Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Research Paper (National Research Council of Canada. Division of Building
Research); no. DBR-RP-566, 1972-08-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=e852fbb6-fa5a-447f-b0a9-c7b9a62d1f8a https://publications-cnrc.canada.ca/fra/voir/objet/?id=e852fbb6-fa5a-447f-b0a9-c7b9a62d1f8a
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001751
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
A new look at compartment fires, Parts I and II
Fire Technology,
.-
8 (3)
196-217 (1972)
A
New Look a t Compartment Fires,
Part
I
T. Z.
HARMATHYFire Research Section
Division of Building Research
National Research Council of Canada
This is the first part of a two-part paper in which the author has endeavored to present all available information on compart- ment fires in a consistent theoretical framework. This well-docu- mented review of the state of the art should serve as a valuable reference for researchers for some time t o come.
I
T
HAS been realized for some time that the so-called "fie load con- cept" propounded in the pioneer work of Ingbergl did not provide a satisfactory basis for the correct design of buildings for fire endurance, and sooner or later it will become desirable to revise the philosophy on which the present practices of fire protection have been built." Naturally, making f i e protection more economical and still adequate is not con- ceivable without knowing, with reasonable accuracy, what is going on in a burning compartment.Although experimental studies concerned with compartment fires have been conducted for several decades, there still are many disturbing gaps and contradictory points in the interpretation of the experimental results. The reason may well be that, during the years, several inade- quately justzed assumptions became firmly embedded in the description of certain phenomena and turned the research in wrong directions.
I n this paper, an attempt will be made to interpret the information available on the decomposition and combustion of cellulosic materials in unconfined and confined spaces in such a way as to obtain a more or less consistent, though not necessarily accurate, picture of the hundreds of phenomena that make up a compartment fire.
Because of the generally poor reproducibility of compartment fires, it would seem illogical to develop sophisticated mathematical models for the description of some of the complex phenomena. For this reason, rigorous investigations will often be bypassed by tentatively introduced simplifying assumptions.
The word "compartment" should be interpreted as a confined space in a building, communicating with the unconfined atmosphere through one or more vertical openings to be referred to hereafter as "windows" for simplicity.
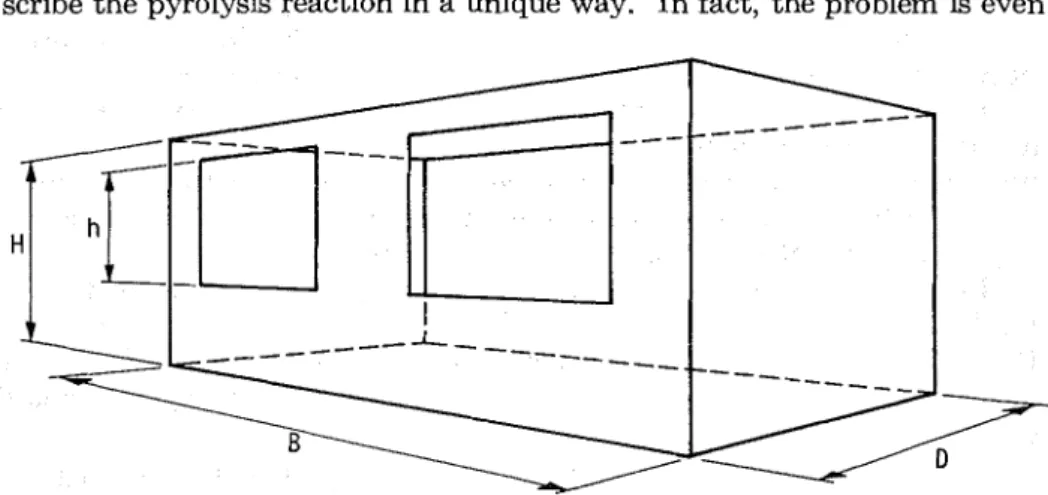
A
typical "compartment" is shown in Figure 1. The conclusions arrived a t in this paper are normally not applicable to very large or very deep compartments without supplementary experimental verification.Only those aspects of compartment fires that are connected with structural fire protection problems will be discussed here. Other aspects, such as detection, use of sprinklers, toxicity of combustion products, and smoke movement, will remain outside the scope of this paper.
P Y R O L Y S I S O F C E L L U L O S I C M A T E R I A L S In the common usage of the language, the "burning" of cellulosic materials is interpreted as the simultaneous development of two processes - pyrolysis and combustion of a t least some of the decomposition products.
Irrespective of whether the model of competitive reactions by Kilzer and Broido3 or the model proposed by Parker and Lipska4 is accepted for describing the pyrolysis, there seems to be substantial agreement in regard to the following statements:
.
The decomposition of cellulose into volatiles and char is primarily an endothermic process up to about 320" C and an exothermic process a t higher temperatures.Higher heating rates enhance the exothermic reactions and produce less char and more volatile products which are richer in carbon and greater in calorific value.
0 The decomposition process is not noticeably affected by the presence or absence of oxygen in the ambient atmosphere.
I n the light of these statements, it seems somewhat arbitrary to de- scribe the pyrolysis reaction in a unique way. I n fact, the problem is even
more complicated than it first appears, since before attempting to formu- late the decomposition reaction, one is faced with the problem of de- fining the "composition" of a "typical" wood.
*
Yet, for practical reasons, this practice is hardly avoidable.On the basis of available data the following chemical formula has been selected to represent a typical wood:
Here the attached 0.233 molecules of water account for the presence of
15 percent moisture, referred t o overall weight, under normal atmospheric conditions.
Assuming that the charcoal yield is 15 percent, referred to oven-dry weight (or 12.76 percent, referred to overall weight), and that the chem- ical formula of charcoalj is approximately CHo.200 az, the pyrolysis reac- tion can be written as follows:
The first two terms on the right side of the equation represent the vola- tiles formed in the process.
Although Equation 1 is reasonably accurate in characterizing the end products of pyrolysis, it may be grossly misleading when applied to in- termediate phases of the process. The composition of the mixture of volatiles changes and its calorific value increases as the pyrolysis pro- gressesG v 7 .
The overall balance of t h e pyrolysis reactions is slightly on the exo- thermic side. The heat of decomposition8 is of the order of 3.0
x
10J/kg. I n studies relating t o the development of building fires, knowing the rate of decomposition, or rather the rate of volatile formation, is of prim- ary importance. It is usual to study the rate of volatilization, U,, by con- ducting experiments on wood samples of some simple geometry exposed to high radiant energy fluxes in a normal or inert atmosphere.It
ap- pears from these experiments that U, is a function of the species of wood, moisture content, geometry, and net heat flux, q~ (i.e. the flux actually absorbed by the wood). For the hypothetical "typical" wood under normal atmospheric conditions, the first two factors lose their significance, and thus the following equation applies:U , =
f
(geometry, q ~ )t
(2)Surprisingly enough, even with the aid of an obviously oversimplified model, according to which the pyrolysis develops without latent heat
*In accordance with the accepted practice, all cellulosic materials will be expressed
hereafter in terms of calorifically equivalent wood quantities. The total mass of the so-interpreted wood quantities in a compartment is usually referred to a s "fire load".
effects and the rate of volatilization is a function of the rate of penetra- tion of a critical decomposition isotherm into the wood, often fairly ac- curate values call be derived for U , , 9~ ll. Although for the entire pyrol-
ysis process the absorbed and evolved heats nearly cancel each other, the latent heat effects can be quite significant a t transient stages of the decomposition.
As said earlier, toward the end of the decomposition process, exo- thermic reactions are dominant. Although much of the evolved heat leaves with the volatiles, still, after a while, sufficient amounts of heat may accumulate in the wood to produce a so-called "pre-ignition" tempera- ture distribution! Thereafter, the decomposition will proceed even if t h e net heat flux is zero or slightly negatives. Unfortunately, information concerning the explicit form of Equation 2 is not available, since it has been usual t o study the volatilization of wood as a function of irradiance (References 6 and 12) instead of the heat flux absorbed by the wood. C O M B U S T I O N O F D E C O M P O S I T I O N P R O D U C T S I t call be readily calculated that 5.11 kg air is required for the perfect combustioil of every kilogram of the defined "typical" wood. If the de- compositioll prior to combustion proceeds as described by Equation 1 , the air requirement is 4.19 kg/kg separately for the volatiles and 11.41 kglkg for the charcoal. Of course, the combustion is never perfect. I n addition to COs, some CO will also be produced in the process.
From the work of Kawagoe l 3 and Gross and Robertson, l 4 it seems that, in the case of compartment fires, the ratio COt/(COr
+
CO) is about 0.6. This value has been used to calculate the composition of the combustion products resulting from the burning of the volatiles (without excess air). The results are given in Table 1.T A B L E 1. Typical Composition of Combustion Products of Volatiles
Gas Mole(%)
The air requirements corresponding to the ratio C 0 2 / ( C 0 2 $ CO)
= 0.6 are as follows: 4.13 kg/kg for the entire wood, 3.39 kg/kg for t h e volatiles and 9.21 kg/kg for the char.
With respect to the heats of combustion, available information indi- cates that 16.7 X 10"/kg can probably be regarded as a representative overall value for the volatiles, and about twice this value, 33.4 X
loG
J/kg for the charcoal. I t must be clearly understood, however, that assigningfixed values to the heat of combustion is no less arbitrary than describing the wood and its decomposition products by simple chemical formulas.
B U R N I N G O F P I L E S O F W O O D
I N U N C O N F I N E D A T M O S P H E R E
I t is quite common to start experimental studies of compartment
fires with burning tests performed on wood cribs in unconfined atmospheres. Some research workers hoped to gain further insight into the mechanism of burning by supplementing these experiments with others performed on
liquid fuels contained in shallow trays or in
drums.
Unfortunately, itappears now that assuming a close analogy between the burning of wood cribs and of pools of liquid fuels delayed rather than helped the under- standing of the characteristics of crib fires.
I n crib-burning experiments the rate of decrease of weight of the
wood pile is commonly regarded as the measure of the rate of burning.
Since volatilization and thus weight loss can occur in atmospheres that cannot support burning, this practice is not necessarily correct.
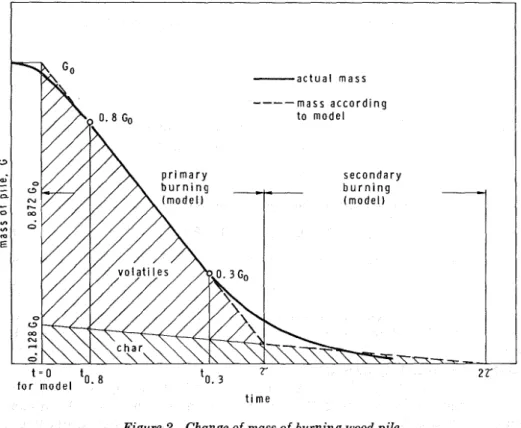
I n Figure 2 a typical mass versus time (G versus t) curve is shown for
a burning wood pile. Numerous experimental results indicated that
the mass of the pile decreases in three stages. I n the first stage the original
t - 0 to.* T
3 f o r m o d e l
t i m e
Compartment Fires
mass, G,, first diminishes slowly, then a t an increasing rate. As the mass of the pile is reduced to about 0.8 Go (at t = the rate of change be-
comes roughly constant and remains so until the mass drops to about
0.3 Go (when t = to.a). This second stage of burning will be referred to in this paper as "primary burning." The constancy of the rate of mass change in this period is mainly a result of the constancy of the rate of volatile fonnation. Finally, in the third stage, to be referred to as "sec- ondary burning," the rate of mass change slows down and approaches zero as the oxidation of charcoal residue progresses toward completion. Figure 2 also shows the mathematical modeling of the experimental curve. I n this model the first stage of burning is disregarded and, there- fore, the origin, t = 0, is slightly displaced. The stage of primary burning
is now extended to cover the entire period of volatile formation. I t is
assumed that by the end of this period (when t = 7) half of the char has
also oxidized. I t is further assumed that both the volatile formation and the oxidation of char proceed, a t all times, at constant rates. These assumptions also imply that, if no ash is left on the oxidation of char, the burning process is completed a t t = 27. I n this model the 7 <t<27 period will be referred to as the stage of secondary burning.
With these assumptions and taking into account the previously intro- duced modeling of wood and its decomposition products, the following equations can be derived:
for the rate of volatile formation during the period of primary burning; -
U ,
= 0.932R
for the duration of primary burning;
7 = 0.936 G,/R
for the rate of mass loss due to char oxidation (which is equal to the rate of mass loss during the period of secondary burning);
for the rate of heat evolution during the period of primary burning;
and for the rate of heat evolution during the period of secondary burning;
Equation 6 is based on the assumption that the volatiles are ignited by the glowing char surfaces and burn entirely in the vicinity of the pile. I t will be seen later that there are certain situations to which this assumption is not applicable.
In the present model, it is essentia! to assert that a significant portion of the char oxidizes during the period of primary burning. To under- stand why this point is emphasized, attention is directed to Equation 2.
According to this equation the rate of volatilization for the individual pieces of wood is a function of the net heat flux and, as said earlier, is independent of the availability of oxygen in the surrounding atmosphere. It is well known, however, that, in the case of burning of piles of wood, there is a definite relation between the airing of the pile and the rate of mass loss during the period of primary burning f l5
-
l6 * l7 18. To recon-cile these two findings, one must assume that the presence of oxygen in
the pile affects the rate of volatilization only in an indirect way - by controlling the rate of combustion of some decomposition products inside the pile and thereby the net heat flux into the individual pieces of wood in the pile.
It
has been customary so far to idealize the process of burning of wood piles by assuming that the glowing combustion of char starts essen- tially after the completion of pyrolysis and flaming combustion of volatiles. This concept was undoubtedly adopted under the impression that the proc-ess of flaming combustion during the period of primary burning is analo- gous to the burning of pools of liquid fuels. For the sake of this analogy some significant differences between these two processes have obviously been overlooked.
I n pool burning the volatiles are vapors of the liquid fuel. Only these vapors are present immediately above the liquid surface. Air enters the rising column of vapors from the sides. Since no combustion is conceivable below the liquid surface, the heat of vaporization must come by "feed- back" from the flaming combustion above. The rate of "volatilization" (i.e. vaporization) a t the liquid surface is fully determined by the nature of the flames and, in turn, by the nature of the liquid fuel and the size of the pool.
I n the case of wood piles, during the period of primary burning, vola- tiles are released not only a t the upper level of the pile, which is now analogous to the liquid surface, but also inside the pile. Air enters the rising columns of volatiles not only from the sides by entrainment but, also to a lesser extent, through the pile; and some oxygen of the secondary air is used up for combustion inside the pile. The gases passing through the upper level of the pile consist not entirely of combustible volatiles, but also contain some combustion products and oxygen.
McCarter and Broidolg proved conclusively that the energy feedback from the flames above the pile could hardly have any effect on the progress of pyrolysis. This finding comes as no surprise. What is surprising, is the fact that the feedback concept, obviously a transplant from pool burning studies, is still often used even though it has been long known that the temperature inside a burning pile is normally much higher than the average temperature of the flames above 9 . 3 0 8 2 1 t 2 2 8 23.
It
seems now that the most plausible model for the mechanism ofpyrolysis and burning of wood piles is as follows.
Fed by air entering the pile, glowing layers of char of about 1000" C
under certain conditions, the pyrolysis can proceed even without external heat supply, the heat released by the oxidation of char (and, to a lesser degree, by volatiles burning inside the pile) and trapped within the pile by the large internal surface areas acts as the factor regulating the rate of volatile evolution. Since the amount of glowing char obviously in- creases with the air flow through the pile, the dependence of the rate of volatilization on the availability of air becomes a natural consequence of the present model.
The heat evolved inside the pile is transferred to the undecomposed portions of the wood either by conduction or by radiation and convection.
It
is extremely importantto
realize that the rate of volatilization is pri- marily determined by these "short-range" high-intensity heat fluxes. Since the flame temperature above the pile is lower than the temperature of the glowing char, the flame cannot feed energy back to the pile but can only act as a guard that moderates the heat losses from the pileto
the surroundings.The average level of energy flux within the pile depends on two factors: The total area of glowing char surfaces, which, in turn, depends on the air flow per unit area of internal surfaces in the pile; and
The external geometry of the pile, which determines the rate of heat loss from the pile to the surroundings.
In the light of this discussion and that in connection with Equation 2, it is now possible to express the rate of volatile formation from a pile of wood during the period of primary burning approximately as follows:
-
U,/G, = f (internal pile geometry, external pile
geometry,
U , / A , ,
Q,/G,) (8)I n this equation the expression "internal geometry" covers variables, such as the characteristic dimension of wood pieces and their specific free surface area, the porosity (fractional voidage) of the pile, its permeability in vertical and horizontal directions, etc. The "external geometry" covers the external dimensions of the pile, the surface area available for air penetration, the position of the pile in relation to a base plane, etc. Although these two groups of variables obviously completely determine the other two "independent" variables in the equation, namely the specific air flow and the rate of heat loss from the pile, for the sake of better under- standing it seemed advisable to retain these variables also.
The last two variables listed have obviously opposite effects on the rate of volatile formation.
It
is probable, therefore, that for those geometric features which affect both of these variables in the same sense, an opti- mum range may exist a t which the rate of volatile formation is maximum. Thus, for example, with the increase of the spacing of individual wood pieces or with the increase of the height-to-width ratio for the pile, the air flow through the pile increases, but so do the heat losses from the pile, especially if the overall sizes of the pile are relatively small. There areindications that the maximum rate of volatilization takes place a t some intermediate values of the spacing of wood pieces or of the height-to- width ratio for the pile (which also depend on the overall sizes of the pile), a t which the beneficial effect of good airing of the pile still outweighs the adverse effect of increased heat losses. For the spacing of wood pieces
the existence of such optimum regime is obvious from Gross' experimental
findingsg.
Admittedly, in its present form Equation 8 cannot be used for more
than "qualitative" predictions. Nevertheless, very often even such a qualitative prediction may prove valuable. The empirical expressions de- veloped by Gross 9, Smith and Thomas 1 6 $ '7, or Nilsson I * for cross piles of
wood are undoubtedly more useful in those cases to which they are ap- plicable. These expressions, however, fail to give any hint in those probably more numerous cases when not the internal, but the external, geometric characteristics of the pile exert the controlling influence on the rate of volatilization.
S I Z E O F F L A M E S I N U N C O N F I N E D A T M O S P H E R E
As mentioned earlier, a portion of the volatiles ignites within the wood pile. The time of residence of the volatiles in the pile is, however, very short, and the air available for mixing is limited. I t is obvious, therefore, that the bulk of the volatiles will leave the pile and burn with the forma- tion of buoyant diffusion flame above the pile where its mixing with air
is readily accomplished by turbulent entrainment.
Theoretical and experimental studies indicated that, for such buoyant
diffusion flames, the flame height is a function of the Froude number
only, and can be described by an equation of the following form 9 r 2 2 $
2 4 , 2 5 . 2 6 . 2 7 . 28:
where the value of
C1
depends on the fuel used.At first sight there seems to be some discrepancy concerning the
value of n. Sunavala 26 presented rather convincing arguments to prove
that the correct value is n = 0.2. This value has also been confirmed by numerous results of experiments conducted on liquid and gaseous fuels summarized in a paper by Seeger and Werthenbach 27. Yet, in the case of burning cribs of wood the value n = 1/3 seems to be more appropriateZ2 + 2" This apparent discrepancy is understandable, however,
in the light of the already discussed differences that exist between the bum-
ing of pools of liquids (or gases emerging slowly from a vertical orifice) and the burning of wood piles. The most significant single difference is probably the fact that, in the case of burning of fluids, the gases entering the base plane of the flame consist entirely of the gaseous molecules of the
fuel, while, in the case of wood fires, the volatiles a t this level are already mixed with some air and combustion products resulting mainly from the glowing combustion of char within the pile. Thus, although on theo- retical grounds a value of n = 0.2 seems to be more justified, there is hardly any doubt that, for burning wood piles, n = 1 / 3 gives better agreement with experimental data.
For a single wood pile, the velocity of the gaseous mixture entering the flame a t the upper level of the pile during the period of primary burn- ing can be expressed as
where the factor C 2 has been introduced to account for the presence of those constituents of the mixture which did not originate from pyrolysis of wood. The area of the flame base can be described with the introduc- tion of a shape factor:
Naturally, for square piles Cg = 1.
By combining Equations 9, 10, and 11 and using the value n = 1/3,
one obtains
Experimental data collected by Thomast2 indicate that, for square cribs, the term CIC2l3 may be combined into a single constant of a value of
about 46.5. Since very little is known about the value of pub, Thomas also
suggested that the pot, = p, approximation be used. Thus Equation 12
becomes
It
should be noted that many experimenters measure the flame heightfrom the base of the pile rather than from the flame base. Under normal practical conditions, however, this discrepancy is not expected to alter the conclusions noticeably.
Thomas 22 and Hinkley et al.29 studied the air entrainment into buoyant
diffusion flames. They found that the amount of air entrained into the flame up to a height z can be described by the following equation
U a e
= C.iPag1'2z3'2,
P
(14)where C4 .= 0.048. Further studies by Thomas et a1.N indicated, however,
ficulties in separating that quantity of the air which actually enters the flame from the amount of air set into motion by the flame. Steward 31
showed that the former quantity is roughly twice the stoichiometric air requirement for the combustion of volatiles. The latter quantity is esti- mated to be one order of magnitude higher30.
B U R N I N G O F W O O D I N C O M P A R T M E N T What makes the mathematical analysis of open fires involved is the difficulty of estimating the ventilation of the wood pile. Fortunately, in the case of compartment fires, this problem is lessened by the fact that the flow rate of air through the windows is readily calculs.ble. Although it is not immediately clear how this information helps in estimating the ventilation of the fuel in the compartment, it is quite obvious that some kind of relation must exist between the ventilation of the contents of the compartment and the compartment ventilation.
Based on the fundamental work of F ~ j i t a 3 ~ and Kawagoe'Y, Thomas
et a1.33 showed that the flow rate of air entering the compartment through
the lower one-third (roughly) of the window area, under the effect of pressure differences brought about by the fire, can be described by the following equation:
where iP is defined as*
and will be referred to as "ventilation Calculations have
show1-13~ * 3 6 s 36 that
U ,
is virtually independent of the value of To, as longas T,,
2
300C.
The letter a is a coefficient; its value depends on the ratio of the window area to the cross-sectional area of the compartment. I t has been usual to assume that a = 0.7. On the basis of some heat balance calculations (to be discussed later), it seems, however, that a = 0.81 is a better choice.Equation 15 shows that the flow rate of air is affected by the rate of burning wood in the compartment; it increases slightly as the rate of burning decreases. Calculations indicated, however, that this effect is
quite insignificant - less than 10 percent under extreme conditions.
I t seems justifiable, therefore, in Equation 15 to regard the term preceding
4, as a contant, as long as the condition T,
>
300C
is fulfilled. The value*It has long been usual to refer to the product Alvhll? as "ventilation parameter." Using 4 instead of A,vhli2 offers the advantage that it helps to eliminate dimensional. constants from some equations to be presented, and thus the annoyance of converting these constants when changing dimensional systems.
tThe method of calculating 4 when the compartment has vertical openings at several height levels has been described in References 34 and 35.
of this constant is about 0.145, thus Equation 15 can be rewritten in the form
If the window area is large in relation to the cross-sectional area of the compartment, the pressure differences between the room and the ambient air may become quite small, and a major portion of the air may enter the compartment as a result of turbulent entrainment by the flames. Thomas et a1.33 showed that Equation 17 remains formally correct even under these conditions, although the value of the constant probably be- comes somewhat smaller.
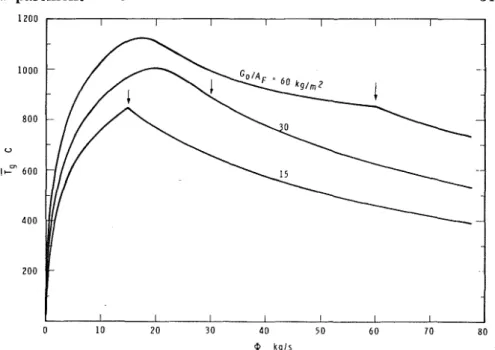
The mass versus time curve for experimental burning of a wood pile in a compartment is usually quite different from that obtained for the same pile when burned in an unconfined atmosphere. The most important cause of the difference is probably that, as noted by Heselden3', the process of pyrolysis in a compartment does not necessarily extend a t any one time to the entire wood contents. If zonal burning occurs, the constant rate portion of the
G
versust
curve may become shorter or may completely disappear. For practical reasons it seems desirable, however, to retain the modeling of theG
versust
curve as shown in Figure 2, and thus t o assume that the burning of wood in all kinds of compartment fires will also consist of a primary and a secondary stage, both occurring a t a con- stant rate.*
Naturally, the period of primary burning is, by far, the most im- portant period of compartment fires. To develop some useful expressions for the rate of burning, three assumptions will now be introduced tenta- tively, pending experimental verification.
The first assumption is concerned with the utilization of the total air
flow, U, (entering through the windows), within the compartment.
A
major portion of this air will obviously be involved in the flaming com- bustion of the volatiles. Some air will still remain, however, to feed the oxidation of surface char layers, the heat of which (as explained in con- nection with Equation 8) controls the rate of volatilization of the wood contents.
It
is assumed that this latter amount of air, U,., is always a constant fraction of the total air flow, i.e. (see also Equation 17):It
has already been mentioned that there is some relation between the area of glowing char surfaces and the rate of volatilization. The second assumption is concerned with fixing the form of this relation.It
is assumed now that the relation between the total char surface, A.,
and the rate of volatile formation U,, is essentially linear. I n virtue of*It may be noted a t this time that the period of primary burning in experimental fires is practically equivalent t o the "period of fully developed fire", and the period of secondary burning, t o the "decay period", in accidental building fires. The different stages of accidental building fires will be further discussed.
Equation 3, this assumption also implies a linear relation between the rate of mass loss during the period of primary burning and the char surfaces:
z
= C & , (19)As mentioned earlier, the process of pyrolysis (or burning) is some- times c o d n e d to one or more zones within the compartment. Since pyrolysis is, in fact, a surface phenomenon, the surface area of wood involved in the volatile formation, in other words, the free surface area of wood in the zones of active pyrolysis, can be expressed as some frac- tion of the total free surface area of wood present in the compartment.
A,, = aA, (20)
where a is, in general, a variable quantity. Furthermore, even in the
zones of active pyrolysis, only a portion of the free surface area of wood is covered with glowing char.
Consequently
A , = CIA^, (21)
where C7 is regarded as a constant. By combining Equations 20 and 21, one obtains
A , = (rC7Af (22)
The third assumption is concerned with defining the factor a. Here a is regarded as a function of
U , ,
and A,,
and it is assumed that the fol- lowing relations hold:In words
. . .
If the rate of air flow available for char oxidation is large in relationto
the total free surface area of wood (larger than some criticalvalue Cs), then there will be no zonal burning in the compartment and the surface area of active pyrolysis will be identical to the total free surface area of the wood contents. If, however,
U ,
, / A , is less than the criticalvalue, the pyrolysis of wood will be limited to one or more zones, the total size of which increases in proportion to the ventilation, more exactly, in proportion to the ratio
U ,
,/A,.
By combining Equations 18, 19, 22, 23a and 23b, the following equation can now be obtained:
where
and Cl1 = 0.145c5c6C7/c8 (27)
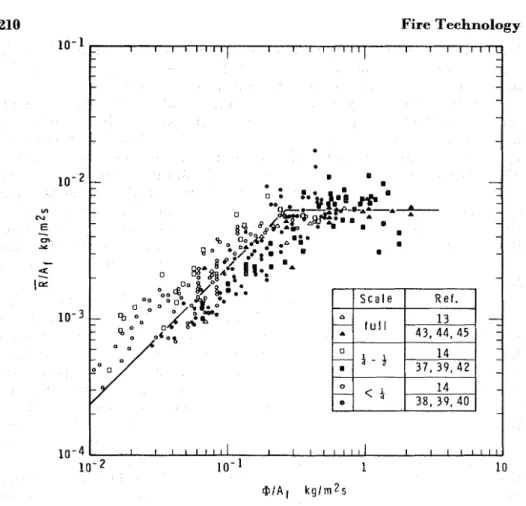
A multitude of experimental data 1 3 , 1 4 * 3 7 - 5 1 has proved conclusively that Equations 24a and 24b are indeed formally correct. This claim is clearly verified by Figure 3 where a plot of RIAl versus @/Al is shown; the plot was prepared by using information on full- and reduced-scale compartment burning tests available from numerous reports. Unfor- tunately, in many cases the proper processing of the test data was rendered difficult by the lack of information from which the free surface area of the wood could be calculated. I n such cases Al was estimated in a way t o be described later.
I n spite of the very significant scatter of points, two distinct regimes are clearly recognizable from Figure 3. The rate of burning appears t o be proportional t o the free surface area of a fuel in one, and t o the ventila- tion parameter in the other, just as predicted by Equations 24a and 24b. Although the existence of these two regimes, one termed "ventilation controlled regime" and the other "fuel bed controlled regime", has been known for a long time 3 J 52 53
,
no consistent model has so far been offered t o derive their existence from a common logical basis.I n Figure 3, the full lines represent the "best" lines through the experi- mental points. With the aid of these lines the constants in Equations 24a and 24b can now be determined. T h e following equations result: for the fuel surface controlled regime*;
for the ventilation controlled regime;
According t o these equations, the transition from one regime t o the other occurs a t a well-defined "critical value" of the ventilation parameter:
*Dimensional constants, i.e. those which have t o be converted when changing t h e dimensional system, are shown in italics.
Figure 3. Correlation of experimental data concerning rate o f burning i n compartment
fires.
This concept is undoubtedly somewhat oversimplified. I t is more likely that the two regimes are separated by a "critical regime". There are strong indications that, in this regime, the rate of burning is often smaller than the values calculable by either Equation 28a or 28b. In this paper the critical regime is defined, somewhat arbitrarily, as
As will be pointed out later, in this critical regime some characteristics of the fire may become rather poorly predictable.
According to Equation 28a, in a fuel surface controlled fire, the rate of burning is 0.0062 kg/m2s, referred to the initial free surface area of wood. The fact that this value is independent of the internal and external geom- etry of the fuel assemblies (e.g. wood cribs in experimental fires) may seem somewhat strange a t first sight.
It
must be remembered, how- ever, that, in a more or less closed compartment, the heat losses from the exterior of fuel assemblies are less significant and thus the heat flux211
level inside these assemblies (which dominates the rate of pyrolysis) is more uniform and independent of the geometric characteristics of the assemblies than in the case of fires burning in the open.
The fact that the geometric characteristics of wood (other than its total free surface area) play rather unimportant parts in the rate of burn- ing within the fuel surface controlled regime was clearly demonstrated by Butcher and co-workers4"n some specially designed experiments. They found no significant differences in the burning characteristics of wood piles of equal weights and built from sticks of identical sizes when rearranging them differently on the floor of a compartment. Even further, they proved 47 that the burning characteristics were practically unchanged if the wood piles were replaced by furniture representing the same fire load (see footnote on page 198) and probably exhibiting roughly the same free surface area.
The value of 0.0062 kg/m2s in Equation 28a is in fair agreement with data derived from some basic experiments on wood and from compart- ment burnout tests. Thomas et al.l2* 54 showed that the rate of mass
loss for various kinds of woods was normally from 0.003 to 0.015 kg/m2s, dependent on the rate of heating. I n compartment burnout experiments Webster et al. 3 9 8 43 found rates of mass losses ranging from 0.0056 to
0.0088 kg/m2s. I n standard fire tests, the rate of burning was observed5js 56 to be about 1/40 in. min-', which corresponds to 0.004-0.007 kg/m2s.
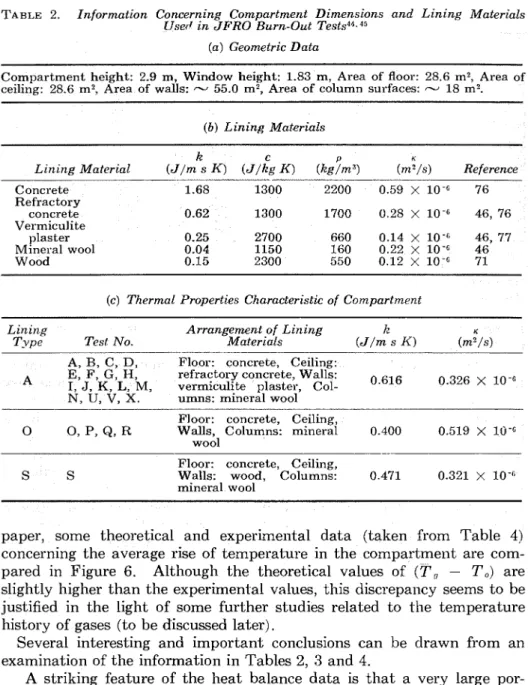
Naturally, there is also some degree of uncertainty about the value of the constant Equation 28b. Small-scale burnout tests indicated 5 7 2 j s that this "constant" depends, to some extent, on both the shape and size of the compartment; it is largest for cubical compartments, decreases slightly with an increase in the breadth of the compartment and somewhat more definitely with an increase in its depth.
It
also decreases slightly as the dimensions of the compartment increase.The reason why (for ventilation controlled fires) the size (scale) of the compartment must have some effect on the value of the "constant" in Equation 28b can be understood in the light of Equation 8. For an as- sembly of wood burning in a compartment (within the zone or zones of active pyrolysis) the heat loss term,
&,/Go,
is less; therefore, the rate of volatile formation is higher. Obviously, as the size of the compartment increases, the rate of volatilization must decrease gradually to the value corresponding to that in an unconfined atmosphere.The fact that the average rate of burning (or volatilization) decreases more definitely with an increase in the depth, D, of the compartment, than with its breadth,
B,
can be explained by the tendency toward zonal burning in ventilation coiltrolled fires. Observatioils have indicated"
that the zone of most intensive pyrolysis recedes slowly from near the window toward the far end of the compartment. Since the air supply to the charring surfaces in the prevailing active zone thus gradually diminishes, there will be a gradual decline in the rate of volatile formation and, naturally, also in the rate of burning.It
is obvious, therefore, that the average rate ofvolatile formation (or burning) must decrease with an increase in the depth of the compartment.
The following empirical equation for this "constant" (Cll according to
Equation 24b) seems
to
describe satisfactorily the results of numerousburnout tests conducted on model compartments of roughly 1/6 to 1/2 of full scale (see Figure 1) :
Unfortunately, this equation does not seem to be applicable to full-size compartments; for these it yields values that are too low. Since, however, the spread of experimental values from which Cll can be calculated greatly overshadows the effect of shape and scale of the compartment*, from a practical standpoint it is justifiable to regard C11 as a true constant. Its
value in Equation 28b represents a fair average of the various values
derivable from the literature58.
Dividing Equation 28b by Equation 17 one finds that
This value of
R / U , ,
although somewhat lower than that derived byThomas et a1.33, seems quite reasonable in the light of the earlier finding that in a realistic combustion process not more than 1/3.39 = 0.295 kg volatiles can be burned with 1 kg air. Of course, the excess air is not as much as it would seem by comparing these two values, since part of the air is used up in the oxidation of the charcoal, often predominantly in some regions near the windows where the stage of secondary burning begins earlier.
S I Z E O F F L A M E S I N C O M P A R T M E N T F I R E S
Because of some differences in the mechanism of air entrainment, one cannot expect that the equation derived for the flame size in open fires is directly applicable to fires burning in compartments. Webster and Smith43 found, however, that Equation 13 is usually applicable to compartment fires as well, if 1 is interpreted as the maximum flame height above the base of the compartment. This finding clearly indicates that, possibly with a different constant, Clz instead of C1, the validity of Equation 12 can be extended to compartment fires.
Thus
To bring this equation into more convenient forms, some relation has to be found between the area of flame base, A ,i.e. the area of the zone in which the active pyrolysis of wood takes place, and the free surface area of wood in that zone.
It
seems reasonable to assume that the re- lation between these two quantities is essentially linear:By combining Equations 11, 33 and 34, the following equation can now be obtained:
where
Equation 35 can further be converted into two different forms with the aid of some previously introduced equations, among them Equations 20, 23a and 23b,* which define the relation between Af, and Af. For the fuel surface controlled regime a: = 1 (by virtue of 23a, and thus, according
to Equation 20, Af, = Af. By expressing
R
with the aid of Equation 28a one obtains:For the ventilation controlled regime, on the other hand, the relation between Af, and Af is defined by Equations 23b and 20. Furthermore, by utilizing Equations 18 and 28b, Equation 35 can finally be brought into the following forms:
Some research workers noted during burnout
tests
that, with an in- crease of the fire load in some cases or with the increase of the ventilation in others, the flames gradually reached the ceiling of the compartment then started to issue through the window. Obviously, when the flames first emerge,1
=H,
and thus, from the known values of Af or 9 pertaining to these conditions, the constants in Equations 37a and 37b can be de- termined.The information provided by Gross and Robertson14, Webster and Smith43 and Butcher et al. 4 4 s 45 was used to evaluate these constants. *In the light of subsequent discussions Equations 23a and 23b can now be recognized
as applicable to the fuel surface controlled and ventilation controlled regimes, re- spectively.
214
With them, Equations 37a and 37b can be rewritten as follows: for the fuel surface controlled regime;
for the ventilation controlled regime:
<
0.263I
=1.17
(38b)A f
N O M E N C L A T U R E
coefficient, dimensionless
area; surface area; free surface area, m2 thickness of boundary elements, m breadth of compartment, m
speciflc heat; without subscript or with numeral subscript: specific heat of lining material, J/kg
K
constant
characteristic dimension of flame base, m depth of compartment, m
acceleration due to gravity, m/s2 mass of wood; fire load, kg height of windows, m
coefficient of heat transfer, J/m2s
K
height of compartment, m enthalpy, J/kg
heat of combustion, J/kg
thermal conductivity of lining material, J / m s
K
length of flame, m
constant exponent, dimensionless perimeter a t the flame base, m
rate of change of mass of fuel (wood), kg/s heat flux; heat loss flux, J/m2s
heat flow; without subscript: rate of evolution of chemical energy, J / s time, s
temperature,
K
mass flow, kg/s
velocity of gas a t flame base, m/s
distance of key component from surface of insulation, m
GREEK
LETTERS
a factor (Equations 23a and 23b), dimensionless
0 factor (Equations 41a and 41b, dimensionless
215
-q empirical factor, dimensionless
K thermal diffusivity of lining material, m2/s
p density; without subscript or with numeral subscript:
density of lining material, kg/m3
a Stefan-Boltzmann constant, J/m2s
K4
T time of primary burning; time of fully developed fire, s
cp specific surface of fuel (wood), m2/kg
+
ventilation parameter, kg/sSUBSCRIPTS of air
a of flame base; a t flame base
n due to burning outside compartment
of char; for feeding char oxidation
c conductive
entrained into flame
E effective
I of fuel (wood)
-
of floorof gaseous mixture (indicates space-averaged values); due to gases leaving
of heat sources
maximum; pertaining to maximum
N net
a t t = 0; a t reference point; a t reference level from pile of wood
R radiant
of bounding surfaces
total (surface area of compartment, heat flux)
T a t temperature T
of volatiles; of gaseous mixture consisting mainly of volatiles of windows
in zones of pyrolysis
T a t t = ~
SUPERSCRIPTS
- bar above symbol or group of symbols: pertaining to period 0
<
t S T ;average over period 0
<
t5
= two bars above symbol: pertaining to period T
<
t5
2~*
criticalfor a compartment of height
H
R E F E R E N C E S
.lngberg, S. H., "Tests of Severity of Building Fires," Quarterly, National Fire Protection Association, Vol. 22 (1928) p. 43.
Harmathy, T. Z., and Lie, T. T., "Fire Test Standards in the Light of Fire Re- search," ASTM Special Technical publication No. 464 (Philadelphia, 1970), p. 85. Kilzer, F. J., and Broido, A., "Speculations on the Nature of Cellulose Pyrolysis,"
216
4 Parker, W. J., and Lipska, A. E., "A Proposed Model for the Decomposition of
Cellulose and the Effect of Flame Retardants", Naval Radiological Defense Labora- tory, San Francisco, Calif., May 1969.
6 Roberts, A. F., "Ultimate Analyses of Partially Decomposed Wood Samples,"
Combustion and Flame, Vol. 8, (1964) p. 345.
CMartin, S. "Diffusion-Controlled Ignition of Cellulosic Materials by Intense Radiant Energy." 10th Symposium (International) on Combustion, T h e Combustion Institute, 1965, p. 877.
7 Brenden, J. J., "Calorific Values of the Volatile Pyrolysis Products of Wood,"
Combustion and Flame, Vol. 11, (1967) p. 437.
8 Roberts, A. F., and Clough, G., "Thermal Decomposition of Wood in an Inert
Atmosphere," 9th Symposium (International) o n Combustion, The Combustion In- stitute, 1963, p. 158.
9 Gross, D., "Experiments on the Burning of Cross Piles of Wood," Journal of
Research, National Bureau of Standards, Vol. 66C, No. 2 (1962) p. 99.
10 Blackshear, P. L., Jr., and Kanury, A. M., "Heat and Mass Transfer to, from,
and within Cellulosic Solids Burning in Air," 10th Symposium (International) on
Combustion, T h e Combustion Institute, 1965, p. 911.
11 Kanury, A. M., "Burning of Well-Ventilated Wood Cribs," Factory Mutual
Research Corporation, Norwood, Mass., F M R C Serial No. 19721-1, 1970.
12 Thomas, P. H., Sirnms, D. L., and Law, M., "The Rate of Burning of Wood,"
JFRO, Fire Research Note No. 657, 1967.
l3 Kawagoe, K., "Fire Behaviour in Rooms," Building Research Institute, Japan,
Report No. 27, 1958.
1 4 Gross, D., and Robertson, A. F., "Experimental Fires in Enclosures," 10th Sym-
posium (International) on Combustion, T h e Combustion Institute, 1965, p. 931.
16 O'Dogherty, M. J., and Young, R. A., "Miscellaneous Experiments on the Burn-
ing of Wooden Cribs," JFRO, Fire Research Note No. 548, 1964.
16 Smith, P. G., and Thomas, P. H., "The Rate of Burning of Cribs of Wood,"
JFRO, Fire Research Note No. 728, 1968.
17 Smith, P. G., and Thomas, P. H., "The Rate of Burning of Wood Cribs," Fire
Technol~gy, Vol. 6 (1970) p. 29.
18 Nilsson, L., "The Effect of the Porosity and Air Flow Factor on the Rate of
Burning for Fire in Enclosed Space," National Swedish Building Research Sum- maries, R22, 1971.
1 9 McCarter, R. J., and Broido, A., "Radiative and Convective Energy from
Wood Crib Fires," Pyrodynamics, Vol. 2 (1965) p, 65.
20 Fons, W. L., Bruce, H. D., and Pong, W. Y., "A Steady-State Technique for
Studying the Properties of Free-Burning Wood Fires," T h e Use of Models in Fire Research, No. 786, National Academy of Science, National Research Council, Wash- ington, D.C., 1959, p. 219.
21 Fons, W. L., Clements? H. B., and George, P. M., "Scale Effects on Propagation
Rate of Laboratory Crib Fnes," 9th Symposium (International) on Combustion, The Combustion Institute, 1963, p. 860.
22 Thomas, P. H., "The Size of Flames from Natural Fires," 9th Symposium ( I n -
ternational) on Combustion, The Combustion Institute, 1963, p. 844.
23 Thomas, P. H., Simrns, D. L., and Wraight, H. G. H., "Fire Spread in Wooden
Cribs, Part 11, Heat Transfer Experiments in Still Air," JFRO, Fire Research Note No. 599, 1965.
24 Thomas, P. H., "Buoyant Diffusion Flames," Combustion and Flame, Vol. 4 ,
(1960), p. 381.
25 Thomas, P. H., Webster, C. T., and Raftery, M. M., "Some Experiments on
Buoyant Diffusion Flames," Combustion and Flame, Vol. 5 (1961), p. 359.
26 Sunavala, P. D., Dynamics of Buoyant Diffusion Flame," Journal of the I n -
stitute of Fuel, Vol. 40, (1967), p. 492.
27 Seeger, P. G., and Werthenbach, H. G., "Diffusionflammen mit extrem niedriger Stram~n~s~eschwindigkeit," Chemie-Ing.-Tech., Vol. 42, (1970), p. 282.
28 Weatherford, W. D., Jr., "Scaling of Flames Above Free-Burning Structural
Models," Combustion and Flame, Vol. 14 (1970), p. 21.
29 Hinkley, P. L., Wraight, H. G. H., and Theobald, C. R., "The Contribution of
Flayes under Ceilings to Fire Spread of Compartments, Part I. Incombustible Ceil- ings, JFRO, Fire Research Note No. 712, 1968.
30 Thomas, P. H., Baldwin, R., and Heselden, A. J. M., "Buoyant Diffusion Flames:
Some Measurements of Air Entrainment, Heat Transfer, and Flame Merging," 10th
Symposium (International) on Combustion, T h e Combustion Institute, 1965, p. 983.
31 Steward, F. R., "Linear Flame Heights for Various Fuels," Combustion and
Flame, Vol. 8 (1964), p. 171.
32 Fujita, K., "Characteristics of Fire Inside a Non-Combustible Room and Pre-
33 Thomas, P. H., Heselden, A. J. M., and Law, M., "Fully-Developed Com-
partment Fires; Two Kinds of Behaviour," JFRO, Fire Research Technical Paper
Nn 1 X lQfi7
-
,-
.
A-
,-
- -
..
34 Kawagoe, K., "Estimation of Fire Temperature-Time Curve in Rooms," Build-
ing Research Institute, Japan, Research Paper No. 29, 1967.
35 Magnusson, S. E., and Thelandersson, S., "Temperature-Time Curves of Com-
plete Process ~f Fire Development. Theoretical Study of Wood Fuel Fires in En- closed Spaces. Acta Polytechnica Scandinavica, Civil Engineering and Building Construction Series No. 65, Stockholm, 1970.
36 Stark, G. W. V., Evans, Mrs. W., and Field P., "Measurements of t h e Flow
of Combustion Gases from Ventilated Comuartments," JFRO, Fire Research Note No. 722, 1968.
37 Heselden, A. J . M., "Some Fires in a Single Compartment with Independent
Variation of Fuel Surface Area and Thickness," JFRO, Fire Research Note No. 469, 1961.
38 Webster, C. T., Wraight, H., and Thomas, P. H., "The Burning of Fires in
Rooms, Part I. Small Scale Tests with Cribs and High Ventilation," JFRO, Fire Research Note No. 389, 1959.
3 9 Webster, C. T., and Raftery,.M. M., "The Burning of Fires in Rooms, P a r t 11.
Tests with Cribs and High Ventilation on Various Scales." JFRO, Fire Research Note No. 401, 1959.
4 0 Simms, D. L., Hird, D., and Wraight, H. G. H., "The Temperature and Duration
of Fires, Part I. Some Experiments with Models with Restricted Ventilation," JFRO, Fire Research Note No. 412, 1960.
4 1 Simms, D. L., and Wraight, H., "The Temperature and Duration of Fires,
Part 11. Analysis of Some Full Scale Tests," JFRO, Fire Research Note No. 413, 1959. Webster, C. T., Raftery, M. M., and Smith, P. G., "The Burning of Well Venti- lated Compartment Fires, Part 111. The Effect of Wood Thickness," JFItO, Fire Re- search Note No. 474, 1961.
43 Webster, C. T., Smith, P. G., "The Burning of W~11 Ventilated Compartment
Fires, P a r t IV. Brick Compartment, 2.4 m (8 f t ) Cube, JFRO, Fire Research Note No. 578, 1964.
4* Butcher, E . G., Chitty, T . B., and Ashton, L. A., "The Temperatures Attained
by Steel in Building Flres," JFRO, Fire Research Technical Paper No. 15, 1966. 45Butcher, E . G., Bedford, G. K., and Fardell, P. J . "Further Experiments on Temperatures Reached by Steel in Buildings," Paper 1, Proceedings of a Symposium held a t Fire Research Station, January 1967, JFRO, 1968, p. 1.
46 Heselden, A. J. M., "Parameters Determining the Severity of Firc," Paper 2,
Proceedings of a Symposium held a t Fire Research Station, January 1967, JFRO, 1968, p. 19.
4 7 Butcher, E. G., Clark, J. J., Bedford, G. K., "A Fire Test in which Furniture
Was the Fuel," JFRO, Fire Research Note No. 695, 1968.
4 8 Beyreis, J . R., "Fire Severity a t ttfe Exterior of a Burning Building and its
Effect on Exposed Structural Members, Underwriters' Laboratories Inc., Report for AISI, January, 1969.
4 9 Thomas, P. H., "The Fire Resistance Required to Survive a Burn-Out," JFRO,
Fire Research Note No. 901, 1970.
Tewarson, A., "Some Observations on Experimental Fires in Enclosures, Part I. Cellulosic Materials," Factory Mutual Research Corporation, Norwood, Mass., Technical Report No. 18305, 1971.
61 Nilsson, L., "Fire Loads in Flats," Lund Institute of Technology, Division of
Structural Mechanics and Concrete Construction, Lund, Sweden, Bulletin 15, 1970. Thomas, P. H., "Studies of Fire in Buildings Using Models," Research, Vol. 13, (1960), pp. 69, 87.
63 Thomas, P. H., "Some Studies of Models in Fire Research," V F D B List, Vol. 9,
(1960), p. 96.
6 4 Thomas, P. H., "On the Rate of Burning of Wood," JFRO, Fire Research Note
No. 446, 1960.
66 Lawson, D. I., Webster, C. T., and Ashton, L. A., "Fire Endurance of Timber
Beams .and Floors," National Building Studies, Bulletin No. 13.
Webster, C. T., and Ashton, L. A., "Fire Resistance of Timber Doors," Na- tional Building Studies, Technical Paper No. 6.
Fire Research 1968, Report of the Fire Research Steering Committee with the Report of the Director of Fire Research, Ministry of Technology - JFRO, London,
1969.
Heselden, A. J . M., Thomas, P. H., and Law, M., "Burning Rate of Ventilation Controlled Fires in Compartments," Fire Technology, Vol. 6, (1970), p. 123.
A
New Look at Compartment Fires,
Part
11
T. Z. HARMATHY
Fire Research Section
Division of Building Research
National Research Council of Canada
This is the second part of a two-part paper in which the author has endeavored to present all available information on compart- ment fires in a consistent theoretical framework. This well-docu- mented review of the state of the a r t should serve as a valuable reference for researchers for some time t o come.
T
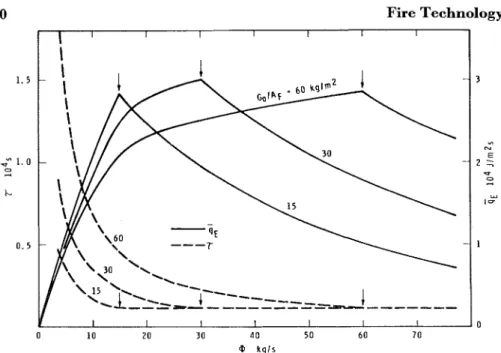
H E first part of this paper dealt with some basic characteristics of the burning of cellulosic materials and developed two equations that describe the burning rate in fuel-surface-controlled fires and ventilation- controlled fires. I n this the final part, we will arrive a t some rather in- teresting, if not unconventional, conclusions.E V O L U T I O N O F H E A T W I T H I N C O M P A R T M E N T Knowing the size of flames in compartment fires is very important from the point of view of estimating the fire severity. As long as
1
5
H,* the combustion of the volatiles will take place entirely inside the compart- ment. If, however,1
>
H, part of the heat of combustion will be evolved outside the window, and thus, other conditions being equal, the severity of fire will lessen.I t seems reasonable to assume t h a t the amount of energy released by the flame up to a certain height is proportional t o the total air en- trained into the flame up to this height. Unfortunately, this concept is not easily applicable to flames burning in a compartment. Tall flames consist of three sections of rather different air entrainment characteristics:
= A primarily vertical section within the compartment, which usually burns under highly turbulent conditions. Although the air available t o this section is often very limited, because of the high turbulence, the air entrainment is probably good and, therefore, the rate of combustion is fair or good.
*See list of nomenclature on page 348. 326
327
A horizontal section floating under the ceiling toward the windows.
It
has been shown 2 9 v 33 that the entrainment of air into this section is very poor, and thus the rate of combustion is presumably only a small fraction of that for the vertical section.A primarily vertical section burning outside the compartment. This section also burns under highly turbulent conditions but, because of the restricted (one-sided) air entrainment caused by the presence of wall above the windows, the rate of combustion is probably not more than fair.
Clearly, no simple mathematical treatment is conceivable without sig- nificant idealization of the conditions.
It
will be assumed here that the air entrainment (and thus the rate of combustion) is negligible in the hori- zontal section of the flame and that the outside vertical section of the flame can be regarded as a direct continuation of the inside vertical section. Thus, with the aid of Equation 14 the following equation can be obtained for the heat released within a compartment of height H by unit mass of the volatiles formed:Keeping in mind that the heat of oxidation of the char is always com- pletely evolved within the compartment, Equation 6 can now be modi- fied to describe the rate of heat evolution within a compartment during the period of primary burning:
Q = R(0.932 @ A H ,
+
0.068 AH,) (40)where
if 1 5 H p = l (41a)
if l > H fi = (H/z)~/' (41b)
and 1 is to be calculated either by Equation 38a or by Equation 38b, dependent on the value of the ratio @/A,.
D U R A T I O N O F C O M P A R T M E N T F I R E S
I t has been customary to divide the duration of compartment fires into three periods; the initial growth period, the period of fully developed fire and the final decay period. Although some work has already been done concerning the initial and final periods of fires, 3" 5 9 3 the plain truth is
that these periods are still not readily amenable to theoretical treatment. Because of this and because the heat evolved during these periods is nor- mally only a small fraction of the total heat evolution, it is usual to re- strict theoretical and experimental investigations to the period of fully developed fires, which, as mentioned earlier (see footnote on page 207), approximately coincides with the period of primary burning for the bulk of fuel in *;he compartment.
I n general, the only experimental information that is readily avail- able from most burnout tests so far conducted is the
T,
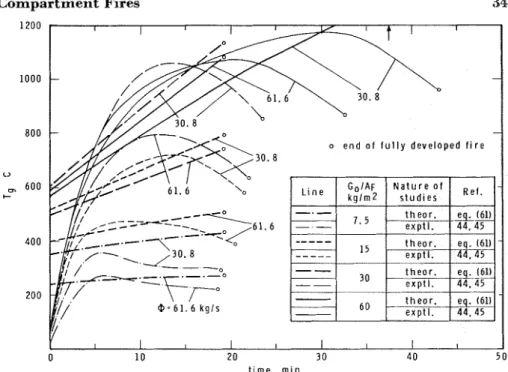
versus t relationship,in other words, the variation of the average temperature of the gaseous mixture in the compartment during the test. I t seems desirable, there- fore, to define the various periods of the fire with the aid of this relationship.
A
typicalTo
versus t curve is shown in Figure 4. The beginning of the fully developed fire can be easily recognized from an abrupt rise in the average gas temperature.It
is indicated by t o in the figure. Since only the period of fully developed fire will be studied in this paper, it seems convenient to regard t o = 0, in other words, to displace the originof the
To
-
t coordinate system to the beginning of the fully developed fire.Figure 4 . Typical temperature history of
gas contents of compartment in fire.
. -- . - . . .
Since the period of fully developed fire coincides roughly with the period of primary burning of the fuel in the compartment, it is reasonable to retain the previously introduced symbol, T, for the length of this period.
It
is not immediately obvious how to define the end point of a fully developed fire. As Figure 2 shows, even for wood piles burning in the open, the actual rate of burning is not constant during the entire period of primary burning. I t slows down considerably as the predominant char- acter of burning gradually changes from flaming combustion of volatile5 to oxidation of the charcoal residue. Furthermore, if zonal burning occurs in a compartment (normally under ventilation controlled conditions), further deviations from the constancy in the average rate of primary burn- ing can be expected. I t is obvious, therefore, that. there is no definite cut- off point for the fully developed fire, and thus it is unavoidable to use some more or less arbitrary definition for determining the value of T.Based on some theoretical considerations, the following definition has been adopted for use in this paper. I n a compartment fire, the end of the fully developed fire is to be taken as the time a t which the average tempera- ture of gases in the compartment, referred to the prefire level, diminishes to 80 percent of i& maximum value. With mathematical symbolism, taking t o = 0,
These conditions are also illustrated in Figure 4.
Theoretical expressions for T can be obtained by combining Equations
specific surface of wood
The results are:
for the fuel surface controlled regime;
for the ventilation controlled regime;
Equation 44a comes somewhat as a surprise. I t indicates that, for fuel surface controlled fires, the duration of the fully developed fire is inde- pendent of the fire load and depends only on the specific surface of the fuel. Since, for larger wood cribs and for conventional furniture, 0.1
<
cp<
0.4 mQg-I (more often between 0.12 and 0.18 m2 kg-'), onecan conclude that, for fuel surface controlled fires, the duration of the fully developed period is normally between 6 and 25 min.
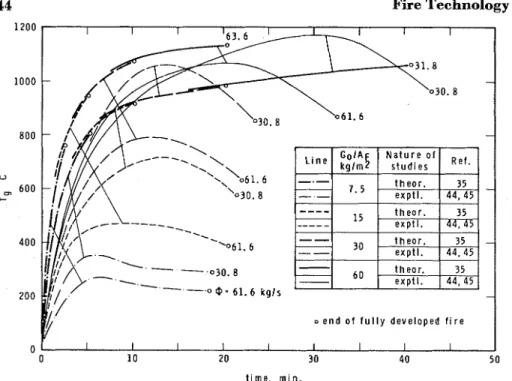
I n Figure 5, theoretical values of T are compared with experimental
values evaluated from References 13, 44, 45 and 48. Because of the lack of information on the free surface area of fuel, only those runs of Reference 13 could be taken into account that clearly related to ventilation controlled fires.
If the fire is, beyond doubt, fuel surface controlled, with the aid of Equation 44a and the experimentally determined value of T (see Equation
42) the specific surface and total free surface area of the fuel contents of a compartment can be estimated.? This procedure may be especially
Figure 5. Comparison of theoretical and experimental information concerning the duration of fully developed fires.
*Such estimated values of A , were used in plotting many experimental data in