Confining a biocatalyst for highly efficient and selective synthesis
of carboxamide derivatives under continuous-flow conditions
The MIT Faculty has made this article openly available.
Please share
how this access benefits you. Your story matters.
Citation
Andrade, Leandro H., et al. “Confining a Biocatalyst for Highly
Efficient and Selective Synthesis of Carboxamide Derivatives under
Continuous-Flow Conditions.” Journal of Flow Chemistry, vol. 6, no.
2, June 2016, pp. 67–72.
As Published
http://dx.doi.org/10.1556/1846.2016.00008
Publisher
Springer International Publishing
Version
Author's final manuscript
Citable link
http://hdl.handle.net/1721.1/116725
Terms of Use
Creative Commons Attribution-Noncommercial-Share Alike
Confining a Biocatalyst for Highly Efficient and Selective Synthesis of Carboxamide
Derivatives under Continuous-Flow Conditions
Leandro H. Andrade
1,2, Bruno A. Sousa
2and Timothy F. Jamison
11Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States 2Institute of Chemistry, University of São Paulo, São Paulo, 05508-000 São Paulo, Brazil
Received: 01 March 2016; accepted: 30 March 2016
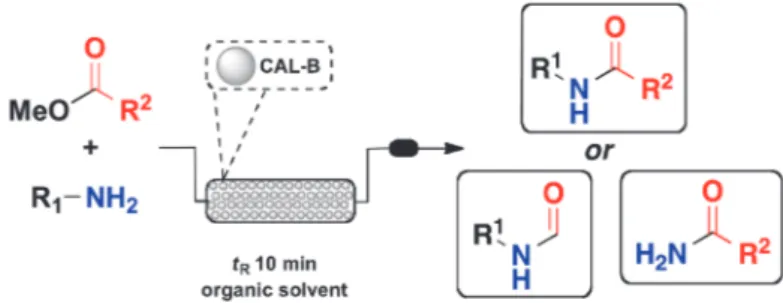
The confinement of a biocatalyst designed to operate under continuous-flow conditions is a strategy developed by nature in order to achieve efficient reactions in biological media. Herein, we present a mimetic model that employs a confined lipase (CAL-B) in the production of several carboxamide derivatives from esters as precursors. The remarkable selectivity of such system is also described whenα,β-unsatured carboxylic substrates are employed. Keywords: lipase, carboxamide, flow conditions
1. Introduction
It is well known that, in nature, enzyme-catalyzed reactions can be completed in a few minutes. Despite being natural substrates, the confinement of biocatalysts inside cellular organelles is one of nature's most impressive strategies to achieve efficient chemical transformations, which technically operate under continuous-flow conditions. On the other hand, enzymatic reactions of non-natural substrates in organic media usually render complications. The pres-ence of xenobiotic compounds such as organic solvents may cause enzyme inhibition or even enzyme denaturation in extreme cases. However, supported enzymes have proven to be reliable biocatalysts for different chemical transformations due to their higher stabilities and tolerance to non-natural environment [1]. Lipase from Candida antarctica (CAL-B) is one of such examples [2].
Lipase-catalyzed reactions are very important for the prepara-tion of several classes of compounds, including amides, esters, carboxylic acids, and alcohols in chiral and achiral form [3]. Amide synthesis using lipases as biocatalysts requires long reac-tion times [4]. In some cases, byproduct formareac-tion can be a problem, especially when uncatalyzed reactions can occur [5].
A literature survey revealed that several synthetic methods have been described to prepare amides (including secondary, primary amides, and formamides) from carboxylic acids and esters [6]. However, in general, those approaches require long reaction times, high temperature, residue generation, and low-or none-catalyst recycling.
Inspired by the natural confinement of enzymes in cells, we envisioned a packed-bed reactor (PBR) filled with lipase in order to create a continuous-flow system for synthesis of carboxa-mides. Despite that flow technology has been used for enzyme-catalyzed reactions [7], no general methodologies for selective synthesis of carboxamides have been described so far.
The types of continuous reactors usually employed for enzymatic processes include membrane reactors, packed-bed reactors, and even microfluidic devices [7]. The choice of the reactor depends on the enzyme and its final application. For example, enzyme membrane reactor can be applied for continuous reactions in which a non-immobilized enzyme is suspended in a solution [7b]. In this case, membrane can retain enzyme, while small molecules, includ-ing substrate and product, can pass through. On the other hand, packed-bed reactor can be applied for immobilized enzymes, espe-cially for reactions which require organic solvents [7c].
Considering that lipase is not a cofactor-dependent enzyme, which can also be used in its immobilized form, we selected
packed-bed reactor to be applied in the synthesis of carboxamides in organic solvent under continuous operation (Scheme 1).
By using this approach, we can ensure low loading of non-natural substrate/product inside the reactor and, as consequence, a negligible enzyme inactivation. In an efficient reaction using organic solvent, the stream, which leaves the PBR system, will contain only the desired product with no aqueous residue (acid or salts). High enzyme recyclability is also expected for PBR, since there is no stir bar or any mechanical agitation, which avoids the destruction of immobilized lipase. For our purpose, we selected CAL-B as biocatalyst, since it has been employed to prepare several chiral and achiral compounds.
2. Results and Discussion
Initially, a comparison between aminolysis reaction under tradi-tional batch and flow conditions was performed to validate our proposal (Tables 1 and 2). Aiming fast enzymatic reactions under continuous-flow conditions, we established that the maximum res-idence time would be no higher than 60 min. Benzylamine (0.023 M in MTBE) and methyl phenylacetate (0.02 M in MTBE) were selected as model substrates for the evaluation of enzyme activity under different temperature and lipase loading (CAL-B) (Table 1).
In 60-min reactions under batch conditions, by increasing temperature (r.t. to 50 °C, Table 1, entries 1–4), the amide conversion was also increased (6 to 38%). We replaced the solvent to toluene, since it is a known solvent for dynamic kinetic resolution via lipase-catalyzed reaction, which requires high reaction temperature under long reaction time. However, only traces of amide were observed (<3% conversion; Table 1, entry 5). In order to push the limits of enzyme efficiency, 10-min reactions with different lipase loading (20–270 mg) were eval-uated. No significant improvement in amide formation was achieved. For example, a high enzyme loading (270 mg) afforded the amide in only 16%. We have clearly evidenced a dependence of temperature and reaction time to achieve reason-able amide yield under batch conditions.
To evaluate the same conditions for amide synthesis under flow conditions, a packed-bed reactor containing CAL-B (270 mg) was prepared (Table 2). The continuous-flow operation involved methyl ester (0.02 M) and amine (0.023 M) in MTBE at 50 °C under different residence time, tR, 10–40 min (BPR: 100 psi).
By employing 10 min as residence time, amide formation was achieved with 76% conversion (Table 2, entry 1; in contrast to 16% under batch condition). However, full conversion was achieved by increasing the residence time to 40 min (Table 2, entry 4). The high enzyme loading and low xenobiotic
*
*
* Authors for correspondence: leandroh@iq.usp.br (L.H. Andrade); tfj@mit.edu (T.F. Jamison)
compounds (substrate, coproduct, and product) concentration inside the packed-bed reactor ensured efficient aminolysis reac-tions under flow condireac-tions. In fact, considering this approach any enzyme inactivation due to the presence of substrate, co-product and co-product was negligible.
It was described by other research groups that lipase-cata-lyzed reactions of α,β-unsaturated esters and amines as sub-strates require long reaction time and mixture of products; aminolysis product and 1,4-addition product are obtained [5]. Motivated by our efficient system under continuous-flow con-ditions, we decided to study those substrates in order to over-come these limitations (Scheme 2, isolated yields were given in brackets). Fortunately, by using our flow system, only after
10 min, the amides 2–6 were efficiently produced in high yield with no byproduct formation (Scheme 2). We can highlight that the fast enzymatic reaction under continuous-flow conditions (tR: 10 min) avoids long exposure of substrate and product to each other, especially under high temperature as employed by other authors. The continuous-flow conditions greatly favor kinetic products, which were obtained from lipase-catalyzed aminolysis reaction.
We also evaluated the behavior of mixed primary alkylamine– aniline functions on the enzymatic acylation system under flow conditions (Scheme 3, isolated yields were given in brackets). Selectivities of lipase-catalyzed acylations of diamines were eval-uated under flow conditions using tRof 10 min. As observed in all Scheme 1.Confined lipase for fast aminolysis reactions
Table 1.CAL-B-catalyzed aminolysis reaction under batch conditionsa
Entry Solvent CAL-B (mg) t (min) T (°C) Conv. (%)b
1 MTBE 100 60 r.t. 6 2 MTBE 100 60 30 12 3 MTBE 100 60 40 31 4 MTBE 100 60 50 38 5 Toluene 100 60 50 <3 6 MTBE 20 10 50 12 7 MTBE 50 10 50 14 8 MTBE 100 10 50 16 9 MTBE 170 10 50 16 10 MTBE 270c 10 50 16 a
Reaction condition: methyl phenylacetate (0.02 M), benzylamine (0.0.23 M), and solvent (4 mL).
bConversions were determined by GC analysis.
cThis lipase amount was selected considering the size of the packed-bed reactor.
Table 2.CAL-B-catalyzed aminolysis reaction under flow conditionsa
Entry tR(min) Amide (%)b
1 10 76
2 20 86
3 30 96
4 40 >99
a
Continuous-flow operation: methyl ester (0.02 M) and amine (0.023 M) in MTBE at 50 °C; residence time (tR): 10–40 min; lipase: CAL-B (270 mg) was
packed into a stainless steel tubing (1/4 in × 4.6 mm × 5 cm; reactor volume with lipase beads: 0.7 mL); back pressure regulator (BPR): 100 psi.
b
Conversion was determined by GC–MS analysis.
Confining a Biocatalyst
reactions, the selectivities were excellent, giving the monoacylated product in high yield as sole product (only the alkylamine was acylated).
By changing the acyl donor to formyl, we were able to easily prepare formamides in high yields with 10 min of residence time at 30 °C (Scheme 4, isolated yields were given in brackets). Aromatic amine as substrate (aniline) gave low yield after 10 min (22%
using methyl formate as acyl donor). However, by an increase of temperature and changing the acyl donor to formic acid, the formamide was obtained in 58% with tRof 30 min.
We also carried out ammonolysis reaction catalyzed by lipase under flow conditions. When this reaction was performed under batch conditions [8], once again, the need for long reaction times in order to achieve reasonable yields is required. Motivated by this Scheme 2.Chemoselectivity of CAL-B-catalyzed aminolysis under flow conditions
issue, we have employed our flow system for this matter and high yields were observed (Scheme 5, isolated yields were given in brackets).
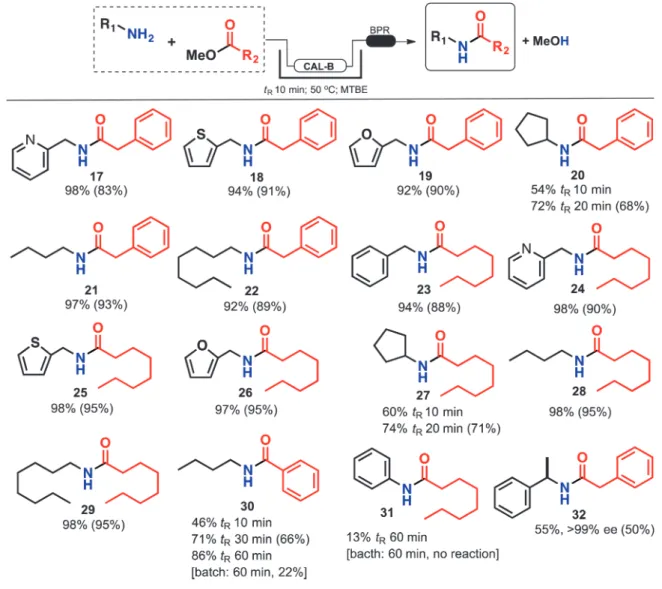
To expand the reaction scope under flow conditions, different amines and esters were used as substrates (Scheme 6, isolated yields were given in brackets). Initially, all reactions were eval-uated with 10 min of residence time at 50 °C. However, for some cases, an increase in tRwas required to achieve excellent yields.
In general, lipase was very tolerant to different esters and amines as substrates. In fact, fast aminolysis reactions gave the corresponding amides in high yields. However, aromatic amines and esters needed high residence times (up to 60 min) to achieve moderate yields. It is important to note that these are less active substrates for lipases. The aniline–methyl octanoate system under batch conditions is completely unreactive even after 60 min. On the other hand, and despite low conversion, the amide 16 was observed under flow conditions.
We also studied the lipase activity during continuous-flow oper-ation (Figure 1). In this case, the same flow conditions described in Scheme 2 for production of amide 28 were selected (tR: 10 min; 50 °C; methyl ester: 0.02 M; n-butylamine: 0.023 M). CAL-B was used for 84-fold residence time under continuous-flow operation with no decrease in lipase activity (98% conv. for amide 28). These results evidenced that CAL-B can easily tolerate organic solvent and high temperature for long period.
Increasing the productivity of enzymatic reactions can be problematic in several cases, since high concentrations of xeno-biotic compounds (substrates, products, and coproducts) can cause negative effects on enzyme activity. In order to further increase the productivity, N-butyloctanamide 28 and 10 min as residence time at 50 °C were selected for this purpose. The
reaction was performed at different substrates concentration (ester: 0.02–0.5 M). As observed in Figure 2, we still can have excellent amide formation up to 0.2 M, a 10-fold higher con-centration than our initial studies.
3. Conclusions
In summary, a confined lipase in a packed-bed reactor under continuous-flow condition was efficiently used to overcome some limitations in the synthesis of several carboxamides derivatives. The described methodology allows a fast approach with high productivity for reversible aminolysis and ammonolysis of esters or acids. A valuable flow chemistry feature was also demonstrated in face of chemoselectivity issues of conjugate- versus direct-addition into α,β-unsatu-rated esters, in which the thermodynamically favored conju-gate addition reaction was completely suppressed under the evaluated conditions.
4. Experimental
4.1. General Information. All the continuous-flow reactions were conducted on a Harvard Apparatus syringe pump equip-ped with 8-mL stainless steel syringes and a 100-psi backpres-sure regulator. Lipase from C. antarctica (CAL-B) is commercially available from Sigma-Aldrich (CAS Number: 9001-62-1; Catalog Number: L4777). Starting materials includ-ing amines and esters were obtained from commercial sources and used as received. Fully packed cylindrical stainless steel tubes of different sizes were employed as reactors for catalyst Scheme 4.CAL-B-catalyzed N-formylation under flow conditions
Scheme 5.CAL-B-catalyzed ammonolysis under flow conditions
Confining a Biocatalyst
compartmentalization. The temperature control was achieved by submerging the reactors in a thermostatic water bath. Before chromatographic analysis and sample acquiring (for all reac-tions), the system was kept under stabilization for three times
the established residence time. Gas chromatography (GC) anal-yses were performed in a Shimadzu GC-17A instrument with a FID detector, using hydrogen as a carrier gas (100 kPa). GC– mass spectrometry (MS) analysis data were recorded on a
Figure 1.Production of 28 under continuous-flow operation
Shimadzu GCMS-QP2010 SE equipment. Nuclear magnetic response (NMR) spectra were recorded on Agilent Inova 300 MHz, Bruker 300 MHz, and Bruker 500 MHz equipment and properly calibrated according to the solvent employed. Infrared spectra were recorded on a Perkin Elmer Frontier FT-IR instrument.
4.2. General Procedure for Aminolysis
4.2.1. Lipase-Catalyzed Aminolysis under Batch Condi-tions. In a 6-mL vial, methyl phenylacetate (0.02 M), benzyl-amine (0.023 M), CAL-B (20–270 mg), and MTBE (4 mL) were stirred for 60–240 min r.t. −50 °C (Table 1). The reaction content was analyzed by GC–MS.
4.2.2. Set Up the Packed-Bed Reactor for Flow Application. The reagent-containing syringe (esters/acids and amines; concen-tration is specified in the tables) was loaded into the syringe pump. The packed-bed reactor: CAL-B beads (270 mg) were packed into a stainless steel tube (1/4 in × 4.6 mm × 5 cm; reactor volume with beads: 0.7 mL). Back pressure regulator (BPR): 100 psi.
Reactions were run at appropriate flow rates to obtain the desired residence time. The effluent was collected in a 20-mL vial and then directly purified by column chromatography (elu-ent: hexane and ethyl acetate) to give the desired amides (1–32). The isolated yields for the carboxamides are given in brackets at the schemes.
Acknowledgment. L.H.A. would like to thank FAPESP (São Paulo Research Foundation; grant number: 2013/04540-0 and 2014/22457-5) for financial support.
Supporting Information
Electronic Supplementary Material (ESM) with supplementary data of compounds 1–32 associated with this article is available in the online version at doi: 10.1556/1846.2016.00004. References
1. (a) DiCosimo, R.; McAuliffe, J.; Poulose, A. J.; Bohlmann, G. Chem. Soc. Rev. 2013, 42, 6437–6474; (b) Franssen, M. C. R.; Steunenberg, P.; Scott, E. L.; Zuilhof, H.; Sanders, J. P. M. Chem. Soc. Rev. 2013, 42, 6491–6533; (c) Datta, S.; Christena, L. R.; Rajaram, Y. R. S. 3 Biotech 2013, 3, 1–9; (d) Netto, C. G. C. M.; Toma, H. E.; Andrade, L. H. J. Mol. Catal. B: Enzym. 2013, 85–86, 71–92. 2. (a) Gotor-Fernandez, V.; Busto, E.; Gotor, V. Adv. Synth. Catal. 2006, 348, 797–812; (b) Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biotech. Adv. 2010, 28,
628–634; (c) Idris, A.; Bukhari, A. Biotech. Adv. 2012, 30, 550–563; (d) Khan, A.; Sharma, S. K.; Kumar, A.; Watterson, A. C.; Kumar, J.; Parmar, V. S. ChemSusChem 2014, 7, 379–390.
3. (a) Gotor-Fernandez, V.; Brieva, R.; Gotor, V. J. Mol. Catal. B: Enzym. 2006, 40, 111–120; (b) Kapoor, M.; Gupta, M. N. Process Biochem. 2012, 47, 555–569; (c) Guan, Z.; Li, L.-Y.; He, Y.-H. RSC Adv. 2015, 5, 16801–16814; (d) de Melo Carvalho, A. C. L.; Fonseca, T. D. S.; de Mattos, M. C.; de Oliveira, M. D. C. F.; de Lemos, T. L. G.; Molinari, F.; Romano, D.; Serra, I. Int. J. Mol. Sci. 2015, 16, 29682–29716.
4. Selected examples: (a) Rebolledo, F.; Brieva, R.; Gotor, V. Tetrahedron Lett. 1989, 30, 5345–5346; (b) Badjic, J. D.; Kadnikova, E. N.; Kostic, N. M. Org. Lett. 2001, 3, 2025–2028; (c) van Pelt, S.; Teeuwen, R. L. M.; Janssen, M. H. A.; Sheldon, R. A.; Dunn, P. J.; Howard, R. M.; Kumar, R.; Martinez, I.; Wong, J. W. Green Chem. 2011, 13, 1791–1798; (d) Adamczyk, M.; Grote, J. Tetrahedron Lett. 1996, 37, 7913–7916; (e) Fernandez-Perez, M.; Otero, C. Enzyme Microb. Tech. 2003, 33, 650–660; (f) Le Joubioux, F.; Bridiau, N.; Ben Henda, Y.; Achour, O.; Graber, M.; Maugard, T. J. Mol. Catal. B: Enzym. 2013, 95, 99–110. For a recent review, see: Goswami, A.; van Lanen, S. G. Mol. Biosyst. 2015, 11, 338–353.
5. (a) Bonte, S.; Ghinea, I. O.; Baussanne, I.; Xuereb, J.-P.; Dinica, R.; Demeunynck, M. Tetrahedron 2013, 69, 5495–5500; (b) Steunenberg, P.; Sijm, M.; Zuilhof, H.; Sanders, J. P. M.; Scott, E. L.; Franssen, M. C. R. J. Org. Chem. 2013, 78, 3802–3813; (c) Domingo Rivera-Ramirez, J.; Escalante, J.; Lopez-Munguia, A.; Marty, A.; Castillo, E. J. Mol. Catal. B: Enzym. 2015, 112, 76–82; (d) Strompen, S.; Weiss, M.; Ingram, T.; Smirnova, I.; Groeger, H.; Hilterhaus, L.; Liese, A. Biotechnol. Bioeng. 2012, 109, 1479–1489; (e) Torre, O.; Gotor-Fernandez, V.; Alfonso, I.; Garcia-Alles, L. F.; Gotor, V. Adv. Synth. Catal. 2005, 347, 1007–1014.
6. Selected review articles: (a) Lanigan, R. M.; Sheppard, T. D. Eur. J. Org. Chem. 2013, 2013, 7453–7465; (b) Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Chem. Soc. Rev. 2014, 43, 2714–2742. Selected examples, see: (c) El Dine, T. M.; Erb, W.; Berhault, Y.; Rouden, J.; Blanchet, J. J. Org. Chem. 2015, 80, 4532–4544; (d) Desrat, S.; Ducousso, A.; Gapil, S.; Remeur, C.; Roussi, F. Synlett 2015, 26, 385–387; (e) Morimoto, H.; Fujiwara, R.; Shimizu, Y.; Morisaki, K.; Ohshima, T. Org. Lett. 2014, 16, 2018–2021; (f) Bai, J.; Zambron, B. K.; Vogel, P. Org. Lett. 2014, 16, 604–607; (g) Mahe, O.; Des-roches, J.; Paquin, J.-F. Eur. J. Org. Chem. 2013, 2013, 4325–4331.
7. Selected review articles: (a) Anderson, N. G. Org. Process Res. Dev. 2012, 16, 852; (b) Giorno, L.; Drioli, E. Trends Biotechnol. 2000, 18, 339–349; (c) Itabaiana Jr., I.; de Mariz e Miranda, L. S.; de Souza, R. O. M. A. J. Mol. Catal. B: Enzym. 2013, 85–86, 1; (d) Bolivar, J. M.; Wiesbauer, J.; Nidetzky, B. Trends Biotechnol. 2011, 29, 333–342; (e) Asanomi, Y.; Yamaguchi, H.; Miyazaki, M.; Maeda, H. Molecules 2011, 16, 6041–6059. Recent articles: (f) de Miranda, A. S.; Miranda, L. S. M.; de Souza, R. O. M. A. Org. Biomol. Chem. 2013, 11, 3332–3336; (g) Du, L.-H.; Ling, H.-M.; Luo, X.-P. RSC Adv. 2014, 4, 7770– 7773; (h) Kim, Y.-J.; Choi, Y.-S.; Yang, S.; Yang, W. R.; Jeong, J.-H. Synlett 2015, 26, 1981–1984.
8. (a) Dezoete, M. C.; Dalen, A. C. K.; van Rantwijk, F.; Sheldon, R. A. J. Chem. Soc. Chem. Comm. 1993, 1831–1832; (b) Garcia-Urdiales, E.; Rebolledo, F.; Gotor, V. Tetrahedron-Asymmetry 1999, 10, 721–726; (c) Wegman, M. A.; Hacking, M.; Rops, J.; Pereira, P.; van Rantwijk, F.; Sheldon, R. A. Tetrahedron-Asymmetry 1999, 10, 1739–1750; (d) Lau, R. M.; van Rantwijk, F.; Seddon, K. R.; Sheldon, R. A. Org. Lett. 2000, 2, 4189–4191; (e) Litjens, M. J. J.; Straathof, A. J. J.; Jongejan, J. A.; Heijnen, J. J. Tetrahedron 1999, 55, 12411–12418; (f) Levinson, W. E.; Kuo, T. M.; Kurtzman, C. P. Enzyme Microb. Tech. 2005, 37, 126–130.
Figure 2.Evaluation of substrate concentration in the synthesis of N-butyloctanamide 28 under flow conditions
Confining a Biocatalyst