Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NACE Northern Area Eastern Conference 2007 [Proceedings], pp. 1-14, 2007-09-24
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=8528e8ec-d4c2-43fd-9d7e-0dc11fb55130 https://publications-cnrc.canada.ca/fra/voir/objet/?id=8528e8ec-d4c2-43fd-9d7e-0dc11fb55130
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Accelerated laboratory and field investigations of corrosion inhibiting systems for concrete bridges
http://irc.nrc-cnrc.gc.ca
A c c e l e r a t e d l a b o r a t o r y a n d f i e l d
i n v e s t i g a t i o n s o f c o r r o s i o n i n h i b i t i n g s y s t e m s
f o r c o n c r e t e b r i d g e s
N R C C - 5 0 0 2 5
Q i a n , S . ; C u s s o n , D .
A version of this document is published in / Une version de ce document se trouve dans: 2007 Northern Area Eastern Conference, Ottawa, Ontario, Sept. 24-26, 2007
The material in this document is covered by the provisions of the Copyright Act, by Canadian laws, policies, regulations and international agreements. Such provisions serve to identify the information source and, in specific instances, to prohibit reproduction of materials without written permission. For more information visit http://laws.justice.gc.ca/en/showtdm/cs/C-42
Les renseignements dans ce document sont protégés par la Loi sur le droit d'auteur, par les lois, les politiques et les règlements du Canada et des accords internationaux. Ces dispositions permettent d'identifier la source de l'information et, dans certains cas, d'interdire la copie de documents sans permission écrite. Pour obtenir de plus amples renseignements : http://lois.justice.gc.ca/fr/showtdm/cs/C-42
ACCELERATED LABORATORY AND FIELD INVESTIGATIONS OF CORROSION INHIBITING SYSTEMS FOR CONCRETE BRIDGES
Shiyuan Qian* and Daniel Cusson
Institute for Research in Construction National Research Council Canada
Ottawa, Canada, K1A 0R6
ABSTRACT
Nine commercially available corrosion-inhibiting systems including concrete admixtures, reinforcing steel coatings, and/or concrete surface coatings/sealers for use in concrete structures, were evaluated in electrochemical cells and on bridge barrier walls by
accelerated methods and actual field conditions. The laboratory investigation included the assessment of the effectiveness of corrosion inhibiting concrete admixtures and rebar coatings in delaying or reducing steel corrosion by measurements of chloride thresholds and corrosion rates. The field evaluation consisted of the measurements of half-cell potential and corrosion rate of the steel reinforcement of concrete barrier walls, which had specially designed rebar ladders embedded in the concrete with thin cover depths, in order to reduce the time needed for chlorides to reach the surface of the reinforcing steel. It was found that the combination of accelerated laboratory investigation and field evaluation on the special rebar ladders could provide a reliable assessment of the effectiveness of corrosion inhibiting systems in reducing steel corrosion in concrete structures. The results showed that the inorganic admixture and some organic admixtures performed very well in the saturated calcium hydroxide solution and on the special rebar ladders in the field.
Keywords: concrete admixtures, corrosion inhibitor, reinforcement corrosion, half-cell potential, polarization resistance.
*
INTRODUCTION
Corrosion of steel reinforcement in concrete structures is a serious problem worldwide resulting in shortened service life and costly maintenance. Corrosion inhibitors are considered as one of the most cost-effective solutions in addressing this problem. They have been increasingly used in the construction of new structures and the rehabilitation of existing structures during the last twenty years. Earlier studies on corrosion inhibitors
focused mainly on sodium benzoate 1,2, on various nitrites (sodium, potassium and
barium) and on chromates/dichromates 3,4 as concrete admixtures for the inhibition of
corrosion in reinforced concrete structures. None of these corrosion inhibitors performed satisfactorily and many of them led to detrimental effects on the strength development of
concrete. A study of the effectiveness of calcium and sodium nitrites by Rosenberg et al.5
revealed the benefits of these corrosion inhibitors in concrete. Since then, calcium nitrite has become commercially available and has been studied and used extensively in
reinforced concrete structures exposed to deicing salts 6,7. During the 1990’s, a number
of organic inhibitors were developed and commercialized, such as amines,
alkanolamines, their salts with organic and inorganic acids 8, and emulsified mixtures of
esters, alcohols and amines 9.
Most of the corrosion-inhibiting materials on the market claim their effectiveness based on either laboratory experiments or periodic field observations. Thorough investigations combining the results of laboratory testing and field performance evaluation to
understand the factors governing the in-service corrosion-inhibiting effectiveness of these materials are limited in the literature.
This paper presents an evaluation of the performance of corrosion-inhibiting systems under accelerated conditions, using laboratory testing in simulated concrete pore
solutions and field testing on special rebar ladders in a reconstructed concrete barrier wall at the Vachon Bridge, located north of Montreal, Canada. These combined corrosion evaluations provided useful information about the effectiveness of corrosion-inhibiting admixtures in reducing steel corrosion in concrete structures.
EXPERIMENTAL PROCEDURES Corrosion-Inhibiting Systems and Concrete Specimens
Corrosion-inhibiting systems containing either concrete admixtures, rebar coatings, concrete coatings/sealers or a combination of the above were installed and evaluated on a bridge barrier wall and in the laboratory. A brief description of the tested corrosion-inhibiting systems is presented in Table 1.
In the laboratory study, the corrosion-inhibiting admixtures (included in Systems B, C, E, F and H) were tested separately from the cementitious coatings (included in Systems A, D and G). Note that Systems B and G share the same concrete admixture and rebar coating. The effectiveness of the admixtures and coatings was evaluated by conducting
corrosion-inhibiting admixtures used in the electrolytes were those suggested by the manufacturers for the laboratory experiments.
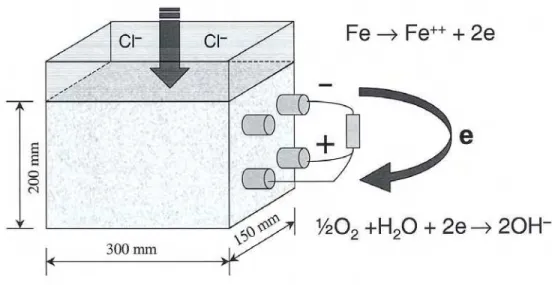
Reinforced concrete prisms (200 x 300 x 150 mm3) containing the tested
corrosion-inhibiting systems were cast on site (at the bridge) for laboratory testing as shown in Figure 1. The concrete had a water-cement ratio (w/c) of 0.36, and an average 28-day strength of 45 MPa. A 100-ohm resistor was connected between the two layers of 15-mm rebars to monitor the galvanic current between the top layer of rebars contaminated by chlorides and the bottom layer of rebars in a passive condition. A 3.5% sodium chloride (NaCl) solution was ponded every second week on the top of the concrete prisms. In the field study, eight spans of barrier wall were rebuilt using standard concrete (as described above) and conventional carbon-steel reinforcement; each span included a different corrosion inhibiting system (Table 1) provided and installed by its manufacturer. The concentration of each corrosion inhibitor in the concrete was the one recommended by its manufacturer for bridge decks. Two other test spans were built using the same concrete but with no corrosion inhibitors: one span with carbon-steel reinforcement (identified as Control), and the other span with epoxy-coated steel reinforcement (identified as Epoxy). The main reinforcement of the barrier wall consisted of eight 15-mm longitudinal bars in the cross-section, and 15-15-mm transverse bars spaced at 230 15-mm along the wall length.
For early detection of corrosion and early performance evaluation of the corrosion inhibiting systems, two sets of rebar ladders were embedded in each test span during construction. Each ladder was made of four 470-mm long horizontal bars (10-mm diameter) spaced at 125 mm center to center and held in place by two thin vertical Plexiglas stiles. The ladder bars had varying concrete cover thicknesses ranging from 13 mm for the upper bar, and 25 mm, 38 mm and 50 mm for the 3 other horizontal bars, respectively.
Steel Electrodes and Electrolytes
For the laboratory experiments, the working electrodes were machined from 10-mm reinforcing carbon steel bars to a size of 8 mm in diameter and 10 mm in length. The electrodes were connected by a 10-gauge copper wire which was isolated from the
solution by a glass tube and embedded in epoxy to cover all surfaces, except one end with
an exposed area of 50 mm2. Before testing, the electrodes were polished with a #600
silicon carbide paper and immersed in the electrolyte. For the tests on the cementitious coatings, the steel electrodes were coated with a 1-mm thickness of the different
corrosion-inhibiting materials. A control steel electrode was coated with normal Type 10 cement paste for comparison.
A saturated Ca(OH)2 solution with a pH of 12.6, and a simulated concrete pore solution
[0.002 M Ca(OH)2 + 0.45 M NaOH + 0.26 M KOH] with a pH of 13.5 10 were used in
this laboratory study. The corrosion-inhibiting admixtures were added to the
solution. Afterwards, NaCl was added to the cells, and its concentration was increased weekly by 0.2% or 0.5% increments (depending on the sample) until significant corrosion developed on the electrode in order to determine the chloride threshold in the presence of each corrosion-inhibiting admixture.
Electrochemical Measurements
In the laboratory, the half-cell potential and linear polarization resistance were measured using a computer-controlled electrochemical interface (Solartron 1280) coupled with a frequency response analyzer (Solartron 1260). The value of the polarization resistance was determined from the average of three measurements and based on the slope of the potential-current relation in the range of ± 15 mV near the open circuit potential.
Macrocell current, polarization resistance and corrosion potential were also measured on the reinforced concrete prisms.
In the field, corrosion surveys of the barrier wall were performed annually during the month of June from 1997 to 2006, including measurements of half-cell potential and corrosion rate. A saturated copper sulphate electrode (CSE) and a multimeter were used to measure the half-cell potential following the ASTM C876 procedure. The
measurements were taken at 110, 345 and 550 mm from the top of the 900-mm high barrier wall, and horizontally at 300-mm intervals over the central 15-m portion of each 34-m long test span. A Gecor 6 device was used to measure the corrosion rate of steel in concrete by the polarization resistance technique. In each test span, measurements were taken on vertical and horizontal bars at cracked and uncracked locations (due to
restrained shrinkage at early age). The details of lab and field investigation can be found
in earlier publications. 11,12
RESULTS AND DISCUSSIONS
Effect of Admixtures on Chloride Threshold and Corrosion Rate
Since the corrosion rate (icorr) is proportional to the reciprocal of the electrochemical
polarization resistance (RP), the value of 1/RP was used to compare the chloride
thresholds and corrosion rates of the carbon steel electrodes in the saturated Ca(OH)2
solution (pH=12.6) with the different admixtures (Figure 2). It was observed that, in the
presence of Admixture B, 1/RP showed a sharp increase at around 1% NaCl. In the
presence of Admixture F, 1/RP started to rise at the low NaCl concentration of 1% but
increased at a slow rate due probably to the adsorption of organic compounds on the steel
electrode. For the control and Admixture C, 1/RP rose at around 2% and 4% NaCl,
respectively, and increased quite rapidly thereafter. On the other hand, with the inorganic
admixture H, 1/RP remained small until the 6% NaCl concentration was reached, and then
increased suddenly. In the case of Admixture E, 1/RP remained very low until 8% NaCl
was reached, without indication of further increases because of strong adsorption of organic compounds on the steel electrode. With these last two admixtures, the chloride thresholds were increased significantly by about 4% and 6% NaCl from the threshold of control concrete, respectively. The effectiveness of these admixtures in delaying the
initiation the corrosion in this solution can be clearly observed in a relatively short time (several weeks).
The development of 1/RP for an increased chloride concentration was also tested in a
simulated concrete pore solution (higher pH of 13.5). The value of 1/RP started to rise at a
6% NaCl concentration for the control and most admixtures. This test clearly showed that the chloride thresholds were high in a high pH solution, even without the addition of a corrosion inhibitor, since the initiation of steel corrosion depends largely on the
chloride/hydroxide ratio13. In fact, similar tests carried out by Mammoliti et al.14 in a
simulated concrete pore solution of a pH of 13.3 also showed that organic and inorganic corrosion inhibitors do not increase the chloride threshold in high pH solutions.
Effect of Rebar Coatings on Chloride Threshold and Corrosion Rate
A rebar coating was considered effective in these tests if the corrosion rate (or 1/RP) was
lower and the chloride threshold higher than those of a general use (type 10) cement
control coating. Figures 3 and 4 show the half-cell potential and 1/RP values,
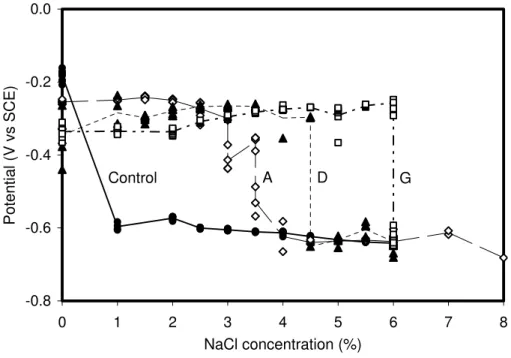
respectively, measured on the steel electrodes with Coatings A, D and G and the control
coating in a saturated Ca(OH)2 solution (pH = 12.6). A drastic potential drop from
approximately –0.2 V to –0.6 V and a sudden increase in 1/RP at approximately 1.0%
NaCl were observed for the control coating indicating that the steel corrosion was initiated. As more sodium chloride was added to the solution, the half-cell potential
almost remained at this level but 1/RP continued to increase significantly showing that
steel corrosion was developing further. For Coatings A and D, the onset of corrosion in the sample was observed at 3.5 % and 4.5% NaCl, respectively, by both half-cell
potential and 1/RP measurements. For Coating G, corrosion initiated on the sample at
6.0% NaCl. The onset of corrosion on these samples was confirmed by observations of corrosion products on the steel electrodes. The three proprietary coatings, especially Coatings D and G, were found more effective than the control cementitious coating in
delaying initiation of corrosion of reinforcing steel when tested in the saturated Ca(OH)2
solution.
Corrosion of Steel Bars in Concrete Prisms
Macrocell currents were measured on the reinforced concrete prisms. It was found that the measured potential drops across the 100-ohm resistor in all concrete prisms (except the control prisms) remained smaller than 0.1 mV, corresponding to a negligible current
(i.e. smaller than 1 μA, or a current density lower than 0.008 μA/cm2
). This small macrocell current is an indication that the top and bottom rebars of most concrete prisms have similar good surface conditions since these prisms were made of a low permeability concrete (w/c = 0.36), had no cracks, and were protected with the full
corrosion-inhibiting systems as listed in Table 1. The potential drops measured on the control prisms, however, were slightly larger than the values measured on the other prisms, indicating possible initiation of corrosion on the top rebars of the control prisms.
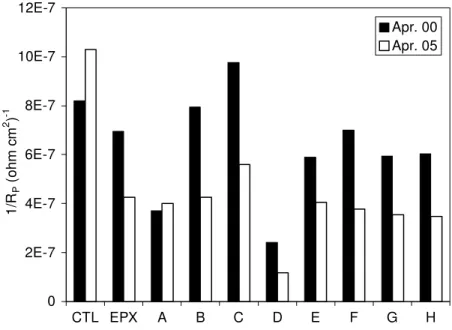
Average half-cell potentials, recorded in 1998, 2000, 2001 and 2005, for prisms ponded with salt water, are shown in Figure 5. The results show that the half-cell potentials of all concrete prisms measured in 2005 were more positive than –200 mV vs. CSE (except the controls), which is an indication of a low probability of corrosion according to ASTM C876. The control prisms had a significant increase in negative potential in 2005, indicating that corrosion possibly initiated on the top rebars. These results are consistent with the measurements of macrocell current discussed previously.
The values of 1/Rp obtained from linear polarization measurements on the concrete prisms are shown in Figure 6. As expected, corrosion currents were very low in most prisms indicating that rebar corrosion has not initiated. The values measured on the control prisms were higher in 2005 than on the other prisms, which are consistent with the half-cell potential readings. It is clear that it will take more time for the development of significant corrosion in these reinforced concrete prisms in order to clearly distinguish the effectiveness of the corrosion-inhibiting systems from the control concrete.
Chloride Content in Concrete Bridge Barrier Walls
The chloride limits for new construction range from 0.1% to 0.20% by mass of cement
depending on the moisture condition of concrete (ACI 222R15). CEB16 recommends a
chloride threshold of 0.4% by mass de cement. Considering a cement content of 450
kg/m3 and a concrete density of 2400 kg/m3 for the concrete used in the barrier wall, the
ACI limits translate into 0.02% to 0.04% by weight of concrete, and the CEB limit becomes 0.08% by weight of concrete. The acid soluble chloride content by weight of concrete was determined on cores taken from the bridge barrier walls from 1997 to 2006, as presented in Figure 7. In general, the chloride content increased over time and
decreased with concrete depth, as expected. In 2006, at depths of 50-75 mm, the
concretes in all test spans had chloride contents below the CEB limit, however, they were slightly above the lower ACI limit (except System B), suggesting that the risk of
corrosion of the 75-mm deep main reinforcement in those systems was low to moderate at uncracked locations. However, localized pitting corrosion was likely to have developed at cracked locations due to the high chloride contents readily available at the rebar level from early age. The concrete in most spans showed quite similar chloride profiles over time. The system in Span G, however, performed very well in 1997, at which time no chlorides penetrated in concrete, due to the added sealer at the concrete surface. It should be mentioned that the chloride content could be used to evaluate the risk of reinforcement corrosion in concretes only if they have similar compositions. For concretes having different corrosion inhibiting systems, however, an evaluation based on the chloride content alone may not necessary reflect the same risk of reinforcement corrosion, since steel reinforcement protected by certain corrosion inhibiting materials may tolerate higher concentrations of chlorides.
Corrosion of Main Reinforcement in Bridge Barrier Walls
In this non-accelerated field test, the cumulative distributions of half-cell potential obtained in 1997 and 2006 showed a moderate increase in the risk of corrosion of the
75-mm deep main reinforcement. In general, all cumulative distributions of potential measured in 2006 were within a narrow range of ±30 mV from the control span, suggesting that corrosion was not advanced enough to differentiate one system from another after only ten years in the field. This is in agreement with the low chloride contents measured at a depth of 75 mm and the lack of active corrosion on the reinforcement (from observations of cored rebar samples).
Corrosion of Rebar Ladders in Bridge Barrier Walls
Figure 8 shows the half-cell potentials measured on the special rebar ladders for each span. It can be seen that the half-cell potentials became more negative with time in all spans, suggesting increased risks of corrosion. In general, half-cell potentials had the most significant change on the top bar (Bar #1 with 13 mm cover, highest chloride content), as expected. Of the ten test spans, System G (for Bar #1) and Systems H and F (for Bar #2, 25 mm cover) had the least negative shifts in half-cell potential, especially on the top bars.
Figure 9 presents the corrosion rates measured on the rebar ladders for each span. In general, the corrosion rates were much higher on Bar #1 than on Bar #2, as expected. In
2006, all corrosion rates on Bar #1 exceeded the threshold value of 0.5 μA/cm2
,
indicating high risk of corrosion17. This is in agreement with the damage observed in
2006 over Bar #1 of the rebar ladders. The best-fit curves of the corrosion rate at Bar #2
show that Systems H and B had the lowest rates in 2006 (≤ 0.2 μA/cm2) for a depth of 25
mm, compared to the ladders of the control system and other systems. Ladders in Spans E
and F also had low corrosion rates (≤ 0.25 μA/cm2) at Bar #2.
The visual assessment of the corrosion-induced damage of the concrete surface of the barrier wall over Bar #1 of the special ladders showed that corrosion was active in all test sections, with a lower degree of damage in Span H and D (few minor horizontal cracks only). Inspection of the concrete cores taken over Bar #2 of the ladders and over the 75-mm deep main reinforcement showed no significant evidence of active corrosion so far.
SUMMARY AND CONCLUSIONS
The long period of time, normally required for evaluating the effectiveness of corrosion-inhibiting systems in concrete structures, has led to the development of accelerated laboratory corrosion testing in electrochemical cells using simulated concrete pore solutions and on actual concrete structures using special embedded rebar ladders with reduced concrete cover. Compared to the non-accelerated corrosion evaluation of the 75-mm deep main reinforcement of the bridge barrier wall, these accelerated lab and field corrosion tests provided very useful information about the effectiveness of the tested corrosion-inhibiting systems:
• Simulated concrete pore solutions were very useful to rapidly identify the individual effects of the inhibitors on the corrosion of reinforcing steel bars. In the saturated
Ca(OH)2 solution (pH of 12.6), Admixtures E, H and C yield chloride thresholds higher than the control. Admixture F enabled to keep the corrosion rate low with further increases in the chloride concentration.
• In the higher pH solution (pH=13.5), the corrosion-inhibiting admixtures did not increase the chloride threshold, as expected. Admixture E obtained a lower chloride threshold than the control, indicating that the performance of corrosion-inhibiting admixtures depends on the solution pH surrounding the steel/concrete interface.
• Concrete coatings A, D, and G provided good performance in the saturated Ca(OH)2
solution by increasing the chloride threshold and reducing the corrosion rate of the reinforcing bars when compared to the control coating made of plain cementitious paste.
• Corrosion measurements on the reinforced concrete prisms showed that the rebars in most prisms were in a passive condition, probably due to the absence of cracks, low concrete permeability and the presence of a corrosion-inhibiting system. Only the control prisms showed some signs of corrosion initiation of the reinforcing bars. It will take more time to compare the performance of the different corrosion-inhibiting systems tested on these concrete prisms.
• Acid soluble chloride contents measured after 10 years at a depth of 50-75 mm in the bridge barrier wall were close to the chloride threshold in all test spans. This suggests that the risk of corrosion of the 75-mm deep reinforcement in the wall was low to moderate at uncracked locations.
• Non-accelerated evaluation of the corrosion over the 75-mm deep main reinforcement could not clearly differentiate one system from another. Inspection of concrete cores taken over 75-mm deep main reinforcing bars indicated no clear evidence of active corrosion in the test spans. This is due to the inherently good quality of the concrete used in the construction of the barrier wall (i.e. very low water permeability). • The visual assessment of the wall surface over the special embedded rebar ladders
identified severe damage of the concrete barrier wall surface over the 13-mm deep bars in all spans, with the lowest level of damage on Spans H and D (only minor cracks). The non-destructive tests showed that on the Bar #2 (25-mm concrete cover), System H (inorganic concrete admixture) provided consistently good performance, with a reduced risk of corrosion, followed by Systems B, E and F (organic concrete admixtures) in comparison to the control system.
ACKNOWLEDGEMENTS
The technical and financial contributions of the following project partners are gratefully acknowledged, namely: Ministère des transports du Québec, the Regional Municipality of Peel, IRAP, and all the product manufacturers who participated in this study. The authors would also like to thank Bruce Baldock, Nathalie Chagnon, Mark Arnott, Rock Glazer, Ted Hoogeveen and Gordon Chan for their valuable technical assistance.
REFERENCES
1 Lewis, J.M., Mason, C.E. and Brereton, D. "Sodium benzoate in concrete," Civil Engineering and Public Works Review, Vol. 51, No. 602, 1956, pp. 881-882.
2 Treadaway, K.W. and Russel, A.D. "Inhibition of the corrosion of steel in concrete - Part 2," Highways and Public Works, Vol. 36, 1968, pp. 40-41.
3 Griffin, D.F. "Corrosion inhibitors for reinforced concrete," Corrosion of Metals in Concrete, ACI SP-49, American Concrete Institute, 1975, pp. 95-102.
4 Berke, N.S. "Corrosion inhibitors in concrete," Corrosion 89, Paper 445, NACE, Houston, Texas, 1989, pp. 1-10.
5 Rosenberg, A.M., Gaidis, J.M., Kossivas, T.G. and Previte, R.W. "A corrosion inhibitor formulated with calcium nitrite for use in reinforced concrete," Chloride Corrosion of Steel in Concrete, American Society for Testing and Materials, Philadelphia, STP 629, 1976, pp. 89-99.
6 Berke, N.S. and Weil, T.G. "World-wide review of corrosion inhibitors in concrete," Advances in Concrete Technology, CANMET, 1992, pp. 899-924.
7 Hope, B.B. and Ip, A. K. C. "Corrosion inhibitors for use in concrete," ACI Materials Journal, Vol. 86, No. 6, 1989, pp. 602-608.
8 Maeder, U. "A new class of corrosion inhibitors," Proceedings of Corrosion and Corrosion Protection of Steel in Concrete, Ed. R. N. Swamy, Sheffield Academic Press, 1994, pp. 851-864.
9 Nmai, C.K., Farrington, S.A. and Bobrowski, G.S. "Organic-based
corrosion-inhibiting admixture for reinforced concrete," Concrete International, Vol. 14, No. 4, 1992, pp. 45-51.
10 Ramirez, C.W., Borgard, B., Jones, D. and Heidersbach, R. "Laboratory simulation of corrosion in reinforced concrete," Materials Performance, Vol. 29, December, 1990, pp. 33-39.
11 Cusson, D.; Qian, S.Y.; Chagnon, N. "Corrosion inhibiting systems for durable concrete bridges - Part 1: Field performance evaluation," Journal of Materials, in press, 1-34,
12 Qian, S.Y.; Cusson, D.; Chagnon, N. "Corrosion inhibiting systems for durable concrete bridges - Part 2: Accelerated laboratory investigation," Journal of Materials, in press, 1-30,
13 Hussain, S. Al-Gahtani, A. and Rasheeduzzafar "Chloride threshold for corrosion of reinforcement in concrete," ACI Materials Journal, 1996, pp. 534-538.
14 Mammoliti, L., Hansson, C.M. and Hope, B.B. "Corrosion inhibitors in concrete, Part II: Effect on chloride threshold values for corrosion of steel in synthetic pore
solutions," Cement and Concrete Research, Vol. 29, 1999, pp. 1583-1589. 15 ACI Committee 222, “Protection of Materials in Concrete Against Corrosion”
American Concrete Institute, 2001, pp 222R-11.
16 CEB, Design Guide for Durable Concrete Structures, 2nd ed., Thomas Telford Publishers, 1992.
17 Rodriguez, J., Ortega, L.M., Garcia, A.M. “Assessment of structural elements with corroded reinforcement,” Proceedings of the International Conference on Corrosion, Sheffield, U.K., July, 1994. p. 16.
Table 1. Generic description of the investigated corrosion-inhibiting systems System
code name†
Generic description
Control - Carbon-steel reinforcement Epoxy - Epoxy-coated reinforcement
A - Rebar coating (water-based liquid blend, Portland cement and fine silica sand) - Concrete coating (polymer-based liquid blend, Portland cement and inert
aggregates)
B - Organic concrete admixture (alkanolamines)
- Rebar coating applied on short bars protruding from slab (water-based epoxy, Portland cement)
C - Organic/inorganic concrete admixture (amine derivatives, sodium nitrite) D - Rebar coating (water-based epoxy, cementitious components)
E - Organic concrete admixture (amines and esters)
F - Organic concrete admixture (alkanolamines and amines, and their salts with organic/inorganic acids)
G - Organic concrete admixture (alkanolamines, ethanolamine and phosphate) - Rebar coating applied on short bars protruding from slab (water-based epoxy,
Portland cement)
- Concrete sealer (water-repellent penetrating silane) H - Inorganic concrete admixture (calcium nitrite)
† The commercial names of these systems are not identified in this paper to maintain confidentiality as requested by the product suppliers.
0E+0 1E-4 2E-4 3E-4 4E-4 5E-4 6E-4 0 1 2 3 4 5 6 7 8 NaCl concentration (%) 1/ Rp (o h m c m 2 ) -1 B Control C E F H
Fig. 2. Values of 1/R measured on carbon steel in saturated Ca(OH)P 2 solution
with increased chloride content in the presence of corrosion inhibiting admixtures
-0.8 -0.6 -0.4 -0.2 0.0 0 1 2 3 4 5 6 7 8 NaCl concentration (%) Potential (V vs SCE) Control A D G
Fig. 3. Half-cell potentials taken on carbon steel with different coatings
0.0E+0 5.0E-7 1.0E-6 1.5E-6 2.0E-6 2.5E-6 0 1 2 3 4 5 6 7 8 NaCl concentration (%) 1/R P (Ohm cm 2 ) -1 0.5E-6 Control D G A
Fig. 4. Values of 1/RP taken on carbon steel with different coatings
in saturated Ca(OH) solution with increased chloride content 2
-0.25 -0.20 -0.15 -0.10 -0.05 0.00 CTL EPX A B C D E F G H P o te n tia l (V v s CS E ) Oct. 98 Apr. 00 Nov. 01 Apr. 05
Fig. 5. Half-cell potentials measured on reinforced concrete prisms pounded with 3.5% NaCl solution
0E+0 2E-7 4E-7 6E-7 8E-7 1E-6 1E-6 CTL EPX A B C D E F G H 1/R P ( ohm c m 2 ) -1 12E-7 Apr. 00 Apr. 05 10E-7
Fig. 6. Values of 1/RP measured on reinforced concrete prisms
pounded with 3.5% NaCl solution
0.0 0.2 0.4 0.6 0.8
Cl Content (% of concrete weight)
0-13 13-25 25-50 50-75 0.0 0.2 0.4 0.6 0.8 Control Epoxy A B C D E F G H 1997-2006 1997-2006 1997-2006 1997-2006 1997-2006 Fig. 7. Total chloride content obtained on concrete cores from bridge barrier wall
-200 -400 -600 -800 P o te nt ia l (m V vs. C S E)
Bar1 Bar2 Bar3 Bar4
-200 -400 -600 -800 Control-1 Control-2 A B C D E F G H 1996 2006 1996 2006 1996 2006 1996 2006 1996 2006
Fig. 8. Half-cell potential measured over embedded rebar ladders in bridge barrier wall
0.0 0.5 1.0 1.5 Current density ( μA/cm 2 ) Bar #1 Bar #2 0.0 0.5 1.0 1.5 Control-1 Control-2 A B C D E F G H 1997-2006 1997-2006 1997-2006 1997-2006 1997-2006 Best-fit