HAL Id: hal-01983207
https://hal.archives-ouvertes.fr/hal-01983207
Submitted on 16 Jan 2019
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Adhesion of Candida albicans to polythene in Sodium
hypochlorite disinfected aquatic microcosm and
potential impact of cell surface properties
Chrétien Djimeli, Antoine Arfao, Olive Ewoti, Geneviève Bricheux, Moïse
Nola, Télesphore Sime-Ngando
To cite this version:
Chrétien Djimeli, Antoine Arfao, Olive Ewoti, Geneviève Bricheux, Moïse Nola, et al.. Adhesion of Candida albicans to polythene in Sodium hypochlorite disinfected aquatic microcosm and poten-tial impact of cell surface properties. Current Research in Microbiology and Biotechnology, Aizeon Publishers, 2014, 2 (5), pp.479-489. �hal-01983207�
Vol. 2, No. 5 (2014): 479-489 Research Article
Open Access
I
ISSSSNN::22332200--22224466
Adhesion of Candida albicans to polythene in Sodium
hypochlorite disinfected aquatic microcosm and
potential impact of cell surface properties
Chrétien Lontsi Djimeli
1, Antoine Tamsa Arfao
1, Olive V. Noah Ewoti
1, Geneviève
Bricheux
2, Moïse Nola
1,*
and Télesphore Sime-Ngando
21 University of Yaoundé I, Laboratory of General Biology, Hydrobiology and Environment Research Unit, P.O. Box 812, Yaoundé,
Cameroon.
2 Université Blaise Pascal, « Laboratoire Microorganismes: Génome & Environnement (LMGE)», UMR CNRS 6023, Complexe
Scientifique des Cézeaux, 24 avenue des Landais, BP 80026, 63171 Aubière Cedex, France.
* Corresponding author:M. Nola; email: moise.nola@yahoo.com / Phone: (+237) 99 43 26 37
ABSTRACT
The adhesion of C. albicans cells harvested from different growth stages to polythene in Sodium hypochlorite (NaOCl) treated water, under static and dynamic conditions was carried out. With cells harvested from the lag, exponential, stationary and decline growth phases, the highest abundance of cells adhered was respectively 0.75, 1.18, 0.85 and 0.95 units (Log (CFU/cm²)), when considering the static and dynamic experimental condition. The hydrophobicity values ranged respectively from 17 to 99, 45 to 93, 8 to 93, and from 10 to 83 %. The increase in incubation duration was significantly correlated (P<0.01) to the decrease in the abundance of adhered C. albicans when considering the NaOCl concentrations used. The increase in the NaOCl concentration in water was significantly correlated (P<0.01) to the increase in the abundance C. albicans adhered to polythene when considering the incubation durations. An increase in NaOCl concentration in the water seems significantly correlated with the decrease of the abundance of C. albicans adhered to the substrate (P<0.01) when considering each cell growth phase. Using the H (Kruskal-Wallis) test to compare the registered mean abundances of cells adhered to polythene after living in water treated with various concentrations of NaOCl with respect to the growth phase, a significant difference (P<0.05) was noted with cells coming from the exponential, stationary and decline growth stages. The adhesion of C. albicans to polythene seems to be impacted by various factors with some depending on the cells status.
Keywords:
Candida albicans, adhesion, NaOCl, Cell growth phases, aquatic microcosm, hydrophobicity.INTRODUCTION
In order to respect the standards of potable water, and preserve public health, one of the major concerns of companies in charge of the treatment of drinking water is to effectively meet the demand and maintain a distribution of water of good quality [1]. Many interactions between disinfectants, pipe walls, free and fixed biomasses always take place and often resulted in many physicochemical and biological reactions. These reactions may be the cause of the degradation of the organoleptic properties of the water supply [2]. The control analyses of drinking water are based not only
on physicochemical parameters, but also on microbiology [3].
The purpose of drinking water treatment is not to produce sterile water, but water that does not pose a risk to public health. These analyses relate mostly to faecal and/or pathogenic bacteria like Escherichia coli and Streptococcus faecalis [4]. The presence of these bacteria species indicates the relatively high probability of the presence of germs that cause waterborne diseases.
Candida albicans is one of the specific germs species on
which research has increasingly being of interest to the scientific community. It has been indicated that C.
albicans is a cosmopolitan yeast. Its isolation frequency
varies with respect to the sampling sites [5]. Its main reservoir is the gut where the frequency of carriage varies with respect to individuals. This yeast is the major cause of opportunistic nosocomial infections of fungal origin. A large majority of deep infections is of endogenous origin and develop from saprophytic yeasts present in the digestive tract of the host and the mucosa. A small proportion of candidiasis may also be of exogenous origin from skin colonization due to equipment care and insertion of catheter into the human body [5].
Previous works allowed the understanding of the mechanisms of emergence of C. albicans in the drinking water distribution system [5]. They also showed that C.
albicans which is a facultative anaerobic microorganism can exceptionally be isolated from soil and water, but this usually resulted from faecal contamination [5, 6].
The adhesion of C. albicans to a surface is a key step in the process of Candida pathogenesis and resistance against current treatments [7]. Many studies have also been focused on measuring the hydrophobicity of C.
albicans at different growth phases [8], monitoring
water supply, treatment plants, and health risks associated with the dysfunctional network of drinking water distribution in Cameroon [9-11]. They showed on one hand that the cells coming from exponential and stationary growth phases appear as some of the important factors impacting the C. albicans adhesion on solid surface [8]. On the other hand they also showed that despite treatment done upstream in factories, in addition to disinfectant residuals in pipes and the oligotrophic nature of the medium, some microorganisms adapt and proliferate in the water distribution network [1]. The latter are sometimes the cause of nests and microbial biofilm formation, among others. In addition, the variation in the abundance of microorganisms in response to disinfectants can be linked to changes in their cell wall which may be due to a change in their growth stage [12] as well as their cell surface properties [13, 14].
Although many studies have been focused on the mechanisms of emergence of C. albicans in drinking water distribution systems [5], there is little information on the importance of growth stages or metabolic processes and cell surface properties in the presence of disinfectants on the cell adhesion process to surface. This study aimed at evaluating in microcosm condition, the effect of cell surface hydrophobicity variation of C. albicans cells on their adhesion at different growth stages to polythene immersed in water treated with different concentrations of Sodium hypochlorite (NaOCl), under stationary and dynamic conditions over time.
MATERIALS AND METHODS
Collection and identification of C. albicans strains, and cell storage
Candida albicans strains was provided by the Microbial
Laboratory of the Yaounde Central Hospital (YCH). Its identification was firstly based on its culture characteristics on a supplemented chloramphenicol Sabouraud culture medium after 48 hours incubation at 37°C, then on cell morphological and growth’s characters under microscope (filamentation test, chlamydosporulation, growth at 45°C) and biochemical properties using ELIchrom fungi gallery (BioMérieux, France). Biochemical tests underlined that C. albicans strains used is able in aerobic conditions to assimilate (auxanogram) or in anaerobic conditions to ferment (zymogram) an organic carbon for its growth. In both conditions, these cells use glucose, maltose, sucrose and galactose. They do not synthesize urease and they develop resistance to acitidione. It was then stored in glycerol at -70°C before use to avoid excessive subcultures.
Assessment of C. albicans cell growth phase
Three replicates of 15 test tubes each containing 10 mL sterile tryptone (Biokar) solution were used. Tubes of each set were labeled t0, t2, t4, t6, t8, t10, t12, t14, t16, t18, t20,
t22, t24, t26 and t28. Prior to the experiment, a frozen vial
containing C. albicans was defrosted at room temperature. The culture (300 μL) was then transferred into 10 mL of nutrient broth (Oxford) and incubated at 37°C for 24 hours. Cells were then collected by centrifugation (8000 rpm for 10 min at 10°C) and washed twice with sterile NaCl (8.5 g/L) solution. The sediment was then diluted in 10 mL of sterile NaCl solution. After dilution, 100 µL of the suspension was added to each of the 15 tubes containing 10 mL of sterilized peptone solution.
The cell suspensions in 3 tubes labeled t0 were
immediately analyzed. Those in the tubes labeled t2, t4,
t6 … t28 were incubated for 2, 4, 6… 28 hours at 37°C.
After each incubation duration, analyses were carried out using plate count method on a supplemented Chloramphenicol Sabouraud culture medium, incubated for 48 hours at 37°C. The colony forming units (CFUs) were then counted. Mean CFUs were calculated from the results of the triplicates and Log(CFU) also calculated. The straight Log(number of CFUs) curve against storage period was plotted and compare to the cell growth curve. The cell growth phases of C. albicans were then determined.
Disinfectant used
Sodium hypochlorite (NaOCl) which belongs to the group of halogen derivatives was used. NaOCl used was from of the Colgate-Palmolive (USA) brand. The ease with which this disinfectant is generally used for drinking water treatment justified its choice for this study. Concentrations of NaOCl used ranged from 0.25 to 0.75‰. These concentrations were evaluated by simple method of dilution of crude solution obtained directly from the manufacturer. To count the surviving
microorganisms after disinfection, the sterile NaCl solution was used as a diluent.
Adsorbing substrate used
The adsorbing substrate used was high dense polythene. It differed from low radical dense polythene and low linear dense polythene by sparsely branched chains of its molecular structure, and its relatively high resistance to shocks, high temperatures and ultraviolet rays [15, 16]. It is a plastic piping material obtained directly from the manufacturer and used in drinking water distribution networks. High dense Polythene resulted from polymerization of macromolecules of polyolefin family. This polymerization is obtained from gaseous ethylene according to the following equation [17, 18]:
The polythene used in this study is commercialized by Goodfellow SARL (France).
Adhesion test of C. albicans on polythene in the solution containing NaOCl
On the basis of previous studies, parallelepipedic shaped fragments of polythene with 13.28 cm² of total surface area suspended to a wire of 0.1 mm diameter were immersed in triplicate in two sets named A and B. Set A contained four subsets each having three Duran’s 250 mL flasks labeled as follows: A1, A1', A1'', A2, A2', A2'', A3, A3', A3'', and A4, A4', A4''. Same for set B with labeling as follows: B1, B1', B1''; B2, B2', B2''; B3, B3', B3'' and B4, B4', B4''. Each flask contained 99 mL of NaCl solution. Meanwhile, controls were made and coded A01, A02, A03, A04 and B01, B02, B03, B04 [19]. The
whole was then autoclaved. Prior to the experiment, a frozen vial containing C. albicans strains was defrosted at room temperature. The culture (300 μL) was then transferred into 10 mL of nutrient broth (Oxford) and incubated at 37°C for 24 hours and the cells latter collected by centrifugation at 8000 rpm for 10 min at 10°C, then washed twice with sterile NaCl solution. The sediment was then diluted in 10 mL NaCl solution. After serial dilutions, the initial concentration of yeast cells (concentration at t=0) in each mother solution was adjusted to 6x108 CFU/mL. This was performed by
reading the optical density (OD) at 600 nm using a spectrophotometer (DR 2800) followed by culture on Sabouraud agar medium [19]. Afterwards, 1 mL of the suspension was added to 99 mL of sterilized NaCl solution contained in Erlenmeyer flasks. For adhesion tests, the final concentrations of NaOCl 0.25, 0.5 and 0.75‰ were respectively prepared in the following three sets of 8 Erlenmeyer flasks: A1, A2, A3, A4, B1, B2, B3, B4, A1', A2', A3', A4', B1', B2', B3', B4' and A1'', A2'', A3'', A4'', B1'', B2'', B3'', B4''.
A set of these erlenmeyer flasks were incubated in triplicates under dynamic condition by stirring at a speed of 60 rpm, using a stirrer (Rotatest brand) and another set under static condition for 180, 360, 540 and 720 min. All these incubations were done at laboratory temperature (25 ± 1°C).
Unhooking cells from fragment
After each incubation period, fragments of polythene were dripped for 10 seconds in a sterile environment created by the Bunsen burner flame and then introduced into 10 mL of sterilized NaCl solution. The unhooking of adherent cells was performed by vortex agitation at increasing speeds for 30 seconds in three consecutive series of 10 mL sterilized NaCl solution. This technique allows the unhooking of maximum adhered cells [20, 21]. The total volume of the suspension containing unhooked C. albicans was 30 mL. Isolation and numbering of unhooked cells were performed using the spread plate method on a supplemented chloramphenicol Sabouraud medium, followed by the incubation of Petri dishes at 37°C for 24 to 48 hours. The disinfectant was not evaluated after incubation.
Assessment of the hydrophobicity cell surface of C. albicans
C. albicans surface hydrophobicity was measured by the
adhesion test on polythene using the MATH (Microbial Adhesion To Hydrocarbons) method [13], as recently described by Jain et al. [14]. After culture on supplemented chloramphenicol Sabouraud medium, cells were centrifuged at 8000 rpm for 10 min at 10°C, washed and resuspended at a concentration of 108
CFU/mL in distilled water (pH 4.9) by reading the spectrophotometer DR 2800. The optical density (OD) of the solution was measured at 400 nm (Ao). Then 1 mL of this solution is introduced into two sets (A and B) of 5 Erlenmeyer flasks each in triplicates A0, A0', A0'' and B0 , B0', B0'', A1, A1', A1'' and B1, B1', B1'', A2, A2', A2'' and B2, B2', B2'', A3, A3', A3'', and B3, B3', B3'', and A4, A4', A4'' and B4, B4', B4'' containing 99 mL sterilized NaCl solution and parallelepipedic shaped fragments of polythene of 13.28 cm² total surface area suspended to a wire of 0.1 mm diameter. The mixture was incubated at room temperature (25±1°C) in stationary and dynamic regimes for 180, 360, 540, and 720 min. After each incubation period, the OD of the aqueous phase was measured (A1). Concentrations of
C. albicans inocula were assessed by turbidity and
expressed by measuring the OD at 600 nm on a spectrophotometer DR 2800. A density of 0.12 to 0.15 corresponded to 1-5 x 105 CFU/mL [22].
The percentage of cells adhered to the polythene was calculated using the following formula [14]:
In this formula, A0 is the OD of the solution measured at 400 nm before the substrate immersion, and A1 is
the OD of the aqueous phase measured after cell adhesion process. According to Rosenberg [13] and Jain
et al. [14], the percentage of cells adhered to the
polythene in this condition is closely proportional to the cell surface hydrophobicity. According to those authors, the more the value obtained is closer to 100% the more cell surface is hydrophobic. The percentages of cells adhered were then compared to the hydrophobicity percentages [14, 23].
Data analysis
Variations in abundance of adhered C. albicans in each experimental condition were illustrated by semi-Logarithmic curves. Standard deviations were not considered because the curves were too close. Spearman "r" correlation test was used to assess the degree of relation between the abundance of adhered cells and other parameters considered. To compare the mean abundance of adhered C. albicans from one experimental condition to another; Kruskal-Wallis H-test and Mann-Whitney U-H-test were used using the statistical software package SPSS 17.0. A p-value of 0.05 was assumed to be statistically significant.
RESULTS AND DISCUSSION
Growth curve
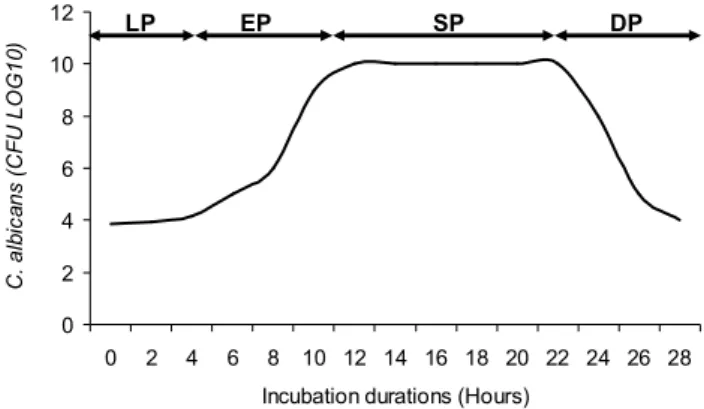
The C. albicans growth in sterile tryptone (Biokar) solution exhibited a hyperbolic curve of 4 phases: a lag growth phase of 5 hours duration, an exponential growth from the 5th to the 13th hour of incubation, a
stationary growth phase of 9 hours duration, and a decline growth phase which begin from the 22nd hour of
incubation (Figure 1). 0 2 4 6 8 10 12 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 Incubation durations (Hours)
C . a lb ic a n s ( C F U L O G 1 0 ) LP EP SP DP
Figure 1. Growth curve of C. albicans (LP: Lag growth phase, EP: Exponential growth phase, SP: Stationary growth phase, DP: Decline growth phase).
Adhesion Kinetics in stationary and dynamic regimes
The hourly C. albicans cells adhesion rate on to the considered substrate was assessed in static and dynamic conditions. It has been noted that it varied from one cell growth phase to another (Table 1). Under static condition, adhesion speeds varied from 1.744 to
17.389 cells adhered/cm2/h whereas under dynamic
condition, it varied from 2.169 to 8.989 cells adhered/cm2/h. Under these experimental conditions,
the lowest adhesion speed (1.744 cells adhered/cm2/h)
was recorded under static condition with the cells harvested from the lag growth phase. The highest adhesion speed (17.389 cells adhered/cm2/h) was
obtained in the same experimental condition but with cells harvested from the stationary growth phase (Table 1).
Table 1. Hourly adhesion speeds (and regression coefficient)
of C. albicans with respect to cell growth phases under static and dynamic conditions.
Cell growth phases Adhesion speed (Cell adhered/cm2 /h)
Static Dynamic Lag 1,7436 (0,8313) 2,1687 (0,9863) Exponential 11,9666 (0,9144) 8,989 (0,8327) Stationary 17,389 (0,9675) 8,811 (0,9117) Decline 2,6444 (0,8342) 2,9777 (0,9672)
Abundance of adhered C. albicans to polythene after a stay period in the NaOCl solution
Abundance of adhered C. albicans in the solution treated with NaOCl sometimes reached 1.18 units (Log (CFU/cm²)). The maximum abundance of cells adhered was recorded during the exponential growth phase under static condition after 180 min in the solution containing 0.25‰ NaOCl. C. albicans was sometimes completely decimated by NaOCl. This result was recorded under static condition during the lag phase in the solutions containing different concentrations of NaOCl and under dynamic condition in the solution disinfected with 0.75‰ NaOCl. The same observation was made in the stationary and decline growth phases under dynamic condition in the 0.75‰ NaOCl solution. With cells harvested from the lag phase, abundance of adhered C. albicans to the control substrate ranged from 1.27 to 1.95 units (Log (CFU/cm²)) and was always higher than those adhered to the substrate in the NaOCl treated solution. Furthermore, they increased with incubation periods. The maximum abundance of cells adhered was recorded after an adhesion test of 720 min under dynamic condition. In the solution disinfected with NaOCl, abundance of adhered C. albicans sometimes reached 0.75 units (Log (CFU/cm²)). The densities of adhered cells in the solution treated with NaOCl decreased with incubation periods. The maximum density was observed under dynamic condition after an adhesion test of 180 min in the solution containing 0.25‰ NaOCl (Figure 2).
Figure 2. Temporal variation of the abundance of cells adhered under static and dynamic conditions in the solution containing
With cells reaching the exponentially growth phase, abundance of adhered C. albicans to the control substrate ranged from 1.85 to 2.59 units (Log (CFU/cm²)) and was always higher than those adhered to the substrate in the NaOCl treated solution and also they increased with incubation periods. The maximum abundance was recorded after an adhesion test of 720 min under dynamic condition. In the solution disinfected with NaOCl, abundance of C. albicans sometimes reached 1.18 units (Log (CFU/cm²)). The densities of adhered cells in the solution treated with NaOCl decreased with incubation periods. The maximum density was observed under static condition after an adhesion test of 180 min in the 0.25‰ NaOCl solution (Figure 2).
The stationary growth phase exhibited abundance of C.
albicans adhered to control substrate ranging from 1.65
to 2.72 units (Log (CFU/cm²)) and was always higher than those adhered to the substrate in the NaOCl treated solution and increased with incubation periods. The minimum and maximum abundance were respectively recorded after an adhesion test of 180 and 720 min under static condition. In the solution disinfected with NaOCl, abundance of adhered C.
albicans often reached 0.85 units (Log (CFU/cm²)). The
densities of adhered cells in the solution treated with NaOCl decreased with incubation periods. The maximum density was observed under static condition after an adhesion test of 180 min in the 0.25‰ NaOCl solution (Figure 2).
With cells harvested from the decline growth phase, the abundance of C. albicans adhered to control substrate ranged from 1.15 to 2.02 units (Log (CFU/cm²)) and was always higher than those adhered to the substrate in the NaOCl treated solution. In addition, they increase with incubation periods. The maximum abundance of adhered cells was recorded after 720 min incubation period under dynamic condition. In the solution disinfected with NaOCl, abundance of adhered C.
albicans sometimes reached 0.95 units (Log (CFU/cm²)). The densities of adhered cells in the solution treated with NaOCl decreased with incubation periods. The maximum density was observed under static condition after an adhesion test of 180 min in the 0.25‰ NaOCl solution (Figure 2).
Surface hydrophobicity of adhered C. albicans cells after a stay period in the NaOCl solution
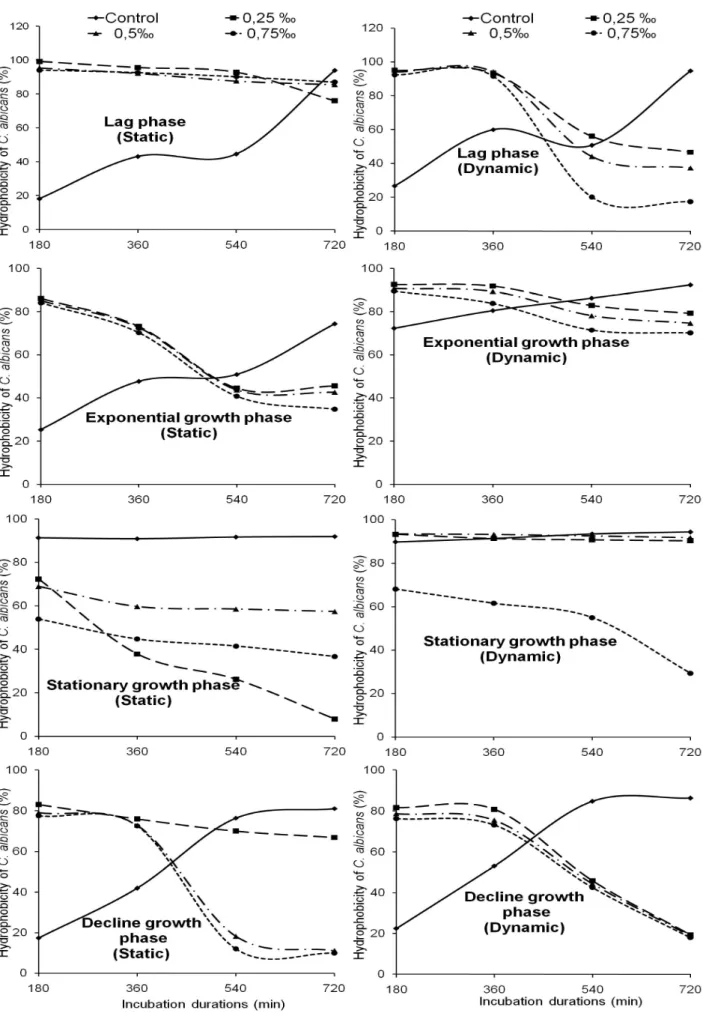
The percentages of cells adhered were calculated and then compared to the hydrophobicity percentages. The percentage of cells adhered to the polythene in experimental conditions is closely proportional to the cell surface hydrophobicity. Without any treatment with NaOCl, the hydrophobicity ranged from 17 to 95%.
It increased with incubation periods. The minimum value was recorded after 180 min under static condition with cells coming from the decline growth phase. During the same growth phase, the maximum value of hydrophobicity percentage was observed after 720 min under dynamic condition. Without any treatment, C. albicans surface seems more hydrophobic in the decline growth phase under dynamic condition after 720 min. In addition, it was observed that the C.
albicans cell surface hydrophobicity varied with the
NaOCl concentrations used.
With cells harvested from the lag phase, the hydrophobicity ranged from 17 to 99% after living in the NaOCl treated solution. It relatively decreased with incubation periods. The lowest value was recorded under dynamic condition after 720 min in the solution disinfected with 0.75‰ NaOCl. The highest value was observed under static condition after 180 min in the solution treated with 0.25‰ NaOCl. Thus in the 0.25‰ NaOCl solution, C. albicans seems more hydrophobic under static condition after 180 min (Figure 3).
Cell surface hydrophobicity of C. albicans coming from the exponential growth phase ranged from 45 to 93% after living in the solution containing NaOCl. It also relatively decreased with incubation periods. The lowest value was recorded under dynamic condition after 720 min in the solution disinfected with 0.75‰ NaOCl. The highest value was noted under static condition after 180 min in the solution containing 0.25‰ NaOCl. In the NaOCl 0.25‰ treated solution, C.
albicans seems is more hydrophobic under static
condition after 180 min (Figure 3).
With cell harvested from the stationary growth phase, the hydrophobicity ranged from 8 to 93% after living in the solution treated with NaOCl. With different NaOCl concentrations, it decreased with incubation periods. The minimum value was observed under static condition after 720 min with NaOCl concentration 0.75‰. The maximum value was recorded under dynamic condition after 180, 360, and 540 min in the solution disinfected with 0.25‰ and 0.50‰ NaOCl. In the 0.25‰ and 0.50‰ NaOCl solutions, C. albicans cells seem more hydrophobic under dynamic condition after 180, 360, and 540 min (Figure 3).
The hydrophobicity ranged from 10 to 83% with the cells coming from decline growth phase. It relatively decreased with incubation periods. The maximum and minimum values were recorded under static condition, respectively after 180 and 720 min in the NaOCl 0.25‰ and 0.75‰ treated solutions. With 0.25‰ NaOCl solution, C. albicans seems more hydrophobic under static condition after 180 min (Figure 3).
Figure 3. Variation of the hydrophobicity of C. albicans cells surface under static and dynamic conditions in the solution
Correlation between the abundance of C. albicans cells adhered and incubation duration or concentrations of NaOCl
Spearman "r" correlation coefficients between the abundance of adhered C. albicans and incubation durations for each concentration of NaOCl and under each experimental condition were assessed and presented in Table 2. It was noted that the increase in incubation duration is significantly correlated (P<0.01) to the decrease in the abundance of adhered C. albicans in the solution disinfected with NaOCl.
Spearman "r" correlation coefficients between abundance of adhered C. albicans and NaOCl concentrations for each incubation period and under each experimental condition were also assessed. Under static as well as dynamic condition, it was noted that the increase in the NaOCl concentration in water is significantly correlated (P<0.01) to the increase in the abundance C. albicans adhered to polythene (Table 3). The degrees of relationship between NaOCl concentrations and abundance of adhered C. albicans harvested from each growth stage were assessed and are presented in Table 4. It resulted that an increase in NaOCl concentration in the water seems significantly correlated with the decrease of the abundance of C.
albicans adhered to the substrate (P<0.01).
Table 2. Spearman "r" correlation coefficients between the
abundance of adhered C. albicans and incubation periods for each concentration of NaOCl and each experimental condition.
Experimental condition NaOCl concentrations 0.25‰ 0.5‰ 0.75‰ Static -0.775* -0.833** -0.500** Dynamic -0.949* -0.400* -0.500** **: P <0.01; *: P <0.05 ddl=15
Table 3. Spearman "r" correlation coefficients between the
abundance of adhered C. albicans and concentrations of NaOCl for each incubation period and under each experimental condition.
Experimental condition
Incubation periods
180 min 360 min 540 min 720 min Static 0.992** 0.976** 0.986** 0.994** Dynamic 0.954** 0.940** 0.873** 0.947** **: P <0.01 ; ddl=15
Table 4. Spearman "r" correlation coefficients between NaOCl
concentrations and abundance of adhered C. albicans harvested from each growth stage.
Experimental condition
Cell growth phases
Lag Exponential Stationary Decline Static -0.993** -0.982** -0.991** -0.979** Dynamic 0.499 -0.957** -0.944** -0.967** **: P <0.01 ; ddl=11
Comparison of mean abundance of adhered C.
albicans amongst the different NaOCl
concentrations used at each growth stage
For each NaOCl concentration used, the mean abundance of adhered C. albicans from the three incubation periods was calculated for each cell growth stage. The mean abundances noted from the three
NaOCl concentrations used were compared using the H test of Kruskal-Wallis. It was noted that with cells harvested from the lag growth phase, no significant difference was registered amongst the mean abundance of C. albicans adhered to polythene (P>0.05). On the contrary, using cells coming from the exponential, stationary and decline growth stages, there was a significant difference (P<0.05) amongst the mean abundance of adhered C. albicans after living in the solutions treated with the various concentrations of NaOCl.
DISCUSSION
This study carried in aquatic microcosm conditions, showed that C. albicans adhered to polythene with varying abundance. Adhesion of microorganisms to surfaces is the first step in biofilm formation which is a form of microbial life in the aquatic environment [24]. It is the source of biocontamination problems in various fields such as health, environment, food industry, water purification [25-27].
It has been indicated that the development of C.
albicans biofilm starts with the adhesion of
blastospores to a surface substrate. This adhesion is ensured by non-specific hydrophobic and electrostatic interactions and specific adhesins on the surface of fungal cells [28-31]. The blastospores layer is in close contact with the anchor surface and the final three-dimensional structure on the substrate colonized. The initial adhesion is followed after about 180 to 240 minutes by the formation of micro-colonies to the colonized surface. After 660 minutes, a thick layer of C.
albicans can be observed. The intermediate phase
(720-1440 minutes) is mainly characterized by the synthesis of the extracellular matrix, which covers the premature
C. albicans biofilm [32]. After 1440 to 2880 minutes, a
complex network of yeast cells, pseudohyphae and true hyphae is established.
The adhesion is governed by physicochemical interactions of Van Der Waals and acid-Lewis base types. The greatest adhesion speed (17.389 cells adhered/cm2/h) was recorded under static condition
with C. albicans harvested from stationary growth phase. Fluctuating velocities of adhesion of cells observed during different growth phases in stationary and dynamic regimes could be due on one hand by changes in physiology of microorganism at each growth stage and on the other hand by changes in the cell surface hydrophobicity of C. albicans after living in NaOCl treated solution [8, 33, 34]. The fight against biofilm formation involves the following steps: (i) the disinfection time before the biofilm develops, (ii) the disinfection of biofilms using aggressive disinfectants, (iii) the inhibition of fixing microbes choosing surface materials that do not promote adhesion [35]. Adhesion to abiotic surfaces is provided primarily by hydrophobic interactions [36].
The hydrophobicity percentages without treatment by NaOCl increased with incubation durations. The
minimum value was recorded under static condition with C. albicans cells harvested from decline growth phase after 180 minutes. The more the incubation period increased, the more C. albicans became hydrophobic and adhered more to substrate. The hydrophobicity percentages of C. albicans after living in the NaOCl disinfected solutions decreased both with incubation periods and concentrations of this disinfectant. It was proven that whatever the cell growth phases or experimental condition considered, a decrease of hydrophobicity percentages was inversely proportional to the NaOCl concentrations and incubation periods. This can be explained by the fact that the increase of cell surface hydrophobicity improves the adhesion of C. albicans to polythene [37]. The abundance of C. albicans adhered to the polythene in the solutions containing NaOCl reached 1.18 units (Log (CFU/cm²)) although sometimes rare. Irrespective of the growth phases or experimental conditions considered, the abundance of C. albicans adhered to the polythene also decreased with incubation periods and different concentrations of NaOCl contained in solution. The considerable differences in antimicrobial susceptibility between planktonic C. albicans and adhered cells were noted. Several mechanisms explain the increased resistance of adhered C. albicans to antimicrobial agents. The first being that environmental gradients within the structure of adhered cells can lead to changes in NaOCl concentrations reaching individual cells. In fact, chemical gradients such as pH can affect the antimicrobial activity [38, 39]. The increased resistance of adhered cells was also explained by a delay of penetration of the antimicrobial agents through the extracellular matrix. Antimicrobial agents must diffuse through the extracellular matrix by means of water channels to reach the cells. The matrix may act as a barrier to antimicrobial compounds because the target cells are in the adhered biomass [40]. The matrix components can also bind directly to antimicrobial agents [41-43]. Another hypothesis is that the adhered cells are in a metabolic rest, grow more slowly than their planktonic counterparts, which makes them refractory to antimicrobial therapy. It is also known that nutrients limitation and the production of toxic metabolites promote biofilm formation [44].
By considering separately each experimental condition, it was noted that increasing incubation durations lead to a significant decrease of the effectiveness of NaOCl (P<0.01). This resulted in a decrease of the abundance of adhered C. albicans. It is indicated that a biofilm can develop within a few hours and allow the microorganisms therein become resistant to external agents causing any contamination [45, 46]. Under static as well as dynamic condition, increased efficiency and concentrations of NaOCl on C. albicans adhered to the polythene was noted. This resulted in a significant decrease of the abundance of adhered C. albicans after living in the NaOCl disinfected solution. According to Ji-Hyoung et al. [47], treatment of biofilms with
antimicrobial agents entails removing adhered cells. Furthermore, the variation of C albicans cells behavior against the action of NaOCl may be related to changes in their surface hydrophobicity due to a change in their growth stage [12]. Some previous studies have shown the effect of pH values on the C. albicans adhesion [8]. Other indicated the effects of disinfectants on the adhesion of some eubacteria [48].
It was also noted that for each incubation period and each growth phase, an increase of the concentration of the disinfectant contained in the solution significantly decreased the abundance of C. albicans adhered to the substrate (P<0.01). C. albicans develop hyphae and form biofilms as a survival strategy to face antimicrobial agent [8, 49, 50]. Several mechanisms are involved in antimicrobial resistance of C. albicans adhered to the substrate namely (i) the slow penetration of the antimicrobial agent into the biofilm, (ii) chemical changes in the microenvironment formed by the adhered cells, leading to areas of slow or zero growth (iii) the adaptation of responses to stress, and (iv) the presence of a small population of highly resistant cells [39, 51, 52].
A significant difference (P<0.05) was observed between the mean densities of adhered C. albicans after living in the solutions treated with different concentrations of NaOCl in the exponential, stationary, and decline growth phases. The effectiveness of any method of disinfection depends on biotic factors such as the physiological state and the intrinsic microbial resistance to lethal agents [53]. It is important to remember that microorganisms contained in a biofilm have very different characteristics from their planktonic counterparts including the production of exopolymers [54], a significant increase in antimicrobial resistance and environmental stress [52, 55]. The exopolymer matrix that acts as a mechanical barrier, reducing the rate of penetration of the compounds through the biofilm environment, thus protecting the cells embedded in the biofilm. This explains the fact that the increase of the concentration of NaOCl in solutions for each growth stage lead to a significant decrease (P<0.01) of the abundance of C.
albicans adhered to substrates. In addition, the
adhesion of C. albicans to substrate is influenced by the cell growth phase. C. albicans harvested from stationary growth phase adhered in greater numbers than the cells harvested from exponentially growth phase [8].
CONCLUSION
This study showed that C. albicans harvested from stationary growth phase and under static condition has a high adhesion speed. The adhesion of C. albicans to polythene was influenced by the cell growth phase. In the solutions containing different concentrations of NaOCl, a significant difference was observed amongst the mean densities of C. albicans adhered in exponential, stationary, and decline growth phases. The hydrophobicity percentages of C. albicans in the absence of NaOCl increased with incubation periods. In
the solution treated with NaOCl, the hydrophobicity percentages decreased both with incubation periods and concentrations of the NaOCl. C. albicans became hydrophobic in the presence of these NaOCl when their concentrations and incubation periods decreased. The increase of cell surface hydrophobicity thus improved the adhesion of C. albicans to polythene. Although C.
albicans adhered to polythene was hydrophobic in the
NaOCl disinfected solution, it was noted that cell surface hydrophobicity was more important under static condition and short incubation periods.
Acknowledgements
We are grateful to Dr Charles Kouanfack (Head of Microbial Laboratory of the Yaoundé Central Hospital (YCH)) for providing us Candida albicans strains.
REFERENCES
1. Gauthier F (2002) Biofilms et qualité biologique de l'eau Potable au cours de sa distribution. Mémoire DESS Université de Picardie-Amiens p.78.
2. Mouchet P, Montiel A and S. Rigal (1992) Dégradations physico-chimiques de l’eau dans les réseaux de distribution. T.S.M. L’eau 87: 299-306.
3. Rodier J (2009) L’analyse de l’eau. 9e édition, Dunod, Paris p.1579.
4. Schoenen D (2002) Role of disinfection in suppressing the spread of pathogens with drinking water: possibilities and limitations. Water Research 36: 3874-3888.
5. Mavor A L, Thewes S, and Hube B, (2005) Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr Drug Targets 6: 863-874.
6. Lagane C (2007) Rôle de l’il-13 et des ligands de PPAR-γ dans la réponse anti-infectieuse des macrophages murins et des monocytes humains vis-a-vis de Candida albicans. Implication de PPAR-γ. Thèse Université Paul Sabatier p.151.
7. Auzeil N, Bonaly R, and Loppinet V (1995) Première approche du drug design d’inhibiteurs de l’adhésion de Candida
albicans aux cellules cibles: relations « structure-activité » en
série thiols polyfonctionnels et dérivés. Adhésion microbienne-Nettoyage-Désinfection 79-86.
8. Verran J, Shakespeare A P, Willcox M D P et al. (1991) The effect of pH on adhesion and hyphal formation by strains of
Candida albicans. Microbial Ecology in Health and Disease 4:
73-80.
9. Tanawa E, Djeuda T H, Ngnikam E et al. (2002) Habitat and protection of water resources in suburban areas in African cities. Building Environment 37: 269-275.
10. Kuitcha D, Kamgang V, Sigha L et al. (2008) Water supply, sanitation and health risks in Yaoundé, Cameroon. African Journal of Environmental Science and Technology 11 (2): 379-386.
11. Ndjama J, Kamgang V, Sigha L et al. (2008) Water supply, sanitation and health risks in Douala, Cameroon. African Journal of Environmental Science Technology 11(2): 422-429. 12. Briandet R (1999) Maîtrise de l’hygiène des surfaces par la création des biofilms-Aspects physico-chimiques. Thèse de Doctorat, Ecole Nationale Supérieure Agronomique de Rennes, Rennes p.197.
13. Rosenberg M, Gutnick D, and Rosenberg E (1980) Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiology Letters 9: 29-33.
14. Jain A, Nishad K K, and Bhosle N B (2007) Effects of DNP on the cell surface properties of marine bacteria and its implication for adhesion to surfaces. Biofouling 23:171-177. 15. Coeyrehourcq K L (2003) Etude de méthodes rapides
d’analyse de la structure moléculaire du polyéthylène, Thèse de Doctorat, Ecole des Mines de Paris Spécialité Science et Génie des Matériaux p.208.
16. Boutaleb N (2007) Etude de la formation de biofilms sur les matériaux couramment utilisés dans les canalisations d’eaux
potables. Thèse de Doctorat, Université de Bretagne-sud p.194.
17. Ratner B D (1993) Plasma deposition of organic thin film-control of film chemistry. ACS Polym Prepr 34: 643-4. 18. Ratner B D (1995) Surface modification of polymers:
chemical, biological and surface analytical challenges, Biosensors and Bioelectronics 10: 797-804.
19. Noah Ewoti O V, Nola M, Moungang L M et al. (2011) Adhesion of Escherichia coli and Pseudomonas aeruginosa on rock surface in aquatic microcosm: Assessment of the influence of dissolved magnesium sulfate and monosodium phosphate. Research Journal of Environmental and Earth Sciences 3(4): 364-374.
20. Dukam S, Pirion P, and Levi Y (1995) Modélisation du développement des biomasses bactériennes libres et fixées en réseau de distribution d’eau potable. In: Adhésion des microorganismes aux surfaces. Bellon-Fontaine M. N., and J. Fourniat (éds), Paris, pp. 149-160.
21. Noah Ewoti O V, (2012) Rétention des bactéries dans le sol et sur des fragments de roches en milieu aquatique: influence du type de cellule et de quelques paramètres chimiques de l’environnement. Thèse de l’Université de Yaoundé I p. 158. 22. Haddouchi F, Lazouni H A, Meziane A et al. (2009) Etude
physicochimique et microbiologique de l’huile essentielle de Thymus fontanesii Boiss et Reut. Afrique Science 5(2): 246 – 259.
23. Pelletier C et Bourlioux P (1995) Effets des concentrations subinhibitrices de nitroxoline sur la surface d’Escherichia coli uropathogènes. In: Adhésion des microorganismes aux surfaces. Bellon-Fontaine M. N., and J. Fourniat (éds), Paris, pp. 101-115.
24. Donlan R M and Costerton J W (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167- 93.
25. Jucker B A, Harms H, and Zehnder A J B (1996) Adhesion of the positively charged bacterium Stenotrophomonas
(Xanthomonas) maltophilia 70401 to glass and teflon. Journal of Bacteriology 178: 5472-5479.
26. Wang I, Anderson J M, Jacobs M R et al. (1995) Adhesion of
Staphylococcus epidermidis to biomedical polymers: Contributions of surface thermodynamics and hemodynamic shear conditions. Journal of Biomedical Material Resources 29, 485-493.
27. Jayasekara N Y, Heard G M, Cox J M et al. (1999) Association of micro-organisms with the inner surfaces of bottles of non-carbonated mineral waters. Food Microbiology 16: 115-128 28. Zhao X, Daniel K J, Oh S H et al. (2006) Candida albicans Als3p
is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology 152: 2287-99.
29. Argimon S, Wishart J A, Leng R et al. (2007) Developmental regulation of an adhesion gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryotic Cell 6: 682-92.
30. Hoyer L L, Green C B, Oh S H et al. (2008) Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family a sticky pursuit. Med Mycol 46: 1-15.
31. Nobile C J, Schneider H A, Nett J E et al. (2008) Complementary adhesion function in C. albicans biofilm formation. Curr Biol 18: 1017-24.
32. Chandra J, Kuhn D M, Mukherjee P K et al. (2001) Biofilm formation by the antifungal pathogen Candida albicans: development, architecture, and drug resistance. Journal of Bacteriology 183: 5385-94.
33. O’Toole G A, and Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Molecular Microbiology 30: 295-304.
34. Parot S (2007) Biofilms Electroactifs: formation, caractérisation et mécanismes. Thèse Institut National polytechnique de Toulouse p. 204.
35. Meyer B (2003) Approaches to prevention, removal and killing of biofilms. International Biodeterioration and Biodegradation 51(4): 249-253.
36. Dunne W M (2002) Bacterial adhesion: seen any good biofilms lately. Clin Microbiol Rev 15: 155- 66.
37. Tribedi P and Sil A K (2013) Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. Journal of Applied Microbiology 12375.
© 2014; AIZEON Publishers; All Rights Reserved
This is an Open Access article distributed under the terms of the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
38. Stewart P S and Costerton J W (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358: 135-8.
39. Stewart P S and Franklin M J (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6: 199-210 40. Gordon C A, Hodges N A and Marriott C (1988) Antibiotic
interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis derived Pseudomonas
aeruginosa. Journal of Antimicrobial Chemotherapy 22:
667-4.
41. Nett J, Lincoln L, Marchillo K et al. (2007) Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother 51: 510-20.
42. Nett J E, Sanchez H, Cain M T et al. (2010) Genetic basis of
Candida biofilm resistance due to drug-sequestering matrix
glucan. J Infect Dis 202: 171-5.
43. Sadovskaya I, Vinogradov E, Li J et al. (2010) High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: the ndvB gene is involved in the production of highly glycerol-phosphorylated beta-(1,3)-glucans, which bind aminoglycosides. Glycobiology 20: 895-904.
44. Kumamoto C A (2002) Candida biofilms. Curr Opin Microbiol 5: 608-11.
45. Beech I B and Coutinho C L M (2003) Biofilms on corroding materials. In Biofilms in Medicine, Lens P, Moran A P, Mahony T, Stoodley P and O’Flaherty V eds.
46. Beech I B and Sunner J (2004) Biocorosion: Towards understanding interaction between biofilms and metals, Journal of Current Opinion in Biotechnology 15:181-186. 47. Ji-Hyoung H, Se-Hee J and Sang-Do H (2011) Synergistic
Effects of Combined Disinfection Using Sanitizers and UV to Reduce the Levels of Staphylococcus aureus in Oyster Mushrooms. Journal of Korean Society in Applied Biology and Chemistry 54(3): 447-453.
48. Lontsi Djimeli C, Nola M, Tamsa Arfao A et al. (2013) Effect of disinfectants on adhered Aeromonas hydrophila to
polyethylene immersed in water under static and dynamic conditions. International Journal of Research in BioSciences 2: 33-48.
49. Stepanovic S, Cirkovic I, Mijac V et al. (2003) Influence of the incubation temperature, atmosphere and dynamic conditions on biofilm formation by Salmonella spp. Food Microbiology 20: 339-343.
50. Stepanovic S, Cirkovic I, Ranin L et al. (2004) Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Letters in Applied Microbiology 38(5): 428-432.
51. Mah T F C, and O’Toole G A (2001) Mechanisms of biofilms resistance to antimicrobial agents. TRENDS in Microbiology 9: 34 -39.
52. Donlan R M (2002) Biofilms: microbial life on surfaces. Emerging Infectious Disease 8: 881-890.
53. Patel R (2005) Biofilms and antimicrobial resistance. Clin Orthop Relat Research 437:41-47.
54. Parsek M R and Greenberg E P (2000) Acyl-homoserine lactone quorum sensing in gram negative bacteria: a signaling mechanism involved in associations with higher organisms, Process Natural Academic Science, 97, 8789-8793.
55. Campanac C, Pineau L, Payard A et al. (2002) Interactions between biocide cationic agents and bacterial biofilms. Antimicrobial Agents and Chemotherapy 46:1469-1474.