Publisher’s version / Version de l'éditeur:
Cement and Concrete Research, 1, 2, pp. 159-176, 1971-03
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/0008-8846(71)90066-4
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Morphology and microstructure of hydrating portland cement and its
constituents II. Changes in hydration of calcium silicates alone and in
the presence of triethanolamine and calcium lignosulphonate, both
with and without gypsum
Ciach, T. D.; Swenson, E. G.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=bd205fc8-a04f-4715-9a91-9d4a802d6cc9 https://publications-cnrc.canada.ca/fra/voir/objet/?id=bd205fc8-a04f-4715-9a91-9d4a802d6cc9
CEMENT
a n d CONCRETE
RESEARCH. Vol.
1
,
p p . 159-1 76, 1971. Pergamon Press, Inc. Printedi n
the United S t a t e s .MORPHOLOGY AND MICROSTRUCTURE O F HYDRATING PORTLAND CEMENT AND ITS CONSTITUENTS I CHANGES IN HYDRATION O F CALCIUM SILICATES ALONE AND IN THE P R E S E N C E O F TRIETHANOLAMINE
AND CALCIUM LIGNOSULPHONATE, BOTH WITH AND WITHOUT GYPSUM
T . D. C i a c h and E . G. Swenson
Division of Building R e s e a r c h , National R e s e a r c h Council of C a n a d a Ottawa 7 , O n t a r i o , Canada
(Communicated by D.
M .
Roy) ABSTRACTS y s t e m a t i c s e q u e n t i a l o b s e r v a t i o n s with the e l e c t r o n m i c r o s c o p e w e r e m a d e of t h e m o r p h o l o g i c a l c h a n g e s which o c c u r r e d d u r i n g h y d r a t i o n of C3S* and C 2 S p a s t e s , w i t h and without t h e p r e s e n c e of gypsum. T h e s p e c i f i c e f f e c t s of e a c h of two a d m i x t u r e s w e r e studied: t r i e t h a n o l a m i n e and c a l c i u m lignosulphonate. W a t e r t o C 3 S o r C,S r a t i o w a s 0 . 5 and a d m i x t u r e d o s a g e w a s 0.5%. T h e a d m i x t u r e s h a d s o m e effect on c h a n g e s i n m i c r o s t r u c t u r e , but t h e y had a pronounced influence on t h e r a t e of t h e h y d r a t i o n p r o c e s s e s . SOMM AIRE D e s o b s e r v a t i o n s au m i c r o s c o p e Clectronique ont e t e f a i t e s e n sCquence systCmatique p o u r o b s e r v e r l e s c h a n g e m e n t s m o r p h o l e g i q u e s q u i p r e n n e n t p l a c e a u c o u r s d e l ' h y d r a t a t i o n de p2te d e C 3 S e t d e C 2 S , e n p r e s e n c e ou non d e g y p s e . L e s effets
spgcifiques de d e u x a d j u v a n t s ont etC Ctudiks: l e t r i g t h a n o l a m i n e e t l e lignosulfonate de c a l c i u m . L a f r a c t i o n eau: C 3 S e t eau: C 2 S Ctait d e 0 . 5 e t l e d o s a g e d ' a d j u v a n t s , d e 0. 5%. L e s
adjuvants ont provoquC un c e r t a i n effet s u r l e s c h a n g e m e n t s d a n s l a m i c r o s t r u c t u r e , e t ont d e p l u s m o n t r i . une influence c o n s i d k - r a b l e s u r l a v i t e s s e d u procCdC d ' h y d r a t a t i o n .
:%Standard c e m e n t n o m e n c l a t u r e i s u s e d ; C 3 S = 3CaO. SiO,; C 2 S = 2CaO. SiO,; C,A = 3Ca0.AJ?,03; C B A F = 4 C a 0 . AJ?,O,. F e 2 0 3 ; w/c = w a t e r : c e m e n t r a t i o by weight.
. - -
MICROSTRUCTURE,
CALCIUM-SILICATE-HYDRATE, MORPHOLOGY
Vol.
1 ,
No.
2
T h i s i s the second of a s e r i e s of p a p e r s r e p o r t i n g the r e s u l t s of l o n g - t e r m s t u d i e s on the r e l a t i o n s h i p between m o r p h o l o g i c a l changes in hydrating portland c e m e n t and i t s m i c r o s t r u c t u r e i n the p l a s t i c and h a r d e n - ed s t a t e s . The f i r s t one (1) dealt with t r i c a l c i u m aluminate: the effects of t r i e t h a n o l a m i n e and c a l c i u m lignosulphonate a d m i x t u r e s in the a b s e n c e and in the p r e s e n c e of gypsum. T h i s one i s c o n c e r n e d with the t r i c a l c i u m and d i c a l c i u m s i l i c a t e components
.
The method involves the o b s e r v a t i o n and i n t e r p r e t a t i o n of a s e r i e s of e l e c t r o n m i c r o g r a p h s of v a r i o u s p a s t e m i x t u r e s during hydration. C a r e i s taken t o s e l e c t r e p r e s e n t a t i v e and c h a r a c t e r i s t i c s t r u c t u r e s and f o r m a t i o n s . X - r a y i s u s e d t o identify c e r t a i n p h a s e s . When changes o c c u r , the c o r r e - sponding s e r i e s of e l e c t r o n m i c r o g r a p h s a r e shown, and when no change o c c u r s , only one o r two a r e p r e s e n t e d . The f o r m a t i o n s shown a r e r e p r e - sentative but of a qualitative n a t u r e .
Many m i c r o g r a p h s showing morphology changes during hydration of cement compounds have been published. Usually t h e s e w e r e taken only at advanced a g e s of hydration; often they r e p r e s e n t e d c a s e s of hydration i n e x c e s s w a t e r , and too often t h e y r e p r e s e n t e d i s o l a t e d e x a m p l e s of m o r p h o l - ogy r a t h e r than the dominant f o r m s .
The p r e s e n t study, c a r r i e d out on a s y s t e m a t i c b a s i s , i s expected t o f i l l a need f o r a s e q u e n t i a l r e c o r d of the c h a r a c t e r i s t i c m i c r o s t r u c t u r e and morphology of c e m e n t and i t s components when hydrating a s a p a s t e with v a r i o u s a d m i x t u r e s . An understanding of t h e s e changes and t h e i r e f f e c t s , and t h e availability of a method of studying a l l s u c h s y s t e m s , a r e of p r a c - t i c a l value t o the c o n c r e t e technologist.
Like the f i r s t p a p e r ( l ) , this c o n s i s t s mainly of observations of changes i n morphology. P a r a l l e l s t u d i e s a r e designed t o m a k e p o s s i b l e a c o r r e l a t i o n between the m i c r o s t r u c t u r e and the . m e c h a n i c a l and o t h e r p r o p e r t i e s of hardened portland cement p a s t e ( 2 ) .
Introduction
T r i c a l c i u m s i l i c a t e (C,S) and d i c a l c i u m s i l i c a t e (C,S) t o g e t h e r constitute s o m e 7 5 p e r cent of portland cement. The hydration of t h e s e
V o l .
1,
No. 2MICROSTRUCTURE, C A L C I U M - S I L I C A T E - H Y D R A T E , MORPHOLOGY
m i n e r a l o g i c a l components l e a d s t o the f o r m a t i o n of the c a l c i u m s i l i c a t e h y d r a t e s which l a r g e l y constitute the s t r u c t u r e of the hardened p a s t e .
Extensive s t u d i e s have been m a d e on the n a t u r e and r e a c t i o n s of C3S and C 2 S , and on the n a t u r e of t h e i r hydration products. Studies of the m o r - phology and m i c r o s t r u c t u r e of the hydration p r o d u c t s and how they a r e affected by v a r i o u s additives have been s u m m a r i z e d in the international symposia on the c h e m i s t r y of cement and c o n c r e t e . The specific effects of the p r e s e n c e of gypsum have been t r e a t e d t h e r e i n by Copeland and Kantro (3) and by T a y l o r (4).
The p r e s e n c e of gypsum can have a n a c c e l e r a t i n g o r r e t a r d i n g effect on the hydration of C3S depending on the concentration p r e s e n t , but both effects a r e r a t h e r s m a l l . Apparently gypsum does not f o r m new p h a s e s with the c a l c i u m s i l i c a t e h y d r a t e s , f o r only the u s u a l p h a s e s of CSH and CH w e r e p r e s e n t a f t e r hydration ( 5 , 6 , 7 , 8 ) . ~ 0 ~ ~ - i o n s can r e p l a c e ~ i 0 4 ~ - i o n s t o a n extent not exceeding 5 p e r cent. I t a p p e a r s t h a t t h i s h a s s o m e influence on morphology because the r o l l e d - u p foils o r s h e e t s do not f o r m under t h e s e
conditions; only the f i b r o u s p a r t i c l e s in a c i g a r - l i k e shape o c c u r (6). The extensive u s e of organic c h e m i c a l s a s a d m i x t u r e s in c o n c r e t e and the v a r i e t y of t h e s e i n u s e m a k e i t i m p o r t a n t to understand the changes that take place due t o t h e i r p r e s e n c e . The p r e s e n t p a p e r d e s c r i b e s e x p e r i - m e n t s with combinations of C3S o r C 2 S and d o s a g e s of triethanolarnine o r
c a l c i u m lignosulphonate i n the absence and in the p r e s e n c e of gypsum. T h e s e two organic c h e m i c a l s a r e commonly used i n m a n y c o m m e r c i a l admixtyre formulations. S y s t e m a t i c sequential observations a r e m a d e with the e l e c t r o n m i c r o s c o p e t o d e t e r m i n e morphological changes f r o m a few m i n u t e s t o 3 months. X - r a y and o t h e r m e t h o d s w e r e used a t t i m e s t o identify c e r t a i n p h a s e s . T h i s study i s p a r t of a continuing, broad study of concrete a d m i x t u r e s a t the Division of Building R e s e a r c h .
M a t e r i a l s and P r o c e d u r e s
The t r i c a l c i u m s i l i c a t e and d i c a l c i u m s i l i c a t e s a m p l e s u s e d i n t h e s e e x p e r i m e n t s w e r e supplied by the P o r t l a n d C e m e n t Association R e s e a r c h L a b o r a t o r i e s . Blaine s u r f a c e a r e a s were: f o r C3S, 3310 s q c m p e r g r a m ;
162 V o l . 1, No. 2
MICROSTRUCTURE, CALCIUM-SILICATE-HYDRATE, M O R P H O L O G Y
f o r C,S, 2770 s q c m p e r g r a m . C o r r e s p o n d i n g f r e e l i m e s w e r e , i n p e r cent: f o r C 3 S , 0.46; f o r C 2 S , not d e t e r m i n e d . T h e C 3 S w a s 9 9 . 2 p e r c e n t p u r e i n t e r m s of s i l i c a and l i m e . T h e p a s t e s w e r e p r e p a r e d on t h e b a s i s of w a t e r t o d r y C,S ( o r C,S) by weight = 0.5. T h e g y p s u m w a s p r e p a r e d f r o m l a r g e , c l e a r n a t u r a l c r y s t a l s which w e r e g r o u n d t o p a s s t h e 200 DIN s i e v e . T h e m i x t u r e of C3S and 10 p e r c e n t by w e i g h t of g y p s u m w a s p r e p a r e d by g r i n d i n g t h e m t o - g e t h e r i n a p o r c e l a i n - a l u m i n a b a l l m i l l . A d m i x t u r e d o s a g e s w e r e 0 . 5 p e r c e n t by w e i g h t of d r y C 3 S a n d C 2 S . T h e t r i e t h a n a o l a m i n e w a s of c h e m i c a l l y p u r e r e a g e n t g r a d e . T h e c a l c i u m l i g n o s u l p h o n a t e w a s a c o m m e r c i a l l y p r e p a r e d p r o d u c t , c h e m i c a l l y t r e a t e d t o d e s t r o y e x c e s s s u g a r s . T o t a l s u g a r s a v e r a g e d 0.08 p e r c e n t and t o t a l r e d u c i n g b o d i e s about 4 p e r c e n t . A d e s c r i p t i o n of m e t h o d s and p r o c e d u r e s u s e d i n p r e p a r i n g and e x a m i n i n g t e s t s p e c i m e n s i s g i v e n i n t h e f i r s t p a p e r i n t h i s s e r i e s ( o n t r i - c a l c i u m a l u m i n a t e ) ( 1 ) :At i n t e r v a l s of 5 m i n u t e s , 1 and 4 h o u r s , 1 , 2, 3,and 7 d a y s , and 1 , 2, and 3 m o n t h s , s a m p l e s w e r e t a k e n and t e s t e d . H y d r a t i o n w a s s t o p p e d by t r e a t i n g w i t h a c e t o n e a t a b o u t - 1 8 " C , t h e n d r y i n g of t h e f r o z e n s a m p l e s i n a v a c u u m . Single s t a g e r e p l i c a s of t h e b r o k e n s u r f a c e s of t h e p a s t e w e r e m a d e u s i n g t h e p l a t i n u m - c a r b o n t e c h n i q u e ( 9 ) . T h e e l e c t r o n m i c r o g r a p h s shown a r e t h o s e t h a t d e p i c t t h e a v e r a g e and g e n e r a l m o r p h o l o g y . E x c e p t i o n a l f o r m s and s t r u c t u r e s w e r e not c o n s i d e r e d . O b s e r v a t i o n s and D i s c u s s i o n 1 . H y d r a t i o n of C 3 S and C,S with n e i t h e r G y p s u m n o r O r g a n i c A d m i x t u r e P r e s e n t In t h e f i r s t 3 d a y s , t h e h y d r a t i o n of C 3 S b e g a n f r o m t h e edge - l i k e p a r t s of t h e g r a i n s and a l s o a t t h e s m a l l p a r t i c l e s f o r m e d by r e c r y s t a l l i z a - tion. V e r y t h i n , s e m i c r y s t a l l i n e c r u m p l e d f i l m s of h y d r a t i o n p r o d u c t s w e r e o b s e r v e d ( F i g , 1). T h e s e s o m e t i m e s f o r m e d a b r i d g e - l i k e s t r u c t u r e b e t w e e n u n h y d r a t e d g r a i n s of C3S.
Vol. 1 , No.
21 6 3
MICROSTRUCTURE, CALCIUM-SILICATE-HYDRATE,
MORPHOLOGY
FIG. 1 FIG. 2
The m i c r o s t r u c t u r e of C3S with no
admixture, w/c = 0. 5. Age 3 days. The m i c r o s t r u c t u r e of C,S tlvith no Loosely crumpled foils of hydration admixture, w/c = 0. 5. Age 23 days. products around unhydrated g r a i n s P l a t e s of hydration products with of C3S. t h r e e -dimensional f i b r e s of CSH. Between 3 and 7 days, the C3S hydration products took on the f o r m of fibrous particles which "radiated" a s a m e s h - l i k e s t r u c t u r e on the surface of the C,S grains. These fibrous particles were probably CSH, which a r e usually described a s fibrous p a r t i c l e s rolled up f r o m semicrystalline, very thin foils o r sheets (5, 10, 11).
Between 7 and 28 days the C3S hydration products did show changes. Some aggregations displaying tabular and s t r i a t e d s t r u c t u r e s appeared t o
cover some of the fibrous particles of CSH observed i n the e a r l i e r stages of hydration. Sometimes these tabular s t r u c t u r e s displayed a mesh-like f r a c - t u r e on the surfaces of the plates. This m e s h - l i k e s t r u c t u r e probably acted a s a nucleus for f u r t h e r formations of hydration products, o r it m a y have been m e r e l y an indication of the appearance of the original pore s y s t e m before filling began (Fig. 2). It was observed that the fibrous p a r t i c l e s of CSH, which w e r e oriented in t h r e e directions a t angles of about 6 0 ° , had formed plates of a "felted," partly interlocking s t r u c t u r e . A s i m i l a r
s t r u c t u r e of C3S hydration products was found by Grudemo ( l l ) , Copelandet a1.(12) and Brunauer (13). These plates of "felted" s t r u c t u r e f o r m striated
1 6 4 V o l
.
1,No.
2 MICROSTRUCTURE, C A L C I U M - S I L I C A T E - H Y D R A T E , MORPHOLOGY F I G . 3 F I G . 4 T h e m i c r o s t r u c t u r e of C 3 S w i t h n o T h e m i c r o s t r u c t u r e of C3S w i t h a d m i x t u r e , w/ c = 0 . 5 . Age 2 no a d m i x t u r e , w / c = 0. 5. Age m o n t h s . P s e u d o m o r p h s of C 3 S 3 m o n t h s . F i b r o u s i n t e r l o c k i n g g r a i n s ; s t r i a t e d p l a t e s t r u c t u r e of and s t r i a t e d p l a t e s t r u c t u r e of h y d r a t i o n p r o d u c t s ; and s o m e h e x a - h y d r a t i o n p r o d u c t s b e t w e e n g o n a l c r y s t a l s of CH. p s e u d o m o r p h s of C 3 S g r a i n s . a g g r e g a t i o n s a n d often d i s p l a y t h e c o l u m n a r c l e a v a g e a s w e l l . B e t w e e n 1 and 2 m o n t h s of C 3 S h y d r a t i o n the s t r i a t e d ( p l a t e ) s t r u c - t u r e b e c a m e bound a s a coating a r o u n d e i t h e r t h e p a r t l y h y d r a t e d C3S g r a i n s o r p s e u d o m o r p h s of t h e m ( F i g . 3 ) . Single c r y s t a l s of c a l c i u m h y d r o x i d e of v e r y good h e x a g o n a l h a b i t c a n a l s o be o b s e r v e d . At 3 m o n t h s ' h y d r a t i o n t h e r e w e r e n o f u r t h e r c l e a r l y defined c h a n g e s in t h e m i c r o s t r u c t u r e of t h e C, S p a s t e . D u r i n g t h i s l a t t e r p e r i o d t h e f e l t e d , p a r t l y i n t e r l o c k i n g s t r u c t u r e of t h e p l a t e s of CSH was a l s o o b s e r v e d ( F i g . 4 ) . T h e h y d r a t i o n p r o d u c t s of C 2 S w e r e found t o be s i m i l a r t o t h o s e of C3S when both w e r e e x a m i n e d i n t h e s a m e w a y , e x c e p t t h a t t h e p r o d u c t s of C 2 S a p p e a r e d l a t e r in a g e , a s w a s e x p e c t e d . As c o m p a r e d w i t h t h e h y d r a t i o n p r o c e s s of C,S, t h e h y d r a t i o n of C,S s t a r t e d m o r e s l o w l y , and t h e s e c o n d f o r m of CSH, w h i c h f o r C,S a p p e a r e d l i k e long f i b r e s , a p p e a r e d r a t h e r n e e d l e - l i k e in t h i s c a s e . T h e t h r e e - d i m e n s i o n a l n e t w o r k which t e n d e d t o f o r m p l a t e s under t h e s e c o n d i t i o n s h a d a l e s s i d e a l a r r a n g e m e n t t h a n t h a t f o r C,S ( s e e F i g s . 2 and 4), and t h e r e f o r e w a s l i k e n e d m o r e t o a f e l t e d s t r u c t u r e ( F i g . 5 ) .Vol.
1 ,
No.2
1 6 5
M I C R O S T R U C T U R E , C A L C I U M - S I L I C A T E - H Y D R A T E , M O R P H O L O G Y
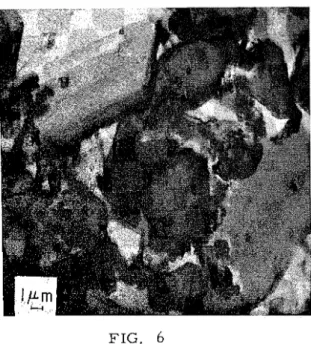
FIG. 5 FIG. 6
T h e m i c r o s t r u c t u r e of C 2 S p a s t e T h e m i c r o s t r u c t u r e of C,S p a s t e with no a d m i x t u r e , w/c = 0 . 5 . Age with gypsum and n o a d m i x t u r e .
3 m o n t h s . F i b r e s of c i g a r -shaped Age 6 h o u r s . L a r g e c r y s t a l s of CSH a r o u n d p s e u d o m o r p h s of g r a i n s gypsum; unhydrat ed g r a i n s of C,S ; of C2S. and s e m i c r y s t a l l i n e c r u m p l e d foils of h y d r a t i o n p r o d u c t s . T h e r a t h e r l i m i t e d n u m b e r of c a l c i u m h y d r o x i d e c r y s t a l s o b s e r v e d i n t h e h y d r a t i o n p r o d u c t s of both C,S and C 2 S s u g g e s t e d t h a t e i t h e r t h e y w e r e o b s c u r e d by o t h e r h y d r a t i o n p r o d u c t s o r that s o m e f o r m of a c c o m m o d a t i o n of t h e CH i n t h e CSH s t r u c t u r e s took p l a c e . X - r a y e x a m i n a t i o n of t h e C,S p a s t e a f t e r two m o n t h s of h y d r a t i o n r e v e a l e d unhydrated C,S and p a t t e r n s t h a t a p p e a r e d t o be c l o s e l y r e l a t e d t o t h o s e of t o b e r m o r i t e . T h e r e w e r e a l s o p a t t e r n s s i m i l a r t o t h o s e of Ca(OH),. H y d r a t i o n of C, S with Gypsum but no O r g a n i c A d m i x t u r e s P r e s e n t
T h e p r e s e n c e of gypsum did not g r e a t l y affect t h e p r o d u c t s of h y d r a t i o n of C,S but i t did affect t h e r a t e s of f o r m a t i o n of t h e s e p r o d u c t s . It a l s o , when c o m p a r e d with t h e h y d r a t i o n of C, S alone, affected t h e s t r u c t u r e of C,S p a s t e .
T h e f i r s t p r o d u c t s , o b s e r v e d a t one h o u r of h y d r a t i o n , a p p e a r e d a s s m a l l , r o u n d e d p a r t i c l e s on t h e s u r f a c e s of u n h y d r a t e d g r a i n s of C,S. At 6 h o u r s t h e r e h a d f o r m e d , i n addition, a n a m o r p h o u s f i l m b e t w e e n t h e C,S g r a i n s and t h e gypsum ( F i g . 6 ).
166 V o l . 1 , N o . 2 MICROSTRUCTURE, C A L C I U M - S I L I C A T E - H Y D R A T E , MORPHOLOGY
FIG. 7 FIG. 8
T h e m i c r o s t r u c t u r e of C3S p a s t e T h e m i c r o s t r u c t u r e of C3S p a s t e with gypsum and no a d m i x t u r e . Age with gypsum and no a d m i x t u r e .
1 day. F i b r o u s p a r t i c l e s of h y d r a - Age 1 month. T h e f i b r o u s s i e v e tion p r o d u c t s of CSH. s t r u c t u r e of h y d r a t i o n p r o d u c t s
with p a r a l l e l c l e a v a g e .
After 1 d a y ' s h y d r a t i o n s m a l l f i b r e s in a r o s e t t e p a t t e r n had developed; t h e y a p p e a r e d on t h e s u r f a c e of t a b u l a r c r y s t a l s of gypsum and C3S g r a i n s At t h e s a m e t i m e , s m a l l , rounded p a r t i c l e s on the g r a i n s of C,S a p p e a r e d ( F i g . 7 ) . T h e s e looked s i m i l a r t o t h o s e r e s u l t i n g f r o m nucleation of CSH f i b r e s between the t h i r d and s e v e n t h day of h y d r a t i o n .
Between 1 day and 1 month t h e h y d r a t i o n p r o d u c t s developed a s a -;l,as- s i v e , p a r t l y s t r i a t e d o r f i b r o u s s t r u c t u r e . F i b r e s of CSH a s s e m b l e d into a g g r e - g a t e s of s i e v e - l i k e and p a r t l y c o l u m n a r f o r m between t h e unhydrated C3S g r a i n s o r t h e p s e u d o m o r p h s of t h e s e ( F i g . 8 ) . After a n etching t r e a t m e n t with ethylene glycol f o r 30 m i n u t e s , p a r t of t h e h y d r a t i o n p r o d u c t s w e r e r e - moved; t h e m i c r o s t r u c t u r e then had a f i b r o u s n e t w o r k in which t h e s e CSH f i b r e s displayed a r o s e t t e - l i k e o r b r u s h a r r a n g e m e n t . Int h e a r e a s w h e r e t h e glycol had a l m o s t c o m p l e t e l y r e m o v e d t h e h y d r a t i o n p r o d u c t s of C3S, t h e r e a p p e a r e d f r a c t u r e s t h a t continued t h r o u g h t h e unhydrated g r a i n s of C3S and t h e f i b r o u s n e t w o r k between t h e m ( F i g . 9 ) .
Vol.
1 ,
No.
2
1 6 7
MICROSTRUCTURE, CALCIUM-SILICATE-HYDRATE, MORPHOLOGY
FIG.
9
FIG. 10T h e m i c r o s t r u c t u r e of C 3 S p a s t e T h e m i c r o s t r u c t u r e of C, S p a s t e w i t h gypsum and no a d m i x t u r e . Age w i t h t r i e t h a n o l a m i n e , w/c = 0. 5.
1 m o n t h . T r e a t e d w i t h glycol. A g e 4 h o u r s . S e m i c r y s t a l l i n e
F i b r o u s h y d r a t i o n p r o d u c t s show c r u m p l e d f o i l s of h y d r a t i o n p r o d u c t s . bundle - l i k e a r r a n g e m e n t .
3. H y d r a t i o n of C3S and C,S i n A b s e n c e of Gypsum but with T r i e t h a n o l a m i n e P r e s e n t
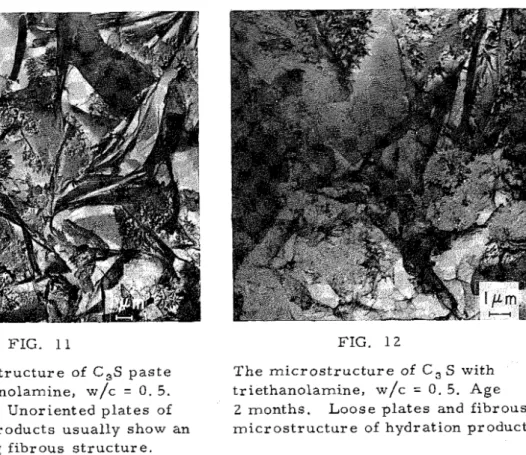
In t h e p r e s e n c e of t h i s a d m i x t u r e , t h e h y d r a t i o n p r o c e s s of C3S a p p e a r e d t o p r o c e e d m o r e r a p i d l y than i t did w i t h n o a d m i x t u r e p r e s e n t . A f t e r only 4 h o u r s t h e g r a i n s of C3S w e r e c o v e r e d b y v e r y t h i n , c r u m p l e d f i l m s which d i s p l a y e d b r i d g e - l i k e bondings s i m i l a r t o t h o s e t h a t h a d f o r m e d , a t 3 days h y d r a t i o n of C3S a l o n e ( F i g . 1 ) . Along with t h e s e s e m i c r y s t a l l i n e f o i l s w e r e s i n g l e f i b r o u s p a r t i c l e s of CSH ( F i g . 1 0 ) . A f t e r 1 day t h e s e s m a l l f i b r o u s p a r t i c l e s of CSH r a d i a t e d o v e r t h e g r a i n s of u n h y d r a t e d C,S. A t 7 d a y s t h e m i c r o s t r u c t u r e a p p e a r e d a s u n o r i e n t e d , p a r t l y r o l l e d s h e e t s and p l a t e s b e t w e e n f i b r o u s p a r t i c l e s and u n h y d r a t e d g r a i n s ( F i g . 11 ). M a n y of t h e s e p l a t e s showed s o m e d e g r e e of f r a c t u r e on t h e s u r f a c e s . At l a t e r a g e s , up t o 3 m o n t h s , n o s i g n i f i c a n t c h a n g e i n t h e m i c r o s t r u c
-
t u r e of C3S p r o d u c t s o c c u r r e d ( F i g . 1 2 ) . A p a r t f r o m t h e r a t e of h y d r a t i o n , t h e o n l y d i f f e r e n c e s b e t w e e n t h e p r o d u c t s f o r m e d i n t h e a b s e n c e and i n t h e p r e s e n c e of t r i e t h a n o l a m i n e w e r e1 6 8 V o l . 1, No. 2 MICROSTRUCTURE, C A L C I U M - S I L I C A T E - H Y D R A T E , MORPHOLOGY
FIG. 11 FIG. 12
T h e m i c r o s t r u c t u r e of C,S p a s t e T h e m i c r o s t r u c t u r e of C, S with with t r i e t h a n o l a m i n e , w/c = 0 . 5. t r i e t h a n o l a m i n e , w/c = 0 . 5. Age Age 7 days. Unoriented p l a t e s of 2 m o n t h s . L o o s e p l a t e s and f i b r o u s h y d r a t i o n p r o d u c t s u s u a l l y show an m i c r o s t r u c t u r e of h y d r a t i o n p r o d u c t s . i n t e r l o c k i n g f i b r o u s s t r u c t u r e .
that in t h e p r e s e n c e of the a d m i x t u r e , t h e f e l t e d , f i b r o u s s t r u c t u r e of t h e p l a t e s w a s c l e a r e r , and m a n y p l a t e s f o r m e d in an u n o r i e n t e d s t r u c t u r e , only
o c c a s i o n a l l y displaying s t r i a t e d c l e a v a g e ( F i g . 1 2 ).
T h e X - r a y examination a t 2 months showed the p r e s e n c e of unhydrated C,S and a CSH c l o s e l y r e l a t e d t o t o b e r m o r i t e ; t h i s had a l s o been t h e c a s e when no a d m i x t u r e w a s p r e s e n t . F r o m a c o m p a r i s o n of t h e s e two s y s t e m s it w a s found that the s i z e of t h e f i b r o u s p a r t i c l e s of CSH was g r e a t e r in t h e p a s t e with t r i e t h a n o l a m i n e p r e s e n t than in t h e p a s t e with no a d m i x t u r e . In both c a s e s X - r a y r e v e a l e d p a t t e r n s s i m i l a r t o t h o s e of c a l c i u m hydroxide, although only a few s i n g l e c r y s t a l s of CH of good hexagonal habit w e r e o b s e r v e d by e l e c t r o n m i c r o s c o p e . T h e s e r e s u l t s a r e s i m i l a r t o t h o s e r e p o r t e d in m a n y o t h e r i n v e s t i g a t i o n s (12, 14, 15, 16, 1 7 ) ; in a l l of t h e s e s t u d i e s , apparently, not a l l of the CH produced d u r i n g h y d r a t i o n can b e accounted for by available m e t h o d s . T h i s situation i s attributed by s o m e to t h e f o r m a t i o n of CSH with a v e r y high C/S r a t i o , and by o t h e r s t o t h e highly a m o r p h o u s s t a t e of t h e CH which can r e a d i l y be a b s o r b e d on t h e s u r f a c e s of CSH.
Vol. 1 ,
No.
2
169 MICROSTRUCTURE,CALCIUM-SILICATE-HYDRATE,
MORPHOLOGY
T h e h y d r a t i o n of C,S and C3S in t h e p r e s e n c e of t r i e t h a n o l a m i n e w e r e v e r y s i m i l a r . T h e p r o c e s s w a s , h o w e v e r , m o r e r a p i d when t h e a d m i x t u r e was p r e s e n t , and t h e m i c r o s t r u c t u r e was, l e s s c o m p a c t ; t h e n e e d l e - l i k e p a r
-
t i c l e s of CSH a p p e a r e d s e p a r a t e l y and m o r e often a s i m p r i n t s on s u r f a c e s of h y d r a t i o n p r o d u c t s . T h e platy f o r m s of t h e h y d r a t i o n p r o d u c t s w e r e r a t h e r unoriented.4. Hydration of C3S with Gypsum and T r i e t h a n o l a m i n e P r e s e n t
T h e m i c r o s t r u c t u r e of the h y d r a t i o n p r o d u c t s of C3S in t h i s combination w a s o b s e r v e d t o be quite s i m i l a r t o that of C3S alone, o r of C3S with gypsum, except that f i b r e s of c a l c i u m s i l i c a t e h y d r a t e displayed a n a r r a n g e m e n t that w a s m a i n l y a t h r e e - d i m e n s i o n a l , felted p l a t e s t r u c t u r e . A t t i m e s one o b s e r
-
ved a l s o t h e r o s e t t e o r b r u s h a r r a n g e m e n t ( F i g . 1 3 ) .5. H y d r a t i o n of C3S and C 2 S without Gypsum but with C a l c i u m Lignosulphonate P r e s e n t
T h e p r e s e n c e of t h i s a d m i x t u r e a p p e a r e d t o r e t a r d d r a s t i c a l l y t h e
h y d r a t i o n p r o c e s s e s of both C3S and C 2 S . T h e only hydration products f o r m e d in 3 m o n t h s w e r e s e m i c r y s t a l l i n e f i l m s o r c r u m p l e d f o i l s ; in t h e c a s e of C3S, f i b r o u s -like p a r t i c l e s of CSH, which u s u a l l y t a k e the f o r m of bundle-like a g g r e g a t i o n s , a l s o developed. T h e s e p r o d u c t s a l l a p p e a r t o be s i m i l a r t o t h o s e that o c c u r r e d in t h e e a r l y s t a g e s of h y d r a t i o n without a n a d m i x t u r e o r with t r i e t h a n o l a m i n e .
In 23 days of h y d r a t i o n of C3S, t h e only p r o d u c t f o r m e d w a s a v e r y thin and c r u m p l e d a m o r p h o u s f i l m . A f t e r 23 days t h e r e w a s a n o b s e r v a b l e i n c r e a s e in t h e amount of t h i s p r o d u c t , which s o m e t i m e s m a n i f e s t e d i t s e l f a s b r i d g e - l i k e p a r t i c l e s between unhydrated g r a i n s of C3S ( F i g . 1 4 ) . Between 1 m o n t h ' s and 2 m o n t h s ' h y d r a t i o n t h e r e could be o b s e r v e d s i n g l e , f i b r o u s - l i k e p a r t i c l e s of h y d r a t i o n p r o d u c t s on t h e s u r f a c e s of C3S g r a i n s , and bundle- l i k e a g g r e g a t i o n s of CSH which a p p a r e n t l y had been f o r m e d f r o m t h e s m a l l f i b r o u s p a r t i c l e s between t h e unhydrated C3S g r a i n s ( F i g s . 1 5 and 16).
T h e s e p r o d u c t s w e r e s t i l l t o be o b s e r v e d up t o 3 m o n t h s ' h y d r a t i o n , but one a l s o o b s e r v e d s m a l l , rounded a g g r e g a t i o n s of amorphous m a t e r i a l ,
1 7 0 MICROSTRUCTURE, C A L C I U M - S I L I C A T E V o l . 1 , N o . 2 -HYDRATE, MORPHOLOGY FIG. 1 3 M i c r o s t r u c t u r e of C3S p a s t e with gypsum and t r i e t h a n o l a m i n e , w/c = 0 . 5. Age 1 month. F i b r o u s , t h r e e - dimensional, interlocking p l a t e s , and a r a d i a l a r r a n g e m e n t of f i b r o u s hydration p r o d u c t s . FIG. 14 T h e m i c r o s t r u c t u r e of C,S p a s t e with calcium lignosulphonate, w/c = 0 . 5. Age 2 3 days. Unhydrated g r a i n s of C3S with c r u m p l e d foils of hydration p r o d u c t s .
FIG. 1 5
T h e m i c r o s t r u c t u r e of C3S p a s t e with calcium lignosulphonate, w/c = 0. 5. Age 1 month. Single, n e e d l e - l i k e p a r t i c l e s of hydration products (CSH) on s u r f a c e s of g r a i n s of p a r t l y h y d r a t e d C3S.
FIG. 16
T h e m i c r o s t r u c t u r e of C3S p a s t e with c a l c i u m lignosulphonate. Age 2 months. Bundles of f i b r e s and s m a l l rounded p a r t i c l e s of hydration p r o d u c t s on t h e s u r f a c e s of p a r t l y h y d r a t e d C3S g r a i n s .
Vol. 1 , No. 2 171 MICROSTRUCTURE, CALCIUM-SILICATE-HYDRATE,
MORPHOLOGY
FIG. 1 7 FIG. 18
T h e m i c r o s t r u c t u r e of C,S p a s t e T h e m i c r o s t r u c t u r e of C, S p a s t e w i t h gypsum and c a l c i u m l i g n o - w i t h g y p s u m and c a l c i u m l i g n o -
s u l p h o n a t e , w / c = 0 . 5. A g e 1 h o u r . s u l p h o n a t e , w/c = 0 . 5. A g e
6
h o u r s . S m a l l r o u n d e d p a r t i c l e s of h y d r a - L a r g e c r y s t a l s of g y p s u m and s m a l l t i o n p r o d u c t s b e t w e e n u n h y d r a t e d r o u n d e d p a r t i c l e s of h y d r a t i o n p r o d u c t s . g r a i n s of C,S.
FIG. 1 9 FIG. 20 T h e m i c r o s t r u c t u r e of C,S p a s t e T h e m i c r o s t r u c t u r e of C,S p a s t e w i t h gypsum and c a l c i u m l i g n o - w i t h gypsum and c a l c i u m l i g n o - s u l p h o n a t e , w / c = 0. 5. A g e 3 d a y s . s u l p h o n a t e , w / c = 0 . 5. A g e 7 d a y s . L a r g e , t h i n p l a t e s of p a r a l l e l S o m e l a r g e h e x a g o n a l - l i k e p a r t i c l e s c l e a v a g e , and r o u n d e d p a r t i c l e s of h y d r a t i o n p r o d u c t s a n d u n h y d r a t e d of h y d r a t i o n p r o d u c t s . g r a i n s of C,S and g y p s u m .Vol.
1 , No. 2
MICROSTRUCTURE, CALCIUM-SILICATE-HYDRATE,
MORPHOLOGY
which was p r o b a b l y CH ( F i g . 1 6 ) . T h e p a s t e h a d not yet h a r d e n e d at t h i s t i m e . T h e h y d r a t i o n of C 2 S i n t h i s c o m b i n a t i o n evinced q u i t e s i m i l a r b e
-
h a v i o u r.
R e s u l t s of X - r a y e x a m i n a t i o n s of C3S p a s t e at 2 m o n t h s showed only t h e p r e s e n c e of u n h y d r a t e d C3S, CSH of v e r y poor c r y s t a l f o r m a n d . s o m e p a t t e r n s s i m i l a r t o t h o s e of Ca(OH),.
T h e f i b r o u s p a r t i c l e s w e r e a f o r m of CSH c l o s e l y r e l a t e d t o t o b e r m o r i t e but, in t h i s c a s e , t h e y had r e t a i n e d t h e s e m i - a m o r p h o u s h a b i t . T h e X - r a y d i a g r a m s f o r a l l t h r e e c a s e s ( w i t h n o a d m i x t u r e , with t r i e t h a n o l a m i n e and with t h e l i g n o s u l p h o n a t e ) , a r e v e r y s i m i l a r , except t h a t in t h e l a s t c a s e t h e p a t t e r n s a r e w e a k e r .6. H y d r a t i o n of C3S i n P r e s e n c e of Gypsum and C a l c i u m Lignosulphonate At 1 hour t h e h y d r a t i o n p r o d u c t s a p p e a r e d a s s m a l l rounded p a r t i c l e s on t h e s u r f a c e of C3S g r a i n s ; a m o r p h o u s f i l m s a l s o o c c u r r e d . T h e s e w e r e s t i l l the dominant f o r m s a t 3 d a y s h y d r a t i o n ( F i g s . 17 and 1 8 ) , although one a l s o o b s e r v e d i n t h e h y d r a t i o n p r o d u c t s s o m e p l a t e - l i k e f o r m s of p a r a l l e l c l e a v a g e c o v e r e d by s m a l l p r e c i p i t a t i o n s ( F i g . 19 ). F r o m t h e s e v e n t h u n t i l t h e f o u r t e e n t h day of h y d r a t i o n , l a r g e g r a i n s of h e x a g o n a l - l i k e c l e a v a g e w e r e o b s e r v e d . T h e s e m u s t be d i s t i n g u i s h e d f r o m t h e t a b u l a r , m o n o c l i n i c g r a i n s of gypsum ( F i g . 20). A f t e r 1 m o n t h ' s h y d r a t i o n t h e p a s t e w a s not yet h a r d e n e d . At t h i s t i m e t h e 3 0 - m i n u t e t r e a t m e n t with glycol d e s t r o y e d a l l h y d r a t i o n p r o d u c t s .
O b s e r v a t i o n s j u s t p r i o r t o etching t r e a t m e n t showed t h a t t h e component p r e s e n t h a d been t h e unhydrated C3S g r a i n s , p a r t l y d i s s o l v e d c r y s t a l s of gypsum and s e m i c r y s t a l l i n e f i l m s , and n e e d l e - l i k e p a r t i c l e s of h y d r a t i o n p r o d u c t s on t h e s u r f a c e of u n h y d r a t e d C 3 S ( F i g s . 21 and 2 2 ) .
X - r a y d i f f r a c t i o n p a t t e r n s m a d e a t 1 m o n t h of h y d r a t i o n showed t h e p r e s e n c e of t h e following p h a s e s : u n h y d r a t e d C3S, gypsum, and c a l c i u m h y d r o x i d e of v e r y poor c r y s t a l l i n i t y .
D i s c u s s i o n
In g e n e r a l , t h e findings of t h e s e s t u d i e s a g r e e w e l l with r e p o r t e d o b s e r v a t i o n s by o t h e r s .
V o l . 1, No. 2 173 MICROSTRUCTURE, CALCIUM-SILICATE-HYDRATE,
MORPHOLOGY
F I G . 21
T h e m i c r o s t r u c t u r e of C3S p a s t e with gypsum and c a l c i u m ligno- sulphonate, w/c = 0. 5. Age 1 month. E r o d e d s u r f a c e s of a l a r g e c r y s t a l of gypsum and g r a i n s of C3S with amorphous hydration p r o d u c t s on the s u r f a c e .
F I G . 2 2
T h e m i c r o s t r u c t u r e of C3S p a s t e with gypsum and c a l c i u m ligno-
sulphonate, w/c = 0 .
5 .
Age 1 month. C r u m p l e d f o i l s and a s i n g l e c i g a r-
shaped f i b r o u s p a r t i c l e of h y d r a - tion p r o d u c t s . T h e p r e s e n c e of gypsum without t h e o r g a n i c c h e m i c a l a d m i x t u r e s a p p e a r e d t o a c c e l e r a t e t h e nucleation of p a r t i c l e s of CSH i n t h e hydration of C3S. O b s e r v a t i o n s i n t h e s e s t u d i e s did not m a k e i t p o s s i b l e t o c l e a r l y d e t e r m i n e the r e p o r t e d f i r s t hydration p r o d u c t s of C3S of v e r y high C/S r a t i o ( 4 , 7 ) . T h i s f o r m p r o b a b l y a d h e r e s v e r y s t r o n g l y to t h e C3S g r a i n s ( 4 ) and cannot be distinguished f r o m t h e m by e l e c t r o n m i c r o s c o p i c e x a m i n a - tion (7). One possibility is that t h i s f o r m c o n s t i t u t e s t h e zonal s t r u c t u r e of CSH around unhydrated g r a i n s of C,S of v e r y s i m i l a r optical p r o p e r t i e s .-
T h e e a r l i e s t s t r u c t u r e o b s e r v e d c o n s i s t e d of t h e s e m i c r y s t a l l i n e f i l m s d e s c r i b e d during hydration of C,S i n the a b s e n c e of gypsum.After 3 d a y s ' hydration i n t h e a b s e n c e of gypsum, and 1 day's i n t h e p r e s e n c e of gypsum, f i b r o u s p a r t i c l e s w e r e o b s e r v e d which often r a d i a t e d i n a m e s h - l i k e s t r u c t u r e .
At l a t e r s t a g e s of hydration when no a d m i x t u r e , o r only triethanolamine, w a s p r e s e n t , t h e f i r s t a m o r p h o u s CSH developed a s p s e u d o m o r p h s of C3S
Vol. 1, No. 2
M I C R O S T R U C T U R E , C A L C I U M - S I L I C A T E - H Y D R A T E , M O R P H O L O G Y
g r a i n s ; t h e l a t t e r w e r e s u r r o u n d e d by o t h e r h y d r a t i o n p r o d u c t s of f i b r o u s a n d p l a t e - l i k e f o r m s . In t h e p r e s e n c e of c a l c i u m l i g n o s u l p h o n a t e t h i s a m o r p h o u s CSH, a l o n g with s e m i - a m o r p h o u s c r u m p l e d f o i l s a r o u n d p s e u d o m o r p h s of g r a i n s of C,S, w e r e t h e d o m i n a n t f o r m s of CSH d u r i n g t h e whole t i m e of h y d r a t i o n . T h e f i b r e s o r n e e d l e f o r m s of CSH a p p e a r only a s s i n g l e p a r t i c l e s . It i s n o t a b l e t h a t i n a l l t h e s e o b s e r v a t i o n s , c r y s t a l s of l i m e w e r e a l m o s t e n t i r e l y a b s e n t . A p o s s i b l e e x p l a n a t i o n is t h a t t h e l i m e , l i b e r a t e d by h y d r a - t i o n of t h e c a l c i u m s i l i c a t e s , r e m a i n s a t t a c h e d i n s o m e m a n n e r t o t h e CSH p r o d u c t w h e n t h e p r o p o r t i o n of w a t e r p r e s e n t is low. With high w a t e r c o n - t e n t s , a s i n t h e c a s e of s u s p e n s i o n s , t h e l i m e m a y b e d e t a c h e d a n d c r y s t a l s m a y t h e n f o r m . U n d e r t h e l a t t e r c o n d i t i o n s a p r o d u c t of low C/S is f o r m e d ( 4 ) . It h a s a l s o b e e n o b s e r v e d t h a t t h e C/S r a t i o s of t h e f i n a l p r o d u c t s i n - c r e a s e s a s t h e w a t e r - c e m e n t r a t i o s d e c r - e a s e ( 1 7 ) . T h e e x p e r i m e n t s with e t h y l e n e g l y c o l r e v e a l e d a m a r k e d d i f f e r e n c e i n r a t e s of e t c h i n g on CSH 2 t t s u r f a c e s . It m a y b e a r g u e d t h a t if M g,
~ 1 ~ t, a n d ~ e ' c a n p e n e t r a t e t h e CSH s t r u c t u r e i n o t h e r t h a n d i s p l a c e m e n t r e a c t i o n s , i t s h o u l d b e ~ o s s i b l e t t f o r C a t o do s o a l s o . T h i s a c c o m m o d a t i o n of l i m e i n t h e CSH s t r u c t u r e u n d e r c o n d i t i o n s of l i m i t e d w a t e r c o n t e n t i s w o r t h y of f u r t h e r s t u d y . T h e i n i t i a l p r e s e n c e of a l a r g e p r o p o r t i o n of C a (OH), a s , f o r e x a m p l e , i n t h e e x p e r i m e n t s of C o p e l a n d a n d K a n t r o ( 3 ) i n which l i m e w a s p r e m i x e d with t h e C3S, c a n b e e x p e c t e d t o a f f e c t a t l e a s t t h e r a t e of h y d r a t i o n . T a y l o r ( 4 , p . 6 0 ) p o i n t s out t h a t t h e C/6 r a t i o is c o n s i d e r e d a r a t e - g o v e r n i n g f a c t o r due t o d i f f e r e n c e s i n diffusion r a t e s . In t h e p r e s e n t s t u d i e s , n o l i m e w a s p r e m i x e d with t h e C 3 S o r C 2 S . S u m m a r y and C o n c l u s i o n s T h i s s t u d y p r o v i d e s a s e q u e n t i a l r e c o r d of t h e m o r p h o l o g i c a l a n d m i c r o s t r u c t u r e c h a n g e s w h i c h a c c o m p a n i e d h y d r a t i o n of C 3 S a n d C 2 S with and without g y p s u m p r e s e n t , a n d with a n d without t h e p r e s e n c e of two o r g a n i c a d m i x t u r e s : t r i e t h a n o l a m i n e a n d c a l c i u m l i g n o s u l p h o n a t e . It c o n t r i b u t e s t o t h e p o s s i b i l i t i e s of i d e n t i f i c a t i o n a n d c l a s s i f i c a t i o n , a n d p r o v i d e s a b a s i s f o r r e l a t i n g t h e m o r p h o l o g i c a l c h a n g e s of c e m e n t h y d r a t i o n t o t h e p r o p e r t i e s of p l a s t i c and h a r d e n e d c o n c r e t e .Vol. 1 , No.
2
175
MICROSTRUCTURE, CALCIUM-SILICATE-HY DRATE, MORPHOLOGY
In t h e h y d r a t i o n of C,S u n d e r t h e c o n d i t i o n s of t h e s e e x p e r i m e n t s , t h e p r e s e n c e of g y p s u m did not a p p e a r t o h a v e a n y s i g n i f i c a n t e f f e c t , e x c e p t i n p r o v i d i n g s o m e a c c e l e r a t i o n of n u c l e a t i o n of p a r t i c l e s of CSH. T h e f i r s t p r o d u c t of h y d r a t i o n , t h e s e m i c r y s t a l l i n e f i l m , a p p e a r e d t o c o n v e r t d i r e c t l y t o t h e n e e d l e - l i k e o r c i g a r - s h a p e d , s t r u c t u r e s of c a l c i u m s i l i c a t e h y d r a t e . T h e a d m i x t u r e t r i e t h a n o l a m i n e a p p e a r e d t o h a v e a n a c c e l e r a t i n g e f f e c t when n o g y p s u m w a s p r e s e n t in C,S o r C 2 S p a s t e . With C3S a n d g y p s u m s u c h a c c e l e r a t i o n w a s e i t h e r v e r y s m a l l o r a b s e n t . In t h e l a t t e r c a s e t h e f i b r o u s p a r t i c l e s w e r e g e n e r a l l y s m a l l e r t h a n in t h e c a s e s w h e r e n o t r i e t h a - n o l a m i n e w a s p r e s e n t , a n d e t c h i n g e x p e r i m e n t s i n d i c a t e d a h i g h e r C/S r a t i o . L i g n o s u l p h o n a t e r e t a r d e d h y d r a t i o n in a l l c a s e s : C3S a n d C,S w i t h n o g y p s u m , and C3S w i t h g y p s u m . T o a g e s u p t o 1 m o n t h t h e only d i s t i n g u i s h - a b l e p r o d u c t s w e r e a n a m o r p h o u s film s u r r o u n d i n g u n h y d r a t e d g r a i n s of c a l c i u m s i l i c a t e , and a f e w r o u n d e d p a r t i c l e s on the s u r f a c e s .
F r o m 7 d a y s on, d e p e n d i n g on the r a t e of h y d r a t i o n i n e a c h c a s e , the f i b r o u s , o r c i g a r - s h a p e d , p a r t i c l e s d e v e l o p e d i n t o a t h r e e - d i m e n s i o n a l n e t - w o r k which t e n d e d t o f o r m p l a t e s . T h e s e p l a t e s often had a f e l t - l i k e a p p e a r - a n c e w i t h a s t r i a t e d s u r f a c e . W h e r e s u c h s t r i a t i o n s w e r e not i n i t i a l l y p r e s e n t , t h e y could be m a d e t o a p p e a r b y e t c h i n g w i t h g l y c o l . I n a l l c a s e s t h e r e w a s a r e m a r k a b l e a b s e n c e of a n a p p r e c i a b l e q u a n t i t y of Ca(OH),
.
T h i s s u g g e s t e d a n a c c o m m o d a t i o n within t h e CSH l a t t i c e of C a O . Acknowledgemefit s We a c k n o w l e d g e g r a t e f u l l y t h e i n v a l u a b l e c o n t r i b u t i o n of M r . E . Quinn i n p r e p a r i n g r e p l i c a s f r o m t h e s a m p l e s f o r e l e c t r o n m i c r o s c o p i c e x a m i n a t i o n . T o P o r t l a n d C e m e n t A s s o c i a t i o n f o r supplying t h e p u r e c o n s t i t u e n t s of c e m e n t we a r e a l s o m o s t g r a t e f u l . T h i s i s a c o n t r i b u t i o n f r o m t h e D i v i s i o n of Building R e s e a r c h , N a t i o n a l R e s e a r c h C o u n c i l of C a n a d a a n d i s p u b l i s h e d w i t h t h e a p p r o v a l of the D i r e c t o r of t h e D i v i s i o n .1 7 6
Vol.
1 ,
No.2
MICROSTRUCTURE, CALCIUM-SILICATE-HYDRATE, MORPHOLOGY
R e f e r e n c e s
1. T. D. C i a c h and E . G. Swenson, Submitted t o Cement and C o n c r . Res.,
1, (1971).
-
2. T.D. Ciach, J . E . Gillott, E.G. Swenson, and P. J . S e r e d a , Cement and Concr. Res.
,
i,
13 (1971).3. L. E . Copeland and D. L. K a n t r o , F i f t h Int. Sy-mp. on the C h e m . of C e m e n t , Tokyo,
-
11, 387 (1968).4. H. F. W. T a y l o r , F i f t h Int. Symp, on the Chem. of C e m e n t , Tokyo,
-
11, 1 (1968).5. S. Diamond, T o b e r m o r i t e and T o b e r m o r i t e - l i k e C a l c i u m Silicate Hydrates: T h e i r P r o p e r t i e s and Relationships t o C l a y M i n e r a l s . P h . D. T h e s i s , Univ. M i c r o i i l m s I n c . , Ann A r b o r , Michigan (1963). 6. L. E . Copeland, E . Bodor, T. N. Chang and C. H. Weise, J. Res. Dev.
L a b s P o r t l d Gem. A s s .
9,
61 (1967).7. J . G . M . de Jong, The I n t e r a c t i o n of T r i c a l c i u m Silicate and T r i c a l c i u m Aluminate during t h e i r Hydration. P h . D. T h e s i s , Technische
Hoge schol, Eindhoven ( 1968).
8. G . L . Kalousek, ASTM, M a t e r . Res. Stan. 5, 292 (1965).
-
9. L . E . Copeland, E . Bodor, T.N. Chang and C.H. Weise, J. Res. Dev. L a b s P o r t l d C e m . A s s . 9, 61 (1967).
-
10. H. F. W. T a y l o r , T h e C h e m i s t r y of C e m e n t s . Academic P r e s s , London (1964).
11. A. G r u d e m o , K. Tek. ~ 8 g s k . Handl. N r . 242 (1965).
12. L . E . Copeland and E.G. Schulz, Bull. P o r t l a n d C e m . A s s . 135 (1962). 13. S. B r u n a u e r , P r o c . 8 t h Conf. Silicate I n d u s t r y , Budapest (1966). 14. S. B r u n a u e r and S.A. G r e e n b e r g , 4th Int. Symp. Chem.of C e m . ,
Washington, 135 (1960).
15. H.G. Kurzyk and H . E . Schwiete, 4th Int. Symp. Chem. of C e m . , Washington, 349 (1960).
16. F. W. L o c h e r , S y m p . , S t r u c t u r e of P o r t l a n d Cem. P a s t e and C o n c r Washington, H. R. B . , Spec. Rept 90, 300 (1966).
17. D. L. Kantro, C . H. Weise and S. B r u n a u e r , Symp. S t r u c t u r e of
P o r t l a n d Cern. P a s t e and C o n c r . , Washington, H. R. B . , Spec. R ~ F 90, 300 (1966).