Radiochimica Acta, 65, 1 8 1 - 1 8 7 (1994) © R. Oldenbourg Verlag, München 1994
Sorption of Nickel and Cobalt on a Size-Fraction of Unconsolidated
Glaciofluvial Deposits and on Clay Minerals
By A. Griitter, H. R. von Gunten, E. Rössler and R. Paul Scherrer Institut, CH-S232 Villigen PSI, Switzerland (Received March 10, 1994; revised May 4, 1994)
Sorption / Nickel / Cobalt / Glaciofluvial deposits / Clay minerals / Cesium / Strontium / Barium
Abstract
In earlier studies we investigated the sorption behaviour of cesi-um, barium and strontium on size fractions of glaciofluvial ma-terials and on the clay minerals montmorillonite, illite and chlo-rite. Here, we report on sorption and desoiption experiments with nickel and cobalt in synthetic groundwater on the < 3 2 μπι size-fraction of a glaciofluvial deposit, on the clay minerals montmorillonite and illite, and on synthetic S i 02 and CaC03. In
general, sorption and desorption equilibrium is not reached for nickel and cobalt, which is in striking contrast to the behaviour observed for strontium and barium. A significant increase in the distribution ratios was observed for nickel and cobalt up to sorp-tion times of 182 and 56 days, respectively. In most cases, the sorption process was largely irreversible. The sorptive power of the < 32 μπι materials with artificially changed mineralogies is considerably reduced as compared to that of the < 3 2 μπι materi-al with the originmateri-al minermateri-alogicmateri-al composition, i. e., during the removal of the carbonates a significant decrease in the sorptive power occurred. A comparison of the sorption behaviour of nickel and cobalt with that of cesium, strontium and barium is also shown.
1. Introduction
The radiation hazard of the activation product 59Ni
(half life: 75 000 years) is taken into account in esti-mates for nuclear waste repositories [e. g. 1]. The en-vironmental behaviour of nickel ions is only poorly known and no data are available about its migration in glaciofluvial deposits. Quaternary glaciofluvial materi-als were selected for the present investigation, since such deposits abound in pre-alpine countries and act as the final barrier before radionuclides released from an underground storage facility enter the biosphere. The glaciofluvial deposits consist of gravel, sand, silt and clay. The clay minerals montmorillonite and illite, and synthetic Si02 and CaC03 are included in our
studies for comparative purposes. Clay minerals are constituents of glaciofluvial materials, are powerful sorbents and will be used as back-fill materials in nu-clear waste repositories.
Cobalt was investigated mainly because unexpect-ed effects were observunexpect-ed for the sorption of nickel. Under our experimental conditions, cobalt and nickel form stable solutions of soluble compounds up to con-centrations of 100 μηιοΙ/1.
Keil
In the literature sorption isotherms are presented in Refs. [2, 3] for nickel and in Refs. [2—6] for cobalt. The results were obtained on widely varying solid ma-terials and under very different conditions and do not allow to draw conclusions of general validity even with respect to the shape of the isotherms: nearly lin-ear isotherms are reported in [2, 3], whereas isotherms of the Freundlich type are shown in [6] and composite ones in [4, 5].
2. Experimental M a t e r i a l s
The glaciofluvial materials were obtained from the water-saturated part of a percussion drilled core at Glattfelden (20 km north of Zürich). This material had been deposited by the Rhine glacier during the ice ages and was subsequently rearranged by rivers. The <32 μπι size fraction was isolated by wet-sieving from the section of the core which was situated 22 to 24 m below the surface. Its mineralogical composition was: 31% quartz, 36% calcite, 7% dolomite, 11% al-bite, 9% illite, 5% chlorite and 1% montmorillonite (Tj. Peters, private communication). Experiments were performed with <32 μπι material treated
— with 1 Μ NaCl solutions in order to reduce the amounts of interfering cations present at low concen-trations.
— to remove carbonates, then free iron and manganese (hydr)oxides, and finally organic com-pounds.
For details concerning these treatments see Ref. [7].
Experiments were also conducted with a montmo-rillonite from Crook County, Wyoming, USA and an illite from Puy-en-Velay, France. In addition, the sorp-tion of nickel was also investigated on two synthetic Si02 powders (Johnson Matthey Alpha Products
400251: crystalline 99.999 % and 88316: <325 mesh, 99.9 %), and the sorption of nickel and cobalt on CaC03 (Merck product No. 2066). These materials
were used as obtained from the suppliers.
The cation-exchange capacities C were determined by sodium saturation in 1 Μ sodium acetate (pro-cedure of Chapman [8], adapted for small samples us-ing Na-22). The results were summarized in Table 1 of Ref. [7],
S y n t h e t i c g r o u n d w a t e r
All experiments were performed with synthetic groundwater (SGW) of the following composition (in meq/1): Ca2 +, 4.50; Mg2 +, 1.69; Na+, 0.67; K+, 0.084; CI", 6.52; alkalinity, 0.42; pH, 7.9. This composition is typical for groundwaters of calcite-rich aquifers, ex-cept for H C 03 which was largely replaced by the chloride ion to allow investigations at ambient C 02 pressure. This synthetic groundwater is slightly un-dersaturated with respect to calcite.
P r o c e d u r e a n d c a l c u l a t i o n s
In a batch method 200 mg of solid material were con-tacted with 30 ml of synthetic groundwater in a closed 40-ml centrifuge tube. The tubes were turned head-over-end at 1 rpm. The solid material remained within this tube throughout the sequence: (1) equilibration with synthetic groundwater, (2) sorption, (3) desorp-tion and (4) treatment with 1 Μ HCl. For the sorpdesorp-tion experiment, either 63Ni- or 57Co-tracer was added to the synthetic groundwater. The tracers were obtained from Amersham (England). Concentrations up to 100 μηιοΐ/ΐ were achieved by adding appropriate amounts of nickel or cobalt chloride. The procedure is described in more detail in Ref. [7]. The duration of the sorption and desorption experiment was 56 days for cobalt and in most cases 182 days for nickel. To follow the time dependence of sorption and desorp-tion, one of the parallel samples was centrifuged from time to time. After each interval, a sample of 5 ml was withdrawn in the experiments with cobalt, counted on a Ge-detector and returned to the centrifuge tube. In the case of nickel, a sample of 1 ml was withdrawn, mixed with 10 ml of Insta-Gel cocktail and counted on a liquid scintillation spectrometer. The centrifuge tubes were weighed to determine the losses of liquid during these operations.
If we denote the concentration of the element un-der consiun-deration in the solid material by [Me"+]s (mmol/g) and that in solution by [Me"+] (mmol/ml), then the distribution ratio is defined by RD = [Me"+]s/ [Me"+] (ml/g) and the loading of the solid material by [Me"+]s*«/C. C is the cation exchange capacity in meq/g. The distribution ratios were calculated using equations (1) to (5) in Ref. [9]. Two different approaches were used to evaluate the concentrations of nickel and co-balt in the solutions:
(1) It was assumed that the specific activity, i. e., Bq/g of 63Ni or 57Co, remained unchanged during the experiment and the concentrations were calculated from the measured radioactivities of 63Ni and 57Co; this yields the lowest possible concentrations, since contributions of inactive nickel or cobalt originating from the solid are not taken into account.
(2) At the end of the sorption and desorption experiment the concentrations of nickel and cobalt were determined in the solutions using ICP-AES. However, the lowest concentrations were below the
Table 1. Speciation of nickel and cobalt ions in solution. The first value in the given range refers to synthetic groundwater and a nickel or cobalt concentration of 100 μπιοΐ/ΐ, the second, which is independent of concentration, to synthetic groundwater saturated with calcite.
The following pK-values were used in the calculations: NiHC03+: 13.41 NiC03: 5.78 CoHC03+: 12.87 * CoC03: 5.08* Nickel Cobalt PH 7.5--8.1 7.7 - 8 . 1 Me2+ 66%--44% 86% - 7 8 % MeHCOJ 19%--14% 8% - 7% MeC03 14%--38% 6% - 1 3 % others 1%-- 4% 1% - 2%
* extrapolated value, see text
detection limit of 0.01 μπιοΐ/ΐ. In these cases the con-centrations were calculated using the specific activities of 63Ni or 57Co determined in extracts obtained by treating the solid materials with 1 Μ HCl at the end of the experiments. The amount of tracer desorbed during this treatment amounted to 2:94 % for nickel and co-balt. The concentrations of nickel or cobalt based on the specific activities in the HCl-extracts represent up-per limits, since nickel and cobalt initially present in the solid materials, but not involved in the sorption process, may have been dissolved by HCl.
The geochemical computer code PHREEQUE [10] was used to calculate the formation of complexes of nickel and cobalt ions in the synthetic groundwater. The results are presented in Table 1. The formation constants for the carbonato-complexes are also given. Since no values were available for cobalt, the means of the pK-values for iron and nickel, the two elements adjacent to cobalt in the periodic table, were used as a rough and possibly somewhat erraneous approxi-mation.
3. Results and discussion
T i m e d e p e n d e n c e of s o r p t i o n and d e s o r p t i o n
The evolution of the distribution ratios (RD) for nickel and cobalt with time is shown in Fig. 1 for the clay minerals montmorillonite and illite and in Fig. 2 for two of the < 3 2 μπι materials, namely the NaCl-treated material and the material " f r e e " of carbonates, oxides and organics. The time dependence of the two < 3 2 μπι materials not shown in Fig. 2, i. e., that free of carbon-ates, and that free of carbonates and oxides, resembled the behaviour of the < 3 2 μπι material free of carbon-ates, oxides and organics. The counting rates for the samples with initial concentrations of 100 μηιοΐ/ΐ of nickel and 1 μπιοΐ/ΐ of cobalt were too low in the case of the NaCl-treated < 3 2 μπι material to yield mean-ingful results in the desorption experiments. The vari-ations in the data for the desorption of nickel from the NaCl-treated < 3 2 μπι material are also due to low counting rates.
Sorption of Nickel and Cobalt on a Size-Fraction of Unconsolidated Glaciofluvial Deposits and on Clay Minerals 183 -| ι 1 , 1 I , , m o n t m o r i l l o n i t e -• 0.12 μΓηοϊ/ΐ -Δ 1.0 μιτιοΙ/Ι j -O 10.0 /xmol/l : -• 100. μιτιοΙ/Ι ! desorption _ sorption 2000 -, • 1 lite 6000 -4000 τ 0.12 μιτιοΙ/Ι Δ 1.0 μπ\ ol/1 __ Ο 10.0 μι-nol/l ι Η 100. μΓηοΙ/Ι i 6000 4000 2000 I I I 1 I ι ι ι ι ι < ι — δ 1.0 μπιοΐ/ι m o n t m o r i l l o n i t e - -Ο 10.0 μηηοΙ/Ι j 0 30.0 μηηοΙ/ί |Δ • 100. ftmol/l j Δ~.Α Δ -1 ' ^ » j desorption ^ >*" sorption ι©ο-θ- -e—-Q Ο ® Θ- - ο - -4 , 1^6-6 Ό — -—-ο·-—ψ life 6000 -• 0.10 jtimol/l · Ο 10.0 μιτιοΙ/Ι I Ο 30.0 μπηοΐ/l | • 100. μηηοΙ/Ι | sorption 4000 -2000 . er -cr
Θ-α )
150 200 250 time ( d )n i c k e l
300 350 400 ί<κ>-ο-- Ύ— ί<κ>-ο--τ • desorption -θ —G Ο ...Ο · — _1_ 20 40 60 80 time (d)b) cobalt
100 120Flg. 1. Time dependence of sorption and desorption of nickel and cobalt at different concentrations on the clay minerals montmorillon-ite and illmontmorillon-ite. The samples are characterized by the initial nickel or cobalt concentration in the sorption solution.
60000 ι—1—ι—1—ι—1—ι—1—ι—1—r < 3 2 - μ ι η material, NaCI-treated 0 desorption Q- _crj sorption f ^ ' - r - r r l · ϊ-Ι&'ίf Δ • 0.13 μσιοΙ/Ι Δ 1.0 μπηοΙ/Ι Ο 10.0 μιτιοΙ/Ι 100. μΓηοΙ/Ι " < 3 2 - μ ι τ > material, free of " c a r b o n a t e s , o x i d e s a n d o r g a n i c s y 30000 20000 10000 —V 0.01 μπιοΙ/Ι j 0.12 μΓηοΙ/Ι ! -Δ 1.0 μιτιοΙ/Ι i —Ο 10.0 μπιοΙ/Ι j y;' • 100. μmol/l SSj^* sorption V -•'V, • -T V
>
Ä' -Δ" *' , 1 , 1 r ·<32-μ.ηη material, NaCI-treated Δ 1.0 μπιοΙ/Ι (Rn/2.0) Ο 10.0 μη-ιοΐ/l u ; -Ο .30.0 μπιοΙ/Ι J^ ^ • ι <3ο" i -• 100. μηηοΙ/Ι sorption , 15000 10000 5000 -< 3 2 - μ ( τ ι material, free of carbonates, oxides and organics_T• 0.10 μτηοΙ/Ι J Ο 10.0 μΓηοΙ/Ι ! τ--Ο 30.0 μπιοΙ/Ι I a 100. μτηο\/\ j sorption 100 150 200 250 300 350 400 time ( d )
α) nickel
40 60 80 time ( d )b) cobalt
120Fig. 2. Time dependence of sorption and desorption of nickel and cobalt at different concentrations on < 3 2 μπι glaciofluvial materials. The samples are characterized by the initial nickel or cobalt concentration in the sorption solution.
From Figs. 1 and 2 it is evident, that equilibrium was not reached during the sorption experiment (182 days duration for nickel and 56 days for cobalt). At initial nickel concentrations of 100 μπιοΐ/ΐ unexpected high sorption rates were observed for the montmoril-lonite, the illite and the NaCI-treated <32 μπι
materi-al. Such an effect was neither observed for the sorption of nickel on the <32 μπι materials with changed min-eralogy nor for the sorption of cobalt on any of the materials investigated.
During the desorption experiment the distribution ratios continue to increase, sometimes after an initial
10000 5 0 0 0 σ> \ Ε ο cc 40 -η 1 1 , 1 1 ρ fine g r a i n e d • 0.13 jumol/l ! Δ 1 . 0 μπιοΙ/Ι Ο 1 0 . 0 μπηοΙ/Ι • 1 0 0 . μιτιοΙ/Ι o p t i o n (Rp*5.0) desorption c o a r s e g r a i n e d - - • 0.13 μη-ιοΙ/Ι • —Δ 1.0 μΓποΙ/Ι — Ο 10.0 μιποΙ/l — • 1 0 0 . μηποΙ/Ι ]1 I sorption desorption 100 150 200 250 300 350 400 time ( d )
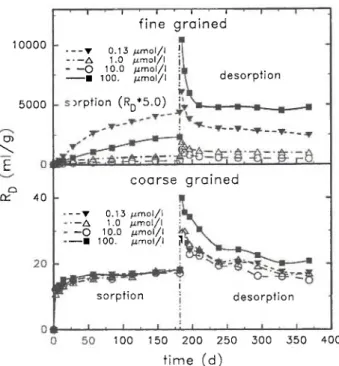
Fig. 3. Time dependence of sorption and desorption of nickel at different concentrations on two synthetic Si02 powders of
differ-ent grain-size. The samples are characterized by the initial nickel concentration in the sorption solution.
decrease, i. e. the tracer continues to be sorbed. Devi-ations from this behaviour were only detected in the experiments with cobalt on montmorillonite and illite at the lowest concentration. By far the largest increase in the distribution ratios was observed at an initial nickel concentration of 100 μηιοΙ/1 for all materials, even for the < 3 2 μπι materials with changed miner-alogy. The desorption data, extrapolated to the begin-ning of the desorption experiments, are in most cases distinctly higher than the distribution ratios at the end of the sorption experiments. This indicates that con-siderable amounts of sorbed tracer do not particpate in the processes occuring during the desorption experi-ments, i. e., are irreversibly sorbed.
Experiments with nickel were also performed on two synthetic Si02 powders. The results are shown in
Fig. 3. For the fine grained Si02 a behaviour similar
to the other materials is observed: the RD-values for sorption continue to increase up to 182 days. The RD's for desorption are much higher than those for sorption (notice different scales for sorption and desorption) demonstrating considerable irreversibilities; moreover at the initial Ni concentration of 100 μηιοΐ/ΐ an en-hanced sorption is observed.
For the coarse grained (crystalline) Si02 the RD
-values for sorption are almost constant after 30 days. At the end of desorption the RD- values are of the same magnitude as at the end of sorption indicating that irre-versibilities are small and are practically independent of the Ni concentrations; the scatter in the desorption data is due to the experimental uncertainties.
Since the decrease in grain-size between the coarse and fine Si02 leads not only to increased ÄD-values,
but also to a very different sorption behaviour, ad-ditional factors must play a role, presumably structural and morphological irregularities. The results with the fine grained Si02, furthermore, suggest that similar
factors may also govern the sorption behaviour on the other materials investigated in this paper.
S o r p t i o n and d e s o r p t i o n c u r v e s
In Figs. 4—7 the distribution ratios are presented in log-log plots as a function of the loadings of the solid materials with nickel or cobalt. We call the resulting curves "sorption and desorption curves". Since equi-librium was not reached, the positions and shapes of the sorption and desorption curves depend on the time interval chosen. In Figs. 4—7 two sets of data are pre-sented for nickel and cobalt (curves and symbols; for details see "Procedure and calculations"). Non-equi-librium conditions and irreversibilities cause ad-ditional uncertainties at low loadings, since it cannot be excluded that the specific activities of the tracers in the solid material differ from those in solution.
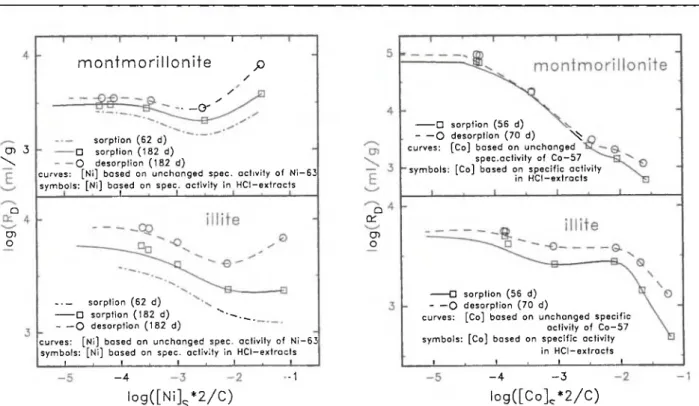
The sorption and desorption curves for the clay minerals are presented in Fig. 4 and for two of the < 3 2 μπι materials in Fig. 5. Only the curves based on unchanged specific activities are presented for the sorption of nickel at an intermediate time interval of approximately 60 days and for the data on the < 3 2 pm material free of carbonates, oxides and organics, since no experimentally determined concentrations are available.
The sorption and desorption curves for nickel bend upwards at high concentrations; the only exceptions are the sorption curves for the < 3 2 pm materials with changed mineralogy. As already mentioned in the chapter "Time dependence", this strange behaviour of nickel is most likely caused by structural and morpho-logical irregularities. On the other hand, the distri-bution ratios for cobalt often show a marked decrease at high concentrations. Approaching saturation of the sorption sites could have caused this decrease. The sorption and desorption curves for cobalt on the mont-morillonite show an unexpected, pronounced depend-ence of the distribution ratios on concentration. This contrasts with the behaviour observed in our experi-ments for all other eleexperi-ments on the montmorillonite (see Fig. 7). The differences between sorption and de-sorption curves are especially large for the de-sorption of cobalt at low concentrations on the NaCl-treated < 3 2 pm material. They are lowest for the data of nickel and cobalt on the montmorillonite. The sorption curves for of all < 3 2 pm materials valid at a time in-terval of approximately 60 days are presented in Fig. 6. To give an idea of the uncertainties at low con-centrations of nickel, values based on the specific ac-tivities of 63Ni in the HCl-extracts are also shown. The
following conclusions can be drawn:
— The NaCl-treated <32 pm material, which has the same mineralogical composition as the original material, shows the highest distribution ratios for
Sorption of Nickel and Cobalt on a Size-Fraction of Unconsolidated Glaciofluvial Deposits and on Clay Minerals 185 cn 3 \ α D1 Ο Τ T m o n t m o r i l l o n i t e ρ / / s • o -sorption (62 d ) • sorption (182 d ) Ο desorption (182 d)
curves: [Ni] bosed on unchanged spec, activity of Ni—63 symbols: [Ni] based on spec, activity in HCI-extracts
_1_ _L
sorption ( 6 2 d )
• sorption (182 d ) ""· Ο desorption (182 d )
curves: [Ni] bosed on unchanged spec, activity of Ni—63 symbols: [Ni] based on spec, activity in HCl—extracts
ι ι , ι ι 1 . 1— a a: cn ο Q sorption (56 d) Ο desorption ( 7 0 d ) ^ curves: [Co] based on unchanged
spec.activity of Co—57 symbols: [Co] based on specific activity
in HCI-extracts
• sorption (56 d) - — Ο desorption ( 7 0 d)
curves: [Co] bosed on unchanged specific activity of Co—57 symbols: [Co] based on specific activity
in HCI-extracts ι . ι . t , I - 4 •1 l o g ( [ N i ]s* 2 / C ) - 4 - 3 log([Co] * 2 / C )
Fig. 4. Sorption and desorption curves for nickel and cobalt obtained for the clay minerals montmorillonite and illite. Curves: concentrations based on the assumption of unchanged specific activities of the tracers. Symbols: experimentally determined
concen-trations, at low concentrations based on the determined in HCI-extracts (see text).
ο cc cn ο 1 < 1 Τ 1 Τ 1 1 < 3 2 - μ Γ η m a t e r i a l , © N a C I - t r e a t e d Q d -sorption (57 d) D sorption (182 d) Ο desorption (182 d)
curves: [ N i ] based on unchanged spec, activity of N i - 6 3 symbols: [Ni] based on specific activity in HCI-extracts I I I ι I I . < 3 2 - μ ι η m a t e r i a l , f r e e of c a r b o n a t e s , oxides a n d o r g a n i c s ' sorption (56 d) sorption (182 d) desorption (182 d )
[Ni] bosed on unchonged specific activity of Ni—63 I . I • I . I . ' < 3 2 - μ η Ί m a t e r i a l , \ N a C i - t r e a t e d \ a on σ> ο <£>. Ο - • sorption (56 d ) ^ Ό - O - — Ο desorption ( 5 6 d )
curves: [Co] based on unchanged specific activity of Co—57
symbols: [Co] based on specific activity in HCI-extracts
ι , I I I » 1 . < 3 2 - μ η η m a t e r i a l , f r e e of c a r b o n a t e s , oxides a n d o r g a n i c s - Φ - © - — - o — © . • sorption (56 d) Ο desorption (56 d )
curves: [Co] bosed on unchanged specific activity of Co—57 symbols: [Co] bosed on specific activity in
HCI-extracts ι . ' · ' . l _
- 1 - 3 - 2 - 1
l o g ( [ N i ]s* 2 / C ) l o g ( [ C o ] * 2 / C )
Fig. 5. Sorption and desorption curves for nickel and cobalt obtained for two of the < 3 2 μτη glaciofluvial materials. Curves and
symbols', for explanation see Fig. 4.
nickel and cobalt. This suggests that the contribution of the carbonates might be significant. However, ex-periments investigating the sorption of nickel and co-balt on a synthetic calcium carbonate did yield very low distribution ratios (for nickel: 20 ml/g at [Ni] = 0.095 mol/1, 4.8 ml/g at [Ni] = 100 μηιοΙ/1; for cobalt: 28 ml/g at [Co] = 0.0087 mol/1, 4.7 ml/g at [Co] = 95 mol/1). Therefore, it is more likely, that structural
and morphological irregularities with a high affinity to sorb nickel and cobalt disappeared during the removal of the carbonates.
— For both nickel and cobalt the sorption curves for the < 3 2 μπι materials with changed mineralogy are similar, as well with respect to the magnitude of the distribution ratios as to the shape of the curves. However, at low concentrations, the curve for the
sorp-Q a: σ> ο n i c k e - . δ • N a C l - t r e a t e d - — Δ f r e e of c a r b o n a t e s ^ f r e e of c a r b o n a t e s a n d o x i d e s f r e e of c a r b o n a t e s , oxides a n d o r g a n i c s c u r v e s : [ N i j b a s e d o n u n c h a n g e d s p e c i f i c a c t i v i t y s y m b o l s : [ N i l b a s e d on s p e c i f i c avtivity i n H C i - e x t r a c t s I . I 1 ! 1 1 1 c o b a l t \ Ο • N a C l - t r e a t e d - — Δ f r e e of c a r b o n a t e s \ \ , ^ f r e e of c a r b o n a t e s a n d o x i d e s Ο f r e e of c a r b o n a t e s , o x i d e s a n d o r g a n i c s c u r v e s : [ C o ] b a s e d on u n c h a n g e d s p e c i f i c a c t i v i t y v s y m b o l s : [ C o ] b a s e d on s p e c i f i c a c t i v i t y in H C i - e x t r a c t s I , I , 1 . 1 ' kO -- 1 l o g ( [ M e ] * 2 / C )
Fig. 6. Influence of changed mineralogy (chemical treatment) on the sorption of nickel and cobalt on all < 3 2 μπι glaciofluvial materials. The sorption time is approximately 60 days. Curves
and symbols: for explanation see Fig. 4.
tion of cobalt on the < 3 2 μπι material free of carbon-ates is steeper than those for the other < 3 2 μηι materi-als with changed mineralogy. Probably a small fraction of the sorption sites causing the high distribution ratios for the NaCl-treated material are still present in the material free of carbonates. Since this effect is not ob-served for the data of nickel, these sites have a higher affinity for cobalt than for nickel.
σι \ Ε α D£ cn ο .... μ - j . 3 3 ι ' 1 ' 1 ' 1 - — Τ -cobalt - m o n t m o r i l l o n i t e nickel \ ν -Δ. • - A- ^ Q . .Qc e s i u m S ^ s t r o n t i u m .J I ι L
%
illite \ c e s i u m ••^--^-Anicket barium^ V „ c o b a l t \ s t r o n t i u m Θ Β - B - β N a C ! - t r e a t e d χ c e s i u m \ <32—μηι \ m a t e r i a nickel A J ' g r ^ ' ' · · ^ __ c o b a l t nickel u V - 6 - 5 - 4 - 3 - 2 log([Me] * n / C )Fig. 7. Data for the sorption of cesium, strontium, barium, cobalt and nickel on montmorillonite, illite and < 3 2 μηι glaciofluvial materials. For cobalt and nickel the < 3 2 μηι materials with changed mineralogical compositions are represented by the ma-terial free of carbonate and oxides (see Fig. 6). The sorption time is approximately 60 days. Curves and symbols: for explanation
see Fig. 4.
C o m p a r i s i o n of the s o r p t i o n of n i c k e l and c o b a l t with t h a t of c e s i u m , s t r o n t i u m and b a r i u m
In Fig. 7 the sorption data of the present work for nickel and cobalt are compared with those for cesium [9], strontium [11] and barium [7]. The data for cesium are included for the sake of completeness.
The sorption of the divalent ions strontium and ba-rium can be characterized in the following way: equi-librium was reached within 14 days and the sorption process was reversible on all solid materials. The dis-tribution ratios of both elements on the montmorillon-ite are almost constant with concentration (linear iso-therm) and the distribution ratios are of similar magni-tude. For the other materials the isotherms for stron-tium are also nearly linear, those for barium on the other hand are strongly non-linear with considerably higher distribution ratios than those of strontium. Changes in the mineralogy of the < 3 2 μπι materials (removal of the carbonates, oxides or organic sub-stances) affected the sorption curves only slightly. The main process governing the sorption of strontium and
barium was ion exchange on the clay minerals [12, 13, 14].
The sorption curves for nickel and cobalt depend on the time interval chosen (in Fig. 7: approximately 60 days), since equilibrium was not reached. The sorp-tion process seems to be largely irreversible. The dis-tribution ratios for nickel and cobalt are distinctly higher than those for strontium and barium. Consider-able differences between the sorption curves of nickel and cobalt are only observed for the montmorillonite and the NaCl-treated < 3 2 μπι material. Processes other than ion exchange, probably reactions on struc-tural and morphological irregularities of the solid ma-terials, are dominant in the sorption of nickel and co-balt.
The comparison of the isotherms and sorption curves in Fig. 7 for different solid materials evidences very drastic differences for the sorption behaviour of these predominantly divalent (i. e. not sensitive to re-dox processes) elements. It demonstrates in an im-pressive way that meaningful predictions for the sorp-tive and desorpsorp-tive behaviour and retardation of ele-ments with a relevance for radioactive wastes can only
Sorption of Nickel and Cobalt on a Size-Fraction of Unconsolidated Glaciofluvial Deposits and on Clay Minerals 187
be made by careful measurements of isotherms (or sorption curves) with the real host rocks. Therefore big care should be taken to make such predictions as conservative as possible.
Acknowledgements
The authors acknowledge H. Gäggeler for discussions, the Institute for Aquatic Sciences (EAWAG) and Tj. Peters for supplying some of the materials.
Literature
1. Nagra NTB 93-26, Beurteilung der Langzeitsicherheit des Endlagers SMA am Standort Wellenberg, Sept. 1993. 2. Cornell, R. M., Aksoyoglu, S.: J. Radioanal. Nucl. Chem.,
Letters 164, 389 (1992).
3. Legoux, Y., Blain, G., Guillaumont, R., Ouzounian, G., Bril-lard, L., Hussonnois, M.: Radiochim. Acta 58/59, 211 (1992).
4. Benes, P., Jurak, M., Kuncova, M.: J. Radioanal. Nucl. Chem., Articles, 132, 209 (1989).
5. Polzer, W. L., Fuentes, H. R., Essington, Ε. H., Roensch, F. R.: Equilibrium Sorption of Cobalt, Cesium and Strontium on Bandelier Tuff: Analysis of Alternative Mathematical Modeling, in: Waste Management '85, Proc. Symp. Waste Management, Tücson, Arizona, Vol. 3, pp. 167—173, (Post, R. G„ ed.), 1985.
6. Ohe, Τ.: Nucl. Technol. 67, 92 (1984).
7. Griitter, Α., von Gunten, H. R., Rössler, Ε.: Radiochim. Acta
58/59, 259 (1992).
8. Chapman, H. D.: Cation-Exchange Capacity by Sodium Saturation, in: Methods of Soil Analysis, Part 2 (Black, C. Α., ed.), Am. Soc. Agron. Publ., Madison 1965.
9. Griitter, Α., von Gunten, H. R., Köhler, Μ., Rössler, Ε.: Radiochim. Acta 50,177 (1990).
10. Parkhurst, D. L., Thorstenson, D. C., Plummer, L. Ν.: A computer program for geochemical calculations, NTIS Tech. Rep. PB81 167801 (1980).
11. Griitter, Α., von Gunten, H. R„ Rössler, E„ Keil, R.: Radio-chim. Acta. 64, 247 (1994).
12. Weiss, Α., Sextl, E.: Clay Minerals as Ion Exchangers, in:
Ion Exchangers (Dorfner, Κ., ed.), Walter de Gruyter, Berlin
1991.
13. Amphlett, C. B.: Inorganic Ion Exchangers. Elsevier Pub-lishing Co, Amsterdam 1964.
14. Clearfield, Α.: Inorganic Ion Exchange Materials. CRC Press, Inc., Boca Raton, Florida 1982.