Publisher’s version / Version de l'éditeur:
Special Report - Highway Research Board, 90, pp. 58-73, 1967-03-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Effect of sorbed water on some mechanical properties of hydrated
portland cement pastes and compacts
Sereda, P. J.; Feldman, R. F.; Swenson, E. G.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=fb83d668-1d05-464f-9cd2-9231b51e6a55 https://publications-cnrc.canada.ca/fra/voir/objet/?id=fb83d668-1d05-464f-9cd2-9231b51e6a55Effect of Sorbed Water on Some Mechanical
Properties of Hydrated Portland Cement
Pastes and Compacts
byP. J. SEREDA, R. F. FELDMAN, a n d E . G. SWENSON
Reprinted f r o m Special Report 90 (1966) Highway Research Board, Washington, D. C.
Effect of Sorbed Water on Some Mechanical
Properties of Hydrated Portland Cement
Pastes and Compacts
P . J . SEREDA, R . F . FELDMAN, and E . G . SWENSON, Research Offices, Division of Building R e s e a r c h , National Research Council, Canada
The chief aim of t h i s work was t o observe any change in the m e - chanical p r o p e r t i e s of cement pastes and compacts when water is sorbed. Thin d i s c s of normal cement paste were cut f r o m 1%-in. diameter cylinders made of 0 . 3 t o 0 . 7 w/c r a t i o s . Com- pacts of s i m i l a r shape were p r e p a r e d f r o m bottle-hydrated c e - ment a t p r e s s u r e s of 15,000, 40,000 and 110,000 p s i . All s a m p l e s w e r e conditioned t o different levels of RH from 0 to 98 percent and back t o 0; a t each levelthe modulus of elasticity of a l l s a m p l e s and the strength of s o m e were determined by m e a n s of a miniature testing machine, which is described. Special precautions were taken t o avoid any carbonation of the s a m p l e s by conditioning them over solutions of sodium hydrox- ide and measuring in gloved conditioned boxes. No change in elastic modulus occurred between 0 and 50 percent RH, but. an i n c r e a s e was observed above 50 percent RH. T h i s was main- tained on desorption, and appeared t o be associated with entry of interlayer water in the tobermorite gel, which supports the mechanism of length change previously proposed by the authors. The maximum f r a c t u r e -strength f o r s a m p l e s of given porosity was attained a t 0 percent RH, and the l a r g e s t reduction of strength was observedfrom 0 t o 1 5 percent RH. P l o t s of Young's modulus v s porosity and f r a c t u r e -strength vs porosity gave common curves f o r compacts and p a s t e s .
oTHE E F F E C T of relative humidity on building m a t e r i a l s h a s long been observed through change i n dimensions. Meeham
(I),
however, was the f i r s t t o r e l a t e t h i s length change t o physical adsorption, and he did t h i s by studying the adsorption of v a r - ious g a s e s on carbon. Since that time, length change a r i s i n g f r o m adsorption h a s been used a s a method of examining and testing various t h e o r i e s concerning the solid-gas interaction. Yates(2,3)
concluded that because length changes do occur during adsorp- tion, the adsorbent is "perturbedtt and a change in the elastic properties of the material will occur. Other w o r k e r s(4,5)
have concluded that the change in thermodynamic s t a t e of an adsorbent a r i s i n g f r o m physical adsorption is wholly equivalent to a change in i t s s t a t e of s t r e s s . Thus, a change in the e l a s t i c p r o p e r t i e s would not be expected f o r changes of s t r e s s within the e l a s t i c range.Extensive work by P o w e r s in defining the s t r u c t u r e of portland cement paste and the r o l e that water plays in i t s physical behavior has laid the foundation for detailed study of i t s mechanical p r o p e r t i e s .
The measurement of the variation of e l a s t i c and f r a c t u r e strength p r o p e r t i e s of hydrated cement with adsorption was initiated because of the fundamental importance it now a s s u m e s . The lack of experimental work in t h i s field on high s u r f a c e - a r e a solids, and m o r e specifically on hydrated cement, f u r t h e r enhances the necessity of t h i s work.
Much has already been published
(5,1,8,2)
on the length changes of compacted specimens of bottle-hydrated cement, and r e s u l t s of measurements of these various mechanical properties will be discussed together a s f a r a s their mechanisms will allow.This work includes the study of both compacted specimens at different porosities and pastes hydrated at different water-to-cement r a t i o s . A comparison of r e s u l t s from the different types of specimens i s discussed to provide information concerning the origin of the cohesive f o r c e s of the hydrated cement system and the mechanism of f r a c t u r e . The mechanism of length change due to adsorption i s further elucidated and conclusions a r e drawn concerning the thermodynamics of the adsorbent-adsorbate s y s - t e m .
EXPERIMENTAL Sample Materials
A batch of Type 1 portland cement (3280 Blaine, our laboratory designation M-2) was procured for a l l samples used in this and future investigations. The m a t e r i a l was stored in sealed containers.
Compact Samples
Preparation of compacts from powdered material has been described in s e v e r a l previous publications (f3,z). The powdered hydrated cement was obtained by hydrating it in rotating polythene bottles, a s described by Brunauer et a l .
(10).
The filtered hydrated cement was dried to 30 percent RH in vacuum desiccators, then screened through a 100-mesh sieve. The fraction not passing 100-mesh, mostly Ca(OH), c r y s - t a l s , was ground and recombined with the remainder of the material. Some of i t was dried in vacuum over magnesium perchlorate f o r compaction under dry conditions. Uniform distribution of powder into the mold was achieved with the aid of a vibrating screen, assembly. All compacts were made to the same thickness of 0.050 ; 0.002 in. Thickness of individual samples varied about 0.0015 in.A total of 75 compacts were prepared at t h r e e compaction p r e s s u r e s , giving three groups of 25. All the compacts were dried in vacuum over magnesium perchlorate and their modulus of elasticity was measured. Several of each group were then broken to determine breaking strength. This procedure was repeated with a l l remaining compacts successively f o r equilibrium conditions of 15, 25, 35, 50, 75, 98, 50, 25 and 0 p e r - cent RH (except that no samples were broken on adsorption at 25, 35 and 50 percent RH). The conditioning was c a r r i e d out in vacuum desiccators over solutions of NaOH, which were s t i r r e d for intervals of one out of every five minutes.
P a s t e Samples
Cement and water were mixed under partial vacuum conditions according t o the p r o - cedure described by Powers et a l . (=) t o give pastes of 0 . 3 , 0.4, 0.5, 0.6 and 0 . 7 w/c r a t i o s . The freshly mixed paste was placed in a tightly stoppered plastic tube 1 . 2 5 i n . in diameter and allowed to rotate horizontally on r o l l e r s at the r a t e of 0.7 r p m . The rolling was continued for 24 hours and achieved a high degree of uniformity in density (determined by taking sections from different p a r t s of the cylinder), especially with high w/c ratio p a s t e s . The paste cylinders were removed after 24 hours and allowed to hydrate in the presence of water for 130 days.
At the end of the hydration period the paste cylinders were cut to yield discs 1 . 2 5 in. in diameter and 0.050
+
0.002 in. thick (variation in thickness of individual samples being 0.001 in.); these were immediately placed in water. A total of 40 samples of each w/c ratio was prepared. Soon after cutting the modulus was determined f o r a l l the samples and four of each w/c ratio were broken t o determine their breaking strength. Measurements were made in a gloved chamber a t near-saturation conditions.~ h k
remaining samples were then conditioned to 0 percent RH in vacuum desiccators over magnesium perchlorate and the modulus was determined f o r a l l the samples in a glovedTABLE 1A
SPOT ANALYSIS FOR CARBONATION-PERCENT CO, OF PASTES a
-- --
Condition of Storage, RH
'Based on actual weight of sample at condition of storage.
TABLE 1B
SPOT ANALYSIS FOR CARBONATION-PERCENT CO, O F COMPACTSa Condition of Storage, RH psi 0 1 5 2 5 75 98 5 0 25 110,000
-
-
1 . 0 1 . 3 0.8 1 . 0 1.1 40,000 1 . 1 1.1-
--
-
1 . 8 15,000 1 . 4 1 . 5 1 . 6 2.0-
2.6 3 . 3 'Based on actual weight o f sample a t c o n d i t i o n of storage.box a t 0 percent RH. Again, four samples of each group w e r e broken to determine strength. This procedure was repeated successively for conditions of 12, 29, 37.5, 47.5, 77.5, 98, 52 and 26 percent RH by conditioning in vacuum desiccators over various concentrations of NaOH t o give the desired conditions of relative humidity while maintaining C0,-free conditions (breaking strength was not determined at 29 and 47.5 percent conditions). Before storage the desiccators were evacuated to i n c r e a s e the r a t e of equilibration with water.
At conditions of high relative humidity (when NaOH concentration was low, affording little protection f r o m diffusing COP) the evacuated desiccators were placed i n chambers controlled to about 25 percent RH with a strong NaOH solution. Air in t h e chamber was circulated over the solution in order t o reduce f u r t h e r the chance of carbonation. Gloved boxes where measurements on the s a m p l e s w e r e made were also conditioned to the desired relative humidity by NaOH solutions to avoid carbonation. These precautions proved effective; on analysis t h e r e was little change in the amount of carbonation after the long s e r i e s of experiments (Table 1). The final condition was 0 percent RH when a l l remaining samples were broken to determine strength.
Apparatus
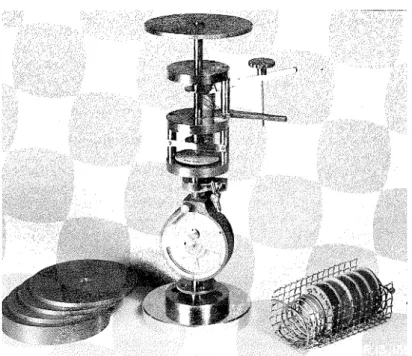
Figure 1 shows the desiccators for conditioning compacts and discs of paste, a s well a s the conditioned gloved box where the various measurements were performed.
Figure 2 shows the miniature testing machine that was used to determine the load- deflection characteristics of the compacts and d i s c s of paste f r o m which the Young's modulus was calculated. T h i s testing machine (Fig. 2a) was equipped with a platform for easy loading with dead weights. T o determine strength a s i m i l a r testing machine
Figure 1. Gloved conditioned box and test apparatus.
Figure 2(a). Miniature testing machine with dead weights for loading used for determining Young's modulus.
I 1 1 1 1 1 1 1 1 I I I I l l I I I 1 1 1 1 1 1 1 - -
k-)
;>*
-
- - - --
LEGEND Conditioned at 0% R.\\
H.-
- o Conditioned at 30% R. H. --
1 I 1 1 1 1 1 1 1 I I 1 1 1 1 1 1 1 I 1 1 1 1 1 1 1 10 1cm C O M P A C T I O N P R E S S U R E , ~ ~ 1 x 1 0 'Figure 3. Compaction characteristics of bottle-hydrated cement.
that this ratio should be the same a s the ratio of their Young's modulus (assuming the s a m e Poisson's ratio). On this basis the modulus of one can be determined when the other i s known.
For calibrating discs of s t e e l and cement, using the known value of E for this steel and the ratio of loads for the common deflection, a value of E f o r cement of 1.68 X l o 6 i s obtained that compares well with the value of 1 . 7 0 x l o 6 obtained by loading the cyl- inder. This result also indicates that appreciable differences in Poisson's r a t i o be- tween samples have little influence on the final result of Young's modulus when i t i s determined in this way.
For compacts the material may not be isotropic because of possible preferred orientation; this would be reflected in the Poisson's r a t i o . Because the change in Poisson's ratio i s not in direct proportion t o the change in E, a s determined by bending the sample a s a plate, it i s expected that this effect will not be very g r e a t . No value can be suggested for this effect at the present time, however.
RESULTS Porosity v s Compaction P r e s s u r e
The porosity of compacts was calculated from the measured external dimensions and the weight. Because the compacts a r e made to precise dimensions, and because the cement was completely hydrated and i t s density determined to be 2.60, i t was possible for this determination to yield a reliable value.
Figure 3 shows r e s u l t s f o r porosity a s a function of compaction p r e s s u r e f o r bottle- hydrated cement in equilibrium with 30 percent RH and with 0 percent RH. At 30 p e r - cent RH a linear portion for the log plot exists between 12,000 and 120,000 psi; a 0 p e r - cent RH this linear portion extends from 15,000 to 205,000 p s i . It may be noted that the final portion of the curve f o r 30 percent RH gives a smaller decrease of porosity than that for 0 percent RH. This exponential relationship has been observed previously f o r other m a t e r i a l s
(13).
The character of the lower p r e s s u r e p a r t of the curve has been attributed to packing of particles and that of the high p r e s s u r e region to the p r o -c e s s of fracture and plastic flow. The effect of adsorbed water is clearly shown, although the exact mechanism is not known. It may involve the process of fracture o r
' . O F
1
T_ - - - A - - - A ---
+-
-
~l----p--G
--I ----
----
--
- A M - - - 7$im psii
L L I20 40 60 80 100
R E L A T I V E H U M I D I T Y . 5.
Figure 4. Young's modulus as a function o f r e l a t i v e humidity f o r compacts.
a more direct interference of adsorbed water (approximately a monolayer) in the com- paction process
(2).
At 205,000 psi the void fraction that can be reached i s 15.5 per- cent.Young's Modulus vs Relative Humidity
Figure 4 shows Young's modulus a s a function of relative humidity f o r compacts fabricated at three different pressures. Approximately 25 samples at each conditioning humidity for each fabrication pressure were used in the measurements. In this plot the confidence interval of results from the arithmetic mean is shown at the 95 percent limit. This indicates that the results at a particular compaction pressure vary by approximately & 10 percent.
From these results it i s clear that within the confidence value of the points there i s no variation from 0 to 50 percent RH. Beyond 50 percent RH for the 110,000-psi com- pacts, there i s a significant increase in E . Although there i s a suggestion of the same result f o r the 40,000-psi compacts it is not in itself significant. For the compact fabricated at 15,000 psi there i s a significant increase in E for 98 percent RH over
50 percent RH, and this trend appears to s t a r t after 50 percent RH. Thus, on the basis of the three different s e r i e s of compacts within the stated significance of the results, E shows no change f r o m 0 to 50 percent RH, but there is a definite trend to an increase in E beyond this. At 0 percent RH f o r the 110,000-psi compacts the value of E i s
S a t u r a t e d 0 2l 40 60 80 lm 1 s t Drying R E L A T I V E H U M I D I T Y . S S a t u r a t e d 0 20 40 M 80 lm 1 s t D r y l n g R E L A T I V E HUM1 D I T Y . S
Figure 5. Young's modulus as a function of relative humidity for paste.
Figure 6. Young's modulus as a function o f com- puted porosity.
Variation of Young's Modulus With Porosity
On desorption t h e r e i s a further increase in E down to 26 percent RH. On drying to nearly the starting condition of dryness the E decreased to the starting value for the 15,000- and 40,000-psi compacts and below it for the 110,000-psi compacts.
The s a m e experimental procedure was repeated for paste samples hydrated a t w/c r a t i o s of 0.3, 0.4, 0.5, 0.6 and 0.7; qualitatively, the r e s u l t s have agreed in a l l details with r e s u l t s obtained f o r the compacts shown in Figure 5.
The paste samples were measured in the wet condition after hydration and before drying prior t o conditioning. This gave values of Young's modulus for the f i r s t drying cycle. This could not be obtained f o r the compacts. It i s evident that the value of E i s unchanged during adsorption in the region 0 to 50 percent RH and that a t higher humidities E increases, being highest near saturation. On desorption the high value of E p e r s i s t s to a condition approaching dry s t a t e . Final measure- ments were made before complete equi- librium was reached with magnesium p e r - chlorate
.
The porosities of compacts were determined from measurements of the dimensions and weights a s described e a r l i e r . The porosities of pastes were calculated on the
7.0;-
0
1
Cornpattion 0
-
a A Adsorption ValuesA Desorptlon Values
\
----
Mean Value Lines\ 8
\0_
--- ---
110,000ps18
RELATIVE HUM1 DITY,
+
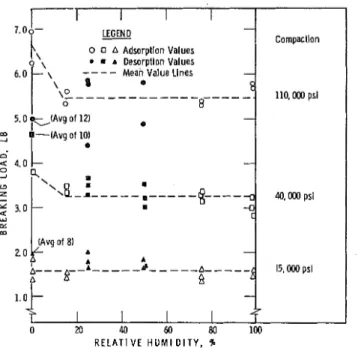
Figure 7. Breaking load vs relative humidity for compacts.
b a s i s of m e a s u r e m e n t s of evaporable w a t e r , non-evaporable water (lost between 120 a n d 800 C ) , density of h y d r a t e d cement ( 2 . 6 0 ) , and a s s u m e d density of unhydrated c e - ment (taken a s 3 . 1 5 ) . In making t h e s e m e a s u r e m e n t s t h e d e g r e e s of hydration of t h e p a s t e s w e r e d e t e r m i n e d (no significant change f r o m beginning t o end of t e s t s ) ; t h e s e w e r e 58, 68, 73, 78 and 77 p e r c e n t , corresponding t o the w/c r a t i o s of 0 . 3 , 0 . 4 , 0 . 5 , 0 . 6 a n d 0 . 7 , r e s p e c t i v e l y .
T h e Young's modulus of t h e c o m p a c t s and t h e p a s t e s f o r 50 p e r c e n t RH (adsorption)
is plotted i n F i g u r e 6 a s a function of total p o r o s i t y . T h e r e a p p e a r s t o b e a c o r r e l a - tion, and within t h e significant l i m i t s of t h e s e d a t a t h e p a s t e s and c o m p a c t s have t h e
s a m e value of E a t t h e s a m e p o r o s i t y .
Effect of Relative Humidity on F r a c t u r e - S t r e n g t h
T h e r e s u l t s f o r f r a c t u r e - s t r e n g t h of t h e c o m p a c t s a r e p r e s e n t e d i n F i g u r e 7 . Only two o r t h r e e s a m p l e s w e r e broken a t any point w h e r e f r a c t u r e - s t r e n g t h w a s d e t e r m i n e d . F r o m t h e s e r e s u l t s alone, it is c l e a r that although t h e r e a r e not enough points included f o r a s t a t i s t i c a l a n a l y s i s , no significant change o c c u r s in t h e f r a c t u r e c h a r a c t e r i s t i c s f r o m 1 5 t o 98 p e r c e n t RH; a c l e a r distinction of t h e f r a c t u r e - s t r e n g t h d a t a f o r e a c h group is shown. Mean values f o r the breaking load f r o m 1 5 t o 98 percent RH m a y be taken a s approximately 5-, 3 - a n d 1 - l b load f o r t h e c o m p a c t s f a b r i c a t e d a t 110,000, 40,000 and 15, OOOpsi, r e s p e c t i v e l y . T h e r e is a n indication that t h e highest f r a c t u r e s t r e n g t h is obtained f o r c o m p a c t s in t h e initially d r y s t a t e , although the r e s u l t s a r e
I
not conclusive because of t h e limited d a t a . It m a y b e noted that desorption points a r e included i n t h i s g r a p h a t 0 , 26 and 52 p e r c e n t RH, a l s o indicating no significant change except f o r 110,000-psi c o m p a c t s a t f i n a l value f o r the d r y s t a t e .
R e s u l t s f o r t h e p a s t e s a m p l e s a r e shown i n F i g u r e 8 , w h e r e t h e effect of f i r s t drying
is c l e a r l y shown; a n i n c r e a s e in t h e s t r e n g t h of the s a m p l e s o c c u r s f o r all the w/c r a t i o s except 0 . 7 . T h i s effect s e e m s t o d e c r e a s e a s the w/c r a t i o is i n c r e a s e d . Be- tween 0 and 1 5 p e r c e n t RH t h e r e is a d e c r e a s e in s t r e n g t h f o r a l l w/c r a t i o s except 0 . 7 . T h i s effect d e c r e a s e s with w/c r a t i o ( F i g . 8 ) . T h i s reduction in strength was a l s o indicated i n the e x p e r i m e n t s on t h e c o m p a c t s , especially t h e 40,000- and 1 1 0 , 0 0 0 - p s i g r o u p s . F o r t h e p a s t e s , t h e r e a p p e a r s t o be a slight f u r t h e r reduction between 1 5 a n d 98 p e r c e n t RH f o r a l l g r o u p s , especially t h o s e with a w/c r a t i o of 0 . 4 and 0 . 5 .
WIC = 0.6 (Avg of 10) 3
.
.
.
@ 2 values 2 Values'
o/ \ / '8--
-"- -&--.-"V"'UeS----
~ 4 ' 3 Values a 3 values-zL
WIC = 0.7 $9 01 131 2 Values 2 values 2 m - - -8-
-8-
-.-1--8-- 8 / - 8 --
-
-
1
1!-
.
S a t u r a t e d 0 10 20 30 40 50 60 70 80 90 1001st D r y i n g RELATIVE HUM1 DITY,
%
Figure 8. Breaking load vs r e l a t i v e h u m i d i t y for paste.
Taking t h e data f o r a l l the w/c r a t i o s into consideration, however, one must conclude that t h e change is slight. T h i s a g r e e s with the r e s u l t s obtained f o r t h e compacts i n t h e region 15 t o 98 percent RH. Desorption points f a l l c l o s e t o t h e adsorption points and again show the g r e a t e s t change on drying f r o m 26 to 0 p e r c e n t RH.
Variation of Breaking Strength With P o r o s i t y
In o r d e r t o m a k e a m o r e statistically significant comparison of the f r a c t u r e - s t r e n g t h r e s u l t s between compacts and p a s t e s , a l l f r a c t u r e values between 15 and 9 7 . 5 percent RH (for each porosity o r w/c ratio) w e r e u s e d t o obtain a n a r i t h m e t i c m e a n value r e p - r e s e n t i n g each point. T h e validity of t h i s was based on the previous conclusion that no significant reduction in s t r e n g t h o c c u r r e d in t h i s r e g i o n . F i g u r e 9 shows t h e r e s u l t s of f r a c t u r e - s t r e n g t h against porosity f o r t h e compacts and p a s t e s that f a l l together, and shows that the c h a r a c t e r of t h e relationship is s i m i l a r t o that of the plot of E v s porosity i n F i g u r e 6 .
M o i s t u r e Content of Samples
10 20 30 40 50 60 70 POROSITY, V O L % 5 m -I a - 4 - u S 0 5 3 - x u Y @z m 2 I
Figure 9. Breaking load as a f u n c t i o n of the computed porosity. (Mean Values for Conditions 15 to 98% R. H. I
6 - LEGEND
\ I
-
A paste Samples-
Compacts \ \ \ \-
-
i
\\I\
\ 24 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 L E G E N D - o Adsorption Values-
Desorption Values1
R E L A T I V E H U M I D I T I E S , %Figure 10. Moisture conditions a t which the mechanical properties were determined for compacts.
relative humidity conditions of storage at which the mechanical p r o p e r t i e s w e r e d e t e r - mined. Two t o four weeks were allowed f o r equilibrium to be reached. It is believed that most of t h e s e values a r e close to the equilibrium values, with the exception of the final drying when t h e experiments had t o be concluded by a certain date.
DISCUSSION
Use of Compacts i n R e s e a r c h and Comparisons of R e s u l t s With P a s t e s
The u s e of compacts in cement r e s e a r c h , a s h a s previously been suggested, is
0 0 0 0 0 20 40 60 80 100 R E L A T I V E H U M I D I T Y , %
Figure 11. Moisture conditions at which the mechanical properties were determined for pastes.
compaction of hydrated cement (Fig. I ) , especially the high density obtainable a t high p r e s s u r e s , cannot be reproduced by n o r m a l paste hydration. Although the effect of compaction on hydrated cement-possible c r y s t a l orientation and other effects-is not a s yet known, t h r e e significant f a c t s have been uncovered by this work:
1. High values of f r a c t u r e strength and Young's modulus a r e obtained by the m e r e compaction of bottle-hydrated cement powder.
2 . When Young's modulus o r f r a c t u r e strength is plotted a s a function of porosity, the values for both paste and compacts f a l l close together, despite the fact that in the paste samples (especially the 0 . 3 and 0 . 4 w/c r a t i o s ) up t o 42 percent of the cement r e m a i n s unhydrated, while the compacts a r e f o r m e d by a compaction of a fine powder of fully hydrated cement. In these r e s u l t s porosity is the main factor, and the degree of hydration o r method of fabrication a p p e a r s t o b e secondary.
3. All effects of adsorbed water on Young's modulus and f r a c t u r e - s t r e n g t h a r e the s a m e f o r both compacts and paste samples.
T h e relationship between porosity and Young's modulus a s well a s strength is not s u r p r i s i n g . Various relationships have been found f o r a number of polycrystalline c e r a m i c s y s t e m s such a s alumina, magnesia and thoria
(9).
Powers and Brownyard (16,17) applied t h i s concept t o cement paste where "gel-space" r a t i o is used a s the porosity f a c t o r .The similarity of the r e s u l t s f r o m compacts and p a s t e s m u s t lead t o the conclusion that the unhydrated m a t e r i a l i n the sample does not greatly affect the r e s u l t s , because the particle-to-particle bonds a r e made through the hydrated cement. The s i m i l a r i t y of r e s u l t s a l s o leads to the conclusion that during compaction many chemical bonds may be formed, o r that both the compacts and the p a s t e derive much of t h e i r strength f r o m physical f o r c e s brought into play by the proximity of much of t h e v e r y l a r g e s u r f a c e .
Effect of Relative Humidity on Young's Modulus
Adsorption of vapors, including water vapor, has a profound effect upon the elastic constants of t h e porous organic s y s t e m s (such a s wood, p a p e r , cotton). Studies by Zhurkov
(18)
with f i b e r s of acetate silk have shown a d e c r e a s e of Young's modulus t o almost one -third of i t s original value. The explanation f o r t h i s is that t h e adsorbed molecules s c r e e n and neutralize the e l e c t r i c a l f i e l d of t h e polar groups of t h e polymer. As a r e s u l t , t h e molecular cohesion between t h e polymeric macro-molecules is weak- ened, and t h e e l a s t i c modulus d e c r e a s e s simultaneously as length changes occur .Thus, t h e interaction of t h e s u r f a c e with t h e adsorbed molecules is considerable.Although no published work e x i s t s on the effect of adsorbed g a s e s on Young's mod- ulus of polycrystalline inorganic m a t e r i a l s , much work on length change due t o a d s o r p - tion h a s been r e p o r t e d
(1,8,B).
Yates
(2,3)
considered that because length changes do occur during the adsorption of i n e r t g a s e s , t h e adsorbate-adsorbent interaction c a u s e s "perturbation" of t h e adsorbent. F r o m t h i s he concluded that equations derived f r o m the assumption that t h e thermodynamic p r o p e r t i e s of the adsorbed g a s a r e those of a one-component s y s t e m will be of doubtful validity; he suggested that t h e bulk modulus of t h e solid may be a l t e r e d by adsorption, a s was t h e c a s e f o r organic fibrous s y s t e m s . Flood and Heyding(4)
distinguished between essentially chemical p r o c e s s e s and those that may be con- s i d e r e d physical. They considered that t h e change in thermodynamic s t a t e of an a d - sorbent a r i s i n g f r o m physical adsorption is wholly equivalent t o a change in i t s volu- m e t r i c mean s t a t e of s t r e s s . It is c l e a r f r o m t h i s that the bulk modulus (or any elastic properties) of the adsorbent should not change due to physical adsorption. F r o m t h e above and other assumptions that will not be dealt with h e r e , t h e s e authors derived an equation s i m i l a r t o the Gibbs adsorption equation f o r c e r t a i n r a n g e s of concentration of adsorbate on adsorbent.Work by Feldman and Sereda (!,8,2) on length changes of compacts of bottle-hy
-
d r a t e d cement due t o adsorption of water shows that the Gibbs-Bangham equations w e r e obeyed f r o m low humidities (below 5 percent RH) to approximately 30 percent RH. Beyond t h i s humidity i t was suggested that s o m e water entered the lattice of the t o b e r - m o r i t e gel, t h i s occurring in l a r g e r quantities above 50 percent RH. In t h e s e p a p e r s Feldman and Sereda concluded that the mechanism of length change up t o approximately 30 percent RH was due t o a change in s t a t e of s t r e s s of the individual c r y s t a l g r a i n s of t h e t o b e r m o r i t e analogous to t h e elastic volume change experienced when a m a t e r i a l issubjected to a change in the hydrostatic p r e s s u r e . T h i s is c o n t r a r y to t h e concept of t h e separation of t h e s e units @)-similar t o the mechanism of expansion of organic fibrous s y s t e m s . Feldman and Sereda considered that i n t e r l a y e r hydration commenced above 30 percent RH and played a m o r e dominant r o l e in expansion a t humidities above
50 percent RH.
T h e findings f r o m the present work, where t h e r e is no change in t h e Young's mod- ulus in t h e region 0 t o 50 percent RH a s f a r as the accuracy of the r e s u l t s (; 10 p e r
-
cent) show, verify t h e assumptions of Flood(2)
and the theory of length change f o r hydrated cement as outlined above. Above 50 percent RH the i n c r e a s e in Young's mod- ulus may be associated with i n t e r l a y e r hydration, which should have s o m e influence on t h e e l a s t i c p r o p e r t i e s of the hydrated cement. T h i s effect should be m o r e p r o - nounced f o r the higher density paste o r compacts (lower porosity) because the possible number of such bonds o r reinforcements of l a y e r s would i n c r e a s e p e r unit volume of the m a t e r i a l . T h e r e s u l t s described h e r e support t h i s conclusion where the g r e a t e s t effect on E is observed at the lowest porosity. The fact that t h e i n c r e a s e in E is retained by t h e sample on desorption, even under conditions n e a r z e r o RH, f u r t h e r supports the idea that t h e effect is caused by i n t e r l a y e r water, which will not be r e - versibly removed ( 7 , 8 , 2 ) . F i g u r e s 10 and 11 show that the m a t e r i a l retained between1 and 2 percent of water (after drying f o r one month in vacuum over magnesium p e r - chlorate) above the z e r o r e l a t i v e humidity condition a t t h e s t a r t of t e s t s . E x c e p t i o ~ l s w e r e the 110,000-psi compacts where the water was removed almost t o t h e original condition. I t may b e that the f i r s t drying of t h e s e dense compacts was not a s coln- plete a s i t was f o r the other compacts. In any c a s e , t h e r e t u r n of t h e 110,000-psi
compacts t o the original m o i s t u r e condition is the only apparent explanation f o r t h e i r d e c r e a s e in E on desorption, in contrast t o the r e s u l t s of t h e other compacts and paste s a m p l e s .
When considering the other possible c a u s e s f o r the observed i n c r e a s e in E , c a r b o - nation w a s r e j e c t e d on the grounds that no significant difference in t h e amount of c a r - bonate was found before o r after the s e r i e s of t e s t s (Table 1); capillary condensation likewise had t o b e d i s m i s s e d a s a f a c t o r because the i n c r e a s e in E was constant over a wide range of RH, and especially on desorption was retained t o a very low level of RH. Joffe's effect
(2)
can a l s o be d i s m i s s e d because f r a c t u r e - s t r e n g t h does not i n c r e a s e with E.
On f i r s t drying, paste s a m p l e s experience a d e c r e a s e in Young's modulus. T h i s d e c r e a s e in E may b e associated with t h e withdrawal of i n t e r l a y e r hydrate water that o c c u r s a t low humidities. During t h e f i r s t drying, however, i t a p p e a r s t h a t i r r e v e r s i b l e physical s t r u c t u r a l changes occur
(2)
and i t is not clear how t h i s will affect the above interpretation.Because Young's modulus does not change in t h e region of 0 to 50 percent RH, one may be led t o the conclusion that hydrated cement p a s t e s and compacts derive t h e i r rigidity f r o m chemical bonds, which a r e not affected by s o r b e d water, o r that s t r o n g physical bonds unaff ected by sorbed water a r e f o r m e d when solid s u r f a c e s a r e brought close together. Ln t h i s a r e a of study i t h a s been considered
(16)
that s o r b e d water would influence t h e s e physical bonds.Effect of Relative Humidity on Fracture-Strength
Much work has been done on the effect of sorption on f r a c t u r e - s t r e n g t h . The t h e o r i e s of f r a c t u r e developed w e r e based on a concept of flaw propagation, p r i m a r i l y due to Griffith
(2).
He a s s u m e d the p r e s e n c e of flaws o r m i c r o - c r a c k s and defined strength in t e r m s of t h e depth of the c r a c k a s well as the Young's modulus and surface energy. Modern t h e o r i e s founded on solid-state physics provide adequate mechanisms f o r c r a c k initiation in crystalline s y s t e m s , and for glassy s t a t e s kinetic theories have been d e - veloped.In an attempt t o account f o r the reduction in strength of solids by various agents, Rehbinder and Lichtman
(3)
and Orowan(3)
have considered that the adsorption of vapors o r surface-active agents reduces the s u r f a c e - f r e e energy of s o l i d s . Hence, through an equation such a s that of Griffith the d e c r e a s e in strength can be accounted f o r . T h i s hypothesis c o n s i d e r s the reduction of the f r e e energy on t h e newly formed s u r f a c e after the c r a c k o c c u r s . It is difficult to understand how t h i s change in f r e e energy wouldaffect the propagation of the c r a c k in the f i r s t place u n l e s s i t affected t h e value of Young's modulus. F r o m t h e r e s u l t s of much work (25,26, 27) on a variety of m a t e r i a l s and using many a d s o r b a t e s , i t is concluded that physical adsorption plays a minor r o l e , if any, in the strength reduction of solids. It may be stated that where t h e a d s o r b a t e i n t e r a c t s with the adsorbent s o that t h e l a t t e r c a n b e considered perturbed (the interaction is not s i m i l a r t o that of an externally applied f o r c e , causing m e r e l y a change in t h e mean volumetric s t a t e of s t r e s s of t h e solid), then the f r a c t u r e strength may be reduced. T h i s is t h e c a s e f o r many fibrous organic m a t e r i a l s (24).The mechanism of strength reduction in the p r e s e n c e of g a s e s a p p e a r s to involve chemisorption o r chemical interaction of g a s molecules with t h e molecules of the solid f r e s h l y exposed, thus influencing the breaking of other bonds a t t h e apex of t h e c r a c k . The r u p t u r e of the s t r a i n e d bonds may b e affected directly by the foreign mol- e c u l e s , a s can b e deduced f r o m the experiments of Campbell
(29).
C h a r l e s(30)
p r o - posed a s i m i l a r mechanism f o r t h e delayed f r a c t u r e of g l a s s involving a p r o c e s s of s t r e s s corrosion a t the t i p s of s u r f a c e c r a c k s in t h e solid.T h e above mechanism f o r f r a c t u r e - s t r e n g t h is supported by t h e r e s u l t that strength is d e c r e a s e d mainly when the sample is f i r s t exposed t o water in the region 0 t o 1 5 p e r - cent RH.
On f i r s t drying t h e r e is a l s o a strength i n c r e a s e f o r the paste sample consistent with t h e above hypothesis, although t h e a s p e c t of s t r u c t u r a l change must not be d i s m i s s e d .
F r o m the above considerations the highest strength f o r the hydrated cement should occur a t 0 percent RH and t h e f i r s t increment of s o r b e d water should have the maximum
effect in reducing the strength because a n interaction of t h e w a t e r molecules with a f r e s h l y r u p t u r e d s u r f a c e is postulated. T h i s explains why t h e r e is no g r e a t e r strength reduction a t any humidity above 1 5 p e r c e n t . It is postulated that c r a c k propagation o c c u r s a t s i t e s other than a t the l a y e r s ; cleavage of the t o b e r m o r i t e c r y s t a l s does not o c c u r because t h e i n t e r l a y e r hydrate water causing the i n c r e a s e in Young's modulus does not affect t h e f r a c t u r e - s t r e n g t h of the hydrated cement.
CONCLUSIONS
1. When considering the e f f e c t of s o r b e d water on t h e mechanical p r o p e r t i e s of hydrated cement, compacts give the s a m e r e s u l t s a s p a s t e s a m p l e s .
2. Young's modulus r e m a i n s constant within t h e a c c u r a c y of the r e s u l t s (* 10 p e r - cent) in t h e region of 0 t o 50 percent RH. T h i s conclusion s u p p o r t s t h e hypothesis f o r length change based on t h e i d e a that c r y s t a l l i t e s a r e in a s t a t e of s t r e s s and that they t h e m s e l v e s expand when adsorption o c c u r s . T h i s a l s o s u p p o r t s the assumption of Flood who considered that t h e change in the thermodynamic s t a t e of a n adsorbent a r i s i n g f r o m physical adsorption is wholly equivalent t o a change in i t s v o l u m e t r i c , m e a n s t a t e of s t r e s s .
3. M e r e compaction of hydrated cement powder a t t a i n s high values of f r a c t u r e - strength and Young's modulus.
4. P o r o s i t y is t h e b a s i c p a r a m e t e r determining t h e s t r e n g t h and Young's modulus. 5. An i n c r e a s e in Young's modulus was observed in the region 50 t o 100 percent RH. T h e r e - e n t r y of water into t h e lattice of t h e t o b e r m o r i t e is suggested a s a n e x - planation.
6. Highest s t r e n g t h f o r s a m p l e s of a given porosity is attained a t 0 p e r c e n t RH, and t h e l a r g e s t reduction of strength is experienced in going f r o m 0 t o 15 p e r c e n t RH. In going t o any humidity above 1 5 percent RH, t h e r e is only a slight f u r t h e r reduction in s t r e n g t h .
T h e r e s u l t s obtained in t h i s investigation a r e b a s e d on equilibrium data, avoiding g r a d i e n t s (use of thin s a m p l e s ) and all extraneous p r o c e s s e s such a s carbonation by t h e u s e of a s p e c i a l p r o c e d u r e .
REFERENCES
1. Meeham, F. T . T h e Expansion of C h a r c o a l on Sorption of Carbon Dioxide. P r o c . Roy. Soc. ondo don), S e r . A, No. 115, p . 199, 1927.
2. Y a t e s , D. J . C. Volume Changes in P o r o u s Glass Produced by the Physical Adsorption of G a s e s . Advances in C a t a l y s i s and Related Subjects, Vol. 9, p . 481, 1956.
3. Yates, D. J . C . T h e Influence of t h e P o l a r Nature of t h e Adsorbate on Adsorption Expansion. J o u r . P h y s . C h e m . , Vol. 60, p. 543, 1956.
4. Flood, E . A.
,
and Heyding, R. P . S t r e s s e s and Strains in Adsorbent-Adsorbate S y s t e m s . Can. J o u r . C h e m . , Vol. 32, p. 660, 1954.5. Flood, E . A. S t r e s s e s and S t r a i n s in Adsorbent-Adsorbate S y s t e m s 11. Can. J o u r . C h e m . , Vol. 35, p . 48, 1957.
6. S e r e d a , P . J . , and Feldman, R . F. Compacts of Powdered M a t e r i a l s as P o r o u s Bodies f o r U s e in Sorption Studies. J o u r
.
Appl. C h e m . , Vol. 13, p. 150, 1963. 7. Feldman, R. F . , and S e r e d a , P . J . T h e U s e of Compacts t o Study t h e SorptionC h a r a c t e r i s t i c s of Powdered P l a s t e r of P a r i s . J o u r . Appl. C h e m . , Vol. 13, p. 375, 1963.
8 . Feldman, R. F . , and Sereda, P . J . Sorption of Water on Compacts of Bottle- Hydrated Cement. I. T h e Sorption and Length-Change I s o t h e r m s . J o u r
.
Appl. C h e m . , Vol. 14, p . 87, 1964.9 . Feldman, R. F . , and Sereda, P . J . Sorption of Water on Compacts of Bottle- Hydrated Cement. II. Thermodynamic Considerations and T h e o r y of Volume Change. J o u r . Appl. C h e m . , Vol. 14, p . 93, 1964.
10. B r u n a u e r , S . , Kantro, D. L . , and Copeland, L . E
.
T h e Stoichiometry of t h e Hydration of B-Dicalcium Silicate and T r i c a l c i u m Silicate a t Room T e m p e r - a t u r e . Arner. Chem. Soc. J o u r . , Vol. 80, p . 761, 1958.P o w e r s , T . C . , Copeland, L . E . , Hayes, J . C . , a n d Mann, H. M. P e r m e a b i l i t y of P o r t l a n d Cement P a s t e s . P r o c . ACI, Vol. 51, p . 285, 1954.
Timoshenko, S
.
T h e o r y of P l a t e s a n d Shell. McGraw-Hill, New Y o r k a n d London, 1940.Cooper, A. R . , a n d Eaton, L . E
.
Compaction Behavior of S e v e r a l C e r a m i c P o w d e r s . J o u r . A m e r . C e r a m i c S o c . , Vol. 45, p . 97, 1962.Van Olphen, J . Second National Conf. on Clay a n d Clay M i n e r a l s , Columbia, M i s s o u r i , 1953. P r o c . , pp. 418-438.
Spinner, S . , Knudsen, F. P . , and Stone, L . E l a s t i c Constant-Porosity Relations f o r P o l y c r y s t a l l i n e T h o r i a . J o u r
.
R e s.
National B u r e a u of S t a n d a r d s , C , Vol. 67, p . 39, 1963.P o w e r s , T . C.
,
a n d Brownyard, T . L . Studies of t h e P h y s i c a l P r o p e r t i e s of Hardened P o r t l a n d Cement P a s t e . J o u r . ACI ( P r o c . ) , Vol. 43, p . 101, 1947; a l s o , PCA Bull. 22.P o w e r s , T . C
.
Fundamental A s p e c t s of t h e Shrinkage of Concrete. T h i r d I n t e r n a t . C o n g r e s s of t h e P r e c a s t Concrete Ind.,
Stockholm, 1960.Zhurkov, S . N. T h e Influence of Volume Sorption on t h e Mechanical P r o p e r t i e s of P o l y m e r s . Dokl. Akad. Nauk USSR, Vol. 49, p . 198, 1945.
A m b e r g , C . H . , a n d McIntosh, R . A Study of Adsorption H y s t e r e s i s b y M e a n s of Length Changes of a Rod of P o r o u s G l a s s . Can. J o u r . C h e m . , Vol. 30, p . 1012, 1952.
Joffe, A. On t h e Cause of t h e Low Value of Mechanical Strength. I n t e r n a t . Conf. on P h y s i c s . P h y s i c a l S o c . , London, p . 72, 1934.
Helmuth, R . A. P r i v a t e communication.
Griffith, A. A . T h e Phenomena of Rupture and Flow i n Solids. P h i l . T r a n s . Roy. Soc. London, S e r . A, No. 221, p . 163, 1921.
Rehbinder, P . , and Lichtman, V. Effect of S u r f a c e Active Media on S t r a i n s a n d Rupture i n Solids. Second Internat. Conf
.
on S u r f a c e Activity, P r o c . ,p . 563. Butterworth, London, 1957.
Orowan, E . T h e Fatigue of G l a s s Under S t r e s s . N a t u r e , Vol. 154, p . 341, 1944. Lunsford, J . H. L o s s i n F r a c t u r e S t r a i n of B o r o s i l i c a t e G l a s s i n Vapor of
Different Dipole Moments. J o u r . A m e r . C e r a m i c S o c . , Vol. 47, p . 309, 1964. Dollimore, D . , a n d Heal, G . R. T h e E f f e c t of V a r i o u s V a p o r s on t h e Strength
of Compacted S i l i c a . J o u r . Appl. C h e m . , Vol. 11, p . 459, 1961.
Schoening, F. R . L . On the Strength of G l a s s in Water Vapor. J o u r . Appl. P h y s . , Vol. 31, p. 1779, 1960.
Feughelman, M . , a n d Haly, A . R . T h e P h y s i c a l P r o p e r t i e s of Wool F i b e r s a t V a r i o u s R e g a i n s . VI. T h e Mechanism of S t r e s s Relaxation a n d Length R e c o v e r y . J o u r . Textile R e s . , Vol. 32, p . 227, 1962.
Campbell, R . B . Reactivity of Deformed Metal Surfaces. Nature, Vol. 197, p. 374, 1963.
C h a r l e s , R . J . Static Fatigue of G l a s s . J o u r . Appl. P h y s . , Vol. 29, p . 1549, 1958.