application of electrode rotation
evidently progressively prevents the solidification of a thin film of KF.XHF (X < 2) at the electrode surface, depending on the rotation rate (Figs, 5 and6),
Effects of progressiveiy increasing rotation rate on the cathodic hyperpolarization are shown in terms of
BBLBNMMMMMMMMMMMMMMMMMセ
",
..
,,---,
2600 2600 -2.9 t800 -e.0....
i
"s .•
セ>
-- -4.!J
3000 (1600) e.,Bto,LMMヲMMMMLARMMBBGZMMMNセMMUセMMAMsMNNNNNNLiL
Time/min.,
ᄋGッlMMヲMMMMLARMMKMMMANMMMMゥGGMMMMゥVセMセ Time/minFigure 6. Reversibility of electrode rotation-rate effect with
In-crease, then decrease of rate, on the cathodic hyperpolarization,
asin Fig. 5.
Figure 8. Progressive decrease of cathodic hyperpolarization
<uponsuccessiveincrease of electrode rotation in seven stages
B.E. CONWAY,at al.: UNUSUAL KINETIC BEHAVIOUR IN.. Chern. Ind. 58 (6) 259·270 (2004)
Figure 9. Effects of increasing u/trasonica.tion intensity on
cathodic hyperpolar;zab'on in the her at the mild-steel electrode
in terms of distance of hom from electrode surface.
Figure 10. Potentiostatic current vs. potential relations for carthodic H2 evolution ata mild steel electrode in the KF.2HF
melt nearthecritical condition for onsetofhyperpolarization. Ae-gions indicated asA, B, C,etc are identified in thetext.
A more detailed examination of the onset of
hyperpolarization, studied under potentiostatic conditions, resulted in the current vs potential relations shown in Fig. 10. In the region A .... B, polarization progressively increases up to region B at which onset of hyperpolarization takes place at a potential Vhp = 1,0 V vs Fe/FeF2, Continuation of the polarization in time results in a drop of current (B .... C) by two magnitudes
(2)
""'
""'
F"
+
HF .... HF+
F"with increasing potential to an high value at and beyond C (Fig. 10). At this point. solid film formation has occurred and the limiting current is reached at a value of ca. 10--3·3 A. Decrease in potential from C results in the steep decline of the voltage vs current curve from C down to D, below which the original voltage vs current curve can be retraced towards A,
If the solid film formation (ct, the phase diagram in Fig. 7) at C is allowed to continue for some minutes, followed by tracing the potential vs.current profile for decreasing potentials, a descending and then almost identical ascending potential vs. current profile can be traced; once the film is established at some, steady-state (determined by approximately equal rates of film formation under electrolysis and dissolution-melting-into the bUlk), then a rapidly descending / re-rising potential vs. current can be followed. Thus, from Fig. 10 it is seen that in the presence of the supposed solid-state film of KFxHF(x<2), currents in the presence of the solid film are reduced by ca. 2,5 magnitudes (log scale), butGセイ。ョウMー。ウウゥカ・B types of current can still pass through the film once it has become established into region BC, since the solid fluoride film remains partially conducting (see below).
The hyperpolarization potential vs current profiles in its "solid-state" film region (Fig. 10) are obviously quite different from the Tafel-type polarization lines of Fig. 2. The former have the shape of curves where linear ohmic polarization effects dominate over the logarithmic Tafel polarization for the cathodic H2 evolution process. Thus, it is instructive to re-plot the data for the "solid-state" polarization region in Fig. 10 on a linear current scale; this is shown in Fig, 11 from which it is clear that the hyperpolarization behaviour is ascending and descending changes of current is largely due to SUbstantial, regUlar (rather than BィゥァィMヲゥ・ャ、Gセ ohmic resistance, IR-drop, effects in the solid fluoride film.
It is clear from the curves of Fig, 10 that significant. potential-dependent currents can still pass through the deposited fluoride film which does not completely block passage of charge for discharge of HF (2HF
+
2e .... H2+
F). Since the electrolyte, and probably also the film, contain excess HI': the conduction mechanism could be analogous to that in aqueous KOH Where proton-jumping provides the well known anomalous conductance [6] of that system, Thus, in the HF/KF system, a similar proton transfer conductance mechanism can be envisaged as:which can b'e continuously repeated between the gener-ated F" ion on the right-hand side of process (2), as in the aqueous case of OH-/H20 [21].
In the above conductance mechanism, probably the bifluoride ion, FHF", is an involved intermediate in
lOG
-,
-,
7rnm iNセ 13mm*-.:«
'-... 1.1
I' Nセ|G no ultrasonication ";."t.\I
""'-....! /
.
'.
, ...
セNゥBLエG .. セGゥQM..
.
\'\
I •.•• \ .' f •+ Current densityIA cmo2.
セiN : ... iNセ セU -.-3-Log(II A em")
.
•. c
ZセMM Sol1d HIm formation
g
ャ|セ
"セ
..
.
";
".
.
=/=I ....
NBBGG[[LZセiセセエゥGB
Solidslste:- .
: .. B.-. .-.
.
. .
.
. .
.
..
.
_ .
.
:- ; Mottenstatl).". . . . :
...
••• 0 : •...
NNセN A • •,j' セNG E ' -I.S -1.7 -1.5 u:'セ
-1.3..
-1.1 セ >-.'
セ
-.7セ
-.S D--.3 -, I..
t,•
.1 iNセ iNセdecrease in cathode potential as rotation rate is sequentially increased from 1800 up to 2500 r,p.m. in seven stages (Fig. 8).
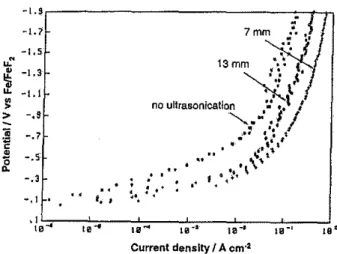
Ultrasonication has a similar effect in diminishing the tendency for onset of hyperpolarization, as illustrated in Fig. 9. Local ultrasonication intensity at the electrode surface was varied by fixing the horn at two different distances, 13 mm and 7 mm, from the electrode and comparing the effects with the polarization
behaviour in the absence of ultrasonication(* points on
Fig. 9). "'I. S -1.7 --1.5
..
"-La
•
セ
-LI セ-,'
> -,7セ
-.S J!l 0 -.3 D--.1 .L7-,
264B:E; CONWAY,at al.:UNUSUAL KINETIC BEHAVIOUR IN ...
.
",- - - . . . ,
Cham.Ind.58 (6) 259-270 (2004)
1.0E-G4
•
2.0E.()5 4.0e.Q5 ME·OS 8.0E-QS Current densityIA em'!
....
...
MM⦅MMセMMMセMM⦅MM⦅ャ o.OE+OQ ·'.6 ... ·1.4セ
.'2.
..
セ.,.•
>i
-0,6.!i .•.•
o Cl. -0.4Figure 11. Indication of linear ohmic polarization in the "solid-state" polarization of Fig. 10.
the KF.2HF melt that becomes starved of HF at the electrode interface during hyperpolarization.
These considerations confirm that the physical and electrochemicai significance of cathodic hyperpoiarization is quite different from that which arises anodica!iy in F2 generation at the carbon anode (Figs. 1 and 2), as treated in other earlier literature from our own [12,16,17] and other [9,10,11
J
laboratories.iii) Electroiyte wetting effects in cathodic polarization in the KF.2HF meil
Unusuai effects, of another kind, were discovered in further studies on cathodic hyperpolarization: electrolyte wetting effects and sluggish disengagement of generated H2 bubbles from a Ni-Mo a!ioy electrode, analogous to the similar effects that arise in F2 -bubble generation at the C anode. These cause enhanced poiarization and erratic current fluctuations, phenomena that are also encountered in industrial practise in the
"cell-room",
In order to pursue these effects further, contact angles of H2 bubbles at a Ni-Mo-Cd composite electrode surface and at a smooth, bulk 94% Ni-B% Mo a!ioy (see section iv) were measured by means of a contact-angle goniometer. Comparisons were made between the contact angle of H2 bubbles generated from the KF.2HF melt and thet for bubbles generated from an aquo-analogue melt, KOH.2H20, at the same temperature, BOoC. Photographic pictures of an H2 bubble generated at a Ni-Mo-Gd composite electrode in KF.2HF (Fig. 12a) are compared with that in a KOH.2H20 (Fig. 12b) melt, both at BOoC. The advancing contact angle in the fluoride melt is 102° while that inthe KOH.2H20 melt Is substantially more, 136°, a difference which leads to visibly different facility of H2 bubble disengagement in the two melts, that in the fluoride melt being much less facile. Note for comparison, Hg on Au has zero contact angle (complete wetting) while on glass it has> ca. 150°.
Figure 12. Comparison of wetting effects of the KF.2HF melt vs the KOH.2H20 melt at the Ni-Mo composite electrode asゥョ、ゥセ
catedby difference of contact angles at recumbent H2 bubbles:
aJ
contact angle in KF.2HF; bJ in KOH.2H20.The geometry and adherence of a drop or bubble at a three-phase interfacial contact is determined by Young's equation [22] which relates the surface tension values for the three, two-phase interfaces (solid/liquid, solid/gas and liquid/gas) to the contact angle of a bUbble, as visually measured in this part of the work.
In the photographs in Figs. 12a and 12b, the spherical forms are, of course, the bubbles while the flat bottom parts are the surfaces of the electrodes, both of them in contact with the electrolyte melts (the bright upper regions). From the "shapes" of the recumbent bubbles at the electrodes (i.e. the extent that they appear spherical) it can be seen how It is possible for the bubbles to be more easily detached from the electrode surface when the latter is in contact with the "aquo"-melt than with the fl uoride melt.
The average residence time of recumbent H2 bubbles (much greater in the fluoride melt than in the "aquo"-one) on the electrode surface will certainly influence the active accessible areas of the electrodes in the two melts and also influence the uniformity of the diffusion-fieid. This conclusion ',s also supported by measurements of the interfacial double-layer
B.E. CONWAY, at a/.: UNUSUAL KINETIC BEHAVIOUR IN;"
capacitance of the two types of interlaces (see section v below).
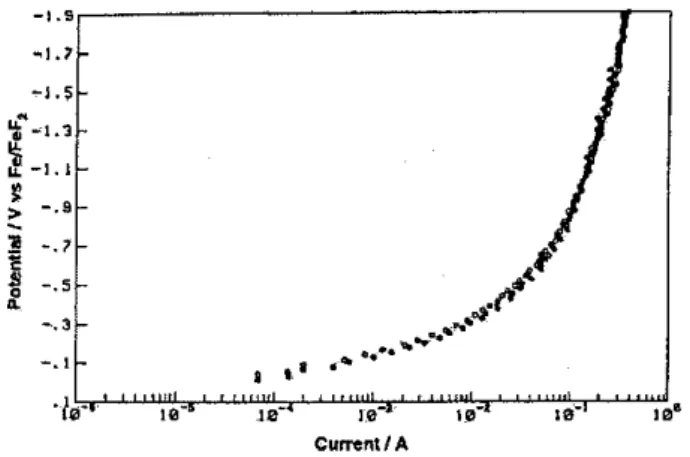
In the light of the above results, it was of interest to compare directly the Tafel relations for the her from the "aqua" and ''fluoro'' melts under otherwise the same conditions (B50C here) and using the same Ni-Mo-Cd composite cathode. Figure 13 shows this comparison, directly on an "overpotential" scale, over 3.5 decades of current-density. Cathodic H2 evolution can evidently be achieved at rates which are ca. two orders of magnitude greater in the KOH.2H2 0 melt than in the KF.2HF one, at the same tempenature, independent of iR corrections that 'can be made to the two "parallel" but curved ascending current-density vs potential Tafel plots in Fig.13. A contributing factor determining this large difference is, we believe, the microscopic accessibility of the two electroiyte melts to the rough, porous interface and the porous interior of the composite electrode arising from the different wettabilities of, or contact angles of H2 bubbles (as in Fig. 12) to, the electrode. This conclusion is supported by the clearly different interfacial specific capacitances of the composite electrode in the two melts, as shown in section v. However, while electrode rotation in the KF.2HF melt doeS lead to some increases of current at high current-densities (as in Fig. 3), it is insufficient to account for the large differences of currents in the two Tafel plots in Fig. 13. Therefore an electrode-kinetic effect must be sought as a basis for the difference.
Such an explanation could be given if the activation energy for proton dissociation and discharge from HF were substantially different from that from H20 in the aqua-system, also taking into account the expected difference of solvation energy of the resulting F ion in HF from that for the corresponding OH- ion in H20, based on the principles of Butler's treatment of proton discharge at electrodes [23]. Thus, the H-to-F
-0.4
,
I
,
/ 00.3i
>,
,
./
セ
l•
..
'セ
-Q.7' セBGゥ.'
セ.
0.
..
セoNQ.
..
..
[セ ·3.,
.,
•
LoglilAem'')
Figure 13. Comparison of currentvs potential relations for the
cathodic H2 evolution at the Ni-Mo-Cd composite electrode
(be-low onset of hyperpolarization) from the KF.2HF snd the KOH.2H20 melts each at85°C: +in KF.2HF; * in KOH.2H20.
286
Cham. Ind. 58 (6) 259·270 (2004)
bond-energy in HF is some 160 kJ mole-1greater than that of H-to-O in H20 (due to the difference of electronegativity between F and 0) which could account for a significant fundamental electrode-kinetic effect but this would be difficuit to evaluate properly since it should also take into account the "medium-€ffect" giving rise to the difference of solvation energy of F and OH- in the two melts, an unknown quantity.
iv) Electrocatalysis aspects
The anomalous electrolyte effects observed (see Figs. 9 and 10) at mild steel restrict any reliable electrode-kinetic analysis of the her from the KF.2HF ュ・セ being made since those effects largely influence the current vs potential behaviour in unusual ways. Thus, mechanistically-signifieant Tafel slopes are difficult to evaluate though, under electrode-rotation conditions (Fig. 3) at the mild-steel electrode, an approach to linear Tafel behaviour is seen with Tafel siopes having values between --{I.l1 and --{I.13\( i.e. normal values, e.g. for protor>-discharge control.
Because of the commercial importance of minimizing the much higher overvoltages (both anodic and cathodic) encountered in the industrial process of F2 production from KF-HF melts, we made some attempt to evaluate an improved cathode electroeatalyst.
Much interest has arisen regarding the preparation of electrocatalysts for electrolytic H2 generation in various works of Jaksic et al. [24,25J based on concepts of Brewer [26J concerning electronic structures of alloys made from elements on the left-hand side of a given transitior>-metal series with those from the right-hand side.
Along such lines, we have prepared bulk and composite Ni-Mo alloy electrodes as cathodes for cathodic H2 evolution from the KF.2HF melt and from aqueous solution. A bUlk, single-phase alloy of composition Ni (94 at.%) - Mo (19 at.%) and an electrolytically prepared Ni (BO at.%) - Mo (19 at. %) - Cd (1 at.%) composite were compared. Such composites have been found to give
< (-)
100 mV of cathodic over-potential at 100 rnA cm-2 [20J.Using the composite electrode, current - densities in the KF.2HF melt, at given overpotentiais, are about two orders of magnitude greater than those at the mild-steel or the bulk Ni-Mo electrode under otherwise identical conditions (BO°C). This difference corresponds mainly to the much larger specific area (roughness or porosity factor) of the composite electrode in comparisen with the specific areas of the mild- steel or the bulk Ni-Mo alloy electrode. Thus the "reai-area" factor in electrocatalysis(ct.ref. [16]) seems to dominate over electronic alloying effects, based on Brewer principles [26], at least when the real-area factor is large as it is here, based on interfacial capacitance measurements (see section v below).
B.E. CONWAY.et al.:UNUSUAL KINETIC BEHAVIOUR IN... Cham. Ind. S8 (8)259-270 (2004)
Figure 14. Lack of poisoning effect of AsF4- ion on the her at the mild-steel cBthode alter addition of As-species (0) as As20S,
relativetocontrol experiment in its absence (.).
Ie' o I
;
.e-·1 -4 -3 -2Log(IIAem.)
a
.1
-loS ... 1.7 .,.l.\i セBGMGQNGSセ
-1.1 セ -.9::
セ
-.7 Sa -. S a -.J-.'
./.-
••
u...-1.5•
セ
-1.1 セ >..
-0.71
..
-0.3 0.1 -8 -5interference with chemisorption of the adsorbed H intermediate in the mechanism of cathodic Hz evolution [20,30]. The action of As in this way can arise from its electrolytic deposition as As (0) on the electrode and, further, its subsequent reduction to AsH. (arsine) which is itself a strong poison like HzS.
Experiments were conducted at an Fe cathode in the presence of As added as KASF4 and, comparatively, as KAsFe, relative to a control experiment using the pure melt without As. Figure 14 shows the polarization behaviour 3.9 h after addition of KAsFe at 0.07M; it was indistinguishable from that in the control experiment. However, As added as KAsF4 or AszO., at the same concentration, gave rise to major effects on the cathodic polarization curve as shown in Fig. 15. Not only is there a substantiai inhibition effect in the ascending direction of polarization but hysteresis becomes exhibited between the ascending and descending curves after approach to a limiting current at ca.10-2.3A (Fig. 15).
Figure 15.Substantial poisoning effect on the her (compare Fig. 14) when the As-species is in the +'1/1oxfdBtionstBte, BS KAsF4, Brising fromAsaddition asAsiJ3(curves for ascending and de-scending chBnges of logI).
v) Interfacial capacitance
A sensitive measure of the properties of interfaces
is their double-layer capacitance. In the present work. in
the context of polarization measurements, the
double-layer capacitance of the composite Ni-Mo-Cd cathode was compared with that of a smooth buik Ni (94%)-Mo (6%) single-phase alloy in the KF.2HF meit at BOoC. The capacitance was determined during the polarization measurements by deriving the initial slope of fast potential-relaxation transients following interruption of the polarization current, following the procedure validated in refs, [27] and [28J, and based on the principles of ref. 23.
A striking difference was found in the double-iayer capacitance value for the Ni-Mo-Cd electrode in the KOH,2H20 melt (73 mF cm-2) compared with that for the same electrode in the KF.2HF melt (810± 10 f1F cm-2). The smooth Ni-Mo bulk-alloy electrode in the same fluoride melt gave a value of 5.9± 0.2 f1F cm-2 indicating that the composite electroplated electrode had a real-to-apparent-area ratio of 135. This was consistent with the appearance and morphology of the composite
electrode viewed under a scanning electron microscope
at high magnifications.
The large difference between the capacitance
values for the composite electrode in the two melts must
be attributed, we believe, to the curious poor wetting characteristics of the KF.2HF melt, indicated by the bubble contact angles and high surface tension, so that the high specific area, composite electrode. having micro-roughness is not so well wetted in the fluoride
melt as in the "aqua" melt.
vi) Electrosorptive and interfacial effects of As species on H2 evolution behaviour
The commercial process of anodic F2 generation is
operated with continuous feed of HF into the electrolytic cells. This HF originates from preparation from a fluorapatite mineral which commonly contains traces of As. This appears in the cell as AsF3 with some AsF5. Both of the5e fluorides become immediately complexed as AsF4- and AsFe-, respectively, by reaction with excess F ions in the melt. It has been found that the presence of traces of As species in the HF feed, arising in this way, leads to deleterious effects in the operation of the electrolytic process of F2 production.
The study of inhibiting effects of strongly chemisorbing species is an important aspect of catalysis and electrocatalysis and indeed has formed an historic feature of the SUbject. As a concluding aspect of this paper. we therefore report the results of some experiments on the electrocatalytic poisoning of the her at Fe electrodes in the KF.2HF melt by As. especially in relation to its speciation (As.11I or As. V) in the melt. It is well known that As acts as a catalytic poison in hydrogenation processes and raises cathodic polarization at transition metals, e.g. Ni, Fe, PI by its
B.E. CONWAY,et al.:UNUSUAL KINETIC BEHAViOUR IN... Chem. Ind. 58 (6)259·270 (2004)
-0.3.---,
Table 1. Comparison of rate-constants for simulationofthe potential-relexEltion behaviour of the her in KF.2HF (Fig. 16) in the absence .nd presence of As (III) species .t1.4x10-5M .
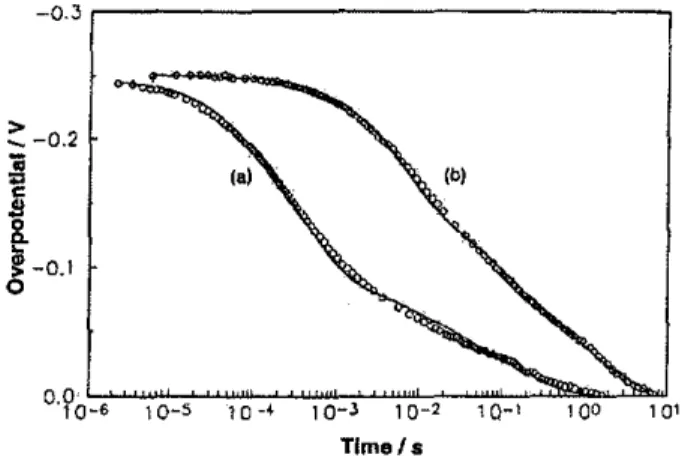
Figure 16. Potential-relaxstion transients associated with
ad-sorbed H pseudocapacitance ata Pt electrode in the KF.2HF melt (80oG) in the absence(CUNSa)and presence (curve b) of
AsasKAsF4 (origInating from addedAS203Jataconcentfationof
1.4x10-5 M. Thin lines represent the simulation behaviour ac-cording to rate-constant values in Table 1.
10' 10-5 ャoMセ 10-J 10-2 10- 1 100 Tlme/s :: -0.2
I
セMoNi
o
Rate contant* Unpoisoned melt Melt poisonedwith As (Iii)
k1/cm s1 2.0x 10-7 1.6x10 10
k_l/s-1 3.0X10-" 2.1 X10-10
k2lcm s-1 3.8 x 10-10 1.0X10-15
k3lmol- 1m s-1 3.8 x 10-14 2.1
X10-11
*k1, k_1 are for the forward and reverse Volmer steps; k2 is 10r the Heyrovsky step andk3for the Tafel step in the her.
proton-discharge イ。エセッョウエ。ョエ from 2.0 x 10-7to 1.6X 10-'0 mol cm-2s-', leading to diminished coverage by H in the presence of As III species as poison.
The potential relaxation transients can be kinetically simulated according to the principies of refs. [27,31) invoiving the rate constants of the three steps of the her. The reSUlting rate constants, giving best fit to the curves a and b of Fig. 16, are shown in Table 1. The simulation takes into account the kinetics of proton discharge and Its reverse, and parallel Heyrovsky and Tafel desorption steps, the latter being the principal H desorption pathway under the conditions of the experiments described here.
From the kinetic rate-constant data the H pseudocapacitance and H coverage in the her at the PI electrode in the absence and presence of the As Iii species (at a concentration of 1.4 x 10-5M) can be derived, as shown in Figs. 17a and 17b. Comparison between those curves demonstrates the major effect of traces of As species on the chemisorption of Hand related kinetics of eiectrocataiysis in these experiments.
Probabiy in the actual experiment, the As species becomes AsH3, as mentioned eartier. Similar results were found for the her at PI in aqueous, 2M KOH in the The lack ofeffect of As when in the
+
V oxidationstate as AsFa- ion is, we believe, due to the greater
stability of this octahedrally complexed and resonance-stabilized ion compared with that when As is present as AsF,-. Under the latter conditions, we have found, by analysis of the evolving H2 gas, that AsH3 is formed (Marsh's test), so that this is probably the species which poisons the H2 evolution process by chemisorption through its donative electron-pair.
Additionally, under conditions where AsH3 can be formed, H2 bubble detachment becomes sluggish with the same consequences as those described insection
iii.
Independent experiments by Gao [31,32J in this laboratory on poisoning of a Pt cathode by As species gave clear indications of effects of chemisorption of the As on the state of chemisorbed H, the coverges by which were substantially diminished already in the presence of low concentrations of As species. Although cyclic voltammetry can be used to reliablY measure changes of coverage by underpotentially deposited (UPD) H at PI, caused by As poisoning, the state and coverage of the kinetically-involved, chemisorbed H intermediate in mechanisms of the her cannot be determined directly by means of cyclic voltammetry as is possible for the UPD H at e.g. PI or Rh. Thus, under conditions of Faradic evolution of H2, the overall currents are orders of magnttude greater than those for UP deposition of H or for the potential-dependent changes of overpotential deposited (OPD) H coverages. Hence, special non-steady-state procedures are required in order to elucidate the coverage by the OPD H that is of interest and primary significance in studies here of electrocatalysis and catalyst poisoning in that process. Therefore, in the present work, the method of recording and anaiysis of ッー・ョセゥイ・オゥエ potential-relaxation transients, taken from various cathodic overpotentials, developed by Conway et al. [27,31,33J, was employed. A more indirect procedure, requiring complex analysis, is impedance spectroscopy.
The above method (ct. refs. 27, 31) of recording potential-relaxation transients, following cathodic polarization at constant current, was therefore used. It provides information on the adsorption pseudo-capacitance arising from the potential-dependence of the kinetically involved, (OPD) H species that is the intermediate in the reaction steps of the cathodic H2
evolution reaction and whose surface coverage is
diminished by competitive adsorption of As species. Figure 16 shows the overpotential-relaxation behaviour at a PI electrode in KF.2HF melt at eooc in the presence of AsF4-poison at 1.4 x 10-5M concentration (curve b) compared with the transient recorded under the same conditions (from an overpotential of -o.25V, RHE) in the As-free, pure melt (curve a). The substantial shift to longer times in the transient for the presence of AsF'- (curve b) is interpreted as a diminution of the
B.E. CONWAY, et al.: UNUSUAL KINETIC BEHAVIOUR IN... Chem. Ind. 58 (8) 259·270 (2004)
the fluoride melt, not observed in the corresponding aquo-system, KOH.2HzO and d) electrode-kinetic effects associated with the greater H-F than H-O bond energy due to the electronegativity difference.
Minimization of cathodic hyperpolarization effects can be achieved by electrode rotation and/or application of local ultrasonication near to the electrode surface in the melt.
Additionaliy, traces of As-species in the HF-feed in the commercial process of F2 production also enhance cathodic hyperpolarization due to electrocatalytic poisoning and to changes of bubble contact angle but the effects are shown to be dependent on the speciation (As Vor As Iii) of the As impurities which determines their adsorption and stability to reduction to AsHs. Ultimately, upon reduction, the poisoning species is
AsH3,arsine.
ACKNOWLEDGMENTS
Grateful acknOWledgment is made to the Natural Sciences and Engineering Research Council of Canada and to Cameco Corporation, Canada, for support of this research. We also acknowledge results of work on As poisoning carried out earlier in our laboratory by Dr.L.Gao. This paper was presented verbally at the Electrochemical Society meeting in Paris, May, 2003.
REFERENCES
(1) W.R. Busing andW. Kauzmann, J.Chem. Phys. 20 (1952) 1129.
(2) A. Parsons, Trans. Faraday Soc. 34(1958) 1053.
[3J P. Dabo, B. Mahadavi, H. Menard and J. Lessard, Electrochim. Acta, 42 (1997) 1457.
[4J J.O.M. Bockris, Disc. Faraday Soc. 1(1947) 95. [51 J.O.M. Bockri$, J.Chim. Phys. 49 (1952) C41.
[61 B.E. Conway, J.O.M. Bockris and H. Linton, J.Chem. Phys.
24 (1956) 634.
[7] S. Trasattl, Electrochim.Acta33 (1987) 369.
[8J D.M. Novak, B.V. lilak anda.E.Conway, Modern Aspects of Electrochemistry 14(1982) 95, Eds. B.E.Conway and J.O.M Bockrls, Plenum Publ.Co, New York.
19} D. DevUliers, F. Lantelme and M. Chern la, Electrochim.
Acta31 (1968) 1235.
[10] N. Watanabe, M. Inoue and S. Yoshizawa,J.Electrochem.
Soc., Japan31 (1963) 168.
[llJ N. Watanabe.J.Fluorine Chern. 22(1983)205.
[12] L. Bai and B.E.Conway, J.Applied Electrochem.18(1988) 839.
[13] J.W. Dewald. J. Phys. Chem. Solids 2 (1957) 65. [14] R.E. Meyer, J. Electrochem. Soc. 107 (1960) 847.
[lSI J.J. MacDonald and
a.E.
Conway. Proc. Roy. Soc.London, A2 69 (1962) 419.
[16[ L. Bal and B.E. Conway, J. Applied Electrochem. 20 (1990) 916.
[17] L. Bal end B.E. Conway, J. Applied Electrochem. 20 (1990) 925.
[181 R. Simpraga, L. Bai and B.E. Conway, J. Applied Electrochem. 25 (1995) 628. 1.00 (.) 0.95 .0.90 セ
•
0.85 0.80 -0.4 .O.S (h) 0.4 .0.3•
セ 0.2 0.10.0
-Q.4 y £ u セ 10 セ "-<t 5 0 0.0Figure 17. Pseudocapacit8.nce (CljIJ and coverage (BI-{) profiles for overpotentiaJ-deposited H at a Pt electrode in KF.2HF (BOoC) in the absence (curves a) and presence of (curves b)As
spe-cies, asAsF4-, derived using the rate constants of Table 1.(Note the scale differences forCtjlandGJHin the two plots).
O"r---c:::====---...,
0.0
0..0G[[MMM⦅Nセ]M⦅NセMM⦅NZZ⦅[⦅MMMMMZA -0.1 -0'.2 -0:.3 Ov.rpotentlal!Vpresence of dissolved AszOs. Note that poisoning effects in the KF:2HF melt are much slower when As is added as KAsFe, due to the high stability of the compiex anion, referred to earlier. The eventual poisoning Is due to slow reductive generation of AsF.- species that are reduced to AsHs at the electrode.
RPイMMMMMMMセMMセMMMML
-0.1 -0.2 -0.3
Overpolentlall V
0.1 _ _
CONCLUSIONS
Anomalous polarization behaviour in the process of electrolysis of KF/HF melts for Fz production arises at both the anode (of carbon) and the cathode (of mlld-steei, Fe, or NI-Mo alloys) where Hz evolution Is the co-reaction. At the C anode, much work has been conducted on the origins of the anomalous high polarization exhibited in anodic Fz evolution. However, hyperpolarization also arises in the her at the cathode but has been little characterized or its origins understood; they are largely different in kind and origin from the anode effects.
The present work reveals that the cathodic hyperpolarization effect originates for severai reasons: a) starvation of HF at the cathode due to HF consumption during electrolysis, when the KF:2HF melt is near the eutectic composition, leading to film formation at the electrode; b) unusuai interfacial capacitance behaviour in the melt; c) sluggish Hz - bubble disengagement from
270
Brian E. Conway
Dr, Conway has held the Natural Sciences and Engineering Research Council Chair of Electrochemistry at University of Ottawa, Canada, where he has led a leading research group since 1956. He is currently Professor Emeritus and continues active research In the fields of interfacial electrochemistry and Its applications to development of double-layer electrochemical capacitors and the fundamentals of electrosorption and electro-catalysis. He Is the single author of four monographs In electrochemistry and ionic solutions, and has published some450 papers in chemistry journals and has received a variety of awards and medals in recognition of his research activities.
Shlyuan Y. Qian
Or. Cian came to Canada to Or. Conway's research group in 1988 from China on a Chi-nesa Government Visiting Scholarship for two years, He remained at University of Ottawa as a Scientific Research Associate, Jater as Senior Research Associate working on the electrochemistry of modified manganese dioxide for Mn02lZn batteries and later on the electrochemistry of fluorine production, supported by Gameco Corporation, Port Hope, Canada. In the period 1996 to 1999, he studied at Ottawa for a Ph. D. degree (granted in 1999), jointly with the University of Sherbrooke. He is now Research Scien-tist at the National Research Council, Institute for Construction laboratories in Ottawa. He is the author of some 12 publications in applied electrochemistry.
Chem, Ind. 58 (6)259-270 (2004)
[271
a.E.
Conway. L. 8ai and D.F. Tessier, J. Electroanal. Chem. 161 (1984) 39,[281
a.E.
Conway and L. Bai,J.ElectroanaL Chem. 198 (1980) 149.[29J J.A.V. Butler and G. Armstrong. Proc. Roy. Soc" London, A137 (1932) 804.
[30) J.O,M. Bockris and B.E. Conway, Trans. Faraday Soc. 45 (1949) 989.
1311 L.Bai, L. Gao and
a.E.
Conway, J. Chern. Soc., Faraday Trans. 89 (1993) 235 and 243.[32} L. Gao, Ph,D. thesis in chemistry, University of Ottawa, 1994.
(33J D.A. Harrington and B.E. Conway, J.Electroanal. Chern. 221 (1987) 1.
BE CONWAY, et el.: UNUSUAL KINETIC BEHAVIOUR IN,,,
(191
a.E.
Conway and L. Bai. J. Hydrogen Energy, 11 (1986) 533.120] B,V, Tilak and B.E. Conway, Adv. In Catalysis 38 (1992) 1, Eds D.O. Eley, H. Pines and RB. Weisz, Academic Press Inc., New York.
[21] a,E. Conway and M. Salomon, chapter 24 in Chemical Physics of Ionic Solutions. Eds. B.E. Conway and R.G. Barral:i8s, John Wiley and Sons, New York. 1966. (22) T. Young, Miscellaneous Works, vol. 1, p. 418, Ed. I.G.
Peacock, Murray, london (1855).
[23] J.A.v. Butler, Proc. Roy. Soc.. London, A157 (1936) 423. (24J M. Jaksic, IntI. J, Hydrogen Energy. 11 (1986) 519. [25[ M. Jaksic, Mat. Chem. Phys.. 22 (1989) 1. [26) L.Brewer, Science, 161(1968) 115,